Abstract
In this study, we observed the effect of the application of soil dust enriched with risk elements (Cd, Pb, As and Zn) to leaf surfaces of lettuce (Lactuca sativa var. capitata) while it was grown under hydroponic conditions. This study aimed to determine how low soil dust particulate matter (PM) doses affected the activity of or damaged the photosynthetic apparatus and how the uptake of risk elements was associated with both epigenetic changes (5-methylcytosine content, i.e., 5mC) and stress metabolism. During the study, we obtained many results pertaining to risk element contents and biochemical (total phenolic content (TPC), malondialdehyde (MDA) content and the amount of free amino acids (AAs)) and physiological (photosynthesis parameters: net photosynthetic rate, transpiration rate, intercellular CO2 concentration, stomatal conductance, instantaneous water-use efficiency, maximum quantum yield of PSII, chlorophyll and carotenoid contents, and leaf water potential (WP)) plant features. The results showed an increase in MDA and 5mC. However, the transpiration rate, WP and free AAs decreased. In conclusion, contamination by very low doses of soil dust PM had no direct or significant effect on plant fitness, as shown by the TPC and 5mC content, which indicates that plants can overcome the oxidative stress caused by the accumulation of risk elements. From the above, we propose the use of epigenetic changes as biomarkers of potential changes in the activation of plant metabolism under stress caused by environmental pollution.
Subject terms: Physiology, Plant sciences
Introduction
The sources of risk elements in dust can be from natural localities as well as from localities with anthropogenic contamination due to current or past mining activities. The problems caused by dust applied to plant leaves have been examined in many studies1,2. Risk elements can be absorbed by leaves via wet or dry deposition of atmospheric fallout3. The absorption and accumulation of risk elements can influence crop safety and human health4.
As published by Salih and Aziz5, leaves are considered good bioindicators of air pollution. According to Bendel and Weigel6, short-term exposure to soil dust particulate matter (PM) at relatively high concentrations generally leads to acute visible foliar injury. Chronic exposure to lower concentrations results in alterations in physiological and biochemical processes that may manifest as chlorosis, premature senescence, growth and yield reductions. The absorption and effects of PM are influenced by foliar morphology and physiology, e.g., cuticular waxes, stomatal numbers, frequency and length of trichomes and length of petioles, as well as physicochemical properties of PM, such as the particle size, the content of toxic active ingredients, solubility, volatility, polarity, reactivity, hydrolysis, the stability to ozone, UV radiation and atmospheric oxygen7–9. The level of damage that can be caused by dust depends on plant resistance against dust particles and on the ecotoxicological availability of the dust through exposure processes7. Of course, the whole effect is dependent on the resistance, tolerance or sensitivity of the plant9.
Dust has different physicochemical properties that determine bioavailability and thus toxicokinetic processes4,10. Dust particles can change the optical properties of a leaf, especially the surface reflectance in the visible and shortwave infrared radiation range11. Soil dust features are especially influenced by their origin12. Therefore, soil dust can contain metals/metalloids such as Cd, Pb, Zn and As that can accumulate in plant leaves after foliar application13. These risk elements in dust can pose a threat to plants by causing oxidative stress through an imbalance in reactive oxygen species (ROS) production14,15. However, Turan et al.16 showed a positive role of Zn in minimising the negative effects of ROS because Zn is the main cofactor of the antioxidative enzyme superoxide dismutase17. The uptake of contaminants affects the primary metabolism of plants18,19. A study by Gajbhiye et al.2 showed that the results and consequences of foliar uptake must be derived from the interactive role between plant and metal type. In addition, the dose of contaminant plays an important role. Despite considerable progress in recent years about the foliar absorption of risk elements by plants, there still exists a gap in knowledge3. Soil dust particles can enter the inside of a leaf by a stomatal or cuticular pathway13. If the PMs are so large that they do not pass through the stomata, they can clog them20. The persistence of particles is also dependent on weather conditions. Rainwater or wind may remove these particles21. Wet surfaces increase deposition potential, notwithstanding that rain may partly carry away the deposited dust22.
The dust particles on leaf surfaces limit stomatal function23, increase temperature24, contribute to some physiological changes25 and cause changes in photosynthetic pigment ratios26 and mineral contents27. Atmospheric compounds and air pollutants that interact with other abiotic and biotic factors have a complex effect on crop performance6 as well as soil quality28.
The main aim of our experiment was to determine the influence of low soil dust application on lettuce leaves growing under defined conditions. The experimental plants were grown under hydroponic conditions to eliminate the effect of soil29 and the absorption of risk elements by roots. The effects of risk elements in soil dust PMs, which cause stress after absorption by leaves, were investigated in the lettuce plants. Therefore, we determined how the uptake of risk elements was associated with both epigenetic changes and stress metabolism and how low soil dust doses affected the activity/damage of the photosynthetic apparatus.
Materials and methods
Plant material and hydroponic experiment
A hydroponic pot experiment was carried out under greenhouse conditions (natural photoperiod; temperature day/night 23/19 °C; relative humidity ~ 60%; light and dark period 10/14 h) using lettuce (Lactuca sativa var. capitata). After pre-cultivation in Rockwool, lettuce plants were grown in 15 l standard Hoagland solution for 14 days. Two treatments were designed: control and soil dust application. On the leaves of the treatment with soil dust, 1 g of sieved soil (mesh size 1 × 1 mm) from a contaminated location (Table 1) was applied by dusting, and then rain was simulated by the spraying of demineralized water (10 ml/plant). In the experiment, there were twelve plants per pot per treatment. The plants were harvested in four replicates per treatment (three plants were randomly selected per replicate). After harvesting, the leaves were washed with demineralized water, dried by cellulose wadding and partitioned, with one portion being immediately frozen in liquid nitrogen and kept in a deep freezer (− 80 °C) until DNA isolation and free AA analysis, while the second portion was oven-dried to a constant weight (three days at 40 °C) and homogenised for elemental analysis.
Table 1.
Characteristics of the contaminated soil used for soil dust application.
| Location | Litavka (49° 43′ N, 14° 0′ E) |
|---|---|
| Soil type and subtype | Gleyic fluvisol |
| Soil texture | Clay loamy sand |
| pH (–) | 5.3 |
| CEC (mmol(+)/kg) | 57 ± 4 |
| Corg (%) | 1.8 |
| Astotal (mg/kg)/Asws (mg/kg) | 286 ± 14/1 ± 0.2 |
| Cdtotal (mg/kg)/Cdws (mg/kg) | 37 ± 0.8/0.2 ± 0.02 |
| Pbtotal (mg/kg)/Pbws (mg/kg) | 2344 ± 57/5 ± 1 |
| Zntotal (mg/kg)/Znws (mg/kg) | 3515 ± 54/16 ± 1 |
CEC is the cation exchange capacity, Corg is organic carbon, (As, Cd, Pb or Zn)total is the pseudototal content of elements determined by ICP–MS (extraction by aqua regia), and (As, Cd, Pb or Zn)ws is the water-soluble content of elements determined by ICP–OES (24-h extraction by demineralized water, 1:5 w/v). Czech legislation limits for pseudototal contents of elements in light-textured soils (mg/kg): As—15, Cd—0.4, Pb—55 and Zn—10530.
Analysis of elements
Homogenised plant material (0.5 ± 0.05 g of dry weight) was digested in 10 ml of a 4:1 (v/v) mixture of HNO3 and H2O2 in an Ethos 1 device (MLS GmbH, Germany). After cooling, the digested sample was diluted to 50 ml with demineralized water. The contents of As, Cd, Mg, Pb and Zn were determined using an inductively coupled plasma-optical emission spectrometer (ICP–OES; Agilent 720, Agilent Technologies Inc., USA). A certified reference material (CRM NIST 1573a tomato leaves) was mineralized under the same conditions for quality assurance.
Determination of lipid peroxidation
The malondialdehyde (MDA) content was measured based on a modified thiobarbituric acid (TBA) method31. Fresh leaves (0.4 g) were homogenised with liquid nitrogen and 80% ethanol and centrifuged in 2 ml microcentrifuge tubes for 5 min at 6000 rpm. Aliquots of 0.7 ml of each supernatant were mixed with 0.7 ml of 0.65% TBA in 20% TCA (trichloroacetic acid) and 0.01% BHT (butylated hydroxytoluene), and a second set of 0.7 ml samples was mixed with 0.7 ml 20% TCA and 0.01% BHT. The microcentrifuge tubes were incubated at 95 °C for 25 min, and after cooling, they were centrifuged for 5 min at 6000 rpm. The absorbance at 440 nm, 532 nm, and 600 nm was read on a UV–Vis spectrophotometer (Evolution 210, Thermo Scientific), and the concentration of MDA (nmol/g FW) was calculated using the equation of Heath and Packer32.
Determination of the total phenolic content
The total phenolic content (TPC) was determined according to the original method described by Singleton and Rossi33, with slight modifications, using ethanolic extracts from the lipid peroxidation assay with tenfold-diluted Folin–Ciocalteu reagent (Sigma–Aldrich) and a UV–Vis spectrophotometer (Evolution 210, Thermo Fisher Scientific) at wavelength 760 nm. The content was calculated as equivalents of gallic acid (GAE mg/g FW).
Isolation of DNA and determination of relative DNA methylation status based on the % of 5-methylcytosine
Plant DNA was isolated by a NucleoSpin Plant II Molecular Kit (Macherey–Nagel GmbH & Co. KG, Germany) as described by Zemanová et al.34. The global DNA methylation status of DNA was determined using 100 ng of isolated DNA and a MethylFlash Methylated DNA Quantification Kit (Fluorometric; Epigentek Group Inc., Farmingdale, USA) according to the manufacturer’s instructions. A spectrophotometer (Tecan Infinity M200, Tecan Deutschland GmbH) with excitation at 530 nm was used to measure the fluorescence at 590 nm using the Magellan program.
Analysis of free amino acids
Free amino acids (AAs) were extracted, derivatized and quantitated as described by Pavlíková et al.35. Extracts were derivatized using an EZ:faast kit (Phenomenex, USA). The prepared samples were analysed on a Hewlett Packard 6890 N/5975 MSD gas chromatography–mass spectrometry (GC–MS) system (Agilent Technologies, USA).
Determination of gas-exchange parameters
The portable gas exchange system LCpro+ (ADC BioScientific, Ltd., UK) was used for in situ determination of the net photosynthetic rate (Pn; µmol/CO2/m2/s), transpiration rate (E; mmol H2O/m2/s), intercellular CO2 concentration (Ci; µmol CO2/mol), stomatal conductance (gs; mol H2O/m2/s) and calculation of instantaneous water-use efficiency (WUE; WUE = Pn/E). All measurements were conducted between 8:00 and 11:30 Central European Time (CET). The duration of each individual measurement was 10 min after the establishment of steady-state conditions inside the measurement chamber. The conditions in the chamber were 25 °C, ambient CO2 concentration (550 ± 50 µl/l), airflow rate of 205 ± 30 µmol/s and photosynthetically active radiation of 650 ± 50/µmol/m2/s.
Determination of pigments
The pigment content in the leaves was measured by a UV–Vis spectrophotometer (Evolution 210, Thermo Fisher Scientific Inc., USA). Fresh leaf segments (0.5 cm2) were incubated in the dark in 1 ml dimethylformamide, with shaking for 24 h. The absorbance of the extract was measured at wavelengths of 480, 646.8 and 663.8 nm. Absorbance values at 710 nm were subtracted from these measurements. From these data, pigment contents were calculated using the Porra et al.36 equations for chlorophylls and the Wellburn37 equations for carotenoids:
The pigment contents were normalised to the leaf area.
Determination of fluorescence
Chlorophyll fluorescence was measured using a portable fluorometer (OS1-FL; Opti-Sciences, ADC, BioScientific, Ltd., UK). A fresh leaf was shaded for 20 min using clips to set up a dark-adapted state. Chlorophyll fluorescence was excited by a 660 nm solid-state light source, with filters blocking radiation longer than 690 nm. The saturation of the measured photosystem was achieved by using a filtered 35 W halogen lamp (350–690 nm) with a pulse of 15,000 µmol/m2/s for 0.8 s. The maximum quantum yield of PSII (Fv/Fm) was calculated as Fv/Fm = (Fm − F0)/Fm.
Determination of the leaf water potential
Leaf water potential (WP; MPa), as a measure of the energy status of the water in a system, was measured using a dew point PotentiaMeter (Decagon Devices, Inc., Pullman, USA). The leaves of the plants were placed in a disposable syringe, the air was drawn off from the syringe, and the syringe was tightly closed with Parafilm. The specimen was frozen at –18 °C, and then thawed, and the sap was pushed into the measuring chamber of the PotentiaMeter.
Statistical analyses
Statistical analyses were performed with Statistica 12.0 software (StatSoft, USA). All data were checked for homogeneity of variance and normality (Levene’s and Shapiro–Wilk tests). One-way analysis of variance (ANOVA) followed by a post hoc comparison with Fisher’s LSD test (p ≤ 0.05) was used to identify significant differences between treatments. Correlation analysis was performed using Pearson’s linear correlation (r; p ≤ 0.05). The present study complies with relevant institutional, national, and international guidelines and legislation.
Results
Content of risk elements in leaf biomass and their influence on leaf yield
The application of soil dust influenced the dry weight (DW) of the leaves as shown in Table 2. The plant DW in the soil dust PM treatment was 16.7% lower than that of the control, but the difference was not significant. The contents of the analysed risk elements were under the limit of detection in the control except for Zn (Table 2). The application of soil dust increased the contents of As, Cd, Pb and Zn in the dust treatments 126-, 780- and 309.5-fold, respectively, while Zn was only threefold higher compared with the control. The highest increase from these risk elements was shown for Cd. All mentioned risk elements correlated with each other (r = 0.95–0.99; p ≤ 0.05). The application of soil dust increased the content of the analysed risk elements in the leaves, but the leaf yield was not significantly decreased by the toxicity of these elements.
Table 2.
Contents of risk elements and leaf yields in the control and soil dust treatments.
| Parameter | Control | Soil dust |
|---|---|---|
| As (mg/kg DW) | < 0.03 | 3.79 ± 0.65 |
| Cd (mg/kg DW) | < 0.001 | 0.78 ± 0.21 |
| Pb (mg/kg DW) | < 0.02 | 6.19 ± 1.37 |
| Zn (mg/kg DW) | 36.49 ± 7.62a | 111.22 ± 15.76b |
| DW (g) | 10.80 ± 2.23a | 9.03 ± 0.06a |
DW represents the dry weight per treatment. Values are means ± SD (n = 4). Lower-case letters indicate significant differences based on Fisher’s LSD test (p ≤ 0.05). Data with the same letter are not significantly different.
Leaf stress response after the application of contaminated soil dust particulate matter
The application of soil dust to leaf area decreased leaf WP compared with the control (Fig. 1). The average values of leaf WP in the dust and control treatments were − 1.75 MPa and − 1.27 MPa, respectively. In comparison with the control, the soil dust treatment leaf water potential was decreased by 27.4%.
Figure 1.
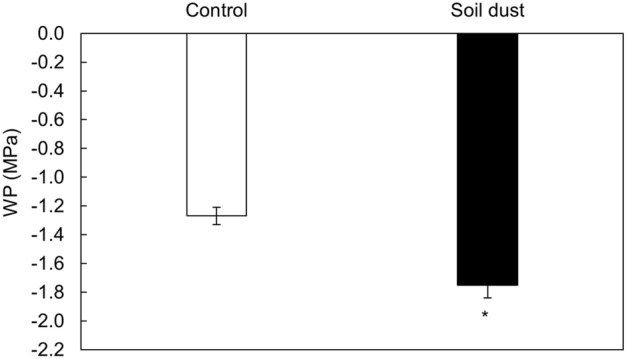
The effect of soil dust application on leaf water potential (WP) in lettuce grown under hydroponic conditions. Significant differences between treatments are indicated by asterisks (ANOVA; Fisher’s LSD test; p ≤ 0.05).
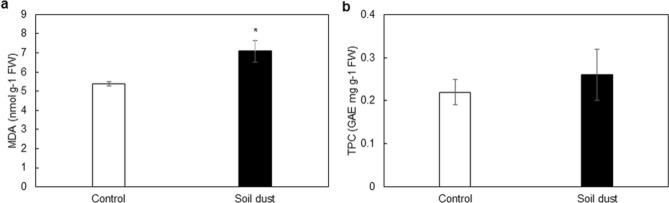
MDA is a well-known bioindicator of the degree of membrane damage because it is caused by oxidative stress after the degradation of unsaturated fatty acids. The application of soil dust increased the content of MDA in the dust treatment by 24.1% compared to the control (Fig. 2a); however, the increase in TPC caused by the application of soil dust PM to leaves in the dust treatment was not significant compared to the control (Fig. 2b).
Figure 2.
The effect of soil dust application on the content of malondialdehyde (MDA) (a) and the total phenolic content (TPC) (b) in lettuce grown under hydroponic conditions. Significant differences between treatments are indicated by asterisks (ANOVA; Fisher’s LSD test; p ≤ 0.05). Columns without asterisks are not significantly different.
Monitoring epigenetic changes in plants is an innovative way to characterise the response of plants to the effects of abiotic and biotic stress factors. The content of 5mC influenced by soil dust application to leaves is shown in Fig. 3. The content was significantly higher in the dust treatment than in the control. The 5mC content increased by 24.9%. It is clear from the increasing methylation of DNA that the toxicity of the absorbed risk elements was not relevant based on the insignificant reduction of the DW leaf yield.
Figure 3.
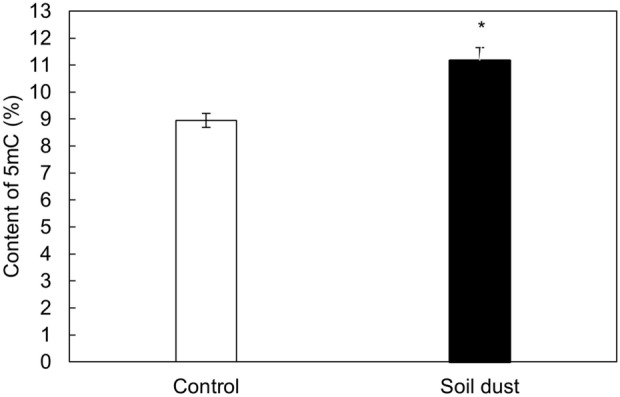
Effect of the soil dust application on the content of 5-methylcytosine (5mC) in lettuce grown under hydroponic conditions. Significant differences between treatments are indicated by asterisks (ANOVA; Fisher’s LSD test; p ≤ 0.05).
The free AA content was affected by soil dust application, i.e., there were changes in the free AA metabolism of the lettuce. Soil dust application decreased the total content of free AAs, which consisted of 19 free AAs and related compounds, by 20.4% compared with the control (Table 3). Furthermore, the content of transport and storage AAs (tAAs), which contain glutamate (Glu), glutamine (Gln), aspartate (Asp) and asparagine (Asn), was lower in the soil dust treatment than in the control; however, the difference was not significant (Table 3). The results clearly showed an effect of soil dust application on free Glu, free proline (Pro) and free ornithine (Orn) in lettuce leaves when compared with the control, and the contents of these AAs decreased by 15%, 12% and 11.5%, respectively. The content of tAAs correlated with the total free AA content in the control (r = 0.97, p ≤ 0.001) and in the soil dust application treatment (r = 0.90, p ≤ 0.01). A higher correlation was observed between the total free AA content and Pro (r = 0.99, p ≤ 0.001) in the soil dust application treatment than in the control (r = 0.89, p ≤ 0.01). The same trend was observed for the total free AA content and Orn (r = 0.99, p ≤ 0.001 and r = 0.91, p ≤ 0.01 under soil dust application and in the control, respectively) (Table 3).
Table 3.
Content of selected free amino acids in the lettuce leaves of the control and soil dust treatments.
| Parameter | Control | Soil dust |
|---|---|---|
| Σ AAs (µmol/kg FW) | 10,380.61 ± 1645.33b | 8259.34 ± 850.99a |
| tAAs (µmol/kg FW) | 2752.09 ± 925.89a | 2095.11 ± 177.44a |
| Glu (µmol/kg FW) | 857.01 ± 127.88b | 735.08 ± 69.82a |
| Pro (µmol/kg FW) | 439.73 ± 42.37b | 387.17 ± 43.80a |
| Orn (µmol/kg FW) | 575.30 ± 55.09b | 508.93 ± 59.38a |
Σ AAs: total content of free amino acids, tAAs: sum of transport and storage free amino acids, Glu: free glutamine, Pro: free proline, Orn: free ornithine. Values are means ± SD. Lower-case letters indicate significant differences based on Fisher’s LSD test (p ≤ 0.05). Data with the same letter are not significantly different.
Response of the photosynthetic apparatus after application of contaminated soil dust particulate matter
The application of soil dust to the leaf area affected some gas-exchange parameters (Table 4). Soil dust decreased the E of plants, which was 22.2% lower in the dust treatment than in the control. In contrast, the application of soil dust increased the WUE by 31.6% compared with the control. Furthermore, other gas-exchange parameters were affected by soil dust application, but the results of Pn, Ci and gs were not significant (Table 4). The application of soil dust to the leaves did not affect the fluorescence parameter (Table 4) because the decrease in the maximum quantum yield of PSII in the dust treatment was not significant compared to the control. These results show that the effect of toxic elements in dust on photosynthetic parameters is consistent with an insignificant reduction in the DW of the leaves.
Table 4.
Gas-exchange parameters and chlorophyll fluorescence in the control and soil dust treatments.
| Parameter | Control | Soil dust |
|---|---|---|
| Pn (µmol CO2/m2/s) | 9.34 ± 2.05a | 9.75 ± 0.95a |
| E (mmol H2O/m2/s) | 1.79 ± 0.44b | 1.42 ± 0.21a |
| gs (mol H2O/m2/s) | 0.16 ± 0.05a | 0.16 ± 0.03a |
| Ci (µmol CO2/mol) | 340.55 ± 22.71a | 335.28 ± 13.90a |
| WUE (µmol CO2/mmol H2O) | 5.24 ± 0.74a | 6.91 ± 0.81b |
| Fv/Fm (–) | 0.83 ± 0.01a | 0.83 ± 0.01a |
Pn: net photosynthetic rate, E: transpiration rate, gs: stomatal conductance, Ci: intercellular CO2 concentration, WUE: water use efficiency (Pn/E), Fv/Fm: maximum quantum yield of photosystem II (Fv/Fm = (Fm-F0)/Fm). Values are means ± SD. Lower-case letters indicate significant differences based on Fisher’s LSD test (p ≤ 0.05). Data with the same letter are not significantly different.
Of the pigments extracted by dimethylformamide, only the contents of Chl a and Chlt were significantly lower in the dust treatment than in the control (Table 5). The contents were decreased by 26.4% and 23.5%, respectively. Chl a/b and Chlt/Car were calculated from the measured values. The difference between the Chl a/b ratio in the soil dust treatment and the control was 13.5% (3.62 and 3.13, respectively). The value of the Chlt/Car ratio in the soil dust treatment decreased by only 4.6% compared with the control (4.8 and 4.6, respectively). Neither ratio differed significantly. Furthermore, the content of Mg was significantly decreased by 9.1% compared with the control (Table 5).
Table 5.
Contents of chlorophyll, carotenoids and magnesium in the control and soil dust treatments.
| Parameter | Control | Soil dust |
|---|---|---|
| Chl a (mg/m2) | 22.65 ± 2.49b | 16.69 ± 1.10a |
| Chl b (mg/m2) | 6.27 ± 0.85a | 5.44 ± 1.08a |
| Chlt (mg/m2) | 28.92 ± 3.31b | 22.13 ± 2.09a |
| Car (mg/m2) | 6.07 ± 0.96a | 4.88 ± 0.79a |
| Mg (g/kg DW) | 4.37 ± 0.32b | 4.00 ± 0.24a |
Chl a: chlorophyll a, Chl b: chlorophyll b, Chlt: total chlorophyll, Car: carotenoids. Values are means ± SD. Lower-case letters indicate significant differences based on Fisher’s LSD test (p ≤ 0.05). Data with the same letter are not significantly different.
Discussion
According to Maletsika and Nanos38, the application of dust originating from contaminated soil increases the aboveground biomass. In contrast, the low dose of applied soil dust in the present study did not affect the DW yield of the leaves in the two treatments (Table 2), while the risk elements contained in soil dust applied to the leaf surfaces increased the content of elements in the plant biomass (Table 2), similar to the results reported by Xiong et al.8. Additionally, the study of Gajbhiye et al.2 showed a link between the application of soil dust to the foliar surface, plant growth and the content of elements in the leaves. The results of Žalud et al.39 showed that the application of a PM suspension to leaf surfaces increased the content of the elements, including Cd. According to these authors, the effect of PM size on the foliar absorption of elements was negligible.
Heavy metals such as Cd40,41 and Pb19 and metalloids such as As41 can cause oxidative stress because they induce an increase in reactive oxygen species (ROS) production at the subcellular level and increase MDA production14. The plant responses described above are in perfect agreement with our results (Fig. 2). Oxidative stress and associated stress metabolism are secondarily induced by soil dust PM42. Our results showed that lettuce leaves respond to risk elements in soil dust PM after the absorption of these elements into the leaf (Fig. 2, Table 2). Increased MDA is a product of oxidative stress, which subsequently increasing leaf senescence, including premature tissue ageing43, by signalling molecules44.
Zn, Cu, Mn, Fe and Ni play key roles as cofactors of various metalloenzymes45. During oxidative stress, these elements, especially Zn together with Mn, Cu and Fe, play a role in the mitochondrial, chloroplast and cytosolic enzyme superoxide dismutase, which is irreplaceable in the plant antioxidant defence system17. Zinc also plays an important role in the synthesis of photosynthetic pigments46. In this experiment, a high amount of As and very high amounts of Zn and Pb were measured in the applied soil dust (Table 1). A high Zn content in the analysed plants compared to the control (Table 2) was therefore available for the functioning of the antioxidant system, which is an integral part of the ascorbate–glutathione cycle45. Therefore, the presence of Zn has a positive effect by reducing the oxidative stress caused by Cd47. Thus, a significant decrease in Chl b and Car was not confirmed (Table 5).
The content of photosynthetic leaf pigments is reduced by the presence of some risk elements in dust PM5. A negative effect on the content of pigments, especially chlorophyll, occurred in soybean due to Cd14, in Pistia stratiotes due to Pb48 and in sunflower due to As49. However, the opposite result was reported by a very important study conducted by Gusman et al.50, who examined the effect of As on lettuce pigments. The increased content of risk elements defines the response of a plant’s fitness costs, such as the pigment contents, photosynthetic parameters and epigenetic changes34. Therefore, these plant responses are determined by the adaptation/resistance/tolerance/sensitivity of the plants to different contents of toxic elements. When a plant depletes its C resources, pigments are reduced, and negative changes in photosynthetic parameters are observed51. Another important indicator of plant fitness cost is the accumulation of bioactive forms of cytokinins that delay senescence and increase chlorophyll biosynthesis52. Nevertheless, the Pteris hyperaccumulator continues to accumulate both As and bioactive and transport forms of cytokinins, which delay senescence by activating chlorophyll biosynthesis. However, even if the C sources are depleted and chlorophyll fluorescence decreases, irreversible leaf senescence is induced35. It follows from the above that the presented results of the photosynthetic parameters (Table 4) and contents of photosynthetic pigments (Table 5) indicate reversible leaf senescence53.
The decline in the chlorophyll content also has a close connection with the decrease in Mg27 because there was a significant difference in the Mg content of the lettuce leaves between the soil dust treatment and the control (Table 5). This is consistent with the published results of Ciećko et al.54, who showed a negative correlation between Cd and Mg. Magnesium deficiency and concomitant accumulation of risk elements in lettuce, compared to the control, reduced both the chlorophyll content and the photosynthesis parameters, as discussed earlier in two Noaccaea species55. This is also in agreement with the results of Küpper et al.18, who showed that in plants, Mg2+ is substituted in chlorophyll by heavy metals (Cd, Cu, Ni, Zn and Pb). This replacement, i.e., Cd, leads to the inhibition of photosynthesis and, in particular, to the breakdown of chlorophyll. At the same time, Cd after penetration into chloroplasts is known to disrupt their function and inhibit enzymes associated with chlorophyll biosynthesis and photosystem II responses40.
ROS accumulation degrades unsaturated fatty acids56 into a number of products such as oxylipins, carbonyls and reactive electrophilic products. These include odd-carbon metabolites such as MDA, 2-propenal, propanal, 2-pentenal and others, which are potential precursors of odd fatty acids57 or 12-oxo-phytodienoic acid and jasmonic acid58. At the same time, high levels of peroxidation of unsaturated bonds reduces the content of unsaturated fatty acids, the decrease of which causes a violation of the permeability and fluidity of the biomembrane59.
Increased ROS production in chloroplasts causes a reduced rate of photosynthesis processes (e.g., CO2 fixation), damage to thylakoid function and ultrastructural changes in the thylakoid system in barley or tomato chloroplasts by Pb19 and in lettuce by Cd60. Because of the action of accumulated ROS, plant growth is inhibited, photosynthetic pigments are reduced, the photosynthetic apparatus is destroyed and nutrient homeostasis is impaired.
Subsequently, stress phytohormones (e.g., jasmonic, salicylic and abscisic acids) and antioxidant secondary metabolites are induced61. One group of important antioxidant secondary metabolites is phenolic compounds (Fig. 2b), which include phenylpropanoids, lignans, naphthoquinones, stilbenes, flavonoids, isoflavones and others62. In lettuce, phenolic compounds (Fig. 2b) and other parameters that did not show significant differences in the reduction of Pn, gs, Ci, Chl b, Car, Fv/Fm, and DW (Tables 2, 4 and 5) conflict with the increase in MDA (Fig. 2a) as well as damage to the photosynthetic apparatus (E, WUE, Chl a; Tables 4 and 5). This indicates only partial damage to the photosynthetic apparatus and partial activation of the antioxidant plant system63, which explains the insignificant reduction in antioxidant phenolic metabolites (Fig. 2b). This is also consistent with the results of Agati et al.64, who linked an increase in phenolic compounds to a decrease in growth by a reduction of the growth phytohormone auxin.
Increases/decreases in phenolic compounds are related to the duration of stress and the degree of damage of the photosynthetic apparatus, induction of the onset of reversible/irreversible leaf senescence and potential changes in epigenetic gene silencing that cause gene inactivation/reactivation after DNA methylation/demethylation65. The results showed that there was no significant reduction in most of the photosynthetic parameters and pigments. The effect of stress caused by toxic elements after the application of soil dust PM on lettuce leaves was small, and therefore, the lettuce plants had a good fitness status. Lettuce fitness and increased DNA methylation (Fig. 3) are associated with efforts to increase growth, while DNA demethylation is associated with decreased growth, accelerated flowering and leaf senescence66. Changes in DNA methylation in seedlings are very specific because as these very young leaves age, DNA methylation must increase, which in turn leads to increased growth of the examined seedlings67. The increase in 5mC also supports the fact that the application of very low doses of Cd to seedlings first increases DNA methylation, while after a further increase in Cd there is already a decrease in DNA methylation68. In plants, an increase/decrease in % 5mC and good/poor fitness, as well as the degree of stress caused by the accumulated toxic elements, are inextricably linked34,35.
Due to the application of soil dust, WUE increased even though the transpiration rate was limited by narrowed stomata. This probably affected the decrease in the amount of CO2 in mesophyll cells and subsequently decreased the photosynthetic performance. This is in line with the results of the foliar application of Zn on wheat, which enhanced WUE69. However, the other observed risk elements caused the opposite effect. For example, Chaneva et al.70 stated that the transpiration rate rapidly decreased in the presence of Cd in hydroponic systems. Similar results were found in soybean and lettuce in the presence of Pb71, which is assumed to be connected to root damage. The next element with the same impact is As. Arsenic limits stomatal conductance, thus decreasing the transpiration rate50. Maletsika and Nanos38 carried out a similar study on plants exposed to dust. From their results, it is obvious that the applied dust had a negative influence on the transpiration and photosynthetic rates as well as stomatal conductance and the chlorophyll content. From the above results, it is clear that the composition of the risk elements in the applied soil dust (Table 1) influenced synergistic and antagonistic relationships between the risk elements, thus affecting the resulting increase/decrease in WUE.
Although dust did not have a direct effect on leaf water potential72 in the present study, a significant decrease in leaf WP was observed in the soil dust treatment (Fig. 1). The explanation is the same as that for transpiration and WUE. The decrease in leaf WP could be explained by the increased accumulation of toxic elements present in the leaves of plants73.
Low photosynthesis causes lower amounts of assimilates, thus leading to limited biomass74. In this study, a significant decrease in free AAs, especially Glu, Pro and Orn, was observed (Table 3). Proline, which is synthesized from Glu or Orn1,75, is an essential AA for the synthesis of proteins rich in Pro or Hyp. These proteins are contained in the cell wall and are associated with cell growth, cell wall structure functionality and pollen fertility75,76. In contrast, the importance of Glu in plants results from the biosynthesis of chlorophyll and glutathione, which are subsequently used for the biosynthesis of phytochelatins. These thiol nonprotein metabolites subsequently detoxify the risk elements. Glutathione is an essential metabolite of the plant antioxidant system, which includes the ascorbate–glutathione cycle77. Glucose produces ascorbic acid, which is an irreplaceable component of the antioxidant system. Through glucose, the antioxidant system of plants is closely linked through the Calvin cycle to photosynthesis63. Under oxidative stress caused by toxic elements, a lack of ascorbic acid and Glu cause the antioxidant plant system to collapse. Glutamic acid, through the biosynthesis of glutathione and phytochelatins, is irreplaceable for the functioning of the plant's defence system and the detoxification of the toxic effects of risk elements78. Therefore, in lettuce leaves, the decrease in free AA was related to the defence response of the plants to oxidative stress, as evidenced by the increase in MDA (Table 3, Fig. 2a).
Conclusions
The results of our study showed that contamination by very low doses of soil dust PM had no significant or direct effect on plant fitness. However, the lettuce leaf condition was affected by risk elements in soil dust PM. The degree of damage caused by oxidative stress was demonstrated by the increased level of MDA. The effect of oxidative stress caused by the increased content of toxic elements in lettuce leaves did not have a significant effect on the fitness of the plants because the decreases in DW, most photosynthetic parameters and pigments were not significant. The reduction of transport AAs, especially Glu, is related to the protection of plants against the increased oxidative stress caused by the accumulation of risk elements. The reduction in phenolic substances, especially the increase in the 5mC percentage, showed that the plants overcome oxidative stress caused after further accumulation of risk elements in soil dust PM. Therefore, this manuscript draws attention to the danger of soil dust PM containing risk elements through induced epigenetic changes in the tested plants. We propose the use of epigenetic changes as biomarkers of the possible change in activation of a stressed plant’s metabolism caused by environmental pollution.
Author contributions
All authors conceived the research plans; M.L. and V.Z. performed the experiment under the supervision of D.P. and F.H.; M.L., V.Z. and M.Pa. wrote the article with contributions from other authors. M.Po. is responsible for analysis linked to DNA methylation/demethylation.
Funding
This research was funded by the Ministry of Education, Youth and Sports from the European Regional Development Fund-Project “Centre for the investigation of synthesis and transformation of nutritional substances in the food chain in interaction with potentially harmful substances of anthropogenic origin: comprehensive assessment of soil contamination risks for the quality of agricultural production” (Grant no. CZ.02.1.01/0.0/0.0/16_019/0000845).
Data availability
The data used to support the findings of this study are included within the article. The datasets generated and/or analysed during the current study are not publicly available due apprehension of unauthorized use of data and plagiarism but are available from the corresponding author on reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Pavlík M, et al. Trace elements present in airborne particulate matter—Stressors of plant metabolism. Ecotox. Environ. Safe. 2012;79:101–107. doi: 10.1016/j.ecoenv.2011.12.009. [DOI] [PubMed] [Google Scholar]
- 2.Gajbhiye T, Pandey SK, Kim K-H. Factors controlling the deposition of airborne metals on plant leaves in a subtropical industrial environment. Asian J. Atmos. Environ. 2016;10:162–167. doi: 10.5572/ajae.2016.10.3.162. [DOI] [Google Scholar]
- 3.Shahid M, et al. Foliar heavy metal uptake, toxicity and detoxification in plants: A comparison of foliar and root metal uptake. J. Hazard. Mater. 2016;325:36–58. doi: 10.1016/j.jhazmat.2016.11.063. [DOI] [PubMed] [Google Scholar]
- 4.Luo X, Bing H, Luo Z, Wang Y, Jin L. Impacts of atmospheric particulate matter pollution on environmental biogeochemistry of trace metals in soil-plant system: A review. Environ. Pollut. 2019;255:113138. doi: 10.1016/j.envpol.2019.113138. [DOI] [PubMed] [Google Scholar]
- 5.Salih Z, Aziz F. Heavy metals accumulation in leaves of five plant species as a bioindicator of steel factory pollution and their effects on pigment content. Pol. J. Environ. Stud. 2019;28:4351–4358. doi: 10.15244/pjoes/99304. [DOI] [Google Scholar]
- 6.Bender J, Weigel HJ. Changes in atmospheric chemistry and crop health: A review. Agron. Sustain. Dev. 2011;31:81–89. doi: 10.1051/agro/2010013. [DOI] [Google Scholar]
- 7.Prusty BAK, Mishra PC, Azeez PA. Dust accumulation and leaf pigment content in vegetation near the national highway at Sambalpur, Orissa, India. Ecotox. Environ. Safe. 2005;60:228–235. doi: 10.1016/j.ecoenv.2003.12.013. [DOI] [PubMed] [Google Scholar]
- 8.Xiong T-T, et al. Foliar uptake and metal(loid) bioaccessibility in vegetables exposed to particulate matter. Environ. Geochem. Health. 2014;36:897–909. doi: 10.1007/s10653-014-9607-6. [DOI] [PubMed] [Google Scholar]
- 9.Ram SS, et al. A review on air pollution monitoring and management using plants with special reference to foliar dust adsorption and physiological stress responses. Crit. Rev. Environ. Sci. Technol. 2015;45:2489–2522. doi: 10.1080/10643389.2015.1046775. [DOI] [Google Scholar]
- 10.Rai PK. Impacts of particulate matter pollution on plants: Implications for environmental biomonitoring. Ecotox. Environ. Safe. 2016;129:120–136. doi: 10.1016/j.ecoenv.2016.03.012. [DOI] [PubMed] [Google Scholar]
- 11.Zhao Y, et al. Study on spectral response and estimation of grassland plants dust retention based on hyperspectral data. Remote Sens. 2020;12:2019. doi: 10.3390/rs12122019. [DOI] [Google Scholar]
- 12.Kuki KN, Oliva MA, Pereira EG. Iron ore industry imissions as a potential ecological risk factor for tropical coastal vegetation. J. Environ. Manage. 2008;42:111–121. doi: 10.1007/s00267-008-9093-7. [DOI] [PubMed] [Google Scholar]
- 13.Schreck E, et al. Metal and metalloid foliar uptake by various plant species exposed to atmospheric industrial fallout: Mechanisms involved for lead. Sci. Total Environ. 2012;427–428:253–262. doi: 10.1016/j.scitotenv.2012.03.051. [DOI] [PubMed] [Google Scholar]
- 14.Hashem H. Cadmium toxicity induces lipid peroxidation and alters cytokinin content and antioxidant enzyme activities in soybean. Botany. 2014;92:1–7. doi: 10.1139/cjb-2013-0164. [DOI] [Google Scholar]
- 15.Turan V, et al. Promoting the productivity and quality of brinjal aligned with heavy metals immobilization in a wastewater irrigated heavy metal polluted soil with biochar and chitosan. Ecotox. Environ. Safe. 2018;161:409–419. doi: 10.1016/j.ecoenv.2018.05.082. [DOI] [PubMed] [Google Scholar]
- 16.Turan V, et al. Alleviation of nickel toxicity and an improvement in zinc bioavailability in sunflower seed with chitosan and biochar application in pH adjusted nickel contaminated soil. Arch. Agron. Soil Sci. 2018;64:1053–1067. doi: 10.1080/03650340.2017.1410542. [DOI] [Google Scholar]
- 17.Cakmak I. Tansley review No. 111—Possible roles of zinc in protecting plant cells from damage by reactive oxygen species. New Phytol. 2000;146:185–205. doi: 10.1046/j.1469-8137.2000.00630.x. [DOI] [PubMed] [Google Scholar]
- 18.Küpper H, Parameswaran A, Leitenmaier B, Trtílek M, Šetlík I. Cadmium-induced inhibition of photosynthesis and long-term acclimation to cadmium stress in the hyperaccumulator (Thlaspi caerulescens) New Phytol. 2007;175:655–674. doi: 10.1111/j.1469-8137.2007.02139.x. [DOI] [PubMed] [Google Scholar]
- 19.Legocka J, Sobieszczuk-Nowicka E, Wojtyla Ł, Samardakiewicz S. Lead-stress induced changes in the content of free, thylakoid- and chromatin-bound polyamines, photosynthetic parameters and ultrastructure in greening barley leaves. J. Plant Physiol. 2015;186–187:15–24. doi: 10.1016/j.jplph.2015.07.010. [DOI] [PubMed] [Google Scholar]
- 20.Li Y, Wang Y, Wang B, Wang Y, Yu W. The response of plant photosynthesis and stomatal conductance to fine particulate matter (PM2.5) based on leaf factors analyzing. J. Plant Biol. 2019;62:120–128. doi: 10.1007/s12374-018-0254-9. [DOI] [Google Scholar]
- 21.Prajapati SK, Tripathi BD. Seasonal variation of leaf dust accumulation and pigment content in plant species exposed to urban particulates pollution. J. Environ. Qual. 2008;37:865–870. doi: 10.2134/jeq2006.0511. [DOI] [PubMed] [Google Scholar]
- 22.Sundar S, Naresh R. Modeling the effect of dust pollutants on plant biomass and their abatement from the near earth atmosphere. Model. Earth Syst. Environ. 2017;3:42. doi: 10.1007/s40808-017-0302-3. [DOI] [Google Scholar]
- 23.Arvin AA, Cheraghi S. Evaluation of dust effect on the quantitative and qualitative growth of sugarcane varieties CP57-614. Phys. Geog. Res. 2013;45:95–106. [Google Scholar]
- 24.Zia-Khan S, et al. Effect of dust deposition on stomatal conductance and leaf temperature of cotton in northwest China. Water. 2015;7:116–131. doi: 10.3390/w7010116. [DOI] [Google Scholar]
- 25.Chaturvedi RK, et al. Effect of dust load on the leaf attributes of the tree species growing along the roadside. Environ. Monit. Assess. 2013;185:383–391. doi: 10.1007/s10661-012-2560-x. [DOI] [PubMed] [Google Scholar]
- 26.Rai, P. Leaf dust deposition and its impact on biochemical aspect of some roadside plants in Aizawl, Mizoram, North-East India. Int. J. Environ. Sci.3, (2014).
- 27.Giri S, Shrivastava D, Deshmukh K, Dubey P. Effect of air pollution on chlorophyll content of leaves. Curr. Agric. Res. 2013;1:93–98. doi: 10.12944/CARJ.1.2.04. [DOI] [Google Scholar]
- 28.Bilen S, Bilen M, Turan V. Relationships between cement dust emissionsand soil properties. Pol. J. Environ. Stud. 2019;28:3089–3098. doi: 10.15244/pjoes/92521. [DOI] [Google Scholar]
- 29.Antonkiewicz J, Jasiewicz C, Koncewicz-Baran M, Bączek-Kwinta R. Determination of lithium bioretention by maize under hydroponic conditions. Arch. Environ. Prot. 2017;43:94–104. doi: 10.1515/aep-2017-0036. [DOI] [Google Scholar]
- 30.Public notice No. 153/2016. Public notice No. 153/2016 for the management of soil protection. Czech Ministry of the Environment, Prague, 2016 [In Czech]. (2016).
- 31.Hodges DM, DeLong JM, Forney CF, Prange RK. Improving the thiobarbituric acid-reactive-substances assay for estimating lipid peroxidation in plant tissues containing anthocyanin and other interfering compounds. Planta. 1999;207:604–611. doi: 10.1007/s004250050524. [DOI] [PubMed] [Google Scholar]
- 32.Heath RL, Packer L. Photoperoxidation in isolated chloroplasts: I. Kinetics and stoichiometry of fatty acid peroxidation. Arch. Biochem. Biophys. 1968;125:189–198. doi: 10.1016/0003-9861(68)90654-1. [DOI] [PubMed] [Google Scholar]
- 33.Singleton VL, Rossi JA. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965;16:144–158. [Google Scholar]
- 34.Zemanová V, et al. Effect of arsenic stress on 5-methylcytosine, photosynthetic parameters and nutrient content in arsenic hyperaccumulator (Pteris cretica L.) var. Albo-lineata. BMC Plant Biol. 2020;20:130. doi: 10.1186/s12870-020-2325-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pavlíková D, et al. Response of cytokinins and nitrogen metabolism in the fronds of Pteris sp under arsenic stress. PLoS One. 2020;15:e0233055. doi: 10.1371/journal.pone.0233055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Porra RJ, Thompson WA, Kriedemann PE. Determination of accurate extinction coefficients and simultaneous equations for assaying chlorophylls a and b extracted with four different solvents: Verification of the concentration of chlorophyll standards by atomic absorption spectroscopy. Biochim. Biophys. Acta-Bioenerg. 1989;975:384–394. doi: 10.1016/S0005-2728(89)80347-0. [DOI] [Google Scholar]
- 37.Wellburn AR. The spectral determination of chlorophylls a and b, as well as total carotenoids, using various solvents with spectrophotometers of different resolution. J. Plant Physiol. 1994;144:307–313. doi: 10.1016/S0176-1617(11)81192-2. [DOI] [Google Scholar]
- 38.Maletsika PA, Nanos GD. Effects of particulate matter contamination on peach leaf physiological functions. Acta. Hortic. 2013;981:643–650. doi: 10.17660/ActaHortic.2013.981.103. [DOI] [Google Scholar]
- 39.Žalud P, Száková J, Sysalová J, Tlustoš P. Factors influencing uptake of contaminated particulate matter in leafy vegetables. Open Life Sci. 2012;7:519–530. doi: 10.2478/s11535-012-0029-0. [DOI] [Google Scholar]
- 40.Molins H, et al. Mutants impaired in vacuolar metal mobilization identify chloroplasts as a target for cadmium hypersensitivity in (Arabidopsis thaliana) Plant Cell Environ. 2013;36:804–817. doi: 10.1111/pce.12016. [DOI] [PubMed] [Google Scholar]
- 41.Yu B, et al. Phytoremediation potential of Youngia japonica (L.) DC: A newly discovered cadmium hyperaccumulator. Environ. Sci. Pollut. Res. 2021;28:6044–6057. doi: 10.1007/s11356-020-10853-6. [DOI] [PubMed] [Google Scholar]
- 42.Dziri S, Hosni K. Effects of cement dust on volatile oil constituents and antioxidative metabolism of Aleppo pine (Pinus halepensis) needles. Acta Physiol. Plant. 2012;34:1669–1678. doi: 10.1007/s11738-012-0962-6. [DOI] [Google Scholar]
- 43.Jakhar S, Mukherjee D. Chloroplast pigments, proteins, lipid peroxidation and activities of antioxidative enzymes during maturation and senescence of leaves and reproductive organs of (Cajanus cajan L.) Physiol. Mol. Biol. Plants. 2014;20:171–180. doi: 10.1007/s12298-013-0219-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nam HG. The molecular genetic analysis of leaf senescence. Curr. Opin. Biotechnol. 1997;8:200–207. doi: 10.1016/S0958-1669(97)80103-6. [DOI] [PubMed] [Google Scholar]
- 45.Zemanová V, Pavlík M, Pavlíková D. Cadmium toxicity induced contrasting patterns of concentrations of free sarcosine, specific amino acids and selected microelements in two Noccaea species. PLoS One. 2017;12:e0177963. doi: 10.1371/journal.pone.0177963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cakmak I, Marschner H, Bangerth F. Effect of zinc nutritional status on growth, protein metabolism and levels of indole-3-acetic acid and other phytohormones in bean (Phaseolus vulgaris L.) J. Exp. Bot. 1989;40:405–412. doi: 10.1093/jxb/40.3.405. [DOI] [Google Scholar]
- 47.Choudhary M, Bailey LD, Grant CA. Effect of zinc on cadmium concentration in the tissue of durum wheat. Can. J. Plant Sci. 1994;74:549–552. doi: 10.4141/cjps94-099. [DOI] [Google Scholar]
- 48.Vesely T, Neuberg M, Trakal L, Szakova J, Tlustos P. Water lettuce Pistia stratiotes L. response to lead toxicity. Water Air Soil Pollut. 2012;223:1847–1859. doi: 10.1007/s11270-011-0989-0. [DOI] [Google Scholar]
- 49.Várallyay S, Bódi É, Garousi F, Veres S, Kovács B. Effect of arsenic on dry weight and relative chlorophyll content in greeningmaize and sunflower tissues. J. Microbiol. Biotechnol. Food Sci. 2015;4:167–169. doi: 10.15414/jmbfs.2015.4.special3.167-169. [DOI] [Google Scholar]
- 50.Gusman GS, Oliveira JA, Farnese FS, Cambraia J. Arsenate and arsenite: The toxic effects on photosynthesis and growth of lettuce plants. Acta Physiol. Plant. 2013;35:1201–1209. doi: 10.1007/s11738-012-1159-8. [DOI] [Google Scholar]
- 51.Zemanová V, Pavlíková D, Hnilička F, Pavlík M. Arsenic toxicity-induced physiological and metabolic changes in the shoots of Pteris cretica and Spinacia oleracea. Plants. 2021;10:2009. doi: 10.3390/plants10102009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cortleven A, et al. Cytokinin regulates the etioplast-chloroplast transition through the two-component signaling system and activation of chloroplast-related genes1. Plant Physiol. 2016;172:464–478. doi: 10.1104/pp.16.00640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kobayashi NI, et al. Leaf senescence in rice due to magnesium deficiency mediated defect in transpiration rate before sugar accumulation and chlorosis. Physiol. Plant. 2013;148:490–501. doi: 10.1111/j.1399-3054.2012.12003.x. [DOI] [PubMed] [Google Scholar]
- 54.Ciećko Z, Kalembasa S, Wyszkowski M, Rolka E. The magnesium content in plants in soil contaminated with cadmium. Pol. J. Environ. Stud. 2005;14:365–370. [Google Scholar]
- 55.Zemanová V, Pavlík M, Pavlíková D, Hnilička F, Vondráčková S. Responses to Cd stress in two Noccaea species (Noccaea praecox and (Noccaea caerulescens) originating from two contaminated sites in Mežica, Slovenia and Redlschlag, Austria. Arch. Environ. Contam. Toxicol. 2016;70:464–474. doi: 10.1007/s00244-015-0198-8. [DOI] [PubMed] [Google Scholar]
- 56.He M, et al. Alleviation of pericarp browning in harvested litchi fruit by synephrine hydrochloride in relation to membrane lipids metabolism. Postharvest. Biol. Technol. 2020;166:111223. doi: 10.1016/j.postharvbio.2020.111223. [DOI] [Google Scholar]
- 57.Pavlík M, Zemanová V, Pavlíková D, Kyjaková P, Hlavsa T. Regulation of odd-numbered fatty acid content plays an important part in the metabolism of the hyperaccumulator Noccaea spp. adapted to oxidative stress. J. Plant Physiol. 2017;208:94–101. doi: 10.1016/j.jplph.2016.09.014. [DOI] [PubMed] [Google Scholar]
- 58.Mano J. Reactive carbonyl species: Their production from lipid peroxides, action in environmental stress, and the detoxification mechanism. Plant Physiol. Biochem. 2012;59:90–97. doi: 10.1016/j.plaphy.2012.03.010. [DOI] [PubMed] [Google Scholar]
- 59.Zemanová V, Pavlík M, Kyjaková P, Pavlíková D. Fatty acid profiles of ecotypes of hyperaccumulator Noccaea caerulescens growing under cadmium stress. J. Plant Physiol. 2015;180:27–34. doi: 10.1016/j.jplph.2015.02.012. [DOI] [PubMed] [Google Scholar]
- 60.Wang Y, Yang R, Zheng J, Shen Z, Xu X. Exogenous foliar application of fulvic acid alleviate cadmium toxicity in lettuce (Lactuca sativa L.) Ecotox. Environ. Safe. 2019;167:10–19. doi: 10.1016/j.ecoenv.2018.08.064. [DOI] [PubMed] [Google Scholar]
- 61.Mithöfer A, Schulze B, Boland W. Biotic and heavy metal stress response in plants: Evidence for common signals. FEBS Lett. 2004;566:1–5. doi: 10.1016/j.febslet.2004.04.011. [DOI] [PubMed] [Google Scholar]
- 62.Harmatha J. Structural abundance and biological significance of lignans and related plant phenylpropanoids. Chem. Listy. 2005;99:622–632. [Google Scholar]
- 63.Smirnoff N. The role of active oxygen in the response of plants to water deficit and desiccation. New Phytol. 1993;125:27–58. doi: 10.1111/j.1469-8137.1993.tb03863.x. [DOI] [PubMed] [Google Scholar]
- 64.Agati G, et al. Functional roles of flavonoids in photoprotection: New evidence, lessons from the past. Plant Physiol. Biochem. 2013;72:35–45. doi: 10.1016/j.plaphy.2013.03.014. [DOI] [PubMed] [Google Scholar]
- 65.Anterola AM, Lewis NG. Trends in lignin modification: A comprehensive analysis of the effects of genetic manipulations/mutations on lignification and vascular integrity. Phytochemistry. 2002;61:221–294. doi: 10.1016/S0031-9422(02)00211-X. [DOI] [PubMed] [Google Scholar]
- 66.Iwase Y, Shiraya T, Takeno K. Flowering and dwarfism induced by DNA demethylation in Pharbitis nil. Physiol. Plant. 2010;139:118–127. doi: 10.1111/j.1399-3054.2009.01345.x. [DOI] [PubMed] [Google Scholar]
- 67.Brown JCL, De Decker MM, Fieldes MA. A comparative analysis of developmental profiles for DNA methylation in 5-azacytidine-induced early-flowering flax lines and their control. Plant Sci. 2008;175:217–225. doi: 10.1016/j.plantsci.2008.03.023. [DOI] [Google Scholar]
- 68.Yanez Barrientos E, Wrobel K, Lopez Torres A, Gutiérrez Corona F, Wrobel K. Application of reversed-phase high-performance liquid chromatography with fluorimetric detection for simultaneous assessment of global DNA and total RNA methylation in Lepidium sativum: Effect of plant exposure to Cd(II) and Se(IV) Anal. Bioanal. Chem. 2013;405:2397–2404. doi: 10.1007/s00216-013-6703-x. [DOI] [PubMed] [Google Scholar]
- 69.Anwar S, et al. Mitigation of drought stress and yield improvement in wheat by zinc foliar spray relates to enhanced water use efficiency and zinc contents. Int. J. Plant. Prod. 2021;15:377–389. doi: 10.1007/s42106-021-00136-6. [DOI] [Google Scholar]
- 70.Chaneva G, Parvanova P, Tzvetkova N, Uzunova A. Photosynthetic response of maize plants against cadmium and paraquat impact. Water Air Soil Pollut. 2010;208:287–293. doi: 10.1007/s11270-009-0166-x. [DOI] [Google Scholar]
- 71.Mensah E, Odai SN, Ofori E, Kyei-Baffo N. Influence of transpiration on cadmium (Cd) and lead (Pb) uptake by cabbage, carrots and lettuce from irrigation water in Ghana. Asian J. Agric. Res. 2008;2:56–60. [Google Scholar]
- 72.FuChun Z, et al. Effect of floating dust weather on leaf photosynthesis and water potential of grapes in Karakash River Basin. Chinese J. Plant Ecol. 2018;26:990–998. [Google Scholar]
- 73.Kaya C, et al. Melatonin-mediated nitric oxide improves tolerance to cadmium toxicity by reducing oxidative stress in wheat plants. Chemosphere. 2019;225:627–638. doi: 10.1016/j.chemosphere.2019.03.026. [DOI] [PubMed] [Google Scholar]
- 74.Kiseleva IS, Kaminskaya OA. Hormonal regulation of assimilate utilization in barley leaves in relation to the development of their source function. Russ. J. Plant Physiol. 2002;49:534–540. doi: 10.1023/A:1016324312244. [DOI] [Google Scholar]
- 75.Pavlíková D, et al. Glutamate kinase as a potential biomarker of heavy metal stress in plants. Ecotox. Environ. Safe. 2008;70:223–230. doi: 10.1016/j.ecoenv.2007.07.006. [DOI] [PubMed] [Google Scholar]
- 76.Battaglia M, et al. Proline-rich cell wall proteins accumulate in growing regions and phloem tissue in response to water deficit in common bean seedlings. Planta. 2007;225:1121–1133. doi: 10.1007/s00425-006-0423-9. [DOI] [PubMed] [Google Scholar]
- 77.Smirnoff N. The function and metabolism of ascorbic acid in plants. Ann. Bot. 1996;78:661–669. doi: 10.1006/anbo.1996.0175. [DOI] [Google Scholar]
- 78.Park S-W, et al. Cyclophilin 20-3 relays a 12-oxo-phytodienoic acid signal during stress responsive regulation of cellular redox homeostasis. Proc. Natl. Acad. Sci. USA. 2013;110:9559–9564. doi: 10.1073/pnas.1218872110. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data used to support the findings of this study are included within the article. The datasets generated and/or analysed during the current study are not publicly available due apprehension of unauthorized use of data and plagiarism but are available from the corresponding author on reasonable request.