Abstract
Tapentadol (TAP) and oxycodone/naloxone (OXN) potentially offer an improved opioid tolerability. However, real-world studies in chronic non-cancer pain (CNCP) remain scarce. Our aim was to compare effectiveness and security in daily pain practice, together with the influence of pharmacogenetic markers. An observational study was developed with ambulatory test cases under TAP (n = 194) or OXN (n = 175) prescription with controls (prescribed with other opioids (control), n = 216) CNCP patients. Pain intensity and relief, quality of life, morphine equivalent daily doses (MEDD), concomitant analgesic drugs, adverse events (AEs), hospital frequentation and genetic variants of OPRM1 (rs1799971, A118G) and COMT (rs4680, G472A) genes, were analysed. Test CNCP cases evidenced a significantly higher pain relief predictable due to pain intensity and quality of life (R2 = 0.3), in front of controls. Here, OXN achieved the greatest pain relief under a 28% higher MEDD, 8–13% higher use of pregabalin and duloxetine, and 23% more prescription change due to pain, compared to TAP. Whilst, TAP yielded a better tolerability due the lower number of 4 [0–6] AEs/patient, in front of OXN. Furthermore, OXN COMT-AA homozygotes evidenced higher rates of erythema and vomiting, especially in females. CNCP real-world patients achieved higher pain relief than other traditional opioids with a better tolerability for TAP. Further research is necessary to clarify the potential influence of COMT and sex on OXN side-effects.
Subject terms: Genetics, Neuroscience, Medical research, Biomarkers, Predictive markers, Prognostic markers
Introduction
In last decades, prescription opioids began to be used for chronic non-cancer pain (CNCP) outside the context of palliative medicine1,2. Since then, there has been an increase in opioid long-term use for conditions that are beyond the evidence base3,4. Efforts are necessary to optimize the opioid benefit/risk balance of opioid use developing best clinical practice guidelines based on researched circumstances5.
New, recently marketed opioid with potentially improved tolerability have opened a more optimistic door. Most clinically relevant opioid analgesics bind to μ opioid receptors in the central and peripheral nervous system in an agonist manner to elicit analgesia6. However, naloxone, a μ receptor antagonist, combined with oxycodone (OXN), a μ and k-opioid receptor agonist, may improve pain control, minimizing opioid-related bowel dysfunction adverse events (AEs)7,8. Another new opioid is tapentadol (TAP), that has a dual mode of action. TAP as a Tapentadol displays μ-opioid receptor agonist and noradrenaline reuptake inhibitor properties and is purported to have comparable analgesic efficacy to controlled-release oxycodone with a better opioid tolerability profile9 and fewer pharmacological interactions10 due others opioids.
There are several well-designed studies comparing both OXN’s and TAP’s analgesic effects, however some of the results seem contradictory11,12. Randomized placebo-controlled studies demonstrated similar analgesic levels between the two in osteoarthritis knee pain cases13, and similar tolerability profiles following minor orthopaedic/trauma surgeries14,15. However, in other CNCP studies, TAP showed better gastrointestinal tolerance and quality of life improvement than OXN did16. The main question is whether this better profile is maintained or changed in real-world pain practice with polymedicated and elderly regular pain patients.
Given this scenario, precision medicine could provide data to understand such variability. Mu opioid receptors (OPRM) and Catechol-O-Methyltransferase (COMT) are candidate genes with a significant influence on morphine analgesic response17,18. There are few validated studies that ultimately call for pharmacogenetic testing to be conducted when initiating opioid therapy in pain management19–23. Results have indicated that many favourable analgesic effects may depend on increased OPRM density24,25 and on higher dopamine concentrations in the prefrontal cortex17. Moreover, the OPRMgene has been widely studied for distinct pain sensitivity phenotypes26 and it seems that OPRM A118G (rs1799971) and COMT G1947A (rs4680) heterozygous patients, need significantly less morphine as compared to A118 mutant homozygous ones27. What´s more, sex-specific effects that have been detected9 could be responsible for modulating the COMT genotype’s effects on synaptic dopaminergic concentrations and emotion modulation capabilities28. These results imply a complex nature in the genotype-phenotypes’ interactions.
The purpose of present study was to compare OXN and TAP analgesic effects and tolerability in real-world CNCP management. Here, genetic variants’ (OPRM1 and COMT genes) impact on clinical outcomes were analysed.
Results
Chronic pain was mostly due to lumbago (nonspecific, associated with radiculopathy, spinal stenosis, or another specific spinal cause), followed by knee pain. Nearly half of them had mixed neuropathic-nociceptive symptoms.
Demographic and clinical outcomes
A summary of the characteristics of the subjects included in the study is presented in Table 1.
Table 1.
Demographic, clinical, and pharmacological data in chronic non-cancer pain patient’s total population, control, and tapentadol (TAP) and oxycodone/naloxone (OXN) cases groups.
| Total (n = 584) | Control (n = 216) | CASE | p-value Effect size |
||
|---|---|---|---|---|---|
| TAP (n = 194) | OXN (n = 175) | ||||
| Sex (female) (%) | 71 | 69 | 74 | 65 | 0.193 |
| 0.002I | |||||
| Age | 65 ± 14 | 65 ± 13 | 65 ± 14 | 64 ± 13 | 0.845 |
| 0.004II | |||||
| VAS pain intensity (0–100 mm) | 64 ± 26 | 60 ± 27 | 61 ± 26 | 64 ± 26 | 0.402 |
| 0.002III | |||||
| Likert pain intensity (%) | |||||
| None | 4 | 7 | 6** | 3** | |
| Mild | 10 | 6 | 15** | 13** | < 0.001 |
| Moderate | 29 | 26 | 24** | 29** | 0.98I |
| Severe | 42 | 34 | 42** | 48** | |
| Extremely severe | 15 | 27 | 10** | 17** | |
| VAS pain relief (0–100 mm) | 37 ± 29 | 31 ± 30 | 36 ± 28** | 40 ± 30** | 0.006 |
| 0.016III | |||||
| Likert pain relief (%) | |||||
| None | 21 | 0.007 | |||
| Mild | 28 | 19 | 24 | 32** | 0.42I |
| Moderate | 37 | 34 | 36 | 35** | |
| Severe | 11 | 11 | 11 | 9** | |
| Extremely severe | 3 | 6 | 4 | 3** | |
| VAS EuroQol (0–100 mm) | 45 ± 22 | 45 ± 24 | 45 ± 22 | 46 ± 23 | 0.968 |
| 0.004III | |||||
| Utilization of hospital services (%) | |||||
| Due to pain | |||||
| Prescription change | 24 | 11 | 19 | 42**† | < 0.001 |
| 0.676I | |||||
| Emergency department visit | 21 | 17 | 16 | 22 | 0.2615 |
| 1.07I | |||||
| Hospital admission | 6 | 5 | 4 | 8 | 0.385 |
| 0.47I | |||||
| Due to other causes | |||||
| Prescription change | 21 | 11 | 18 | 26* | < 0.001 |
| 1.18I | |||||
| Emergency department visit | 24 | 14 | 27** | 25** | < 0.001 |
| 1.34I | |||||
| Hospital admission | 17 | 7 | 10 | 22** | < 0.001 |
| 1.93I | |||||
Data is presented as mean ± SD or as %.
Comparison cases vs. control, *p < 0.05, **p < 0.001 and †p < 0.05 tapentadol vs. oxycodone/naloxone, cell in italics. Chi-square χ2 the effect size was determined using ICramer’s V (effect size < 0.2 small, 0.2 < effect size < 0.6 intermediate and effect size > 0.6 large effect).
IIEta squared for One-Way ANOVA.
IIIEta squared for Kruskal Wallis Test (effect size of 0.01–0.04 small, 0.06–0.11 intermediate and 0.14–0.2 large effect).
Large effect size is written in bold font.
Most of CNCP patients were elderly females (65 ± 14 years, 71% women) who presented moderate chronic pain (VAS, 64 ± 26 mm), mild relief (37 ± 29 mm) and moderate QoL (45 ± 22 mm) at the time of inclusion. A total of 3–6% of them had no pain, and 57% had severe or extremely severe pain. Controls suffered around 10% more extreme severe pain and a statistically significant lower pain relief level (VAS, 31 ± 30 mm) as compared to OXN (40 ± 30 mm) and TAP (36 ± 28 mm) test cases (p < 0.001) without any significant differences on pain relief between test cases groups. On the other hand, any significant clinical difference was found between naïve or opioid switchers (Supplementary Table S1).
A significant positive correlation was evidenced between pain relief and QoL in cases with a negative correlation to pain intensity in all groups. Thus, pain intensity and QoL were predictive values of relief (R2 = 0.3). Pain relief was negatively affected by age in control´s relief and positively by anxiolytics in OXN group, while sex, number of AEs, MEDD, neuromodulators, and analgesics showed no impact (Supplementary Table S2).
Utilization of hospital services
Significantly, OXN cases needed double the percentage of prescription changes due to pain as compared to TAP (42% vs. 19%, p = 0.002), as can be seen in Table 1. Due to causes other than pain, OXN cases needed a significant 15% more prescription changes (p < 0.001, large effect size = 1.93, 95% CI [1.2–2.5]), 11% more EDs visits (p < 0.001, large effect size = 1.34. 95% CI [0.7–2.2]) and 15% more hospital admissions than controls (p < 0.001, large effect size = 1.18, 95% CI [0.7–2.02]). Additionally, TAP needed 13% more EDs than controls (p < 0.001, large effect size = 1.93, 95% CI [1.2–2.5]). Globally, cases showed 11–13% more ED visits (p < 0.001, large effect size = 1.34, 95% CI [0.7–2.2]) than controls.
Pharmacology variables
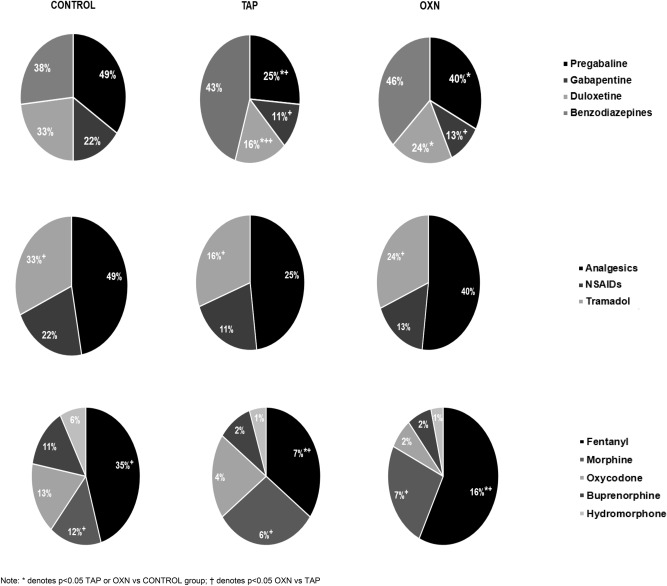
A summary of the prescribed analgesic drugs is presented in Table 2 and Fig. 1.
Table 2.
Analgesic drug prescription in control, and tapentadol (TAP) and oxycodone/naloxone (OXN) cases groups for chronic non-cancer pain.
| Pain medication n (%) | Control (n = 216) | Case | p-value Cramer’s V |
|
|---|---|---|---|---|
| TAP (n = 194) | OXN (n = 175) | |||
| Analgesic | 71 (33) | 73 (36) | 55 (31) | 0.329 |
| 0.044I | ||||
| Tramadol | 92 (43) | 26 (12)* | 13 (7)* | < 0.001 |
| 0.381I | ||||
| NSAIDs | 23 (11) | 25 (12) | 22 (12) | 0.639 |
| 0.023 I | ||||
| Opioids n (%) | ||||
| MEDD (mg/day) | 110 ± 109 | 89 ± 88* | 124 ± 109†† | 0.007 |
| 0.017II | ||||
| Fentanyl transdermal | 75 (35) | 15 (7)** | 29 (16)†* | < 0.001 |
| 0.194I | ||||
| Oxycodone | 28 (13) | 6 (4)* | 3 (2)* | < 0.001 |
| 0.213I | ||||
| Morphine | 27 (12) | 12 (6)* | 4 (2)* | < 0.001 |
| 0.165I | ||||
| Buprenorphine | 23 (11) | 3 (2)* | 4 (2)* | < 0.001 |
| 0.293I | ||||
| Hydromorphone | 14 (6) | 2 (1)** | 2 (1)** | < 0.001 |
| 0.153I | ||||
| Coadyuvants n (%) | ||||
| Pregabalin | 107 (49) | 51 (25)* | 72 (40)† | < 0.001 |
| 0.212I | ||||
| Gabapentin | 48 (22) | 22 (11)* | 24 (13)* | 0.003 |
| 0.138I | ||||
| Duloxetine | 71 (33) | 32 (16)** | 44 (24)† | < 0.001 |
| 0.167I | ||||
| Benzodiazepines | 83 (38) | 88 (43) | 83 (46) | 0.292 |
| 0.064I | ||||
MEDD morphine equivalent daily dose.
Comparison cases vs. control, *p < 0.05, **p < 0.001 and †p < 0.05 tapentadol vs. oxycodone/naloxone, cell in italics denotes also significant differences between tapentadol and oxycodone/naloxone. Effect size was determined as follows: For Chi-square χ2 test using ICramer’s V (effect size < 0.2 small, 0.2 < effect size < 0.6 intermediate and effect size > 0.6 large effect).
IIEta squared for One-Way ANOVA (effect size of 0.01–0.04 small, 0.06–0.11 intermediate and 0.14–0.2 large effect).
Large effect size is written in bold font.
Figure 1.
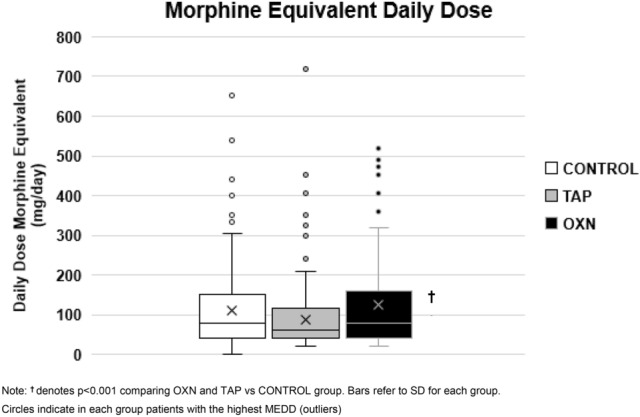
Daily morphine equivalent dose (MEDD) (mean ± SD) depending on control and tapentadol (TAP) and oxycodone/naloxone (OXN) cases groups.
OXN showed the highest MEDD requirements (124 ± 109 mg/day), which were 28% higher than TAP’s (89 ± 88 mg/day, p < 0.001) and 11% higher than controls (110 ± 109 mg/day, p > 0.05). Here, TAP showed the lowest MEDD, even significantly lower than controls. In contrast, the controls required higher tramadol use than cases.
On the other hand, the rates of coadjuvant medication use differed between groups. OXN patients reported a significant 15% higher use of pregabalin (p = 0.002) and 8% higher duloxetine use (p = 0.040), than TAP, as seen at Table 2 and Fig. 2. However, control group needed a significant higher use of 9–24% pregabalin, 9–11% gabapentin and 7–17% of duloxetine (p < 0.001) vs. test cases, but at similar doses requirements. Gabapentin (cases vs. controls, 1600–1800 mg/day vs 1650 [675–2250] mg/day, p = 0.551), antidepressants (above all duloxetine 45 [30–60] mg/day for all groups, p = 0.925), and benzodiazepines (above all lorazepam 3–4 [2–6] mg/day for all groups, p = 0.471) were prescribed in a similar dose-range except for the non-significant higher median dose of pregabalin (cases vs. controls, 300 mg/day vs. 150 [150–300] mg/day, p = 0.236). Other antidepressants (such as amitriptyline or fluoxetine) did not exceed 1–2% of drug prescriptions. Hence, they were not included in the final analysis due to their low prescription rate.
Figure 2.
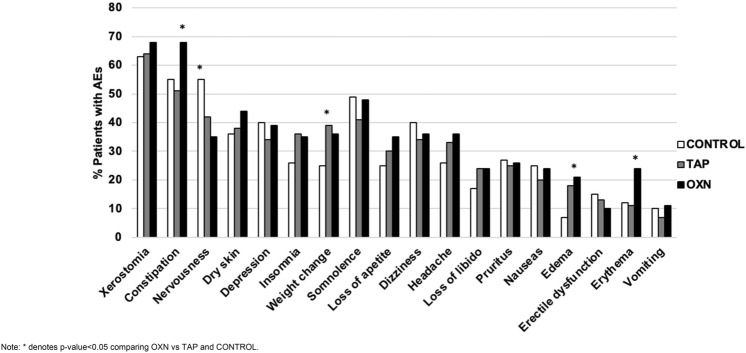
Percentage of patients with adverse events of patients (AEs) self-reported in in total population, control, tapentadol (TAP) and oxycodone/naloxone (OXN) cases groups.
Safety profile
The percentage of patients showing some AE is displayed in Fig. 3 and in Supplementary Table S3.
Figure 3.
Analgesic, opioid and coadjuvant treatment in total population, control, and case (tapentadol and oxycodone/naloxone) groups.
In total, 3131 AEs (incidence rate of 5 AEs/patient) were logged through questionnaires, with most being mild and having disappeared with the withdrawal of the drug. Nearly all patients (94%) reported at least one AE with a median of 6 AEs (IQR 3.5–9) per patient. Most prevalent were dry mouth (65%), nervousness (54%) and constipation (46%). According to MEDRA, the most frequents disorders were: 22% (n = 657) psychiatric (40% nervousness, 29% insomnia, 31% depression), 21% (n = 611) nervous (38% somnolence, 30% headache, 32% dizziness) and 16% gastrointestinal (62.6% constipation, 27% nausea, 10% vomiting).
TAP group referred the lowest number of AEs (4 [0–6] AEs/patient) compared with OXN and control groups. In this context, OXN patients reported more 13–17% frequent constipation (p-value ≤ 0.001, large effect size = 2.4, Odd ratio of OXN vs control 2.46 [1.6–3.8] and OXN vs TAP 2.3 [1.5–3.5]), as well as 12% and 11% higher incidence of erythema than both TAP and control patients (p = 0.002, large effect size = 1.46, Odd ratio OXN vs control 2.37 [1.3–4.2] OXN vs TAP 2.47 [1.4–4.3]).
What´s more, controls showed a significant 7% and 20% higher frequency of nervousness vs. TAP and OXN, respectively (p = 0.002, large effect size = 1.461). Control also developed a higher frequency of edema at 11% and 14% compared to TAP and OXN, respectively (p = 0.001, large effect size = 1.498, Odd ratio of TAP vs control 2.65 [1.3–5.2]; OXN vs control 3.4 [1.7–6.6]). However, TAP patients showed 14% more frequent weight change compared to the control group and 3% compared to OXN (p = 0.047, large effect size = 1.007, Odd ratio of TAP vs control 1.6 [1.03–2.5] and OXN vs TAP 1.02 [0.66–1.56]).
In total, 192 commonly occurring ADRs were noted (ratio of 16 AEs: 1ADR). Mainly systems affected were 25% nervous, 17% psychiatric disorders, and 12% gastrointestinal systems without notable differences between test cases and controls (data not shown).
OPRM1 and COMT genotypes and sex influence
The frequencies of occurrence in the study population of the OPRM1 (rs1799971, A118G) genotypes were 59% for A/A, 38% for A/G and 2% for G/G (Hardy–Weinberg equilibrium (HWE) p = 0.067). On the other hand, the frequency of the COMT (rs4680, G472A) G/G genotype was 26%, G/A 46% and A/A 28% (HWE p = 0.219).
The influence of the OPRM1 and COMT genetic variants over clinical and pharmacological variables in TAP and OXN groups was analysed. In this case, no statistically significant influence was found over these variables.
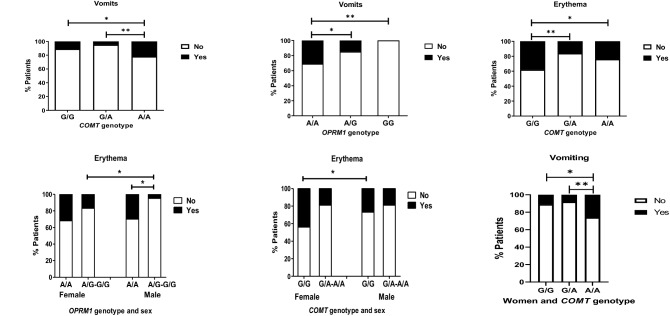
These variants’ significant impacts on frequency of occurrence of erythema (OPRM1 A/A 31%, A/G 15% and G/G 0%, p = 0.037, medium effect size = 0.202), vomiting (COMT G/G 11%, G/A 5%, A/A 22%, p = 0.031, medium effect size = 0.212) and erythema (COMT G/G 38%, G/A 16% and A/A 24%, p = 0.031, medium effect size = 0.210) in OXN patients as can be seen in Fig. 4. Here, females reported a much higher incidence of vomiting, depending on the genotype, with COMT G/G reporting 11%, G/A 8%, and A/A 26% (p ≤ 0.001, medium effect size = 0.221). Incidence of erythema due a higher frequency of flushing was also found to be genotype-dependent in females (COMT G/G 44%, G/A 17% and A/A 26%, p = 0.025, medium effect size = 0.195). Any genetic variant analysed influence on incidence of AEs in cases.
Figure 4.
Difference of frequency of vomiting and erythema adverse events depending on COMT and OPRM1 gene variants in oxycodone/naloxone case groups.
Discussion
Both OXN and TAP achieved a higher pain relief than other traditional opioids with a better improvement in safety profile for TAP. Here, OXN showed the highest pain relief but under a significantly higher MEDD and rates of side-effects compared to TAP. Furthermore, preliminary data indicates a lower opioid tolerability in females OXN group that could vary according to COMT genotype.
These results provide clear directions in terms of clinical practice. Firstly, as expected, control group reported a higher frequency of severe pain29 that together with the QoL, could be predictive for a higher pain relief30. In fact, a stronghold of our data is that provides from CNCP real-world sample of patients, that were 71% middle-aged women, under a multidrug analgesic treatment, who exhibited common pain intensity, tolerability and hospital frequentation rates12,31. Thus, our results would hold for similar pain patients that routinary attends PUs. Secondly, data evidenced that OXN yielded higher pain relief32,33 but with a higher prevalence of side-effects (including constipation rates), drug change requirements due to pain or hospital frequentations due to other causes. What’s more discuss the significant differences concerning coadjuvant medication in OXN and TAP groups, could contribute to the differences observed in analgesic tolerability that should be confirmed in further studies. Our results suggest that TAP could provide better clinical outcomes at lower costs due the lower opioid requirements and incidence of AEs34. It is possible that TAP’s opioid-saving effect due its mechanism of action29 could serve to optimize opioid rotation practices35–37. Here, the TAP more frequent weight change compared to other groups should be deeper analysed. Prior studies have identified an association between obesity and prescription opioid use in the US. However, the pain conditions that are factors in this association remain unestablished38 and this. Unfortunately, the term recorded in our study as “weight change” AE did not specify if was an increase or a loss of weight. Additional larger studies are needed to evaluate these results and, also, whether genotyping COMT, alone or in combination with OPRM1, could be potentially translated to pain practice36.
Our data showed a mild COMT genetic influence on some side-effects, such as erythema and vomiting, especially in females. Previous studies have indicated OPRM1 and COMT genotypes’ significant influence on prevalence of erythema and nausea/vomiting39 such as for acute post-operative pain, CNCP and cancer-related pain40. This can be mediated by dopamine, which is an important neurotransmitter in the postrema area and vomiting centre, when it is used together with catecholamines that can modulate inflammatory processes41. In addition, the remarkably female predominance in this data merits further attention. Mostly of our patients were elderly women, as was previously highlighted in our pain population42. Literature data strongly suggest that men and women differ in their pain responses, potentially due to differences in modulation of the endogenous opioid system43 and sex hormones44, which could, in turn, have differential pharmacogenetic impacts45,46. Awareness about this sex influence should be emphasized in order to improve pain management.
Limitations and strengths
Our data present some limitations. First, the lack of randomization is problematic and raises questions about bias. As noted above, important factors such as duration of pain, type of pain/diagnosis, and psychosocial factors were not controlled. This should be addressed in future studies. What’s more, the diagnoses associated with CNCP were done following clinical routines, but not with other objective measurements. This potentially clouds an understanding of what types of non-opioid analgesics as duloxetine or pregabalin could be appropriate for use. This could have introduced a bias influenced by several other variables, such as sociodemographics, that might be more relevant than pain status47. Second, a convenience sample was assessed based on patients attending the PU. This can affect the population representativeness, especially in genotype variables, and in this way to find significant differences. Thirdly, patients in all groups were able to take multiple opioids, and thus a host of adjuvants from various opioid combinations (such as tramadol with tapentadol) and/or from other non-analgesic prescriptions might have played a role, which was not recorded in the present study. What’s more, patients could receive other concomitant prescriptions due to their comorbidities, they might have independently contributed to the observed side-effects. Thus, AEs could not be always directly attributed to the opioid with the highest prescribed dose. This may limit conclusions related to effectiveness or side-effects. Finally, while a combination of Oxycodone with naloxone may have benefits related to gastrointestinal side effects, potential interactions between these drugs have not been contemplated in this study. All these aspects should be addressed in future studies.
Conclusions
Taken together the findings presented here suggest that opioids of the new generation, OXN and TAP, can control pain intensity than traditional opioids used in pain treatment routines. However, OXN showed a worse tolerability and a higher health resource as compared to TAP. Additionally, COMT genotypes were associated with higher incidence of some opioid side-effects, especially in females. Hence, further studies are warranted to confirm and refine these results on a wider population and finally ascertain the role that pharmacogenetic in terms of improve analgesic tolerability.
Methods
Study design
A real-world observational and cross-sectional study was conducted from November 2014 to November 2017, using CNCP outpatients who required either OXN or TAP prescriptions. CNCP patients were recruited following their routine clinical visits for standard treatment at the Pain Unit (PU, Health Department of the Alicante-General Hospital, Spain). At the time of the enrolment, all participants received information on the design and purpose of the study and provided their written informed consent, allowing their genetic samples and electronic health records (EHRs) to be used for the research. All the methods were carried out in accordance with the ethical guidelines established in the Declaration of Helsinki. The Research Ethics Committee of the Alicante-General Hospital approved the protocol (PI2019/108, 190715), after being classify by Spanish Agency for Medicines and Health Products, which complies with the applicable STROBE guidelines.
Participants
A total of 600 patients were pre-screened, with 7% of patients excluded (due mostly to non-chronic cancer pain or fibromyalgia). Although patients under 18 years old, pregnant women, oncologic pain or any psychiatric disorders that could interfere with the proper development of the study were excluded. Furthermore, other chronic pain syndromes of unclear pathophysiology, such as fibromyalgia, and neuropathic pain, such as painful polyneuropathy, postherpetic neuralgia, trigeminal neuralgia, and post-stroke pain, were not included48.
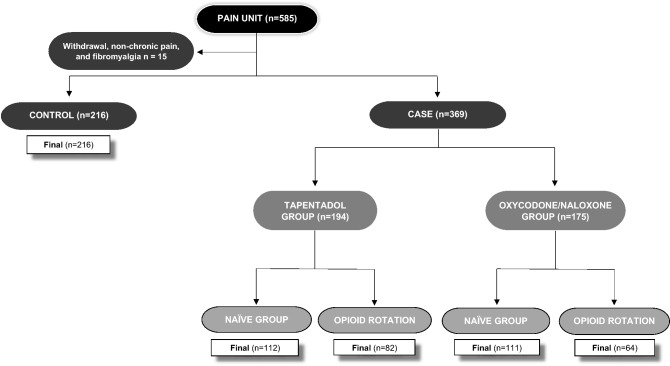
Finally, 585 CNCP patients (mean age 65 ± 14 years old, 71% female and all Caucasian) were included, as displayed in Fig. 5. These patients were included under the following inclusion criteria: adult men and women (≥ 18 years of age) with a stable regimen of regular opioid prescriptions (required opioid prescription for their pain) due to CNCP. There was no minimum pain score required for inclusion in the study.
Figure 5.
Study flow chart of patients’ selection and controls.
Subjects were divided in two groups, cases (n = 369, under routine treatment with TAP or OXN) and controls (n = 216, other opioids except TAP or OXN). Cases could be previously naïve to opioids (n = 223) or who switched from another opioid (n = 146).
First, the researchers reviewed the schedule of PU consultations by patients weekly. Then they pre-screened patients with active OXN or TAP prescriptions and prepared the questionnaires and informed consent forms. In the case that a new patient began an OXN or TAP prescription on the day of the researchers’ visit, PU healthcare notified the researchers for their potential inclusion. For every two cases, one control was included from a concomitant observarional study12 (age and sex-matched patients with the same inclusion criteria yet treated with opioids different from TAP or OXN as fentanyl, morphine or buprenorphine).
The population selected as controls included a total of 1339 clinical records of 753 patients who routinely attend to PU for treat their CNCP. From this group, a subpopulation of 216 patients was extracted who were being treated with a main opioid (excluding OXN, oxycodone without combination or TAP) being main analgesic drug adjuvants prescription rate (gabapentin, duloxentin), similar to those of the test cases studied.
The lack of randomization led to the patients’ being either: 1. under OXN or TAP prescription (the test group previously naïve to opioids), 2. switched to OXN or TAP from a different opioid (minimum a month before), or 3. under another combination of opioids (e.g., morphine or fentanyl plus OXN or TAP), either due to the former’s use as a rescue medication or to aid the switching process. In any case, control group must not under treatment neither with OXN nor TAP and the opioid with the highest MEDD was designated as the main treatment.
Procedure
A consecutive sampling method was used in ambulatory patients. When a patient met the inclusion criteria, he/she was informed about the purpose of the study by the PU healthcare staff. Then, interested individuals were attended to by the research staff for signing of the informed consent paperwork and collection of a saliva sample for the pharmacogenetic analysis.
Demographic data, pain history, drug use and medical history were recorded from EHRs. Clinical data was reported through validated scales and questionnaires completed as part of a standard clinical routine for assessing pain intensity, pain relief, QoL, and most common AEs in pain management49. Outcomes were assessments at a single time point where pain (intensity or relief) and QoL was asked at the present time whilst the cumulative AEs reported since last month.
Validated scales and questionnaires were used to evaluate clinical outcomes and were collected every time a patient was included at a single time point. Pain intensity, relief and QoL were measured using the visual analogue scale (VAS), consecutively by clinical routine. The VAS for each indicator consists of a 100 mm horizontal line ranging from 0 (lowest) to 100 mm (highest), where the patient points on the line to the intensity of pain or relief that he/she feels, respectively49. Specifically, QoL was evaluated through the VAS-EuroQol Scale, which consists of a vertical line from 0 (the worst imaginable health status) to 100 mm (the best imaginable), upon which the patient indicates his/her current health status. Likert pain intensity and relief scales were also registered (4 = extremely intense, 3 = intense, 2 = moderate, 1 = mild, 0 = none) in subsequent questionnaires.
Safety profile
For collection of patients’ reported AEs, a questionnaire with a list of the most frequent adverse drug reactions (ADRs, selected for being “very common” or “common” on the opioids’ Summary of Product Characteristics) and a blank field to add any other AEs was collected. These AEs consisted of: sleepiness, dizziness, nausea, vomiting, constipation, itchiness, sexual dysfunction, loss of libido, weight change, headache, erythema, dry skin, dry mouth, edema, depression, insomnia, nervousness and loss of appetite. Additionally, to the questionnaire, the listed ADRs were recorded from EHRs. Clinical data of AE/ADR reporting were coded according to the medical dictionary for regulatory activities (MedDRA) and the system organ class (SOC)50.
In addition, the percentage of Emergency Department (ED) visits, hospitalizations, or any drug changes due to pain or other causes were registered when patients were included referred to the last month. Prescription changes included: (1) Change in any drug-dosage. (2) Product or generic brand switch. (3) Stopping medication or non-adherence, and (4) starting a new medication51.
The comparison between test cases’ and controls’ opioid benefit/risk profiles was defined as a balance between benefits (decrease in pain intensity and/or increase in pain relief) and tolerability in terms of number of AEs or hospital frequentation52,53.
Drug prescription
Simple analgesics’ use (paracetamol, metamizole and NSAIDs) as well as prescriptions for tramadol and strong opioids like OXN, TAP or others (fentanyl, buprenorphine, morphine, or hydromorphone) were registered. In cases where different opioids were combined, oral MEDD was estimated using available references54.
The use of any other concomitant analgesics most widely prescribed at the PU were also registered from the institution’s EHRs: antidepressants (amitriptyline and duloxetine), anxiolytics (benzodiazepines) and gabapentinoids (pregabalin, gabapentin). For the analysis, these drugs were called “neuromodulators”, given their role as substances that alter the way nerves communicate with each other and, consequently, the overall activity level of the brain55.
Genotyping
Approximately 2 ml of saliva was collected in tubes containing 6 ml of PBS. Once the saliva sample was taken, it was stored at − 80 °C until its processing. Genomic DNA was isolated using the E.N.Z.A. Forensic DNA kit (Omega bio-tek), according to the manufacturer’s instructions. Real-time polymerase chain reaction (RT-PCR) analysis was used to genotype OPRM1 (rs1799971, A118G) and COMT (rs4680, G472A) gene polymorphisms. All PCR amplifications were carried out in a RT-PCR Rotor Gene Q (Qiagen), using specific TaqMan probes MGB® (Applied Biosystems). The amplification parameters were as follows: initial 10 min denaturation at 95 °C, 45 cycles for 15 s at 92 °C, 90 s at 60 °C, and 1 min final extension at 60 °C.
Statistical analysis
Convenience sampling was considered to be more likely to represent the target population. This entailed selecting participants on the basis of availability until the final sample size was achieved56. The assumption of normality was validated with the Kolmogorov Smirnov test using the Lilliefors correction method. Quantitative parametric data is presented as mean ± standard deviation (SD) while non-parametric data and discrete variables are shown using their median values (interquartile range). Categorical data is expressed by percentages, among them the relative frequencies of genotypes and alleles.
Comparisons between any two given groups (case, controls) of data exhibiting parametric distributions was conducted using the independent T-test analysis, and for analyses comparing three groups an ANOVA test was carried out. Outcomes from opioid naïve vs opioid rotation patients were done to wonder any difference. Analysis of non-parametric data was done using U Mann–Whitney and Kruskal–Wallis tests for comparison between two and three groups, respectively. Comparisons for categorical data were conducted using Chi-square (χ2) goodness-of-fit and Fisher’s exact test. A multiple linear regression was performed to generate a predictive risk model and to analyse the influence of the following variables over pain relief: age, gender, VAS pain intensity, EQD, MEDD, number of AEs and the use of neuromodulators, anxiolytics, and analgesics.
In addition to this, the effect sizes were calculated for all the comparisons. Eta-Squared (η2) was used for ANOVA and Kruskal–Wallis analyses (effect size between 0.01 and 0.04 being a small effect, 0.06 and 0.11 intermediate and 0.14 and 0.2 a large effect), while for the chi-square χ2 the effect size was determined using the Cramer’s V method (effect size < 0.2 being a small, 0.2–0.6 intermediate, and > 0.6 large) and using Odd Ratios for AEs between study groups.
Observed gene frequencies were compared to expected values using the chi-square χ2 goodness-of-fit test and the Hardy–Weinberg proportion. Chi-square test analysis was conducted to compare the distribution of genotypes and alleles between the different groups. The subjects were grouped based on their genetic profiles, whether they were homozygotes or heterozygous, and whether they were carriers or non-carriers of a determinate allele. In cases of significant genetic associations, co-dominant, dominant, recessive, and over-dominant models were calculated. Sex analysis of genotypes were grouped according to the presence or absence of the mutant allele (OPRM1 A/A vs A/G-G/G and in COMT gene G/G VS G/A-A/A) when frequencies of mutant alleles were low.
p-values < 0.05 were considered statistically significant. In all cases, multiple testing was adjusted using the Bonferroni correction. Analyses were carried out using the R software package version 4.0.3 and Graph Pad Prism 5.0.
Supplementary Information
Acknowledgements
We would like to thank Pain Unit Nurses Mrs. Andrea Flor and Fernanda Jiménez (Department of Health of Alicante-General Hospital, Alicante, Spain) for their help with patients’ care; Pura Ballester, Ph.D. (Neuropharmacology on Pain (NED) at the Alicante Institute for Health and Biomedical Research (ISABIAL-FISABIO Foundation) in Alicante, Spain) for her support in the laboratory and reviewing this article; as well as Maila Sepri for her help reviewing and correcting this article.
Abbreviations
- CNCP
Chronic non-cancer pain
- OXN
Oxycodone/naloxone
- AEs
Adverse events
- TAP
Tapentadol
- OPRM1
Opioid receptor Mu
- COMT
Catechol-O-methyltransferase
- PU
Pain Unit
- EHRs
Electronic health records
- VAS
Visual analogue scale
- QoL
Quality of life
- ED
Emergency Department
- ADRs
Adverse drug reactions
- MedDRA
Medical dictionary for regulatory activities
- SOC
System organ class
- NSAIDs
Non-steroidal anti-inflammatory drugs
- MEDD
Morphine equivalent daily dose
- SD
Standard deviation
- ANOVA
Analyses of variance
- HWE
Hardy–Weinberg equilibrium
Author contributions
J.B.: This author helped with the conceptualization of the work, data collection, data analysis and interpretation, and drafting the article. C.M.: This author helped with the conceptualization of the work, data collection, interpretation of analysis and drafting the article. J.M.: This author helped with the conceptualization of the work, data collection, and data analysis and interpretation. S.L.G. and V.L.G.: These authors helped with data collection, and data analysis and interpretation. A.V.G.: This author helped with data collection, data analysis and interpretation and drafting the article. B.P.: This author helped with data collection and data analysis and interpretation. M.M.I.: This author helped with data analysis and interpretation and drafting the article. D.M.: This author helped with data analysis and interpretation. A.M.P.: This author helped with the conceptualization of the word, data collection, analysis interpretation, and drafting the article. All the authors reviewed and gave the final approval of the following version to be published and are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Data availability
The datasets generated during and/or analysed during the current study are not publicly available due include medical information of patients but are available from the corresponding author on reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-022-13085-5.
References
- 1.Dowell D, Haegerich TM, Chou R. CDC guideline for prescribing opioids for chronic pain—United States, 2016. MMWR Recommend. Rep. 2016;65:1–49. doi: 10.15585/mmwr.rr6501e1. [DOI] [PubMed] [Google Scholar]
- 2.Hardt J, Jacobsen C, Goldberg J, Nickel R, Buchwald D. Prevalence of chronic pain in a representative sample in the United States. Pain Med. 2008;9:803–812. doi: 10.1111/j.1526-4637.2008.00425.x. [DOI] [PubMed] [Google Scholar]
- 3.Muriel J, et al. Pharmacogenetics and prediction of adverse events in prescription opioid use disorder patients. Basic Clin. Pharmacol. Toxicol. 2019 doi: 10.1111/bcpt.13155. [DOI] [PubMed] [Google Scholar]
- 4.Fernandes K, et al. High-Dose opioid prescribing and opioid-related hospitalization: A population-Based study. PLoS ONE. 2016;11:e0167479. doi: 10.1371/journal.pone.0167479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alexander G, Frattaroli S, Gielen A. The Prescription Opioid Epidemic: An Evidence-Based Approach. Johns Hopkins Bloomberg School of Public Health; 2015. [Google Scholar]
- 6.Pathan H, Williams J. Basic opioid pharmacology: An update. Br. J. Pain. 2012;6:11–16. doi: 10.1177/2049463712438493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ueberall MA, Mueller-Schwefe GHH. Efficacy and tolerability balance of oxycodone/naloxone and tapentadol in chronic low back pain with a neuropathic component: A blinded end point analysis of randomly selected routine data from 12-week prospective open-label observations. J. Pain Res. 2016;9:1001–1020. doi: 10.2147/JPR.S112418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kang JH, Lee GW, Shin SH, Bruera E. Opioid withdrawal syndrome after treatment with low-dose extended-release oxycodone and naloxone in a gastric cancer patient with portal vein thrombosis. J. Pain Symp. Manage. 2013;46:e15. doi: 10.1016/j.jpainsymman.2013.02.009. [DOI] [PubMed] [Google Scholar]
- 9.Langford RM, Knaggs R, Farquhar-Smith P, Dickenson AH. Is tapentadol different from classical opioids? A review of the evidence. Br. J. Pain. 2016;10:217–221. doi: 10.1177/2049463716657363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Biondi DM, Xiang J, Etropolski M, Moskovitz B. Tolerability and efficacy of tapentadol extended release in elderly patients ≥75 years of age with chronic osteoarthritis knee or low back pain. J. Opioid Manage. 2015;11:393–403. doi: 10.5055/jom.2015.0289. [DOI] [PubMed] [Google Scholar]
- 11.Planelles B, et al. Gender based differences, pharmacogenetics and adverse events in chronic pain management. Pharmacogenom. J. 2020;20:320–328. doi: 10.1038/s41397-019-0118-9. [DOI] [PubMed] [Google Scholar]
- 12.Planelles B, et al. Health benefits of an adverse events reporting system for chronic pain patients using long-term opioids. Acta Anaesthesiol. Scand. 2019;63:248–258. doi: 10.1111/aas.13243. [DOI] [PubMed] [Google Scholar]
- 13.Afilalo M, et al. Efficacy and safety of tapentadol extended release compared with oxycodone controlled release for the management of moderate to severe chronic pain related to osteoarthritis of the knee: A randomized, double-blind, placebo-and active-controlled phase III study. Clin. Drug Investig. 2010;30:489–505. doi: 10.2165/11533440-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 14.Abeyaratne C, Lalic S, Bell JS, Ilomäki J. Spontaneously reported adverse drug events related to tapentadol and oxycodone/naloxone in Australia. Ther. Adv. Drug Saf. 2018;9:197–205. doi: 10.1177/2042098618760939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Haeseler G, et al. Combatting pain after orthopedic/trauma surgery-perioperative oral extended-release tapentadol vs extended-release oxycodone/naloxone. BMC Anesthesiol. 2017;17:91. doi: 10.1186/s12871-017-0383-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Baron R, et al. Tolerability, safety, and quality of life with tapentadol prolonged release (PR) compared with oxycodone/naloxone PR in patients with severe chronic low back pain with a neuropathic component: A randomized, controlled, open-label, phase 3b/4 trial. Pain Pract. 2016;16:600–619. doi: 10.1111/papr.12361. [DOI] [PubMed] [Google Scholar]
- 17.Diatchenko L, et al. Genetic basis for individual variations in pain perception and the development of a chronic pain condition. Hum. Mol. Genet. 2005;14:135–143. doi: 10.1093/hmg/ddi013. [DOI] [PubMed] [Google Scholar]
- 18.Kieffer BL, Gavériaux-Ruff C. Exploring the opioid system by gene knockout. Prog. Neurobiol. 2002;66:285–306. doi: 10.1016/S0301-0082(02)00008-4. [DOI] [PubMed] [Google Scholar]
- 19.Hoehe MR, et al. Sequence variability and candidate gene analysis in complex disease: Association of μ opioid receptor gene variation with substance dependence. Hum. Mol. Genet. 2000;9:2895–2908. doi: 10.1093/hmg/9.19.2895. [DOI] [PubMed] [Google Scholar]
- 20.Ablin JN, Buskila D. Personalized treatment of pain. Curr. Rheumatol. Rep. 2013 doi: 10.1007/s11926-012-0298-7. [DOI] [PubMed] [Google Scholar]
- 21.Bonica JJ. The relation of injury to pain. Pain. 1979;7:203–207. doi: 10.1016/0304-3959(79)90012-5. [DOI] [PubMed] [Google Scholar]
- 22.Kambur O, Männistö PT. Catechol-O-methyltransferase and pain. Int. Rev. Neurobiol. 2010;95:227–279. doi: 10.1016/B978-0-12-381326-8.00010-7. [DOI] [PubMed] [Google Scholar]
- 23.Cheung CW, et al. Chronic opioid therapy for chronic non-cancer pain: A review and comparison of treatment guidelines. Pain Phys. 2014;17:401–414. doi: 10.36076/ppj.2014/17/401. [DOI] [PubMed] [Google Scholar]
- 24.Diatchenko L, et al. Catechol-O-methyltransferase gene polymorphisms are associated with multiple pain-evoking stimuli. Pain. 2006;125:216–224. doi: 10.1016/j.pain.2006.05.024. [DOI] [PubMed] [Google Scholar]
- 25.Melia U, et al. Interaction between EEG and drug concentration to predict response to noxious stimulation during sedation-analgesia: Effect of the A118G genetic polymorphism. Annu. Int. Conf. IEEE Eng. Med. Biol. Soc. 2014;2014:4298–4301. doi: 10.1109/EMBC.2014.6944575. [DOI] [PubMed] [Google Scholar]
- 26.Crist RC, Berrettini WH. Pharmacogenetics of OPRM1. Pharmacol. Biochem. Behav. 2014;123:25. doi: 10.1016/j.pbb.2013.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mura E, et al. Consequences of the 118A>G polymorphism in the OPRM1 gene: Translation from bench to bedside? J. Pain Res. 2013;6:331. doi: 10.2147/JPR.S42040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Harrison PJ, Tunbridge EM. Catechol-O-methyltransferase (COMT): A gene contributing to sex differences in brain function, and to sexual dimorphism in the predisposition to psychiatric disorders. Neuropsychopharmacology. 2008;33:3037–3045. doi: 10.1038/sj.npp.1301543. [DOI] [PubMed] [Google Scholar]
- 29.Steigerwald I, et al. Effectiveness and tolerability of tapentadol prolonged release compared with prior opioid therapy for the management of severe, chronic osteoarthritis pain. Clin. Drug Investig. 2013;33:607–619. doi: 10.1007/s40261-013-0102-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.van de Donk T, et al. Tapentadol treatment results in long-term pain relief in patients with chronic low back pain and associates with reduced segmental sensitization. PAIN Rep. 2020;5:e877. doi: 10.1097/PR9.0000000000000877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pergolizzi J, et al. Opioids and the management of chronic severe pain in the elderly: Consensus statement of an International Expert Panel with focus on the six clinically most often used World Health Organization Step III opioids (buprenorphine, fentanyl, hydromorphone, methadone, morphine, oxycodone) Pain Pract. 2008;8:287–313. doi: 10.1111/j.1533-2500.2008.00204.x. [DOI] [PubMed] [Google Scholar]
- 32.Jespersen A, et al. Is neuropathic pain underdiagnosed in musculoskeletal pain conditions? The Danish PainDETECTive study. Curr. Med. Res. Opin. 2010;26:2041–2045. doi: 10.1185/03007995.2010.502748. [DOI] [PubMed] [Google Scholar]
- 33.Finnerup NB, et al. Pharmacotherapy for neuropathic pain in adults: Systematic review, meta-analysis and updated NeuPSIG recommendations. Lancet Neurol. 2015;14:162. doi: 10.1016/S1474-4422(14)70251-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Coluzzi F, Ruggeri M. Clinical and economic evaluation of tapentadol extended release and oxycodone/naloxone extended release in comparison with controlled release oxycodone in musculoskeletal pain. Curr. Med. Res. Opin. 2014;30:1139–1151. doi: 10.1185/03007995.2014.894501. [DOI] [PubMed] [Google Scholar]
- 35.Gatti A, et al. Effects of opioid rotation in chronic pain patients. Clin. Drug Investig. 2010;30:39–47. doi: 10.2165/1158413-S0-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 36.Sarzi-Puttini P, et al. The appropriate treatment of chronic pain. Clin. Drug Investig. 2012;32:21–33. doi: 10.2165/11630050-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 37.Merchant S, et al. Budget impact analysis of tapentadol extended release for the treatment of moderate to severe chronic noncancer pain. Clin. Ther. 2013;35:659–672. doi: 10.1016/j.clinthera.2013.03.016. [DOI] [PubMed] [Google Scholar]
- 38.Stokes A, et al. Association of obesity with prescription opioids for painful conditions in patients seeking primary care in the US. JAMA Netw. Open. 2020;3:e202012. doi: 10.1001/jamanetworkopen.2020.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Aroke EN, Hicks TL. Pharmacogenetics of postoperative nausea and vomiting. J. Perianesthesia Nurs. 2019;34:1088–1105. doi: 10.1016/j.jopan.2019.03.007. [DOI] [PubMed] [Google Scholar]
- 40.Margarit C, et al. OPRM1 gene interaction with sleep in chronic pain patients treated with opioids. Pain Phys. 2019;22:97–107. [PubMed] [Google Scholar]
- 41.Oh J, Fernando A, Muffley L, Honari S, Gibran NS. Correlation between the warrior/worrier gene on post burn pruritus and scarring: A prospective cohort study. Ann. Surg. 2020 doi: 10.1097/SLA.0000000000004235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ranque B, Nardon O. Prise en charge des symptômes médicalement inexpliqués en médecine interne: Un paradigme de la relation médecin-malade en situation d’incertitude. Rev. Med. Interne. 2017;38:458–466. doi: 10.1016/j.revmed.2016.12.005. [DOI] [PubMed] [Google Scholar]
- 43.Pieretti S, et al. Gender differences in pain and its relief. Ann. Ist. Super. Sanita. 2016 doi: 10.4415/ANN_16_02_09. [DOI] [PubMed] [Google Scholar]
- 44.Traub RJ, Ji Y. Sex differences and hormonal modulation of deep tissue pain. Front. Neuroendocrinol. 2013 doi: 10.1016/j.yfrne.2013.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Planelles B, et al. Gender based differences, pharmacogenetics and adverse events in chronic pain management. Pharmacogenom. J. 2020 doi: 10.1038/s41397-019-0118-9. [DOI] [PubMed] [Google Scholar]
- 46.Samulowitz A, Gremyr I, Eriksson E, Hensing G. ‘Brave men’ and ‘emotional women’: A theory-guided literature review on gender bias in health care and gendered norms towards patients with chronic pain. Pain Res. Manage. 2018;2018:1–14. doi: 10.1155/2018/6358624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sharma N, Chakrabarti S, Grover S. Gender differences in caregiving among family—Caregivers of people with mental illnesses. World J. Psychiatry. 2016;6:7. doi: 10.5498/wjp.v6.i1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Baron R, Binder A, Wasner G. Neuropathic pain: Diagnosis, pathophysiological mechanisms, and treatment. Lancet Neurol. 2010;9:807–819. doi: 10.1016/S1474-4422(10)70143-5. [DOI] [PubMed] [Google Scholar]
- 49.Barrachina J, et al. Global pain state questionnaire: Reliability, validity, and gender gap. Arch. Intern. Med. Res. 2021;4:084–106. doi: 10.26502/aimr.0061. [DOI] [Google Scholar]
- 50.Spanish Agency for Medicines and Health Products. Online Information Center of Medicines of Spanish Agency of Medicines and Health Products (AEMPS-CIMA). https://cima.aemps.es/cima/publico/home.html (2016). Accessed 9 Sept 2020.
- 51.Sino CG, et al. The association between prescription change frequency, chronic disease score and hospital admissions: A case control study. BMC Pharmacol. Toxicol. 2013;14:39. doi: 10.1186/2050-6511-14-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rojas GC, et al. Benefit-Risk Methodology Project. Development and Testing of Tools and Processes for Balancing Multiple Benefits and Risks as an Aid to Informed Regulatory Decisions About Medicinal Products. The European Medicines Agency; 2009. [Google Scholar]
- 53.European Medicines Agency . Benefit-Risk Methodology Project Work Package 2 Report: Applicability of Current Tools and Processes for Regulatory Benefit-Risk Assessment. European Medicines Agency; 2010. [Google Scholar]
- 54.Mercadante S, et al. Opioid switching from and to tapentadol extended release in cancer patients: Conversion ratio with other opioids. Curr. Med. Res. Opin. 2013;29:661–666. doi: 10.1185/03007995.2013.791617. [DOI] [PubMed] [Google Scholar]
- 55.Richards BL, Whittle SL, Buchbinder R. Neuromodulators for pain management in rheumatoid arthritis. Cochrane Database Syst. Rev. 2012 doi: 10.1002/14651858.CD008921.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mathieson K. Making sense of biostatistics: Types of nonprobability sampling. J. Clin. Res. Best Pract. 2014;10:1–2. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during and/or analysed during the current study are not publicly available due include medical information of patients but are available from the corresponding author on reasonable request.