Abstract
Intensive cultivation increases the salinity and alkalinity of soil leading to its degradation. Such soil lead to abiotic stress conditions in plants causing ROS-mediated cellular damage. Microbes constitute an important group of bio-stimulants, which are promising alternatives to reduce ROS-mediated abiotic stresses and improve plant growth. In the present study synergistic activity of stress-tolerant Trichoderma koningiopsis NBRI-PR5 (MTCC 25372) and T. asperellum NBRI-K14 (MTCC 25373) (TrichoMix) was assessed in paddy crop under salt stress conditions. Improved soil microbial biomass carbon (MBC), total organic carbon (TOC), and available nutrients N/P/K by 2–3 folds was observed in the pot experiment using the TrichoMix. It restored the heterogeneous microbial population of the paddy rhizosphere during salt stress and modulated the soil enzyme activities. The anatomical distortions in rice roots due to salt stress were stabilized in presence of the TrichoMix. Different stress marker genes (OsMAPK5, OsAPX, OsGST, OsUSP, OsBADH, OsLYSO, OsNRAMP6, and OsBz8) were differentially modulated by the TrichoMix in presence of salt stress as compared to the control. The TrichoMix increased the yield by 10% in marginally stressed fields; however, it enhanced the yield by approximately 60% when used with the 50% recommended dose of NPK. In the integrated treatment, Fe and Zn were fortified by approximately 40% and 29% respectively in the grains. From the present study, it was concluded that the TrichoMix stimulated the rice plants to accumulate osmoprotectants, improved the anatomical features, modulated the plant defense system, and improved the grain yield and quality. Therefore, the NBRI-PR5 and NBRI-K14 mixture may be used as a bio-stimulant to increase productivity in the rapidly deteriorating soil and reduce the NPK inputs.
Supplementary Information
The online version contains supplementary material available at 10.1007/s12298-022-01192-6.
Keywords: Paddy, TrichoMix, Biostimulants, Synergistic, Salt tolerance, T. koningiopsis, T. asperellum
Introduction
The twenty-first century is threatened with global insecurity of water, food, pollution, and decreasing agricultural land. Salinity has stemmed from an agro-economic crisis that is continuously increasing due to human-induced interferences. It has been anticipated that 20% of the cultivated and 33% of the agricultural lands are afflicted worldwide by salinity (Jamil et al. 2011). Machado et al. (2017) have reported that approximately 10 million/year hectares of agricultural land in the world are destroyed due to salt accumulation, low precipitation, high evaporation, weathering of rocks, saline water irrigation, and poor agricultural practices. It is estimated that more than 50% of the agricultural land area is on the verge of salinification by 2050 (Jamil et al. 2011; Kumar et al. 2020). Salinity is introduced by natural processes and largely by the intensive cropping of agricultural lands (Yin et al. 2016). Salinity affects the soil's physicochemical properties, structural properties, microbial diversity, and ecology eventually leading to poor soil health and decreased productivity.
The upland rice or dry-land rice is cultivated in nearly 20-million-hectare area in the world with majority in the Asian countries (60%). Upland rice is grown in tropical areas and is affected by rainfall, temperature, salinity, and drought stress. Rice grown in any salt-affected environment develops osmotic stress, alteration in nutrient uptake, and generation of reactive oxygen species (ROS) (Liao et al. 2014). Therefore, for sustainable upland rice production in the tropics, measures need to be taken to ameliorate these stresses. Microbial application has been a potent candidate to enhance tolerance against environmental stresses. Trichoderma is one such widely used agricultural important microorganism.
Trichoderma is widely used in agriculture and applied as a bio-control and plant growth-promoting agent. It has been increasingly reported for its stress tolerance and abiotic stress amelioration ability in plants (Tandon et al. 2020). Although Trichoderma has versatile properties for many agricultural and industrial applications, these properties are scattered in different species. Therefore, combining them for the synergistic activity to improve salt tolerance in paddy was studied in the current work. Two Trichoderma species Trichoderma koningiopsis (NBRI-PR5) and T. asperellum (NBRI-K14) having different stress-tolerant properties were used in combination to ameliorate salinity stress in paddy under net house and field conditions.
Material and methods
Cultures and growth conditions
Two stress-tolerant strains of Trichoderma, NBRI-PR5 (PR5) and NBRI-K14 (K14) were used in the study (Tandon et al. 2022). T. koningiopsis strain NBRI-PR5 (PR5) was isolated from rice plants (Tandon et al. 2020) and T. asperellum strain NBRI-K14 (K14) was isolated from the Ganga water collected during Kumbh Mela in the year 2013 at Prayagraj, UP. The mixture of these two Trichoderma strains has been referred to as ‘TrichoMix’ throughout the manuscript. The Trichoderma strains were grown and maintained on potato dextrose agar (PDA). For the preparation of the TrichoMix Trichoderma spores were harvested from 5 to 7 days old culture grown on PDA plates. The spores and mycelia were scrapped in 20 ml sterile distilled water (sdw) and mixed in1:1 ratio.
Screening and characterization of the Trichoderma strains for Salt stress tolerance
Salt stress tolerance was assessed by observing the Trichoderma growth in Potato dextrose broth (PDB) supplemented with different concentrations of salt. Sodium chloride salt was used in 10, 25, 50, 100, 150, 250, and 500 mM concentration in 100 ml PDB. The broth was inoculated with1 ml of the Trichoderma spore suspension (106 Log10 CFU ml−1) and incubated at 28 °C ± 2 °C for five days in shaking conditions at 100 rpm. The mycelia were harvested by centrifugation at 8000 rpm for 10 min and washed twice with sterile saline (0.85% NaCl). The mycelia were used for estimating the biomass, proline, and glycine betaine. The mycelia were dried in an oven at 60 °C for 10–12 h and dry biomass was taken. The proline and glycine betaine were determined using the harvested mycelia following the method described for the plant tissue (Sect. 2.4).
Plant test in Net house conditions
The impact of TrichoMix was studied on paddy growth to ameliorate salt stress. The experiment was conducted in earthen pots under the net-house condition at CSIR-National Botanical Research Institute Lucknow, India (latitude/longitude 11°24′ N/79° 044’ E). The rice variety Saryu-52 was grown in July–October 2018. During this period the temperature fluctuated between 25 ± 2 °C (day) and 20 ± 2 °C (night) with a photoperiod of 14/10 h light/ Dark and 65 ± 5% relative humidity. Soil collected from NBRI field premises was used in the present study (pH 7.28), (EC180.1 µS/cm), and TOC (0.25%). Before filling the soil in pots, it was dried, ground, sieved (< 2 mm), and mixed with NaCl solution maintaining a final concentration of 100 mM salt on a dry weight basis of the soil. After mixing the salt solution the soil was allowed to stabilize for 4 days and then filled into pots (6 kg/pot). The soil pH, EC, and TOC were 7.38, 396.4 (µS/cm), and 0.24% respectively. Rice seeds were surface sterilized using 0.1% HgCl2 for 30 s followed by 5 washing with sterile distilled water (sdw), and germinated in dark for 3 days. The germinated seeds were sown in soil for 15 days to raise the seedlings (Chauhan et al. 2019). The seedlings were uprooted; roots were washed with sdw to remove the adhering soil and dipped in the Trichoderma spore’s suspension for 30 min before transplanting. The experiment included two sets of treatments, one set without salt stress having 4 treatments (i) Control (Cont.), (ii) T. koningiopsis (PR5) (iii) T. asperellum (K14), (iv) Trichoderma mixture of PR5 and K14 (TrichoMix) and second set with salt stress of treatments including (v) Salt + Control (S + Cont.), (vi) Salt + PR5 (S + PR5), (vii) Salt + K14 (S + K14), (viii) Salt + TrichoMix (S + TrichoMix) with six replicates of each treatment. Watering was done using 100 mM NaCl solution as and when required for salt treatments. Sampling of the tissues was done at 0, 15, 30, and 45 days after transplantation for biochemical and molecular assays. The sampled tissue was washed with milliQ, separated into roots, and shoots blot dried and crushed in liquid N2 and stored at − 80 °C till further estimation of biochemical parameters. Plant growth and yield parameters were taken at seed maturity.
Biochemical assays
Different biochemical assays in plant tissue as physiological indicators were performed at 0, 15, 30, and 45 days after transplantation under salt stress and control conditions. The percent relative water content (RWC%) of shoot tissue was determined by recording the fresh weight, turgid weight, and dry weight of the samples as described by Teulat et al. (2003).
Proline content was analysed in tissue extract prepared by homogenizing 100 mg fresh tissue in 1 ml of 70% ethanol as described by (Tiwari et al. 2016). Total soluble sugar (TSS) in rice tissue was estimated by the phenol–sulphuric acid method (Dubois et al. 1956). Chlorophyll and carotenoid were estimated by grinding 0.05 g of fresh leaf tissue in 5 ml of 80% chilled acetone and the absorbance was taken at 663, 645, 510, and 480 nm. Chlorophyll calculation was performed as described earlier by (Bruuinsma 1963). The amount of Glycine betaine (GB) was estimated in tissue extract prepared in 20 ml of deionized water as described by Grieve and Grattan (1983).
Enzymatic and non-enzymatic antioxidants analysis
Enzymatic and non-enzymatic antioxidant activities were assayed in the plant extract prepared by crushing the plant tissues in 0.1 M potassium phosphate buffer containing, 0.1 mM EDTA, 1% polyvinyl pyrrolidone (PVP), PMSF, and dithiothreitol. The tissue was centrifuged and the supernatant was used for different enzymatic assays. Superoxide dismutase (SOD) activity was determined by measuring the inhibition in the photochemical reduction of nitro-blue-tetrazolium as described by Srivastava et al. (2018). The activity of ascorbate peroxidase (APX) was determined using the method of Nakano and Asada (1981). Catalase activity was measured by recording the decrease in absorbance of H2O2 (Aebi 1984). Total guaiacol peroxidase (GPX) activity was measured in a reaction mixture using guaiacol as the substrate. The increase in absorbance was measured at 470 nm for 1 min (Hemeda and Klein 1990). The lipid peroxidation (LPx) was estimated by measuring the thiobarbituric acid (TBA) as reacting substance for the formation of Malondialdehyde (MDA) and expressed as nM of TBASRS/g FW (Schmidt and Kunert 1986). Further, Phenol peroxidase (PPO) was estimated by using the 0.1 M catechol and expressed as ΔOD/min/mg of FW (Mohammadi and Kazemi 2002). Non-enzymatic parameters were observed by estimating the total phenolics. The total phenolic content (TPC) in the plant tissues was estimated and expressed in terms of Gallic acid equivalent to g − 1 of plant tissue (Ainsworth and Gillespie 2007).
Quantitative real-time (q-RT) PCR study of Stress marker genes
Real-time qRT-PCR analysis for different abiotic stress-responsive genes was carried out in rice shoot tissue at the flag leaf stage using specific primers viz. OsMAPK5, OsAPX, OsGST, OsUSP, OsBADH, OsLYSO, OsNRAMP6, and OsBZ8. The list of primers used is given in Supplementary Table 1. Total RNA was isolated from rice shoots using RaFlex™ Total RNA isolation Manual (Plant) Kit (GeNei™) and cDNA synthesis was done using H minus first-strand cDNA synthesis Kit (Fermentas, Thermo Scientific). The real-time PCR analysis was carried out with SYBR green mix Ex Taq (Agilent) on Stratagene Mx3000 P systems, using actin as an internal control. The reactions were performed using the cycle conditions of an initial denaturation at 94 °C for 5 min, followed by 35 cycles of 94 °C for 30 s, 60 °C for 30 s, and 72 °C for 30 s. After obtaining the value for each reaction, the fold change was calculated by the delta-delta ct method.
Determination of microbial population in the rhizospheric soil
The heterogeneous microbial populations of the rhizospheric soil were determined by a standard dilution-plating procedure on selective media and the colony-forming units (CFU) per gram of soil were calculated as described earlier (Srivastava et al. 2011). Trichoderma was counted on Trichoderma Selective Media (TSM) (Elad and Chet 1983). The rhizospheric soil used for CFU determination was collected from the soil adhering to the plant roots. The initial CFU of the rhizosphere was determined 3 days after the transplantation and final at the time of harvesting. All the media and media components were used from Hi-media.
Physicochemical analysis of soil
For determining the physicochemical properties of soil samples, the soil was air-dried, sieved (2 mm), and processed immediately or stored at 10 °C until analysis. The bulk density (BD) was determined by the pycnometer method (Blake 1965). The water holding capacity (WHC) of soil was measured gravimetrically using the Keen's box method by weighing water-saturated and oven-dried soil samples for 24 h at 105 °C. Total organic carbon (TOC) was estimated following an established protocol (Walkley and Black 1934) and microbial biomass carbon (MBC) was performed by using the chloroform-fumigation-extraction method (Johansson et al. 2004). Available phosphorus (P) in soil samples was determined by following the method of Olsen (1954). Available potassium (K), and calcium (Ca) contents were measured by a flame photometer (Systronix-128, USA). Available nitrogen was analyzed by Kjeldahl’s method (Bremner 1965). Soil pH and electrical conductivity (EC) were measured using 1:2.5 (w/v) soil suspension prepared in milliQ water.
Soil microbial enzymatic activities
Different microbial soil enzyme activities were assayed in the rhizosphere soil samples collected after 30 days of the plant growth in pots. Dehydrogenase activity (DHA) was determined by the reduction of 2, 3, 5-triphenyl tetrazolium chloride (TTC) as described by Alef et al. (1995). Alkaline phosphatase activity in soil was determined according to Tabatabai and Bremner (1969) based on the utilization of p-NPP (p-nitrophenyl phosphate) using MUB buffer (pH 11 for alkaline phosphatase), and 1 h incubation at 37 °C. After incubation, 1 ml of 0.5 M CaCl2 and 4 ml of 0.5 M NaOH were added and absorbance of the filtrate was observed at 400 nm. Protease activity was measured by Folin-Cocteau reagent (Ladd et al. 1972). Fluorescein diacetate activity (FDA) was estimated by the determination of the released amount of fluorescein (Stubberfield and Shaw 1990). β-glucosidase activity was determined by Eivazi and Tabatabai (1988). Urease enzyme was measured in soil according to the method by Kandeler and Gerber (1988). Arylsulphatase activity in soil was determined according to the method by Alef and Nannipieri (1995).
Root anatomical studies
Rice root samples were fixed, and processed and hand-cut sections were stained as described earlier (Tewari et al. 2021). Briefly, the manually cut sections of root segments of 2–2.5 mm from the root tip region, were differentially stained with Johansen’s safranin and fast green. EVOS FL Cell Imaging System (ThermoFisher Scientific) was used to capture the images.
Field trial
The TrichoMix was further validated for its stress amelioration activity in paddy under field conditions at U.P. State Government Seed farm, Sandila (27.0765° N, 80.5179° E), Lucknow which has marginally stressed soil (pH 9.94 and EC 2.5 dS/m). The experiment was carried out in plots of size 5 m × 5 m with 3 replicates each in RBD (Randomized blocks designs). Row to row spacing was 25 cm and plant to plant spacing was 20 cm. Watering was done as and when required. The treatments included (i) Control (Cont.), (ii) Farmers Practice (FP, where NPK was applied as per recommendation) (iii) TrichoMix (iv) TrichoMix + 50% NPK.The yield data was taken at seed maturity. The quality of seed grains was also determined by estimating Zn, Fe, Mn, and Se. Mineral elements were analysed as described earlier (Kaur et al. 2020). Inductively Coupled Plasma-Mass Spectrometry (ICP-MS) Agilent, 7500 ce was used for the analysis.
Statistical analysis
All experimental data represent the means of three independent biological replicates and their results are expressed as mean with standard deviation (mean ± SD) or standard error (mean ± SE). Analysis of variance (ANOVA) and Duncan’s post hoc test (DMRT) at 95% confidence was performed using SPSS 16.0 on the entire data to identify the significant differences among the treatments.
Results
Stress tolerance of Trichoderma spp.
The Trichoderma strains NBRI-PR5 and NBRI-K14 were selected from 27 Trichoderma isolates screened for salt tolerance (Supplementary Fig. 1). NBRI-PR5 and NBRI-K14 exhibited tolerance to various abiotic stresses such as pH, salt, drought, and a combination of pH and salt. The optimum growth range of the two Trichoderma strains was between pH 4–9 and approximately 50% reduction in mycelia dry-weight was observed above pH 10 (Table 1). Both the strains showed proline accumulation; however, it was observed that in PR5 proline accumulation was optimum above pH 7 (pH 7–11) while in K14 it was optimum below pH 7 (pH 4–7). Mycelia dry weight, proline, and glycine betaine accumulation in PR5 and K14 in salt and combined stress conditions are presented in Table 1. The r2 values show the correlation of the stress conditions with the mycelia dry-weight, proline, and glycine betaine (Table 1).
Table 1.
Accumulation of osmoprotectants in T. koningiopsis (PR5) and T. asperellum (K14) mycelium under abiotic stress condition in-vitro
| Stress | Mycelia dry wt. (g) | Proline (µg/g) | Glycine betaine (µg/g) | |||
|---|---|---|---|---|---|---|
| pH stress | ||||||
| PR5 | K14 | PR5 | K14 | PR5 | K14 | |
| Control | 0.66 ± 0.03d | 1.21 ± 0.06c | 4.6 ± 0.55a | 5.4 ± 0.17a | 172.8 ± 10.49a | 256.0 ± 21.21a |
| pH 4 | 0.73 ± 0.03d | 1.17 ± 0.09c | 7.0 ± 0.29b | 31.2 ± 0.83c | 263.7 ± 17.09b | 258.7 ± 31.76a |
| pH 5 | 0.71 ± 0.04d | 1.14 ± 0.09c | 6.7 ± 0.50b | 15.2 ± 0.50b | 246.9 ± 8.55b | 248.0 ± 8.80a |
| pH 6 | 0.69 ± 0.02d | 1.12 ± 0.02c | 8.1 ± 0.28b | 13.4 ± 0.28b | 291.5 ± 30.99b | 292.8 ± 36.41b |
| pH 7 | 0.58 ± 0.02c | 1.08 ± 0.07c | 9.5 ± 0.39c | 10.5 ± 0.39a | 295.3 ± 47.10b | 295.5 ± 37.80b |
| pH 8 | 0.55 ± 0.03c | 0.57 ± 0.04b | 10.1 ± 0.17c | 7.6 ± 0.17a | 299.7 ± 33.34b | 330.9 ± 48.70c |
| pH 9 | 0.51 ± 0.04b | 0.55 ± 0.05b | 14.7 ± 0.50c | 6.9 ± 0.50a | 404.3 ± 45.60c | 335.5 ± 20.64c |
| pH 10 | 0.45 ± 0.03b | 0.53 ± 0.06b | 20.8 ± 0.58d | 6.2 ± 0.58a | 371.7 ± 27.07c | 326.1 ± 20.77c |
| pH 11 | 0.29 ± 0.02a | 0.44 ± 0.05a | 50.0 ± 0.56e | 5.8 ± 0.56a | 428.3 ± 19.55c | 379.5 ± 43.02d |
| Correlation Coeff. (r2 values; p = 0.5) | 0.93 | 0.86 | 0.61 | 0.71 | 0.84 | 0.88 |
| Drought stress (PEG 6000) | ||||||
|---|---|---|---|---|---|---|
| PR5 | K14 | PR5 | K14 | PR5 | K14 | |
| Control | 2.2 ± 0.02a | 3.2 ± 0.03a | 10.5 ± 0.29a | 32.3 ± 0.44a | 265.1 ± 38.7a | 183.2 ± 21.7a |
| 10% | 3.1 ± 0.09b | 3.8 ± 0.09a | 22.5 ± 0.17b | 43.3 ± 0.29b | 299.2 ± 37.9b | 266.9 ± 43.3b |
| 15% | 3.5 ± 0.10b | 5.4 ± 0.05b | 23.5 ± 0.33b | 43.3 ± 0.69b | 389.6 ± 28.0b | 261.3 ± 45.2b |
| 25% | 4.2 ± 0.03c | 6.4 ± 0.26b | 28.6 ± 0.64b | 44.4 ± 0.11b | 415.5 ± 27.7b | 339.9 ± 30.9b |
| 35% | 4.6 ± 0.10c | 7.0 ± 0.03c | 56.6 ± 0.40c | 47.1 ± 0.34b | 594.7 ± 41.5c | 398.9 ± 31.1c |
| 45% | 4.5 ± 0.04c | 5.1 ± 0.02b | 93.9 ± 0.17d | 48.8 ± 0.36b | 783.2 ± 41.2d | 437.1 ± 34.0c |
| 55% | ND | 4.4 ± 0.03a | ND | 54.5 ± 0.94c | ND | 668.3 ± 33.9d |
|
Correlation Coeff (r2 values; p = 0.5) |
0.84 | 0.96 | 0.82 | 0.90 | 0.92 | 0.87 |
| Salt stress | ||||||
|---|---|---|---|---|---|---|
| PR5 | K14 | PR5 | K14 | PR5 | K14 | |
| Control | 0.48 ± 0.03b | 0.85 ± 0.04b | 44.5 ± 0.23a | 24.7 ± 0.11d | 136.5 ± 24.28a | 106.4 ± 7.63a |
| 10 mM | 0.55 ± 0.01b | 0.89 ± 0.04c | 60.9 ± 0.35b | 40.7 ± 0.64b | 244.0 ± 35.39a | 307.5 ± 21.05b |
| 25 mM | 0.54 ± 0.02b | 0.91 ± 0.12c | 61.0 ± 0.23b | 45.9 ± 0.54b | 267.2 ± 31.75b | 315.2 ± 12.87c |
| 50 mM | 0.62 ± 0.05c | 0.97 ± 0.07c | 73.4 ± 0.50c | 73.5 ± 0.40c | 315.5 ± 15.81b | 395.5 ± 10.86c |
| 100 mM | 0.69 ± 0.04c | 0.92 ± 0.11c | 80.8 ± 0.28c | 75.5 ± 0.42c | 398.7 ± 11.44c | 433.3 ± 37.60cd |
| 150 mM | 0.69 ± 0.06c | 0.72 ± 0.02b | 80.5 ± 0.23c | 85.2 ± 0.17c | 524.5 ± 56.82c | 599.7 ± 52.35d |
| 250 mM | 0.48 ± 0.02b | 0.46 ± 0.04a | 90.8 ± 0.22d | 172.7 ± 0.17d | 593.6 ± 44.41d | 688.5 ± 63.51d |
| 500 mM | 0.31 ± 0.01a | 0.40 ± 0.08a | 97.3 ± 0.33d | 194.8 ± 0.58d | 677.9 ± 35.79d | 886.9 ± 39.47e |
| Correlation Coeff. (r2 values; p = 0.5) | 0.30 | 0.73 | 0.95 | 0.85 | 0.84 | 0.97 |
| Combined stress (pH + salt stress) | ||||||
|---|---|---|---|---|---|---|
| PR5 | K14 | PR5 | K14 | PR5 | K14 | |
| Control | 0.66 ± 0.03b | 1.21 ± 0.06c | 4.6 ± 0.55a | 5.4 ± 0.17a | 172.8 ± 10.49a | 256.0 ± 21.21b |
| pH 5 + 250 mM | 0.49 ± 0.09b | 0.98 ± 0.12b | 5.0 ± 0.56a | 5.1 ± 0.50a | 234.9 ± 30.57b | 262.4 ± 47.71b |
| pH 5 + 500 mM | 0.34 ± 0.06a | 0.81 ± 0.15b | 5.2 ± 0.55a | 4.7 ± 0.55a | 260.8 ± 28.61c | 237.1 ± 36.45a |
| pH 9 + 250 mM | 0.37 ± 0.03a | 0.54 ± 0.08a | 5.1 ± 0.44a | 4.4 ± 0.29a | 302.7 ± 31.77c | 334.1 ± 49.89b |
| pH 9 + 500 mM | 0.29 ± 0.04a | 0.39 ± 0.07a | 5.8 ± 0.73a | 3.5 ± 0.46a | 291.2 ± 44.45c | 280.8 ± 19.71b |
|
Correlation Coeff (r2 values; p = 0.5) |
0.74 | 0.98 | 0.63 | 0.95 | 0.79 | 0.22 |
ND: Not determined. The r2 values in this table shows the correlation of the stress conditions with the mycelia dry weight, proline and glycine betaine. All the values are means of 3 replicates with S.E. bars. Different letters indicate significance among the treatments (DMRT, P value ≤ 0.05)
Effect of stress-tolerant Trichoderma on paddy
Plant growth promotion (PGP)
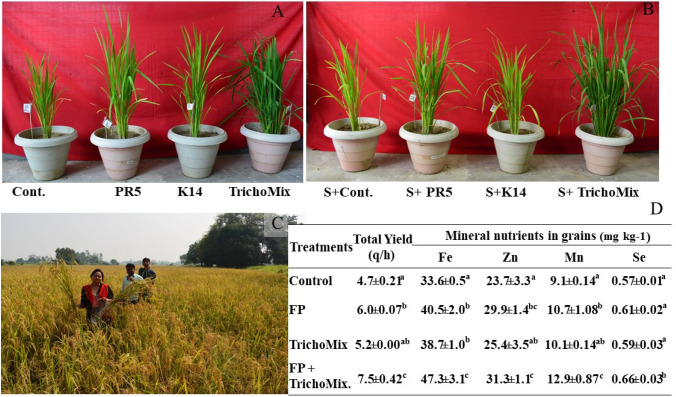
The impact of salt stress and TrichoMix on rice plants was studied under net house conditions in a pot experiment. The biochemical and physiological parameters of the plants were determined at regular intervals, whereas growth and yield parameters were taken after maturity. As compared to the control plants the root length and shoot length were increased by 45% and 35% respectively in the TrichoMix + salt treatment. Flag leaf is one of the most important source for photosynthetic energy which helps in grain filling. Its length and number were increased by 41% and 79% in Salt + TrichoMix in comparison to control. Grain yield parameters like the number of panicles, panicle length, and seed weight showed improved growth in Salt + TrichoMix rice plants as compared to the Salt + Control treatment. The number of panicles increased by 60%, panicle length by 46%, and seed weight was improved by 33%. The number of tillers and spikes were increased by 77% and 70% respectively. These plants showed an approximately 2 folds boost in biomass (Table 2). Thus, the overall plant growth was impaired in salt treatment which was overcome by the TrichoMix (Fig. 1A, 1B).
Table2.
Plant growth parameters of rice plants grown under salt stress condition in net-house experiment
| Plant growth parameters of rice plants | ||||||||
|---|---|---|---|---|---|---|---|---|
| Parameters | Cont | PR5 | K14 | TrichoMix | S + Cont | S + PR5 | S + K14 | S + TrichoMix |
| Root length (cm) | 17.9 ± 1.60a | 28.2 ± 1.76c | 22.2 ± 2.07c | 24.2 ± 2.09c | 17.2 ± 2.26a | 24.3 ± 2.61c | 19.6 ± 1.36b | 25.0 ± 1.95d |
| Shoot length (cm) | 62.6 ± 5.21a | 82.3 ± 3.75c | 66.0 ± 3.62a | 81.5 ± 3.66c | 63.4 ± 3.80a | 79.3 ± 4.98c | 67.9 ± 3.26b | 85.5 ± 2.32cd |
| Fresh wt. (g) | 28.6 ± 1.87a | 36.7 ± 4.49c | 31.5 ± 2.39a | 48.8 ± 10.73c | 28.4 ± 3.53a | 37.3 ± 4.01c | 30.3 ± 2.35a | 57.0 ± 5.11d |
| Dry wt. (g) | 17.0 ± 3.19a | 19.9 ± 2.36b | 18.1 ± 1.90a | 25.8 ± 4.03c | 17.8 ± 3.78a | 20.7 ± 2.01b | 17.7 ± 4.19a | 29.6 ± 3.89d |
| RWC(%) | 68.31b | 84.48c | 74.03c | 88.97bc | 59.47a | 80.6c | 83.75c | 92.31d |
| No. of Tillers | 7.7 ± 0.97a | 12.0 ± 1.48d | 11.3 ± 1.44cd | 10.5 ± 1.38c | 6.9 ± 1.00a | 9.8 ± 1.19b | 7.3 ± 1.37a | 12.2 ± 1.05d |
| No. of Panicle | 7.3 ± 0.98a | 11.1 ± 1.53d | 10.1 ± 1.27c | 11.8 ± 0.94d | 7.2 ± 1.06a | 9.2 ± 1.22c | 8.0 ± 0.85b | 11.5 ± 1.31d |
| No. of Spikelet | 18.7 ± 2.30b | 27.7 ± 3.67c | 20.1 ± 2.59ab | 20.5 ± 1.38ab | 17.2 ± 3.25a | 22.6 ± 3.39c | 18.3 ± 2.67ab | 29.2 ± 1.36d |
| No. of flag leaf | 8.7 ± 0.97b | 11.0 ± 1.31cd | 9.1 ± 2.55b | 10.5 ± 1.68c | 6.3 ± 0.89a | 10.7 ± 0.87c | 9.5 ± 1.78b | 11.3 ± 1.44cd |
| Panicle Length | 13.5 ± 1.93a | 18.2 ± 2.53c | 15.5 ± 2.57b | 19.6 ± 2.15d | 13.7 ± 1.60a | 16.7 ± 2.22c | 15.4 ± 2.91ab | 20.0 ± 2.45c |
| Flag leaf Length | 20.5 ± 1.45b | 24.0 ± 1.86c | 20.7 ± 1.22b | 26.5 ± 2.64d | 19.3 ± 1.72a | 24.3 ± 1.87c | 20.0 ± 1.16a | 27.1 ± 2.37b |
| 100 grain seed wt. (g) | 2.4 ± 0.01a | 3.0 ± 0.01bc | 2.9 ± 0.05b | 3.0 ± 0.07cd | 2.3 ± 0.01a | 2.9 ± 0.02bc | 2.9 ± 0.03b | 3.1 ± 0.01d |
Cont: Control, Trichoderma koningiopsis (PR5) and T. asperellum (K14), TrichoMix: Trichoderma mixture, S + Cont: Salt + Control, RWC: Relative water content.All the values are means of 3 replicates with S.E. bars. Different letters indicate significance among the treatments (DMRT, P value ≤ 0.05)
Fig. 1.
Effect of TrichoMix (T. koningiopsis and T. asperellum) on plant growth under salt stress conditions. A Without salt control set, B set with salt treatment (100 mM), c sodic field trial, D yield and mineral content of the rice grain obtained from field trial. Cont: Control, S + Cont: Salt + Control, S + TrichoMix: Salt + TrichoMix, FP: Farmer’s practice (NPK), TrichoMix: Trichoderma mixture
Biochemical parameters
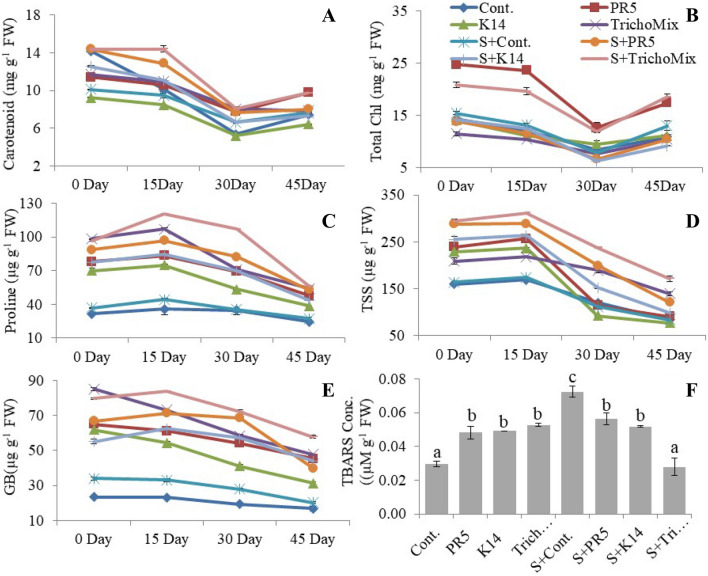
The biochemical status of the plants was monitored at different time intervals. It provided evidence to show the utility of TrichoMix during the seedling stabilization and at different growth periods under stress conditions. It was observed that the stress markers were higher in the initial 15 days of transplantation and declined with the plant growth. The chlorophyll content was increased in Salt + TrichoMix treatment by 50.64% (15d), 54.39% (30d) and 42.21% (45 d) as compared to Salt + Control treatment. The carotenoid content, 45 days after transplantation (dat) was found to be maximum in the rice leaves of PR5 treatment (> twofold) followed by K14 treatment (57.90%) as compared to the control. The carotenoid level was increased by > twofold in Salt + TrichoMix treatment as compared to the Salt + Control. The Trichoderma strains PR5, K14, and TrichoMix increased the plant’s overall proline content in both stress and control sets. Maximum proline concentration (56.38 ± 0.72µg ml−1) in tissues was observed in Salt + TrichoMix treatment in comparison to Salt + Control 45 dat.Tissue proline was reduced in the salt control treatment (27.34 ± 1.71 µgml−1) in comparison to control (24.420.22 µg ml−1) 45 dat. Total soluble sugar (TSS) content in rice plants treated with the TrichoMix and Salt + TrichoMix showed an increase by 67.10% and > twofold respectively as compared to the control, at 45 dat. Glycine betaine content in the Salt + TrichoMix treatment was highest (58.36 ± 0.36) as compared to the control (15.83 ± 1.51).
The cellular toxicity, represented by the increased level of malondialdehyde (MDA) was decreased in the Salt + TrichoMix treatment. The total phenols, representing the stress tolerance were increased by more than2 folds in the Salt + TrichoMix treatment as compared to the control (Fig. 2).
Fig. 2.
Biochemical status of rice plants treated with T.koningiopsis and T. asperellum under salt stress conditions. A Carotenoid, B total chlorophyll, C proline, D TSS (Total soluble sugar), E GB (Glycine betaine), F LPX (Lipid peroxidation). Values are means of 3 replicates. Different letters indicate significance among the treatments (DMRT, P value ≤ 0.05)
Enzymatic and non-enzymatic antioxidants
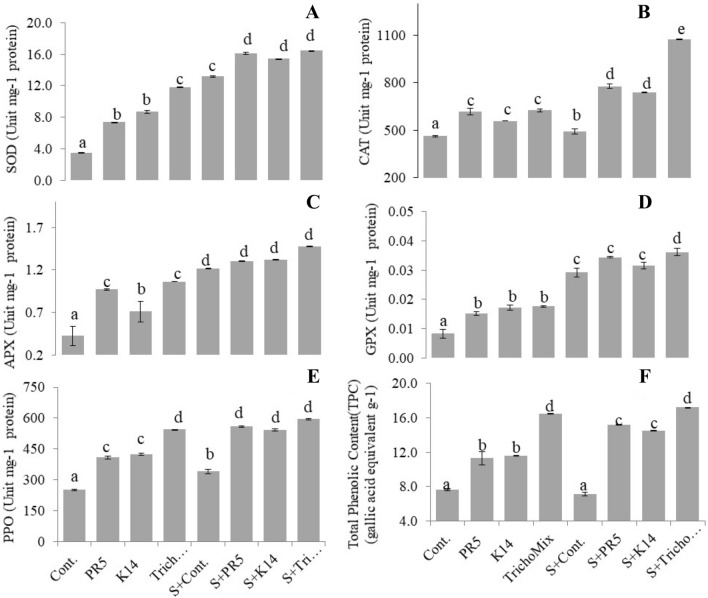
The antioxidant enzyme activity in rice plants increased after salt and Trichoderma treatments (Fig. 3). The SOD activity was found to be maximally increased in the combination of Salt + TrichoMix by approx. fourfold compared to the control. The increased activity for other antioxidant enzymes was in the order of CAT (> twofold), APX (> threefold), GPX (> fourfold), and PPO (> twofold), respectively as compared to the control.
Fig. 3.
Antioxidant enzymes status of rice plants after 30 days of salt stress treatment. SOD: Superoxide dismutase, APX: Ascorbate peroxidase, PPO: Phenol peroxidase, CAT: Catalase, GPX: Guaiacol peroxidase All the values are means of 3 replicates with S.E. bars. Different letters indicate significance among the treatments (DMRT, P value ≤ 0.05)
Modulation of stress-related genes in presence of TrichoMix
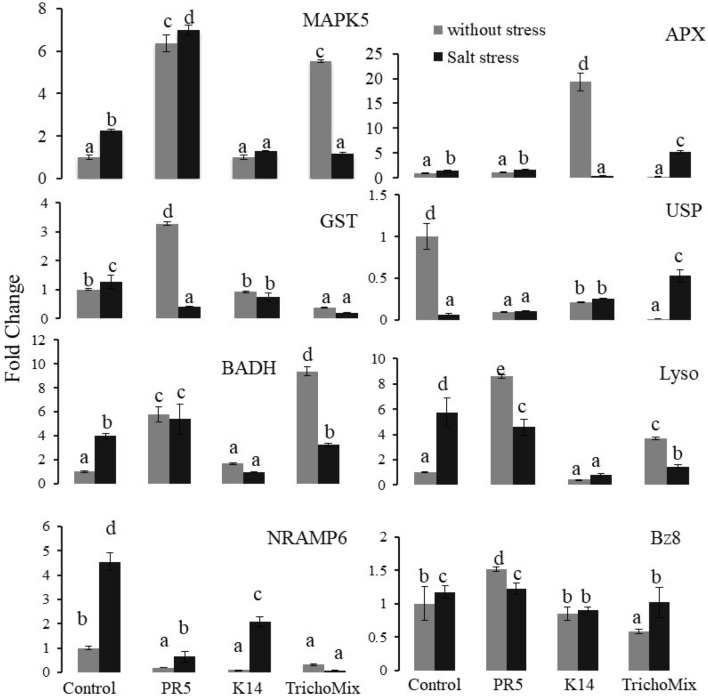
The expression analysis of some of the stress-responsive genes viz. Mitogen-activated protein kinase5(MAPK5), Ascorbate peroxidase (APX), GlutathioneS-transferase (GST), Universal stress protein (USP), Betaine aldehyde dehydrogenase (BADH), Lysophospholipase, Metal transporter OsNRAMP6 and ABA responsive bZIP protein were studied in the rice plants at vegetative stage (45 dat) (Fig. 4). In salt stress conditions the OsMAPK5 gene showed upregulation in Salt + PR5 by 7 folds while its expression was downregulated in presence of Salt + K14 and Salt + TrichoMix. In the control set, the OsMAPK5 showed sixfold increased expression in PR5 and 5.5-fold in TrichoMix treatment. The OsGST gene showed upregulation by 3.3 fold in presence of PR5 as compared to the control. The expression was down-regulated in salt treatment as compared to the control without stress. The expression of the BADH gene was upregulated in PR5 treatment by 5.8 folds, 1.6 folds in K14, and 9.4 folds in TrichoMix treatment. Under salt stress, the expression decreased in all the treatments except in PR5. The expression pattern of Lysophospholipase (OsLYSO) and OsBADH gene were similar, which was upregulated in PR5 and the TrichoMix, and downregulated in K14 as compared to the control. Under salt stress conditions, the expression was downregulated in all the samples in comparison to control rice plants. The expression level of metal transporter OsNRAMP6 was very much reduced in the presence of PR5, K14, and TrichoMix as compared to the control plants. The gene OsBZ8 expression level in the control set was increased to 1.5 fold in PR5 while reduced in K14 and TrichoMix. No significant differences were observed in the salt stress set. The difference in the expression patterns of these stress-related genes suggests that PR5 and K14 modulate these genes and help to alleviate the oxidative stress generated due to saline conditions.
Fig. 4.
Differential expression of stress responsive genes in rice treated with TrichoMix grown under salt stress conditions in net house. The graphs show fold—change values in treatment as compared to the control. All the values are means of 3 replicates with S.E. bars. Different letters indicate significance among the treatments (DMRT, P value ≤ 0.05)
Soil microbial population
The populations of heterogeneous bacteria, actinomycetes, total fungi, and Trichoderma in the rhizosphere region after inoculation (72 h) and at maturity are given in supplementary Table 2. Rhizospheric heterogeneous microbial populations decreased by approximately 1–1.5 Log10 CFU/g soil from initial to the final harvest. Approximately ten times increased population of Trichoderma (1–1.5 Log10 CFU/g) were present in the rhizospheric soil of the inoculated seedlings as compared to the un-inoculated ones. Within the treatments, no significant effect of the salt or the microbial inoculation was observed (Supplementary Table 2). However, a detailed study related to microbial diversity may reveal the role of the dominant micro-flora in rhizosphere modulation leading to the differences observed in the soil biochemical properties.
Physico-chemical properties of soil
The physicochemical properties of the soil treated with individual Trichoderma or TrichoMix improved the overall soil physicochemical properties. The physicochemical parameters of soil treated with the TrichoMix were found to be improved by 39.13%, 73.3%, 72.43%, 77.883%, 68.57%, 10.18%, and 79.90% for available P, TOC, SOM, Ca, N, Bulk density and WHC respectively. While other parameters except pH increased by 2–3-fold (Table 3).
Table 3.
Physicochemical parameters of Rhizosphere soil of paddy grown under salt stress condition in net-house experiment
| Physicochemical parameters of Rhizosphere soil | ||||||||
|---|---|---|---|---|---|---|---|---|
| Parameters | Cont | PR5 | K14 | TrichoMix | S + Cont | S + PR5 | S + K14 | S + TrichoMix |
| pH | 7.61 ± 0.23b | 7.56 ± 0.02b | 8.52 ± 0.01d | 7.15 ± 0.02a | 7.70 ± 0.08c | 7.52 ± 0.02b | 7.61 ± 0.05b | 7.72 ± 0.02c |
| EC (µS/cm) | 106.47 ± 3.80a | 204.07 ± 10.05c | 208.00 ± 2.88c | 220.08 ± 13.91d | 176.87 ± 5.20b | 220.07 ± 13.91d | 229.60 ± 2.25d | 250.47 ± 3.79e |
| Avail. P mg/g | 2.07 ± 0.03a | 2.43 ± 0.01b | 2.11 ± 0.01a | 2.88 ± 0.00c | 2.08 ± 0.02a | 2.87 ± 0.01c | 2.49 ± 0.01b | 2.82 ± 0.01c |
| MBC (µg/g) | 393.59 ± 16a | 1297.5 ± 226c | 1438.53 ± 446c | 1302.01 ± 222d | 794.41 ± 24b | 1565.93 ± 392e | 1561.95 ± 389e | 1570.49 ± 392e |
| TOC (%) | 0.90 ± 0.08a | 1.68 ± 0.22cd | 1.67 ± 0.18cd | 1.56 ± 0.03c | 1.20 ± 0.20b | 1.63 ± 0.15cd | 1.48 ± 0.04c | 1.84 ± 0.08d |
| SOM (%) | 1.56 ± 0.13a | 2.89 ± 0.38cd | 2.88 ± 0.31cd | 2.69 ± 0.06c | 2.06 ± 0.35b | 2.81 ± 0.26cd | 2.55 ± 0.06c | 3.16 ± 0.13d |
| Avail. K (mg/g) | 131.44 ± 0.58a | 396.56 ± 2.68d | 394.12 ± 0.26d | 403.08 ± 1.98d | 161.36 ± 8.13b | 323.36 ± 0.50c | 344.56 ± 0.50c | 475.6 ± 0.24e |
| Avail. Ca (mg/g) | 1366.84 ± 150a | 2110.64 ± 190de | 1735.32 ± 146c | 2271.00 ± 123d | 1506.64 ± 112b | 2120.92 ± 156c | 1902.56 ± 220c | 2172.44 ± 131d |
| Avail. S (mg/g) | 1.11 ± 0.04a | 1.46 ± 0.13c | 1.37 ± 0.18b | 2.24 ± 0.06f | 1.35 ± 0.12b | 2.06 ± 0.09e | 1.77 ± 0.11d | 2.34 ± 0.04f |
| Avail. Nitrogen (gm/kg) | 0.70 ± 0.04a | 1.06 ± 0.06c | 1.1 ± 0.15cb | 1.18 ± 0.17d | 0.69 ± 0.17a | 1.01 ± 0.06b | 1.01 ± 0.06b | 1.16 ± 0.12d |
| Bulk density (gm/cc3) | 1.13 ± 0.03b | 1.12 ± 0.05a | 1.12 ± 0.06a | 1.15 ± 0.01c | 1.13 ± 0.02b | 1.14 ± 0.8 | 1.13 ± 0.06b | 1.12 ± 0.02a |
| WHC (%) | 21.95 ± 8.84a | 38.87 ± 0.34c | 35.83 ± 13.62c | 39.49 ± 0.64d | 19.50 ± 3.15a | 40.11 ± 4.92d | 28.29 ± 7.27d | 41.30 ± 16.65d |
EC: Electrical conductance, Avail. P: Available Phosphorus, MBC: Microbial biomass carbon, TOC: Total organic carbon, SOM: Soil organic matters, WHC: Water holding capacity.All the values are means of 3 replicates with S.E. bars. Different letters indicate significance among the treatments (DMRT, P values ≤ 0.05)
Soil microbial enzymatic activities
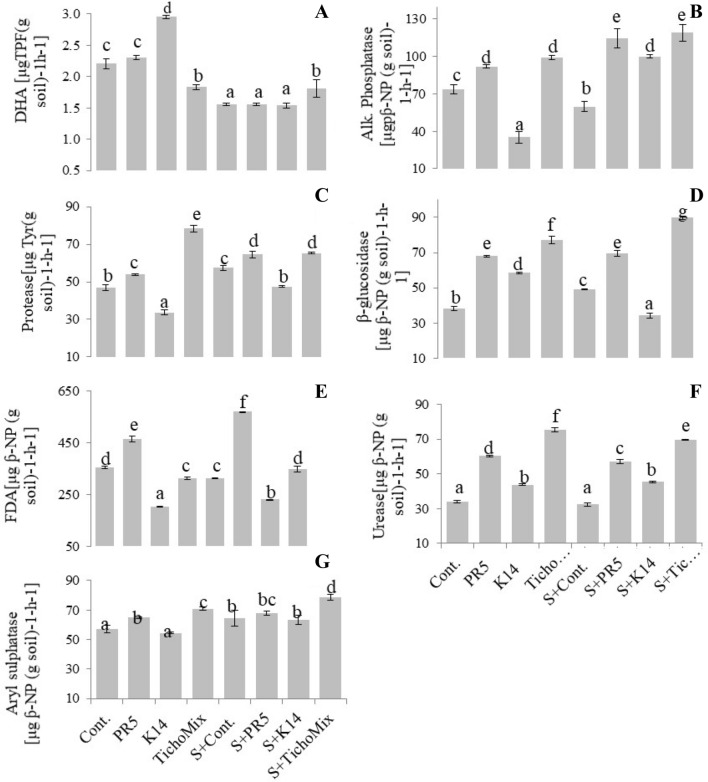
The soil microbial enzymes DHA, phosphatase, protease, β-glucosidase, urease, FDA, and aryl sulphatase were assayed in the treatments at maturity, approximately 90 days after transplantation (Fig. 5). The activity of soil enzyme DHA treated with K14 was found to be increased significantly as compared to the other treatments and control. DHA activity in soil treated with salt was decreased by 41% and no significant change was observed in Salt + PR5 and Salt + K14 treatment. The activity of phosphatase enzyme in soil treated with PR5 and TrichoMix was increased by 24.93% and 34.57%, respectively, as compared to the control. In salt treatment, the phosphatase enzyme activity decreased to 59.821 ± 7.116 [μg þ-NP (g Soil)−1 h−1] as compared to control (73.67 ± 6.89 [μg - NP (g Soil) −1 h−1]. Further, phosphatase activity in soil treated with Salt + PR5, Salt + K14 and TrichoMix with salt was 114.53 ± 13.75 [μg þ-NP (g Soil)−1 h−1], 100.00 ± 2.37 [μg þ-NP (g Soil)−1 h−1] and 118.95 ± 11.81 [μg þ-NP (g Soil)−1 h−1 respectively as compared to phosphatase activity of soil treated with salt control. Protease is an important soil enzyme that hydrolyses the polypeptides into simple amino acids. Protease activity in soil treated with PR5, K14, and their mixture were 53.90 ± 0.77 [μg Tyr(g Soil)−1 h−1], 33.70 ± 2.37[μg Tyr(g Soil)−1 h−1] and 78.27 ± 3.08[μg Tyr(g Soil)−1 h−1], respectively as compared to the control. Results showed that protease activity was significantly high in soil treated with TrichoMix with and without salt. However, protease activity was decreased by 27.92% in K14 and17.39% in Salt + K14 compared with their respective controls.
Fig.5.
Effect of TrichoMix on enzyme activities of rhizosphere soil of paddy grown under salt stress conditions. A DHA (dehydrogenase), B alkaline phosphatase, C protease D β-glucosidase, E fluorescein diacetate hydrolysis (FDA) and F urease, G Aryl sulphatase
FDA activity increased in the PR5 and Salt + PR5 treatments by 31.19% and 82.53%, respectively as compared to their controls. FDA activity in K14 and Salt + K14 exhibited decreasing trend as in the case of protease. Salt + TrichoMix showed enhanced FDA activity by 11.35% compared to Salt + Control treatment.
The soil β-glucosidase activity was increased in all the treatments except Salt + K14. The maximum β-glucosidase activity was observed in the Salt + TrichoMix treatment as compared to the Salt control. The soil urease activity increased in the TrichoMix treatments in both, normal and salt stress conditions. In PR5 and Salt + PR5 treatments, the activity was enhanced by 77.55% and 76.15% respectively, as compared to their respective controls. The result indicates that there is an almost equal impact of salt and PR5 on enzyme activity. The activity of soil enzyme arylsulphatase was found to be increased under all treatments and maximum activity was observed in Salt + TrichoMix (21.64%) whereas, minimum activity was observed in K14 treatment (4.74%) as compared to the control. In salt control treatment the arylsulphatase activity was increased to 64.461 ± 9.58 from 57.109 ± 4.413in control. Overall results concluded that TrichoMix enhanced the soil enzymatic activity in normal as well as salt-stressed soils.
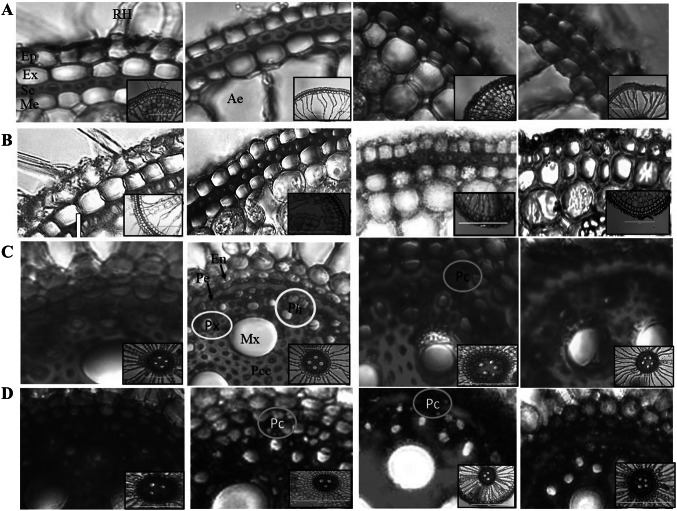
Rice root anatomy
The root anatomical features of the plants grown in the salt and Trichoderma treatments showed differences in the cell size, packing, and lignification (Fig. 6). The exodermis, sclerenchyma, and endodermis showed different patterns of staining, which are summarized in (Supplementary Table 3). The lignification was absent in the longitudinal-transverse intercellular walls in control exodermis and present on lower-surface in stress conditions. Similarly, the sclerenchyma cells which were small with significant lignification on all the walls became comparatively larger with prominent lignification only on the upper wall under stress conditions. The TrichoMix treatment showed more compact and organized cells having equally distributed lignification in exodermis and sclerenchyma in presence of the salt stress. The aerenchyma region was well developed in salt-treated plants showing early PCD (programmed cell death) of the cortical cells in presence of stress compared to the control and the Salt + TrichoMix treatment.
Fig. 6.
Transverse section of rice roots treated with TrichoMix in salt stress conditions showing differences in the lignification of exodermis and endodermis. A, B Show details of the cellular structure of the exodermis region in control (A) and stress (B) condition. C, D The panels show the details of the steler region in control (C) and stress (D) condition. The figures are enlarged portion of the pictures shown in the inset having a scale bar of 200 μm. EVOS digital inverted microscope was used to capture the root images of rice at 20X magnification. Ep(epidermis), Ex (exodermis), Sc (sclerenchyma layer), Me (Mesodermis) Ae (aerenchyma lacunae), En (Endodermis), Pe (Pericycle), Mx (Metaxylem), Mx (Central meta xylem), Px (Protophloem), Pcc (Pith companion cells), Pc (Passage cell), Ph (Phloem complex)
Application of TrichoMix in field
The TrichoMix was applied in the marginally stressed soil conditions to validate its applicability as a bio-stimulant to promote paddy growth and grain quality. Results revealed that application of the TrichoMix in paddy enhanced the yield by 10% and farmer’s practice with recommended NPK dose by 27% as compared to the control. However, in the integrated treatment of TrichoMix and 50% of the recommended NPK dose, the yield increased by approximately 60%. Similarly, improved grain quality was observed in TrichoMix in the presence and absence of NPK as compared to the control. Therefore, the integrated application of the TrichoMix along with the 50% dose of NPK was observed to be the most effective treatment in terms of both yield and nutrient quality of the produce (Fig. 1C and 1D). As compared to the control the integrated application increased the grain Mn and Fe by 40%, Zn by29%, and Se by 16%.
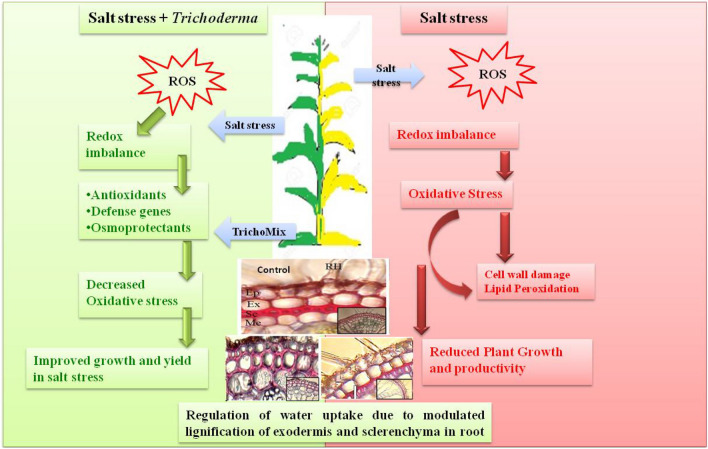
A model summarizing the physiological and molecular studies carried out in the present work has been proposed (Fig. 7).
Fig. 7.
A model summarizing the physiological, molecular and anatomical changes in the salt stressed and Salt + TrichoMix treated plants
Discussion
Trichoderma spp. is a widely used biocontrol agent that provides resistance to plants against various biotic and abiotic stresses (Fiorentino et al. 2018). Their role as a bio-stimulant leading to the elicitation of systemic resistance and stress-responsive genes in plants has also been elucidated (Rouphael et al. 2020). Besides, affecting the plants, the microbial inoculum influences the resident rhizosphere microflora and modulates the activities as per the requirements (Tandon et al. 2018). Therefore, to utilize the synergism between microbes, in the present study a consortium of compatible Trichoderma (TrichoMix) comprising T. koningiopsis (NBRI-PR5) and T. asperellum (NBRI-K14) was used. Their synergy has been earlier reported in rice straw utilization in the alkaline condition in the wheat crop (Tandon et al. 2022). In the present study bio-stimulation of plant growth under salt stress conditions by the synergistic activity of the TrichoMix is reported.
The strains used in the present study were selected based on their complementary activities under stress conditions. In NBRI-PR5, proline was produced at higher pH and salt stress conditions while in K14 proline was produced at acidic pH and glycine betaine in salt stress conditions. Thus, the strains used in the mixture are equipped with different modes of combating abiotic stresses. Complementation was evident from the similar pattern of the proline and glycine betaine production by PR5 and K14 in the combined salt + pH stress. No such combination of Trichoderma has been used earlier as per our knowledge to abate salt stress. The altered biochemical profile of the rice plants in presence of salt and TrichoMix is related to the generation and quenching of the oxidative stress mediators. In most of the previous studies the status of these mediators has been reported immediately after the induction of stress (Ahmed et al. 2021), however, the conditions in nature are continuous and the stress experienced by the plants is also continuous. This was reflected in the present study through observations taken at different time intervals showing gradual adaptation of the plants to the stress. The TrichoMix properties were reflected in rice plants under salt stress conditions which helped the plants to increase the osmolyte concentration. Glycine betaine accumulation helped in modulating the enzyme activities to overcome the ROS. The role of glycine betaine accumulation in Trichoderma treatments to improve productivity and yield parameters has been reported earlier (Carillo et al. 2020). The TrichoMix alleviated the salt-induced ROS stress and damage to cell wall integrity by the increased production of antioxidants and osmoprotectants as also reported earlier (Luo et al. 2008). Similarly, the application of the bioagent Trichoderma hamatum was reported to mitigate the harmful effects of salt stress on plant growth and metabolic processes in Ochradenus baccatus (Hashem et al. 2014). Kumar et al. (2017) have also reported the ameliorative role of Trichoderma strains (TRC3, NRT2, and THB3) in maize under different salinity conditions.
The TrichoMix application stimulated the root and shoots growth, nutrient uptake, production of photosynthetic pigment, and triggered the activity of the antioxidant enzymes. Various authors have reported that under osmotic stress, Trichoderma induces systemic resistance, nitrogen utilization, and osmolyte production in plants (Berg et al. 2013; Per et al. 2017). Increased SOD and catalase activity in rice plants under Salt + TrichoMix stress in this study indicate scavenging responses of the plants towards superoxide radicals. Significant high SOD and catalase activity in the Salt + TrichoMix treatment might be due to the development of a strong association of the plant with the fungi which helped in constantly inducing the stress marker. The presence of higher phenols in Salt + TrichoMix treatment further reinforced the antioxidants by scavenging free radicals. This probably contributed to cell wall formation facilitating the plant’s recovery from the salt stress. The observation corroborates with the decreased MDA level in Salt + TrichoMix indicating improved cell wall integrity. Earlier reports have shown Trichoderma harzianum to improve the growth of cucumber plants under salt stress by increasing ROS scavenging and other metabolic homeostasis mechanisms (Zhang et al. 2019).
High ROS status in salt-stressed rice plants is reported due to the upregulation of genes related to ethylene production and ROS generation (Ahanger et al. 2017). Stabilization of salt stress-induced ACC synthase activity and ROS quenching enzymes have been reported on Streptomyces inoculation in rice (Jaemsaeng et al. 2018). The present study also showed high ROS status in salt-stressed plants as indicated by the upregulated OsMAPK5, OsGST, OsBADH, OsLYSO and OsNRAMP6 genes and also the ROS quenching enzymes in the rice plants. Expression of these genes was considerably reduced in presence of the TrichoMix, whereas the gene expression profile was different in presence of the two Trichoderma species in individually inoculated plants. Overall, the observation showed that in the salt-stressed plants the TrichoMix is maintaining a biochemically elevated status of plants with high activities of oxidative enzymes which is contradictory to the low expression of many of the corresponding genes such as OsMAPK5, OsLYSO, OsBADH, OsNRAMP6 and OsGST in Trichoderma. It is suggested that in Salt + TrichoMix plants the damage to the plant cells was abated despite the elevated ROS due to high amounts of osmoprotectants which helped in maintaining the cell wall integrity (low lipid peroxidation); it corresponds to the low LYSO gene expression. Since the enzymes were stabilized, low gene expression was observed for OsMAPK5 and OsGST and since the osmoprotectants were stable, low OsBADH expression was observed. Highly upregulated OsUSP in Salt + TrichoMix indicates induction of alternative responses in combating the stress which may be unique to the treatment. These alternative responses in Salt + TrichoMix may include the non-enzymatic components such as the increased carotenoids and phenolics which act as antioxidant buffers removing the higher ROS levels. The role of such non-enzymatic components including ascorbic acid, carotenoids, reduced glutathione, phenolics and α-tocopherol present in all subcellular compartments have been reported as antioxidant buffers to remove higher levels of ROS (Miller et al. 2010).
Microflora is an important component of soil that expresses the health status of the soil (Chen et al. 2018). The Salt + TrichoMix treatment exhibited increased bacteria, actinomycetes, and fungi in soil showing a rendering of the salt stress. Increased activity of DHA reflects the positive physiological status and luxuriant growth of the microorganisms present in the soil. Earlier reports have ascribed decreased DHA activity in salt-treated soil to decreased biotic activity, osmotic imbalance, pH alteration, and reduction in carbon availability in soil (Lakhdar et al. 2009). The TrichoMix with salt stress condition showed a maximum increase in phosphatase activity. It has been earlier reported that PR5, one of the TrichoMix components produced organic acids in alkaline conditions and phosphatase enzymes under drought conditions (Tandon et al. 2018). The increased presence of phosphatase in the rhizosphere indicates the presence of drought-like conditions in the rhizosphere due to salt application. Similar mechanisms of phosphatase enzyme production under stress have been reported by many groups (Alori et al. 2017; Naik et al. 2013). A significant increase in β glucosidase activity in Salt + TrichoMix treatment represents high organic carbon in the soil due to increased root biomass. Increased urease activity in soil treated with Trichoderma, either single or in the mixture may be ascribed to the increased nitrogen availability and regulation of the nutrient cycle in presence of incorporated organic manure as reported earlier (Liang et al. 2003).
The roots which are directly involved in the water and nutrient uptake showed altered lignification patterns and packing of the sclerenchyma and exodermis cells in presence of salt and Trichoderma. Such differences in the lignification pattern of root cells have been earlier reported in drought-sensitive and resistant rice varieties (Tiwari et al. 2021). Different colors of the stained lignin showed that the salt-affected the lignin monomer composition that was deposited on the vertical and lower wall of the sclerenchyma cell layer. Deposition of compensatory or chemically distinct stress lignin has been reported which affects the water balance, and mineral nutrient homeostasis (Reyt et al. 2021). In Salt + TrichoMix treatment larger cells having lesser lignification with altered dye coloration on all the four walls of sclerenchyma and exodermis cells were observed. This probably helps reduce the resistance to water and nutrient uptake. In other treatments, the thick and darkly stained lignin on all four walls of small sclerenchyma cells offers resistance to water and nutrient uptake resulting in compromised growth. Comparison of TrichoMix and Salt + TrichoMix treatments, both having equally good growth, show contrasting characteristics of aerenchyma development and lignification of the exodermis, sclerenchyma, and endodermis. Aerenchyma formation is an adaptation to reduce the metabolic costs in stress conditions and increase root porosity (Zhu et al. 2010; Agarwal et al. 2019). Specific differences were also observed in the endodermis lignification, presence of passage cells, development of xylem and their numbers, and lignification of pith parenchyma. Xylem embolism (blockages) was observed in presence of salt which was absent on inoculation with Trichoderma.
The present study infers that the TrichoMix applies different strategies to improve plant growth in control and stress conditions which transmutes into increased grain yield and quality in field conditions as well. The nutrient uptake in stressed soil is inhibited due to NPK fixation (Yanai et al. 2010), therefore, fortification of the grains with important nutrients like Fe and Zn is also an important aspect to be considered in stressed soil. In the present study increased grain quality besides the productivity in the TrichoMix and NPK (50%) integrated treatment in the sodic fields makes it a preferred inoculum for application in sodic and marginally degraded agricultural soil. The study also shows that the application of the TrichoMix is instrumental in reducing the application of NPK without compromising the yield.
Conclusions
A comprehensive approach to tap the plant's optimum yield is required in the current abiotic stress conditions prevailing throughout the world. Apart from the conventional approaches including appropriate irrigation and using resistant crop varieties, the application of microbes-based bio-stimulants is excellent in inducing the plant's stress response systems to maximize its stress tolerance and yield capacity. The present study concludes that the synergistic activity of the two Trichoderma species with complementary activities modulates molecular, physiological, structural, and anatomical features of rice plants to improve plant growth, yield, and quality in salt stress conditions. Therefore, Trichoderma-based bio stimulants can be effectively applied in marginally stressed fields to improve productivity and quality with reduced fertilizer application.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
We thank Director, CSIR-NBRI, for his support and providing the necessary facilities at the institute. This study was financially supported by Council of Scientific & Industrial Research funded project, MLP-49 and Department of Science &Technology funded project (DST-SEED), GAP-3487. UY and IV acknowledge AcSIR and fellowship from UGC, PA acknowledges DST-SEED for fellowship.
Declarations
Conflict of interest
The authors declare no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References:
- Aebi H. Catalase in vitro. Methods Enzymol. 1984;105:121–126. doi: 10.1016/S0076-6879(84)05016-3. [DOI] [PubMed] [Google Scholar]
- Agarwal P, Singh PC, Chaudhry V, Shirke PA, Chakrabarty D, Farooqui A, Nautiyal CS, Sane AP, Sane VA. PGPR-induced OsASR6 improves plant growth and yield by altering root auxin sensitivity and the xylem structure in transgenic Arabidopsis thaliana. J Plant Physiol. 2019;240:153010. doi: 10.1016/j.jplph.2019.153010. [DOI] [PubMed] [Google Scholar]
- Ahanger MA, Tomar NS, Tittal M, Argal S, Agarwal R. Plant growth under water/salt stress: ROS production; antioxidants and significance of added potassium under such conditions. Physiol Mol Biol Plants. 2017;23(4):731–744. doi: 10.1007/s12298-017-0462-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed S, Heo TY, Roy Choudhury A, Walitang DI, Choi J, Sa T. Accumulation of compatible solutes in rice (Oryza sativa L.) cultivars by inoculation of endophytic plant growth promoting bacteria to alleviate salt stress. App Biol Chem. 2021;64(1):1–14. [Google Scholar]
- Ainsworth EA, Gillespie KM (2007) Nature protocols 2(4):875–7. 10.1038/nprot.2007.101 [DOI] [PubMed]
- Alef K, Nannipieri P (1995) Methods in applied soil microbiology and biochemistry (No. 631.46 M592ma). Academic Press. Estimation of total phenolic content and other oxidation substrates in plant tissues using Folin–Ciocalteu reagent. 10.1038/nprot.2007.102 [DOI] [PubMed]
- Alori ET, Glick BR, Babalola OO. Microbial phosphorus solubilization and its potential for use in sustainable agriculture. Front Microbiol. 2017;8:971. doi: 10.3389/fmicb.2017.00971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg G, Alavi M, Schmidt CS, Zachow C, Egamberdieva D, Kamilova F, Lugtenberg B. Biocontrol and osmoprotectants for plants under salinated conditions. Mol Microbial Ecol Rhizosphere. 2013;1:561–573. [Google Scholar]
- Blake GR. Bulk density. Methods of soil analysis: part 1 physical and mineralogical properties. Stat Meas Sampl. 1965;9:374–390. doi: 10.2134/agronmonogr9.1.c30. [DOI] [Google Scholar]
- Bremner JM. Total nitrogen. Methods of soil analysis: part 2. Chemical and Microbiological Properties. J Res. 1965;9:1149–1178. doi: 10.2134/agronmonogr9.2.c32. [DOI] [Google Scholar]
- Bruuinsma J. The quantitative analysis of chlorophylls a and b in plant extracts. Photochem Photobiol. 1963;2(2):241–249. doi: 10.1111/j.1751-1097.1963.tb08220.x. [DOI] [Google Scholar]
- Carillo P, Woo SL, Comite E, El-Nakhel A, Rouphael Y, Fusco GM, Vinale F. Application of Trichoderma harzianum, 6-pentyl-α-pyrone and plant biopolymer formulations modulate plant metabolism and fruit quality of plum tomatoes. Plants. 2020;9(6):771. doi: 10.3390/plants9060771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chauhan PS, Lata C, Tiwari S, Chauhan AS, Mishra SK, Agrawal L, Chakrabarty D, Nautiyal CS. Transcriptional alterations reveal Bacillus amyloliquefaciens-rice cooperation under salt stress. Sci Rep. 2019;9(1):1–3. doi: 10.1038/s41598-019-48309-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Sun X, Zheng J, Zhang X, Liu X, Bian R, Li L, Cheng K, Zheng J, Pan G. Biochar amendment changes temperature sensitivity of soil respiration and composition of microbial communities 3 years after incorporation in an organic carbon-poor dry cropland soil. Biol Fertil Soils. 2018;54(2):175–188. doi: 10.1007/s00374-017-1253-6. [DOI] [Google Scholar]
- Dubois M, Gilles KA, Hamilton JK, Rebers PT, Smith F. Colorimetric method for determination of sugars and related substances. Analyt Chem. 1956;28(3):350–356. doi: 10.1021/ac60111a017. [DOI] [Google Scholar]
- Eivazi F, Tabatabai MA. Glucosidases and galactosidases in soils. Soil Biol Biochem. 1988;20(5):601–606. doi: 10.1016/0038-0717(88)90141-1. [DOI] [Google Scholar]
- Elad Y, Chet I. Improved selective media for isolation of Trichoderma spp. or Fusarium spp. Phytoparasitica. 1983;11(1):55. doi: 10.1007/BF02980712. [DOI] [Google Scholar]
- Fiorentino N, Ventorino V, Woo SL, Pepe O, De Rosa A, Gioia L, Romano I, Lombardi N, Napolitano M, Colla G, Rouphael Y. Trichoderma-based bio stimulants modulate rhizosphere microbial populations and improve N uptake efficiency, yield, and nutritional quality of leafy vegetables. Front Plant Sci. 2018;9:743. doi: 10.3389/fpls.2018.00743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grieve CM, Grattan SR. Rapid assay for determination of water-soluble quaternary ammonium compounds. Plant Soil. 1983;70(2):303–307. doi: 10.1007/BF02374789. [DOI] [Google Scholar]
- Hashem A, AbdAllah EF, Alqarawi A, Al Huqail A, Egamberdieva D. Alleviation of abiotic salt stress in Ochradenus baccatus (Del.) by Trichoderma hamatum (Bonord.) Bainier. J Plant Interact. 2014;9(1):857–868. doi: 10.1080/17429145.2014.983568. [DOI] [Google Scholar]
- Hemeda HM, Klein BP. Effects of naturally occurring antioxidants on peroxidase activity of vegetable extracts. J Food Sci. 1990;55(1):184–185. doi: 10.1111/j.1365-2621.1990.tb06048.x. [DOI] [Google Scholar]
- Jaemsaeng R, Jantasuriyarat C, Thamchaipenet A. Molecular interaction of 1-aminocyclopropane-1-carboxylate deaminase (ACCD)-producing endophytic Streptomyces sp. GMKU 336 towards salt-stress resistance of Oryza sativa L. cv. KDML105. Sci Rep. 2018;8(1):1–15. doi: 10.1038/s41598-018-19799-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamil A, Riaz S, Ashraf M, Foolad MR. Gene expression profiling of plants under salt stress. Crit Rev Plant Sci. 2011;30(5):435–458. doi: 10.1080/07352689.2011.605739. [DOI] [Google Scholar]
- Johansson JF, Paul LR, Finlay RD. Microbial interactions in the mycorrhizosphere and their significance for sustainable agriculture. FEMS Microbiol Ecol. 2004;48(1):1–13. doi: 10.1016/j.femsec.2003.11.012. [DOI] [PubMed] [Google Scholar]
- Kandeler E, Gerber H. Short-term assay of soil urease activity using colorimetric determination of ammonium. Biol Fertil Soils. 1988;6(1):68–72. doi: 10.1007/BF00257924. [DOI] [Google Scholar]
- Kaur J, Anand V, Srivastava S, Bist V, Tripathi P, Naseem M, Nand S, Khare P, Srivastava PK, Bisht S, Srivastava S. Yeast strain Debaryomyces hansenii for amelioration of arsenic stress in rice. Ecotoxicol Environ Saf. 2020;95:110480. doi: 10.1016/j.ecoenv.2020.110480. [DOI] [PubMed] [Google Scholar]
- Kumar P, Sharma PK. Soil salinity and food Security in India. Front Sustain Food Syst. 2020;4:174. doi: 10.3389/fsufs.2020.533781. [DOI] [Google Scholar]
- Kumar K, Manigundan K, Amaresan N. Influence of salt tolerant Trichoderma spp. on growth of maize (Zea mays) under different salinity conditions. J Basic Microbiol. 2017;57(2):141–150. doi: 10.1002/jobm.201600369. [DOI] [PubMed] [Google Scholar]
- Ladd JN, Butler JHA. Short-term assays of soil proteolytic enzyme activities using proteins and dipeptide derivatives as substrates. Soil Biol Biochem. 1972;4(1):19–30. doi: 10.1016/0038-0717(72)90038-7. [DOI] [Google Scholar]
- Lakhdar A, Rabhi M, Ghnaya T, Montemurro F, Jedidi N, Abdelly C. Effectiveness of compost use in salt-affected soil. J Hazard Mater. 2009;171(1–3):29–37. doi: 10.1016/j.jhazmat.2009.05.132. [DOI] [PubMed] [Google Scholar]
- Li LY, WM, and Wang W, Trehalose: protector of antioxidant enzymes or reactive oxygen species scavenger under heat stress? Environ Exp Bot. 2008;63(1–3):378–384. doi: 10.1016/j.envexpbot.2007.11.016. [DOI] [Google Scholar]
- Liang Y, Yang Y, Yang C, Shen Q, Zhou J, Yang L. Soil enzymatic activity and growth of rice and barley as influenced by organic manure in an anthropogenic soil. Geoderma. 2003;115(1–2):149–160. doi: 10.1016/S0016-7061(03)00084-3. [DOI] [Google Scholar]
- Liao W, Ning Z, Chen L, Wei Q, Yuan E, Yang J, Ren J. Intracellular antioxidant detoxifying effects of diosmetin on 2, 2-azobis (2-amidinopropane) dihydrochloride (AAPH)-induced oxidative stress through inhibition of reactive oxygen species generation. J Agric Food Chem. 2014;62(34):8648–8654. doi: 10.1021/jf502359x. [DOI] [PubMed] [Google Scholar]
- Machado RMA, Serralheiro RP. Soil salinity: effect on vegetable crop growth. Management practices to prevent and mitigate soil salinization. Horticulture. 2017;3(2):30. doi: 10.3390/horticulturae3020030. [DOI] [Google Scholar]
- Miller G, Suzuki N, Ciftci-Yilmaz S, Mittler R. Reactive oxygen species homeostasis and signaling during drought and salinity stresses. Plant Cell Environ. 2010;33:453–467. doi: 10.1111/j.1365-3040.2009.02041.x. [DOI] [PubMed] [Google Scholar]
- Mohammadi M, Kazemi H. Changes in peroxidase and polyphenol oxidase activities in susceptible and resistant wheat heads inoculated with Fusarium gramine arum and induced resistance. Plant Sci. 2002;162(4):491–498. doi: 10.1016/S0168-9452(01)00538-6. [DOI] [Google Scholar]
- Naik SK, Maurya S, Kumar R, Sadhna K, Gagrai S, Das B, Kumar S, Bhatt BP. Inorganic phosphate solubilization by phosphate solubilizing fungi isolated from acidic soils. Afr J Microbiol Res. 2013;7(34):4310–4316. doi: 10.5897/AJMR2013.5947. [DOI] [Google Scholar]
- Nakano Y, Asada K. Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol. 1981;22(5):867–880. doi: 10.1093/oxfordjournals.pcp.a076232. [DOI] [Google Scholar]
- Olsen SR (1954) Estimation of available phosphorus in soils by extraction with sodium bicarbonate (No. 939). US Department of Agriculture
- Per TS, Khan NA, Reddy PS, Masood A, Khan HM. Approaches in modulating proline metabolism in plants for salt and drought stress tolerance: phytohormones, mineral nutrients and transgenics. Plant Physiol Biochem. 2017;115:126–140. doi: 10.1016/j.plaphy.2017.03.018. [DOI] [PubMed] [Google Scholar]
- Reyt G, Ramakrishna P, Salas-González I, Fujita S, Love A, Tiemessen D, Lapierre C, Morreel K, Calvo-Polanco M, Flis P, Geldner N. Two chemically distinct root lignin barriers control solute and water balance. Nat Commun. 2021;12(1):1–15. doi: 10.1038/s41467-021-22550-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouphael Y, Carillo P, Colla G, Fiorentino N, Sabatino L, El-Nakhel C, Giordano M, Pannico A, Cirillo V, Shabani E, Cozzolino E. Appraisal of combined applications of Trichoderma virens and a biopolymer-based bio stimulant on lettuce agronomical, physiological, and qualitative properties under variable N regimes. Agronomy. 2020;10(2):196. doi: 10.3390/agronomy10020196. [DOI] [Google Scholar]
- Schmidt A, Kunert KJ. Lipid peroxidation in higher plants: the role of glutathione reductase. Plant Physiol. 1986;2(3):700–702. doi: 10.1104/pp.82.3.700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava PK, Vaish A, Dwivedi S, Chakrabarty D, Singh N, Tripathi RD. Biological removal of arsenic pollution by soil fungi. Sci Total Environ. 2011;409(12):2430–2442. doi: 10.1016/j.scitotenv.2011.03.002. [DOI] [PubMed] [Google Scholar]
- Srivastava S, Srivastava S, Bist V, Awasthi S, Chauhan R, Chaudhry V, Singh PC, Dwivedi S, Niranjan A, Agrawal L, Chauhan PS. Chlorella vulgaris and Pseudomonas putida interaction modulates phosphate trafficking for reduced arsenic uptake in rice (Oryza sativa L.) J Hazard Mater. 2018;351:177–187. doi: 10.1016/j.jhazmat.2018.02.039. [DOI] [PubMed] [Google Scholar]
- Stubberfield LCF, Shaw PJA. A comparison of tetrazolium reduction and FDA hydrolysis with other measures of microbial activity. J Microbiol Methods. 1990;12(3–4):151–162. doi: 10.1016/0167-7012(90)90026-3. [DOI] [Google Scholar]
- Tabatabai MA, Bremner JM. Use of p-nitrophenyl phosphate for assay of soil phosphatase activity. Soil Biol Biochem. 1969;1(4):301–307. doi: 10.1016/0038-0717(69)90012-1. [DOI] [Google Scholar]
- Tandon A, Fatima T, Gautam A, Yadav U, Srivastava S, Singh PC. Effect of Trichoderma koningiopsis on chickpea rhizosphere activities under different fertilization regimes. Open J Soil Sci. 2018;8(10):261. doi: 10.4236/ojss.2018.810020. [DOI] [Google Scholar]
- Tandon A, Fatima T, Shukla D, Tripathi P, Srivastava S, Singh PC. Phosphate solubilization by Trichoderma koningiopsis (NBRI-PR5) under abiotic stress conditions. J King Saud Univ Sci. 2020;32(1):791–798. doi: 10.1016/j.jksus.2019.02.001. [DOI] [Google Scholar]
- Tandon A, Anshu A, Kumar S, Yadav U, Mishra SK, Srivastava S, Chauhan PS, Srivastava PK, Bahadur L, Shirke PA, Srivastava M, et al. Trichoderma-primed rice straw alters structural and functional properties of sodic soil. Land Degrad Dev. 2022;33(5):698–709. doi: 10.1002/ldr.4151. [DOI] [Google Scholar]
- Teulat B, Zoumarou-Wallis N, Rotter B, Salem MB, Bahri H, This D. QTL for relative water content in field-grown barley and their stability across Mediterranean environments. Theor Appl Genet. 2003;108(1):181–188. doi: 10.1007/s00122-003-1417-7. [DOI] [PubMed] [Google Scholar]
- Tiwari S, Lata C, Chauhan PS, Nautiyal CS. Pseudomonas putida attunes morphophysiological, biochemical and molecular responses in Cicerarietinum L. during drought stress and recovery. Plant Physiol Biochem. 2016;99:108–117. doi: 10.1016/j.plaphy.2015.11.001. [DOI] [PubMed] [Google Scholar]
- Tiwari P, Srivastava D, Chauhan AS, Indoliya Y, Singh PK, Tiwari S, Fatima T, Mishra SK, Dwivedi S, Agarwal L, Singh PC, et al. Root system architecture, physiological analysis and dynamic transcriptomics unravel the drought-responsive traits in rice genotypes. Ecotoxicol Environ Saf. 2021;207:111252. doi: 10.1016/j.ecoenv.2020.111252. [DOI] [PubMed] [Google Scholar]
- Walkley A, Black IA. An examination of the Degtjareff method for determining soil organic matter, and a proposed modification of the chromic acid titration method. Soil Sci. 1934;37(1):29–38. doi: 10.1097/00010694-193401000-00003. [DOI] [Google Scholar]
- Yanai J, Nakata S, Funakawa S, Nawata E, Katawatin R, Kosaki T. Effect of NPK application on growth, yield and nutrient uptake by sugarcane on a sandy soil in Northeast Thailand. Trop Agric Dev. 2010;54(4):113–118. [Google Scholar]
- Yin A, Zhang M, Gao C, Yang X, Xu Y, Wu P, Zhang H. Salinity evolution of coastal soils following reclamation and intensive usage, Eastern China. Environ Earth Sci. 2016;5(18):1–1. doi: 10.1007/s12665-016-6095-2. [DOI] [Google Scholar]
- Zhang F, Wang Y, Liu C, Chen F, Ge H, Tian F, Zhang Y. Trichoderma harzianum mitigates salt stress in cucumber via multiple responses. Ecotoxicol Environ Saf. 2019;170:436–445. doi: 10.1016/j.ecoenv.2018.11.084. [DOI] [PubMed] [Google Scholar]
- Zhu J, Brown KM, Lynch JP. Root cortical aerenchyma improves the drought tolerance of maize (Zea mays L.) Plant Cell Environ. 2010;3(5):740–749. doi: 10.1111/j.1365-3040.2009.02099.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.