Abstract
Schisandra chinensis (Omija) is a well-known medicinal plant in East Asia. In this study, Omija oligosaccharide syrup was prepared from sucrose with Omija fruit extract using two glucansucrases of Leuconostoc mesenteroides B-512F/KM and L. mesenteroides B-1355CF10/KM. The degree of polymerization of Omija oligosaccharide syrup was ranged from 2 − 13 by MALDI-TOF–MS analysis. Compared to the Omija syrup, the Omija oligosaccharide syrup reduced 61% calories based on the enzymatic gravimetric method. It also reduced up to 96% insoluble glucan formation from sucrose by mutansucrase of Streptococcus mutans at 500 mg/mL. Additionally, it has 1.78-fold higher oxygen radical absorbance capacity value compared to Omija syrup. Using electronic tongue sensor system, Omija oligosaccharide syrup showed decreased sourness, astringency, and saltiness compared to Omija syrup. Thus, Omija oligosaccharides can be used as functional sweetener in nutraceutical industries.
Supplementary Information
The online version contains supplementary material available at 10.1007/s10068-022-01061-8.
Keywords: Schisandra chinensis, Oligosaccharides, Leuconostoc mesenteroides, Glucansucrase, Omija
Introduction
Schisandra chinensis is red-colored berries found in China, Republic of Korea, Russia, and Japan to treat spontaneous sweating, chronic coughs, spermatorrhea, and palpitation (Bensky et al., 1993). In Republic of Korea, it is recognized as Omija berry which means berry with five flavors (bitter, spicy, sweet, sour, and salty). Omija has been used as a functional and nutritional ingredient in foods like yogurt, jam, bread, sponge-cake, cookie, beer, wine, soybean curd, and high sucrose concentration juice called cheong or syrup (Deng et al., 2020; Wu et al., 2011) because it has high lignan (schisandrin, gomisin A, and gomisin N), phenolic acids, vitamins, tannins, organic acids, phytosterols, and essential oils (Mocan et al., 2014; Wu et al., 2011) that contribute to its biological activities, including anti-obesity, anti-inflammation, liver protection, anti-microbial activity, immune response, and anti-cancer (Szopa et al., 2017). Omija syrup is prepared by mixing equal quantities of fresh Omija berries and sucrose for one to six months in a natural extract manner that is popular in Republic of Korea (Bae and Yoo, 2019). Then, it is used to make tea by diluting with water. However, high consumption of sugar-sweetened beverages has caused long-term obesity, type 2 diabetes, heart disease, and dental plaque and caries formation (Cury et al., 2000; Rippe and Angelopoulos, 2016). Therefore, consumers are finding alternative products with good taste and health benefits.
Glucooligosaccharides composed of 2−12 glucose residues have high prebiotic activities via in vitro and in vivo (Hu et al., 2020a, 2020b). They are widely used in foods, such as candies, cereals, drinks, cookies, soft drinks, and anticariogenic agents (Chung and Day, 2002). In our previous study, mandarin oligosaccharide juice was produced by enzymatic synthesis from sucrose in mandarin juice using single glucansucrase from Leuconostoc mesenteroides NRRL B-512FMCM (Nguyen et al., (2015). Although mandarin glucooligosaccharide juice showed reduction in calories and insoluble glucan formation by mutansucrase from Streptococcus mutans (Nguyen et al., 2015), the digestion of glucooligosaccharides depends on the type of linkages following α-1,4 > α-1,6 > α-1,3 (Hu et al., 2020b). Alternansucrase from L. mesenteroides NRRL B-1355C synthesizes alternan with alternating α-1,6 and α-1,3 linkages (Côté and Fobyt, 1982; Hu et al., 2020a). Therefore, the main objective of this study was to synthesize Omija oligosaccharide syrup by combining glucansucrase from L. mesenteroides NRRL B-512F/KM and alternansucrase from L. mesenteroides NRRL B-1355CF10/KM to reduce its sugars, calories, and enhance its anticariogenic and antioxidant activities. The prevention of insoluble glucan formation and oxygen radical absorbance capacity (ORAC) of the synthesized Omija oligosaccharide syrup were studied. The degree of polymerization (DP), lignans, and total phenolic content of the synthesized Omija oligosaccharide syrup were investigated by MALDI-TOF–MS analysis, ultraperformance liquid chromatography-tandem mass spectrometry (UPLC-MS), and Folin-Ciocalteu method, respectively.
Materials and methods
Enzyme preparation
Glucansucrases were prepared from both constitutive enzyme hyperproducing strains, L. mesenteroides NRRL B-512F/KM and B-1355CF10/KM, in a medium with 0.5% (w/v) yeast extract, 0.5% (w/v) peptone, 2% (w/v) K2HPO4, 2% (w/v) glucose, 0.02% (w/v) MgSO4·7H2O, 0.001% (w/v) NaCl, 0.001% (w/v) FeSO4·7H2O, 0.0013% (w/v) MnSO4·H2O, and 0.0013% (w/v) CaCl2·H2O, and consequently purified using a previously described method (Seo et al., 2007). One unit of glucansucrase activity is equal to the amount of enzyme required to generate fructose (1 μmol/min) at 28 °C. The amount of fructose released from the reaction was calculated using the AlphaEaseFC 4.0 software (Alpha Inotech, San Leandro, CA, USA) with fructose as the standard.
Synthesis of Omija oligosaccharide syrup
Forty °Brix Omija extract was purchased from Jin Seong FM Inc. (Hwaseong-si, Republic of Korea). The Omija oligosaccharide syrup was synthesized in a reaction mixture composed of 50% (w/v) sucrose in 25% (v/v) Omija extract, 10 U/mL of glucansucrase from L. mesenteroides 512F/KM, and 1 U/mL of alternansucrase from L. mesenteroides B-1355CF10/KM at 28 °C. The pH of the reaction mixture was controlled at 4.0 using 10% (w/v) Ca(OH)2 in 25% (w/v) sucrose before adding the enzyme to start the reaction. After 6 h, 1 μL of the reaction mixture was spotted on a silica gel 60F254 thin-layer chromatography (TLC) plate (Merck Co, Darmstadt, Germany) and developed with two ascents of nitromethane/n-propyl alcohol/water (2:5:1.5, v/v/v). The carbohydrates were visualized by dipping the plate in a solution composed of 0.5% (w/v) N-(1-naphthyl)-ethylenediamine dihydrochloride and 5% (w/v) sulfuric acid in methanol, followed by heating at 125 °C for 5 min (Seo et al., 2007). The Omija syrup was prepared as described above without adding enzymes. Each sample was freeze-dried only for analyzing lignan content, total phenolic content, and preventive effect of insoluble glucan synthesis.
MALDI-TOF–MS analysis
The monosaccharides in Omija oligosaccharide syrup were removed using yeast beads at 37 °C for 12 h (Yoon et al., 2003). Then, the polymers were eliminated through precipitation using 50% (v/v) ethanol. MALDI-TOF–MS analysis was conducted at the National Center for Inter-University Research Faculties, Seoul National University, Republic of Korea. The mass spectrum was obtained using the MALDI-TOF 5800 System (AB SCIEX, Framingham, MA, USA) in a positive reflector mode with delayed extraction method (average of 500 laser shots) at 20 kV acceleration voltage using the DHB matrix.
Analysis of lignans in Omija oligosaccharide syrup
Schisandrin, gomisin A, and gomisin N were purchased from TCI chemical (Tokyo, Japan). Schisandrin, gomisin A, and gomisin N in Omija oligosaccharide syrup were analyzed using the UPLC-MS system (Waters Corp. H-Class system equipped with QDa detector, Milford, MA, USA) (Nguyen et al., 2021). The samples were diluted with methanol and filtered using a 0.2-μm membrane syringe filter (Sartorius Stedim, Aubagne, France). Then, 1 μL of the sample was injected into the UPLC-MS Waters BEH C18 column (1.7 μm, 2.1 mm × 150 mm). The mobile phases were 0.1% (v/v) formic acid in acetonitrile (solvent A) and 0.1% (v/v) formic acid in water (solvent B). The elution gradient was as follows: 5% (v/v) solvent A initially, increased to 10% (v/v) solvent A at 20 s; 55% (v/v) solvent A at 30 s, 65% (v/v) solvent A at 3 min; 75% (v/v) solvent A at 4.5 min; 80% (v/v) solvent A at 6 min; 100% (v/v) solvent A at 7 min; and reverted to the initial gradient at 7.6 min and maintained until 15 min to equilibrate at a flow rate of 0.3 mL/min. Electrospray ionization was positive with single-ion recording (schisandrin, 432.50 m/z; gomisin A, 416.46 m/z; and gomisin N, 400.46 m/z). The linear correlation between the standard concentrations (schisandrin, gomisin A, and gomisin N) and area was evaluated (r2 > 0.99; Table S1). The total lignan content in Omija and Omija oligosaccharide syrup was calculated as follow:
Total phenolic content
The total phenolic contents in Omija syrup and Omija oligosaccharide syrup were determined using the Folin-Ciocalteu method, with gallic acid (Sigma-Aldrich, St. Louis, MO, USA) as the standard (Singh et al., 2021). Twenty microliters of 0.2 mg/mL of Omija oligosaccharides, Omija sucrose, or gallic acid (0 − 110 μg/mL) were mixed with 100 μL of Folin-Ciocalteu reagent into a 96-well plate (SPL, Republic of Korea). Then, 80 μL of Na2CO3 (7.5%, w/v) was added, and the mixture was incubated at room temperature for 30 min in the dark. The plate was read at 765 nm using the SpectraMax M3 Microplate Reader (Molecular Devices LLC, San Jose, CA, USA). The results were expressed as μg gallic acid equivalent (GAE)/g dry weight (g dw).
Calorie analysis
The calories of Omija syrup and Omija oligosaccharide syrup were determined using the enzymatic gravimetric method (AOAC, method 2009.01). The enzymatic hydrolysis of Omija syrup and Omija oligosaccharide syrup used three enzymes: α-amylase (Megazyme, Sydney, Australia) to hydrolyze digestible oligosaccharides (7.5 U/mL at 37 °C for 16 h (pH 6.9)), amyloglucosidase to cleave terminal glucoses of α-1,4- or α-1,6-linked polysaccharides (Megazyme) (33 U/mL at 60 °C for 45 min (pH 4.8)), and 500 U/mL of invertase (Sigma-Aldrich) to hydrolyze sucrose at 50 °C for 3 h. The concentration of glucose and fructose in the reaction mixtures was determined by using K-FRUGL kit (Megazyme, Wicklow, Ireland).
Prevention of insoluble glucan synthesis using mutansucrase from Streptococcus mutans
Mutansucrase was prepared by culturing S. mutans in a brain–heart infusion (BHI) medium containing 0.5% (w/v) glucose at 37 °C with shaking at 150 rpm for 8 h (Ryu et al., 2000). After fermentation, the culture supernatant was obtained by centrifugation at 6,000 × g for 20 min. Then, it was concentrated using a 30 K cut-off hollow fiber membrane (Millipore, Burlington, MA, USA) and the concentrated enzyme was stored at − 20 °C for further use. One unit of mutansucrase activity was defined as the amount of enzyme that liberates 1 µmol of fructose per min at 37 °C with pH 6.8. Different concentrations of Omija syrup and Omija oligosaccharide syrup (6.25 − 50%; w/v) were added to the reaction mixture composed of 100 mM sucrose and 0.1 U/mL of mutansucrase in 20 mM sodium phosphate buffer (pH 7.0) at 37 °C. The reaction without adding any test sample was used as the control. After 12 h, the water-insoluble glucan was obtained by centrifugation at 12,000 × g for 10 min. The water insoluble pellets were washed several times using water, and dissolved with 1 M NaOH. One microliter of the sample was spotted on a silica gel 60F254 TLC plate and developed with two ascents of acetonitrile/water (85:15, v/v). The TLC plate was stained as described above. The amount of insoluble glucan was analyzed using IDV by employing AlphaEaseFC 4.0 Image Program.
ORAC assay
ORAC was conducted according to Huang et al (2002) with slight modifications. Ten microliters of diluted sample or Trolox (0 − 100 μM) (Sigma-Aldrich) was mixed with 90 μL of 25 nM fluorescein, then 100 μL of 25 mM 2,2′-azobis(2-amidinopropane) dihydrochloride (AAPH) was added. Relative fluorescent units (RFUs) were measured with SpectraMax M3 at excitation and fluorescence emission wavelengths of 485 and 538 nm, respectively, for 2 h at 37 °C. The net area under the curve (net AUC) was calculated by subtracting the AUC of the blank from the AUC of each tested sample. The antioxidant capacity was expressed as μmol of Trolox equivalent (TE)/100 g of the sample.
Taste sensory evaluation by electronic tongue sensor system
The taste sensory of Omija syrup and Omija oligosaccharide syrup was evaluated using the electronic tongue sensor system TS-5000Z (Insent Inc., Atsugi-Shi, Japan). The umami (AAE), saltiness (CT0), sourness (CA0), astringency (AE1), and bitterness (C00) sensors were used to analyze the taste difference between Omija syrup and Omija oligosaccharide syrup. Eighty milliliters of each sample were prepared by fivefold dilution with water. The taste information was obtained by converting sensor output through taste analysis application SA402B (Insent Inc.) (Kobayashi et al., 2010).
Statistical analysis
All data are shown as mean ± standard error of the mean (SEM) from three independent experiments. In the experiment of relative insoluble glucan reduction, differences between groups were obtained using one-way analysis of variance followed Tukey’s honest significant difference (HSD) method (p < 0.05). In the calorie measurement and ORAC assay, the differences between two groups were evaluated using the independent samples t-test (p < 0.0001 and p < 0.01, respectively). Statistical analysis was performed using SPSS version 23.0 for Windows (SPSS Inc., Chicago, IL, USA).
Results and discussion
Synthesis of Omija oligosaccharide syrup
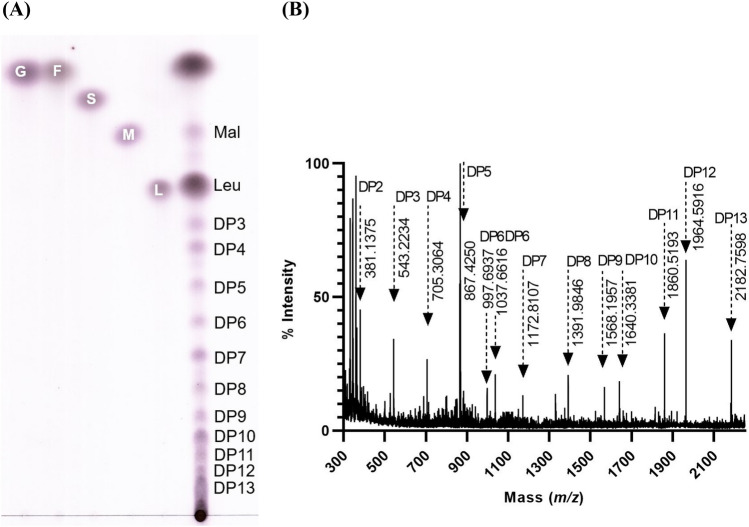
The TLC and MALDI-TOF–MS analyses of the synthesized Omija oligosaccharide syrup are shown in Fig. 1. Based on the MALDI-TOF–MS result, the Omija oligosaccharide syrup contained 2 − 13 glucose residues with their molecular weights of 381.1 (DP2), 543.2 (DP3), 705.3 (DP4), 867.4 (DP5), 997.7 (DP6), 1172.8 (DP7), 1392.0 (DP8), 1568.2 (DP9), 1640.3 (DP10), 1860.5 (DP11), 1964.6 (DP12), and 2182.8 (DP13) (Fig. 1B). Anthocyanins as a group of flavonoid existing in Omija present a spectrum from orange to blue in color (Ma et al., 2012). They contribute as a source of natural colorants for foods, beverages, and pharmaceuticals with numerous health benefits such as anti-inflammatory, antioxidant activities, and inhibition of carcinogenesis and heart disease (Ma et al., 2012). The maximum anthocyanins in the Omija extract was obtained at pH 1 − 4 with bright-red color (Ma et al., 2012). The colors of Omija extract changed from pink to navy blue at pH 7 − 12 (Ma et al., 2012). Glucansucrase and alternansucrase showed over 60% activity at pH 4.0 and higher (Kim and Robyt, 1994; Wangpaiboon et al., 2018). Therefore, pH 4.0 was selected for oligosaccharide synthesis in this study.
Fig. 1.
Thin-layer chromatogram A and MALDI–TOF–MS B of Omija oligosaccharide syrup synthesized by using glucansucrase from L. mesenteroides NRRL B-512F/KM and alternansucrase from L. mesenteroides B-1355CF10/KM. Lane 1, glucose; lane 2, fructose; lane 3, sucrose; lane 4, maltose, lane 5, leucrose, and lane 6, Omija oligosaccharide syrup; DP: degree of polymerization. The Omija oligosaccharide syrup was synthesized in the reaction mixture composed of 50% (w/v) sucrose in 25% (v/v) Omija extract, 10 U/mL of glucansucrase from L. mesenteroides 512F/KM, and 1 U/mL of alternansucrase from L. mesenteroides B-1355CF10/KM at 28 °C for 6 h. The pH of reaction mixture was controlled at 4.0 using 10% (w/v) Ca(OH)2 in 25% (w/v) sucrose before adding enzyme to start reaction
Total phenolic and lignan contents
The total phenolic contents of Omija syrup and Omija oligosaccharide syrup were 700 ± 30 and 780 ± 10 μg GAE/g dw, respectively (Table 1). The lignan contents of Omija syrup and Omija oligosaccharide syrup were 150.6 ± 2.5 μg/g dw and 156.4 ± 0.3 μg/g dw, respectively. The concentration of schisandrin and gomisin A were 140.3 and 10.3 μg/g dw for Omija syrup, and 128.7 and 13.3 μg/g dw for Omija oligosaccharide syrup, respectively (Table 1). The concentration of gomisin N in Omija syrup was not detected, while it was 14.4 μg/g dw in Omija oligosaccharide syrup (Table 1). Although the total phenolic contents in Omija oligosaccharide syrup and Omija syrup were not significantly different from each other, gomisin N was detected at 14.4 μg/g dw in Omija oligosaccharide syrup, while it was not detected in Omija sucrose since the value was under the limits of detection.
Table 1.
Schisandrin, gomisin A, gomisin N, total lignan content, and total phenolic content of Omija syrup and Omija oligosaccharide syrup
| Sample | Schisandrin | Gomisin A | Gomisin N | Total lignan content | Total phenolic content |
|---|---|---|---|---|---|
| µg/g dw | µg GAE/g dw | ||||
| Omija syrup | 140.3 ± 2.2 | 10.3 ± 0.3 | N/D | 150.6 ± 2.5 | 700 ± 30 |
| Omija oligosaccharides | 128.7 ± 0.6 | 13.3 ± 0.3 | 14.4 ± 0 | 156.4 ± 0.3 | 780 ± 10 |
Total phenolic content is expressed as µg gallic acid equivalent (GAE)/g dry weight (dw)
The value indicates mean ± standard error of the mean (SEM)
N/D means not detected
Caloric analysis
The calories of Omija syrup and Omija oligosaccharide syrup were 50.5 ± 0.7 kcal/200 mL and 19.6 ± 0.1 kcal/200 mL, respectively (Fig. 2). The calorie of the Omija oligosaccharide syrup was decreased by 61.1% compared to the Omija syrup, which was due to the conversion of sucrose, fructose, and/or glucose to oligosaccharides by glucansucrases as described by Nguyen et al. (Nguyen et al., 2015). Omija oligosaccharide syrup showed significant reduction in calorie comparing to Omija syrup (p < 0.0001) (Fig. 2).
Fig. 2.

Caloric analysis of Omija syrup and Omija oligosaccharide syrup. Data are standard error of the mean (SEM) of three independent experiments. Asterisk star (****) indicates significant difference at p < 0.0001
Prevention of insoluble glucan formation by Omija oligosaccharide syrup
The prevention of insoluble glucan formation by Omija syrup and Omija oligosaccharide syrup at different concentrations (62.5 − 500 mg dw/mL) were evaluated, as shown in Fig. 3. The relative insoluble glucan reduction compared to the control at 62.5, 125, 250, and 500 mg dw/mL was 46%, 45%, 78%, and 96% for Omija oligosaccharide syrup, and 36%, 35%, 63%, and 88% for Omija syrup, respectively. At 500 mg dw/mL of two kinds of syrup, they were not significantly (p > 0.05) different in reducing insoluble glucan. Omija oligosaccharide syrup at 250, 125, and 62.5 mg dw/mL showed significantly (p < 0.05) higher suppression in forming glucan comparing Omija syrup. In the prevention of insoluble glucan formation by mutansucrase from S. mutans, the relative insoluble glucan formation was decreased when the concentration of Omija syrup and Omija oligosaccharide syrup increased. Seo et al. (2007) and Nguyen et al (2015) reported that oligosaccharides synthesized from sucrose using the glucansucrase from L. mesenteroides inhibited the insoluble glucan formation by mutansucrase. In addition, Yanagida et al. (2000) reported that polyphenols from leaves and fruits have anti-cariogenic ability through the inhibition of enzyme activity for water-insoluble glucan. Thus, it is possible that the presence of polyphenols in Omija is also responsible for its inhibitory effects against water-insoluble glucan formation by mutansucrase. Thus, we could conclude that the higher inhibition of water insoluble glucan formation by Omija oligosaccharide syrup compared to the Omija syrup was due to the oligosaccharides and polyphenols from the Omija extract.
Fig. 3.
Prevention of insoluble glucan formation by mutansucrase from Streptococcus mutans using Omija oligosaccharide syrup and Omija syrup. Data are standard error of the mean (SEM) of three independent experiments. a, b, c, d, e, and f Different superscripts in lower-case letter after values mean significant differences at p < 0.05
ORAC
For the determination of radical scavenging ability and antioxidant activity of compounds, a wide variety of in vitro methods have been established. Among them, we selected ORAC, free radicals and hydrogen atom transfer, that based on the ability of antioxidant to remove the free radicals by donating a hydrogen atom (Munteanu and Apetrei, 2021). The antioxidant capacities of Omija syrup and Omija oligosaccharide syrup are shown in Fig. 4. The ORAC values were 1,247 ± 32 μmol TE/100 g for Omija syrup, and 2,218 ± 136 μmol TE/100 g for Omija oligosaccharide syrup (Fig. 4). Omija oligosaccharide syrup showed significant improvement in antioxidant capacity (p < 0.01) (Fig. 4). Considering the serving size of each syrup (20 g syrup/serving), Omija oligosaccharide syrup and Omija syrup have antioxidant activity values of 444 ± 27 μmol TE/serving and 249 ± 6 μmol TE/serving, respectively. The Omija oligosaccharide syrup had 1.78-fold higher antioxidant capacity than that of Omija syrup. The ORAC values of schisandrin, gomisin A, and gomisin N were 1,512 ± 649 μmol TE/100 g, 75,557 ± 7,083 μmol TE/100 g, and 797 ± 65 μmol TE/100 g, respectively (Fig. S1). Gomisin A has the highest antioxidant capacity, followed by schisandrin, and gomisin N. One proposed theory for the free-radical-scavenging capacity of carbohydrates is that they give anomeric hydrogen to free radicals, after which spirocyclization reaction occurs to extinguish the alkoxyl radical of oligosaccharides (Francisco et al., 2002a, 2002b; Martin et al., 1996). Omija contains lignans (schisandrin, gomisin A, and gomisin N) and polyphenols that can contribute to its antioxidant activity (Park and Lee, 2021; Szopa et al., 2017). However, as shown in Šmejkal et al. (2010), Schisandra lignans alone did not show high scavenging activity of free radicals, in using the DPPH•, ABTS•+, Fenton reaction, and tyrosine-nitration inhibition methods. Instead, the antioxidant activity of dibenzocyclooctadiene lignan is more related with the effect of antioxidative enzymatic system in liver such as glutathione content and glutathione peroxidase activity, resulting in the inhibition of cellular peroxides formation (Kim et al., 2004; Panossian and Wikman, 2008).
Fig. 4.

Antioxidant activity of Omija syrup and Omija oligosaccharide syrup. Data are standard error of the mean (SEM) of three independent experiments. Asterisk star (*) indicates significant difference at p < 0.01
Taste sensory evaluation
The taste information unit of Omija oligosaccharide syrup was 2.23 U for sourness, − 1.26 U for astringency, and − 1.67 U for saltines compared to Omija syrup as the control. The bitterness, aftertaste-B, aftertaste-A, umami, and richness of Omija oligosaccharide syrup were − 0.32, − 0.06, − 0.02, 0.46, and − 0.09 U, respectively, in comparison with Omija syrup as the control. By evaluating the taste sensory, using the electronic tongue sensor system and Omija syrup as the control, the taste information unit of astringency and saltiness of Omija oligosaccharide syrup decreased, and the sourness was increased by over 1 taste information unit. It is estimated that samples with difference of more than 1 taste information unit can be differentiated by any person. The significant difference in taste between Omija oligosaccharides and Omija syrup was shown in the aspects of sourness, astringency, and saltiness. Astringency in beverages is typically associated among phenolic compounds in beverages with salivary proteins to form aggregates and precipitates that lead to reduce of mouth lubrication and cause a sensation of dry and constriction (de Freitas et al., 2003; Ployon et al., 2018). In vitro studies have reported that the reduce of astringency is caused by polysaccharides disrupting the tannin-protein interaction via inhibition of the tannin-protein interaction or of the precipitation and aggregation of tannin-protein complexes (Carvalho et al., 2006; de Freitas et al., 2003; Mateus et al., 2004). But until now, there are only few studies reported the relationship between oligosaccharides and astringency. Quijada-Morín et al. (2014) reported that the structure and size of compounds including oligosaccharides had important effect on astringency perception. However, the relationship of oligosaccharides with perceived astringency is not clear. In another study, Boulet et al. (2016) showed that oligosaccharides had a directed effect on astringency in all their selected models. Based on these studies, the decrease of astringency in Omija oligosaccharide syrup compared to Omjija syrup is due to the formation of oligosaccharides and polysaccharides in Omija oligosaccharide syrup. Melis and Barbarossa (2017) studied the effects of L-arginine supplementation to taste 6-n-propylthiouracil on taste perception of sweet, sour, salty, bitter, and umami, and they found that L-arginine enhanced the 6-n-propylthiouracil taste. Specifically, although L-arginine is weakly sweet, weakly bitter, and pH 9.0 − 9.88 depending on concentration, the supplementation of L-arginine enhanced the saltiness of NaCl and decreased the sourness of citric acid (Melis and Barbarossa, 2017). From these results, they concluded that supplementation with this polar amino acid could modify the taste responses (Melis and Barbarossa, 2017). Therefore, the difference in taste between Omija oligosaccharides and Omija syrup in sourness and saltiness is relative to the difference of the amino acid content during producing of oligosaccharide from sucrose using glucansucrase and alternansucrase. In contrast, the bitterness, aftertaste-B, aftertaste-A, and richness decreased lower than 1 taste information unit means that the taste of the two samples were hard to distinguish by people.
In conclusion, the synthesized Omija oligosaccharide syrup with DP 2 − 13 using two glucansucrase from L. mesenteroides B-512F/KM and alternansucrase from L. mesenteroides B-1355CF10/KM with Omija extract and sucrose showed 61% decrease in calories. The total phenolic and lignan contents of Omija oligosaccharide syrup were 780 μg GAE/g dw and 156.4 μg/g dw, respectively. The Omija oligosaccharide syrup showed enhanced inhibitory effect of water-insoluble glucan formation by mutansucrase, antioxidant capacity through ORAC assay, and mild taste sensory. Therefore, Omija oligosaccharide syrup has potential applications in nutraceutical industry.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
This work was financially supported by a grant from National Research Foundation of Republic of Korea (2018R1D1A1A09083366), by the Nuclear R&D program of Ministry of Science and ICT (MSIT), Republic of Korea, and by the Korean Institute of Planning and Evaluation for Technology in Food, Agriculture, Forestry, and Fisheries (IPET) through the Agriculture, Food and Rural Affairs Convergence Technologies Program for Educating Creative Global Leader, funded by the Ministry of Agriculture, Food, and Rural Affairs (MAFRA) (710012-03-1-HD220), and by the OTTOGI Corporation through the Research and Publication Project.
Declarations
Conflict of interest
The authors declare that there is no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
So-Hyung Kwak, Email: shkwak16@snu.ac.kr.
Hayeong Kim, Email: hara2910@snu.ac.kr.
Seonmin Lee, Email: luck1035@snu.ac.kr.
Juho Lim, Email: juholim@snu.ac.kr.
Kunal Pal, Email: kpal.nitrkl@gmail.com.
Byoungsang Chung, Email: bschung@ottogism.co.kr.
Dong-Hyun Kang, Email: kang7820@snu.ac.kr.
Doman Kim, Email: kimdm@snu.ac.kr.
References
- Bae M-J, Yoo S-H. Changes in oligosaccharide content during the storage period of maesil cheong formulated with functional oligosaccharides. Korean Journal of Food Science and Technology. 2019;51:169–175. [Google Scholar]
- Bensky D, Gamble A, Kaptchuk TJ. Chinese herbal medicine: Materia medica. Seattle, WA, USA: Eastland Press; 1993. [Google Scholar]
- Boulet J-C, Trarieux C, Souquet J-M, Ducasse M-A, Caillé S, Samson A, Williams P, Doco T, Cheynier V. Models based on ultraviolet spectroscopy, polyphenols, oligosaccharides and polysaccharides for prediction of wine astringency. Food Chemistry. 2016;190:357–363. doi: 10.1016/j.foodchem.2015.05.062. [DOI] [PubMed] [Google Scholar]
- Carvalho E, Mateus N, Plet B, Pianet I, Dufourc E, De Freitas V. Influence of wine pectic polysaccharides on the interactions between condensed tannins and salivary proteins. Journal of Agricultural and Food Chemistry. 2006;54:8936–8944. doi: 10.1021/jf061835h. [DOI] [PubMed] [Google Scholar]
- Chung CH, Day DF. Glucooligosaccharides from Leuconostoc mesenteroides B-742 (ATCC 13146): A potential prebiotic. Journal of Industrial Microbiology & Biotechnology. 2002;29:196–199. doi: 10.1038/sj.jim.7000269. [DOI] [PubMed] [Google Scholar]
- Côté GL, Fobyt JF. Acceptor reactions of alternansucrase from Leuconostoc mesenteroides NRRL B-1355. Carbohydrate Research. 1982;111:127–142. doi: 10.1016/0008-6215(82)85013-1. [DOI] [Google Scholar]
- Cury JA, Rebelo MA, Del Bel Cury AA, Derbyshire MT, Tabchoury CP. Biochemical composition and cariogenicity of dental plaque formed in the presence of sucrose or glucose and fructose. Caries Research. 2000;34:491–497. doi: 10.1159/000016629. [DOI] [PubMed] [Google Scholar]
- de Freitas V, Carvalho E, Mateus N. Study of carbohydrate influence on protein–tannin aggregation by nephelometry. Food Chemistry. 2003;81:503–509. doi: 10.1016/S0308-8146(02)00479-X. [DOI] [Google Scholar]
- Deng Y, Lim J, Nguyen TTH, Mok IK, Piao MZ, Kim D. Composition and biochemical properties of ale beer enriched with lignans from Schisandra chinensis Baillon (omija) fruits. Food Science and Biotechnology. 2020;29:609–617. doi: 10.1007/s10068-019-00714-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francisco CG, Freire R, Herrera AJ, Peréz-Martín I, Suárez E. Intramolecular 1, 5-versus 1, 6-hydrogen abstraction reaction promoted by alkoxy radicals in carbohydrate models. Organic Letters. 2002;4:1959–1961. doi: 10.1021/ol025981u. [DOI] [PubMed] [Google Scholar]
- Francisco CG, Herrera AJ, Suárez E. Intramolecular hydrogen abstraction reaction promoted by alkoxy radicals in carbohydrates. Synthesis of chiral 2, 7-dioxabicyclo [2.2.1] heptane and 6, 8-dioxabicyclo [3.2.1] octane ring systems. The Journal of Organic Chemistry. 2002;67:7439–7445. doi: 10.1021/jo026004z. [DOI] [PubMed] [Google Scholar]
- Hu X, Song L, Yang Y, Jin Z, Miao M. Synthesis of potential prebiotic α-glucooligosaccharides using microbial glucansucrase and their in vitro fecal fermentation. Food & Function. 2020;11:1672–1683. doi: 10.1039/C9FO02054C. [DOI] [PubMed] [Google Scholar]
- Hu Y, Winter V, Gänzle M. In vitro digestibility of commercial and experimental isomalto-oligosaccharides. Food Research International. 2020;134:109250. doi: 10.1016/j.foodres.2020.109250. [DOI] [PubMed] [Google Scholar]
- Huang D, Ou B, Hampsch-Woodill M, Flanagan JA, Prior RL. High-throughput assay of oxygen radical absorbance capacity (ORAC) using a multichannel liquid handling system coupled with a microplate fluorescence reader in 96-well format. Journal of Agricultural and Food Chemistry. 2002;50:4437–4444. doi: 10.1021/jf0201529. [DOI] [PubMed] [Google Scholar]
- Kim D, Robyt JF. Production and selection of mutants of Leuconostoc mesenteroides constitutive for glucansucrases. Enzyme and Microbial Technology. 1994;16:659–664. doi: 10.1016/0141-0229(94)90086-8. [DOI] [PubMed] [Google Scholar]
- Kim SR, Lee MK, Koo KA, Kim SH, Sung SH, Lee NG, Markelonis GJ, Oh TH, Yang JH, Kim YC. Dibenzocyclooctadiene lignans from Schisandra chinensis protect primary cultures of rat cortical cells from glutamate-induced toxicity. Journal of Neuroscience Research. 2004;76:397–405. doi: 10.1002/jnr.20089. [DOI] [PubMed] [Google Scholar]
- Kobayashi Y, Habara M, Ikezazki H, Chen R, Naito Y, Toko K. Advanced taste sensors based on artificial lipids with global selectivity to basic taste qualities and high correlation to sensory scores. Sensors. 2010;10:3411–3443. doi: 10.3390/s100403411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma CH, Yang L, Yang FJ, Wang WJ, Zhao CJ, Zu YG. Content and color stability of anthocyanins isolated from Schisandra chinensis fruit. International Journal of Molecular Sciences. 2012;13:14294–14310. doi: 10.3390/ijms131114294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin A, Salazar JA, Suárez E. Synthesis of chiral spiroacetals from carbohydrates. The Journal of Organic Chemistry. 1996;61:3999–4006. doi: 10.1021/jo960060g. [DOI] [PubMed] [Google Scholar]
- Mateus N, Carvalho E, Luis C, de Freitas V. Influence of the tannin structure on the disruption effect of carbohydrates on protein–tannin aggregates. Analytica Chimica Acta. 513: 135–140 (2004)
- Melis M, Barbarossa IT. Taste perception of sweet, sour, salty, bitter, and umami and changes due to l-arginine supplementation, as a function of genetic ability to taste 6-n-propylthiouracil. Nutrients. 2017;9:541. doi: 10.3390/nu9060541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mocan A, Crisan G, Vlase L, Crisan O, Vodnar DC, Raita O, Gheldiu AM, Toiu A, Oprean R, Tilea I. Comparative studies on polyphenolic composition, antioxidant and antimicrobial activities of Schisandra chinensis leaves and fruits. Molecules. 2014;19:15162–15179. doi: 10.3390/molecules190915162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munteanu IG, Apetrei C. Analytical methods used in determining antioxidant activity: A Review. International Journal of Molecular Sciences. 2021;22:3380. doi: 10.3390/ijms22073380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen KN, Kim Y, Maibunkaew S, Park J, Nguyen MT, Oh DB, Kwon OS. Enhanced production of 1-deoxynojirimcin in Bacillus subtilis subsp. inaquosorum by random mutagenesis and culture optimization. Biotechnology and Bioprocess Engineering. 2021;26:265–276. doi: 10.1007/s12257-020-0231-2. [DOI] [Google Scholar]
- Nguyen TTH, Cho J-Y, Seo Y-S, Woo H-J, Kim H-K, Kim GJ, Jhon D-Y, Kim D. Production of a low calorie mandarin juice by enzymatic conversion of constituent sugars to oligosaccharides and prevention of insoluble glucan formation. Biotechnology Letters. 2015;37:711–716. doi: 10.1007/s10529-014-1723-y. [DOI] [PubMed] [Google Scholar]
- Panossian A, Wikman G. Pharmacology of Schisandra chinensis Bail: an overview of Russian research and uses in medicine. Journal of Ethnopharmacology. 2008;118:183–212. doi: 10.1016/j.jep.2008.04.020. [DOI] [PubMed] [Google Scholar]
- Park M, Lee K-G. Effect of roasting temperature and time on volatile compounds, total polyphenols, total flavonoids, and lignan of omija (Schisandra chinensis Baillon) fruit extract. Food Chemistry. 2021;338:127836. doi: 10.1016/j.foodchem.2020.127836. [DOI] [PubMed] [Google Scholar]
- Ployon S, Morzel M, Belloir C, Bonnotte A, Bourillot E, Briand L, Lesniewska E, Lherminier J, Aybeke E, Canon F. Mechanisms of astringency: Structural alteration of the oral mucosal pellicle by dietary tannins and protective effect of bPRPs. Food Chemistry. 2018;253:79–87. doi: 10.1016/j.foodchem.2018.01.141. [DOI] [PubMed] [Google Scholar]
- Quijada-Morín N, Williams P, Rivas-Gonzalo JC, Doco T, Escribano-Bailón MT. Polyphenolic, polysaccharide and oligosaccharide composition of Tempranillo red wines and their relationship with the perceived astringency. Food Chemistry. 2014;154:44–51. doi: 10.1016/j.foodchem.2013.12.101. [DOI] [PubMed] [Google Scholar]
- Rippe JM, Angelopoulos TJ. Relationship between added sugars consumption and chronic disease risk factors: current understanding. Nutrients. 2016;8:697. doi: 10.3390/nu8110697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryu S-J, Kim D, Ryu H-J, Chiba S, Kimura A, Day DF. Purification and partial characterization of a novel glucanhydrolase from Lipomyces starkeyi KSM 22 and its use for inhibition of insoluble glucan formation. Bioscience, Biotechnology, and Biochemistry. 2000;64:223–228. doi: 10.1271/bbb.64.223. [DOI] [PubMed] [Google Scholar]
- Seo ES, Nam SH, Kang HK, Cho JY, Lee HS, Ryu HW, Kim D. Synthesis of thermo- and acid-stable novel oligosaccharides by using dextransucrase with high concentration of sucrose. Enzyme and Microbial Technology. 2007;40:1117–1123. doi: 10.1016/j.enzmictec.2006.08.017. [DOI] [Google Scholar]
- Singh M, Lee KE, Vinayagam R, Kang SG. Antioxidant and antibacterial profiling of pomegranate-pericarp extract functionalized-zinc oxide nanocomposite. Biotechnology and Bioprocess Engineering. 2021;26:728–737. doi: 10.1007/s12257-021-0211-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Šmejkal K, Šlapetová T, Krmenčík P, Kubínová R, Suchý P, Innocenti G, Vančo J, Kalvarová K, Dvorská M, Slanina J. Evaluation of the antiradical activity of Schisandra chinensis lignans using different experimental models. Molecules. 2010;15:1223–1231. doi: 10.3390/molecules15031223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szopa A, Ekiert R, Ekiert H. Current knowledge of Schisandra chinensis (Turcz.) Baill (Chinese magnolia vine) as a medicinal plant species: a review on the bioactive components, pharmacological properties, analytical and biotechnological studies. Phytochemistry Reviews. 2017;16:195–218. doi: 10.1007/s11101-016-9470-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wangpaiboon K, Padungros P, Nakapong S, Charoenwongpaiboon T, Rejzek M, Field RA, Pichyangkura R. An α-1, 6-and α-1, 3-linked glucan produced by Leuconostoc citreum ABK-1 alternansucrase with nanoparticle and film-forming properties. Scientific Reports. 2018;8:8340. doi: 10.1038/s41598-018-26721-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X, Yu X, Jing H. Optimization of phenolic antioxidant extraction from wuweizi (Schisandra chinensis) pulp using random-centroid optimazation methodology. International Journal of Molecular Sciences. 2011;12:6255–6266. doi: 10.3390/ijms12096255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanagida A, Kanda T, Tanabe M, Matsudaira F, Oliveira Cordeiro JG. Inhibitory effects of apple polyphenols and related compounds on cariogenic factors of mutans streptococci. Journal of Agricultural and Food Chemistry. 2000;48:5666–5671. doi: 10.1021/jf000363i. [DOI] [PubMed] [Google Scholar]
- Yoon S-H, Mukerjea R, Robyt JF. Specificity of yeast (Saccharomyces cerevisiae) in removing carbohydrates by fermentation. Carbohydrate Research. 2003;338:1127–1132. doi: 10.1016/S0008-6215(03)00097-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.