Abstract
Ureaplasma and Prevotella infections are well-known bacteria associated with preterm birth. However, with the development of metagenome sequencing techniques, it has been found that not all Ureaplasma and Prevotella colonizations cause preterm birth. The purpose of this study was to determine the association between Ureaplasma and Prevotella colonization with the induction of preterm birth even in the presence of Lactobacillus. In this matched case–control study, a total of 203 pregnant Korean women were selected and their cervicovaginal fluid samples were collected during mid-pregnancy. The microbiome profiles of the cervicovaginal fluid were analyzed using 16S rRNA gene amplification. Sequencing data were processed using QIIME1.9.1. Statistical analyses were performed using R software, and microbiome analysis was performed using the MicrobiomeAnalyst and Calypso software. A positive correlation between Ureaplasma and other genera was highly related to preterm birth, but interestingly, there was a negative correlation with Lactobacillus and term birth, with the same pattern observed with Prevotella. Ureaplasma and Prevotella colonization with Lactobacillus abundance during pregnancy facilitates term birth, although Ureaplasma and Prevotella are associated with preterm birth. Balanced colonization between Lactobacillus and Ureaplasma and Prevotella is important to prevent preterm birth.
Subject terms: Translational research, Reproductive disorders, Microbiology, Medical research, Risk factors
Introduction
Preterm birth (PTB) is characterized as the delivery of newborns at less than 37 weeks of gestation and is a major cause of morbidity and mortality among infants1. In preterm infants, respiratory and cardiovascular complications are generally observed, which can result in neonatal death, as well as long-term complications, such as neurodevelopmental delay, hearing and visual loss, and cerebral palsy, leading to significant socioeconomic loss2. The risk factors for PTB are influenced by various causes such as ethnicity, education level, smoking, obesity, environmental effects, and underlying diseases3. The recent increase in PTB is affected by rising in the number of elderly and multiple pregnancies. This has prompted an increase in research to diagnose and prevent PTB4,5.
Spontaneous PTB (sPTB) accounts for 70–75% of all PTBs1,6. One-third of sPTBs are accompanied by intra-amniotic infections, and the isolated microorganisms, especially Ureaplasma, are similar to those observed in the lower genital tract7. Hence, infection/inflammatory response attributed to ascending infections was thought to be the cause8. Increased levels of inflammatory cytokines in the amniotic fluid, cervicovaginal fluid (CVF), and blood support this hypothesis9–11. Ascending infections contributing to sPTB are related to the vaginal environment, and this risk is high in bacterial vaginosis (BV), which involves the proliferation of harmful bacteria12. BV can be identified using a culture test, by microscopy, or measurement of vaginal pH. With the development of molecular genetic diagnostic techniques, including 16s rRNA metagenomics analysis, it became possible to detect various bacterial species, including anaerobic bacteria, and to obtain genetic information of all bacterial species distributed in bio-fluid13.
In pregnant women, Lactobacillus becomes dominant in the vaginal environment because of marked increases in circulating estrogen14. Specifically, the Lactobacillus group tends to be dominant in women with TB15. According to the dominant Lactobacillus spp., there have also been studies that reported on the relationship with PTB by classifying them into community state types (CST)16,17. In contrast, microbiome dysbiosis, a state of imbalance in the microbial community, particularly in the vaginal microbiome, is related to PTB18. Among various bacteria, several studies have reported on the relationship between Ureaplasma and Prevotella and PTB. In addition, the effect of the microbiome of pregnant women on the prognosis during pregnancy and PTB has been studied19–23.
However, according to previous studies, the vaginal microbiome shows differences according to race and place of residence18,24, and a study has also reported that there is no relationship between PTB and a specific microbiome in some races17. Ureaplasma spp. is known to be associated with PTB, however, vaginal colonization does not universally result in induction of ascending infection and pregnancy complications, even for animal models25,26.
Therefore, in this study, we aimed to evaluate candidates that can be a predictor of PTB among the microbiome of CVF and to determine the association between Ureaplasma and Prevotella colonization with PTB and whether it induces PTB even in the presence of Lactobacillus.
Results
Clinical characteristics
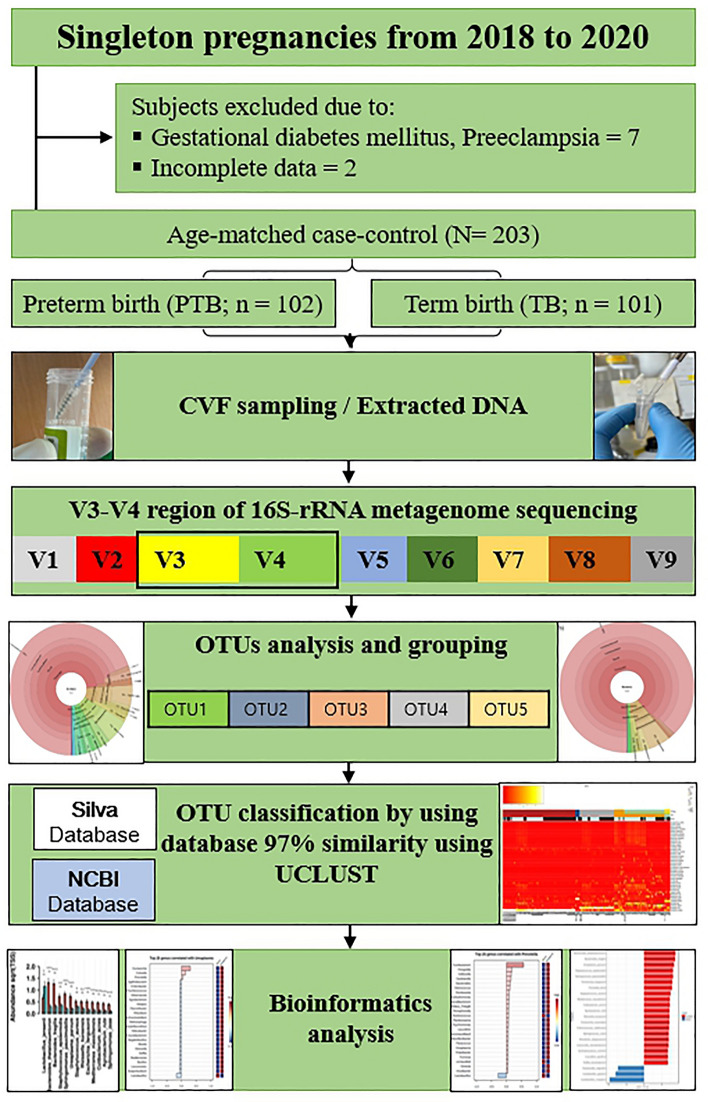
A total of 203 women participated in this age-matched case–control study, excluding nine subjects: 102 women in the PTB group and 101 women in the TB group (Fig. 1). There were no significant differences between the characteristics of the PTB and TB groups, except for the history of sPTB, white blood cell (WBC) count, cervical length (CL), gestational age at sampling (GAS), gestational age at birth (GAB), birth weight, appearance, pulse, grimace, activity, respiration (APGAR) score, and neonatal intensive care unit (NICU) admission rate (P < 0.001, Table 1).
Figure 1.
Flowchart of the study. CVF cervicovaginal fluid, rRNA ribosomal ribonucleic acid, OTUs operational taxonomic units.
Table 1.
Clinical characteristics of the study subjects.
| Characteristics | Preterm birth (n = 102) | Term birth (n = 101) | P-value |
|---|---|---|---|
| Maternal age (year) | 32.32 (± 4.35) | 33.02 (± 3.40) | 0.237 |
| Pre-pregnancy BMI (kg/m2) | 21.60 (± 2.89) | 21.36 (± 2.77) | 0.657 |
| Education level | > 0.999 | ||
| High school graduation or below | 6 (13.3) | 11 (15.3) | |
| University graduates | 39 (86.7) | 61 (84.7) | |
| History of PTB | < 0.015* | ||
| No | 79 (88.8) | 94 (97.9) | |
| Yes | 10 (11.2) | 2 (2.1) | |
| WBC | 10.1 (8.8–12.9) | 8.9 (7.8–10.4) | < 0.001* |
| Cervical length | 20.8 (± 12.1) | 30.4 (± 8.5) | < 0.001* |
| GAS (weeks) | 31.8 (26.2–34.2) | 24.5 (22.1–31.0) | < 0.001* |
| CST | 0.156 | ||
| I | 46 (45.1) | 45 (44.6) | |
| II | 1 (1.0) | 4 (4.0) | |
| III | 20 (19.6) | 22 (21.8) | |
| IV-A | 7 (6.9) | 1 (1.0) | |
| IV-B | 23 (22.5) | 27 (26.7) | |
| V | 5 (4.9) | 2 (2.0) | |
| Delivery mode | 0.004* | ||
| ND | 41 (40.2) | 61 (60.4) | |
| CS | 61 (59.8) | 40 (39.6) | |
| GAB (weeks) | 34.0 (30.4–35.6) | 39.3 (38.2–39.9) | < 0.001* |
| Birth weight (g) | 1975.4 (± 780.5) | 3234.9 (± 316.8) | < 0.001* |
| APGAR score at 1 min | 6.99 (5–9) | 9.31 (9–10) | < 0.001* |
| APGAR score at 5 min | 8.29 (7–10) | 9.74 (10–10) | < 0.001* |
| NICU admission | 86 (84.3) | 13 (12.9) | < 0.001* |
Categorical variables were expressed as frequencies (percentage) and analyzed using the chi-square test and Fisher’s exact test. Continuous variables were expressed as the mean ± standard deviation (SD) or median (interquartile range) and were compared using the t-test or Mann–Whitney U test.
BMI body mass index, PTB preterm birth, WBC white blood cell, GAS gestational age at sampling, CST community-state type, ND normal delivery, CS cesarean section, GAB gestational age at birth, APGAR appearance, pulse, grimace, activity, respiration, NICU neonatal intensive care unit.
*Statistical significance was defined as P < 0.05.
Association between bacteria and preterm birth
Differences in microbial diversity between PTB and TB groups
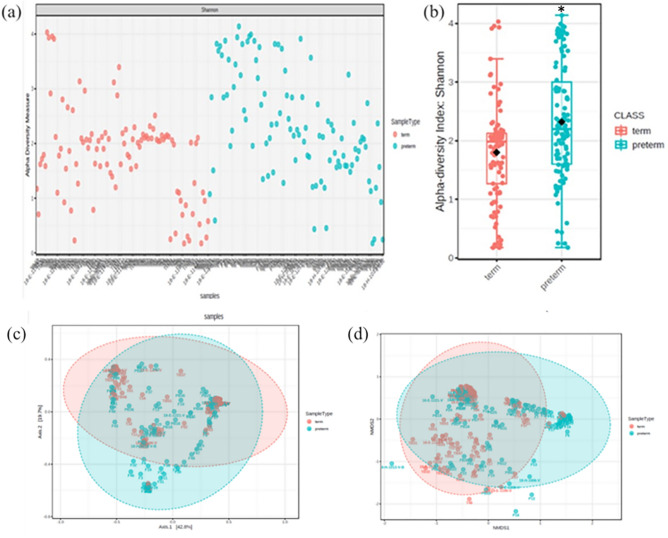
Comparing the Krona chart with PTB and TB, PTB showed that Bacteroidetes, Proteobacteria, and Mollicutes were diversely distributed from the phylum level in addition to Firmicutes and Actinobacteria (Supplementary Fig. 1). In the TB group, 88% consisted of Lactobacillus, and Gardnerella, Bifidobacterium, and Atopobium accounted for a small percentage. In the PTB group, Lactobacillus accounted for 69% and the distribution of other species such as Staphylococcus, Bacteroides, Prevotella, Ureaplasma, Sphingomonas, and Escherichia was shown (Supplementary Fig. 1). When comparing Shannon’s α-diversity index between the PTB and TB groups, the medians (interquartile ranges) were 2.20 (1.27–2.12) and 1.99 (1.60–3.03), respectively. The median was significantly higher in the PTB group (P < 0.001, Fig. 2a,b). In the β-diversity analysis, most of the microbial communities were similar, but a distance was observed between specific microorganisms (Fig. 2c,d). There was no significant difference in the CST type between the PTB and TB groups (P = 0.156, Table 1 and Supplementary Fig. 2).
Figure 2.
Differences in alpha- and beta-diversity between PTB and TB groups. (a,b) Shannon's alpha diversity was significantly higher in the PTB group (PTB, n = 102; TB, n = 101). (c) Principal coordinate analysis plot (d) Non-metric multidimensional scaling plot. Boxes show median and interquartile ranges, black dots represent the average, and whiskers extend from minimum to maximum values. The asterisk symbol shows a significant difference. *Statistical significance was defined as P < 0.05.
Microbiome related to the PTB and TB groups
A total of 157 species were detected, of which 82 species had an adjusted P value of < 0.05, and the median values were compared for the top 20 bacteria (Supplementary Fig. 3). In the analysis of the two groups, the abundances of Lactobacillus jensenii, Bacteroides thetaiotaomicron, B. fragilis, Staphylococcus epidermidis, Sphingomonas paucimobilis, Ureaplasma parvum, S. aureus, Weissella koreensis, Escherichia fergusonii, Mediterranea massiliensis, Cutibacterium acnes, Agrobacterium rubi, and S. zeae were significantly different (Supplementary Fig. 4, P < 0.05, abundance > 0.5%).
A total of 23 significant species were observed, with 20 in the PTB group and three in the TB group (P < 0.05, LDA score of ≥ 4). L. crispatus, L. gasseri, and Gardnerella vaginalis were the major taxa in the TB group. B. thetaiotaomicrom, B. fragilis, U. parvum, S. epidermidis, S. paucimobilis, E. fergusonii, Prevotella bivia, S. aureus, M. massiliensis, C. acnes, A. rubi, W. koreensis, E. marmotae, Chthonomonas calidirosea, S. zeae, Rhizobium daejeonense, Lawsonella clevelandensis, Syntrophaceticus schinkii, Leucothrix pacifica, and Delftia tsuruhatensis were the major taxa in the PTB group (Fig. 3).
Figure 3.
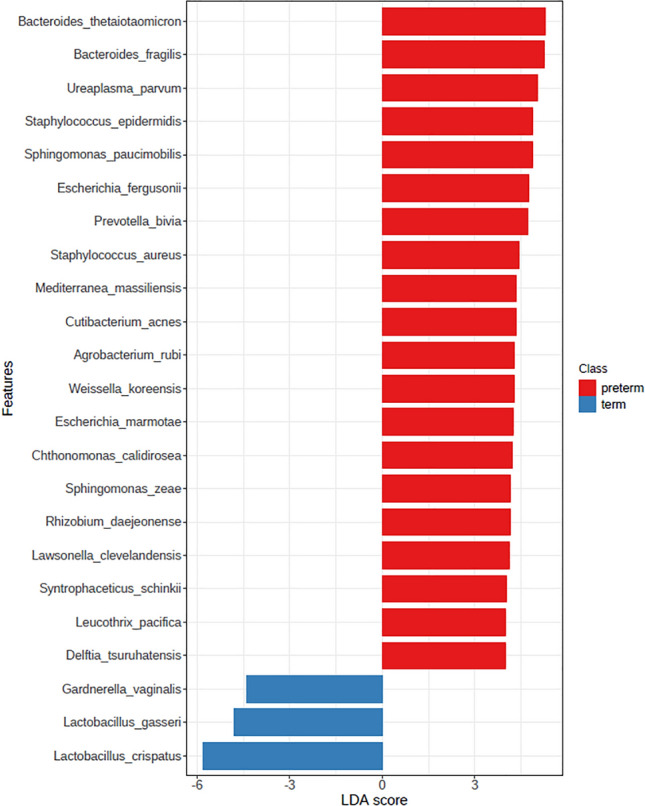
Differential dominant relative abundance of bacterial taxa in the PTB and TB groups. Linear discriminant analysis effect size analysis (LDA score > 4). LDA Linear discriminant analysis.
Pattern search using correlation analysis of the vaginal microbiome
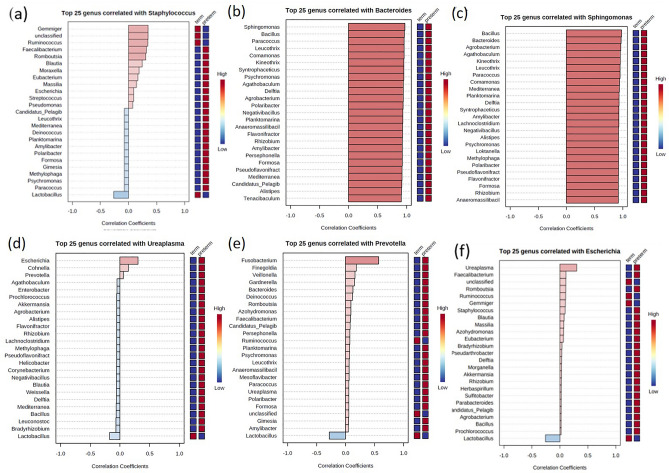
When the examination was performed at the representative genus level to identify significant differences in pattern using correlation analysis, Staphylococcus showed a positive correlation of 0.3 with Gemmiger and Ruminococcus and showed a negative correlation of 0.3 with Lactobacillus, and in this case, the association with TB was high (Fig. 4a). Bacteroides showed a positive correlation of ≥ 0.8 with 43 genera and a negative correlation of 0.6 with Lactobacillus (Fig. 4b). Sphingomonas showed a positive correlation of ≥ 0.8 with 46 genera and a negative correlation of 0.6 with Lactobacillus (Fig. 4c). Ureaplasma had a correlation of 0.3 with Escherichia, and Prevotella had a positive correlation of 0.5 with Fusobacterium (Fig. 4d,e). When Ureaplasma showed a negative correlation with Lactobacillus, the subjects associated with TB were marked in red on the right box line (Fig. 4d). Similarly, in the case of Prevotella, negative correlations with Lactobacillus were highly correlated with TB. In addition, positive correlations with Ruminococcus were also associated with TB (Fig. 4e). Escherichia showed a positive correlation with Gemmiger and Ruminococcus and a negative correlation with Lactobacillus, and in this case, the association with TB was high (Fig. 4f).
Figure 4.
Pattern search using correlation coefficient analysis of the vaginal microbiome of pregnant women at the genus level. (a) Staphylococcus (b) Bacteroides (c) Sphingomonas (d) Ureaplasma (e) Prevotella (f) Escherichia.
Discussion
This is the first study to conduct correlation analysis with bacteria of the vaginal microbiome, and we suggest that the relevance to the PTB should be evaluated by the community, not just the presence of specific bacteria. Although Ureaplasma and Prevotella are related to PTB, if these bacteria coexist with Lactobacillus, pregnancy outcome will be followed by TB.
These results were obtained by comparing the CVF microbiome of the PTB and TB groups using 16s rRNA metagenome sequencing. Through this method, we were able to understand the entire ecosystem of the vagina and identified 20 candidate bacteria related to PTB and three bacteria related to TB among all colonizing microbiomes. The Lactobacillus spp. and Gardnerella vaginalis were dominant in the TB group, whereas Bacterioides spp., Ureaplasma spp., Staphylococcus spp., Escherichia fergusonii, Prevotella spp., and Sphingomonas spp. etc. were increased in the PTB group. However, although bacteria related to PTB were present, there were cases where delivery became TB, which coexisted with Lactobacillus, Ruminococcus, and Gemmiger.
Our study results were similar to those of previous studies that used 16s rRNA metagenome sequencing. When comparing between the PTB with TB groups, Lactobacillus spp., including L. crispartus, L. jensenii, and L. gasseri, were associated with TB18. In pregnant women, the vaginal microbiome is dominated by Lactobacillus from the second trimester, reducing bacterial diversity27. Vaginal Lactobacillus spp. is known to protect the vaginal environment from harmful bacteria as beneficial bacteria, which is thought to be because lactic acid or hydrogen peroxide secreted by Lactobacillus plays a bacteriostatic role. Therefore, in the group with a lower dominance of Lactobacillus, the risk of PTB increases with the result of increasing pathogens15,19,28,29.
In contrast to the findings of previous studies30,31, we found that G. vaginalis was associated with TB. However, controversy exists because there were positive findings in healthy women31. Thus, we realized that G. vaginalis needed to be identified up to the strain level32.
Among the increased bacteria in PTB cases, B. fragilis, U. parvum, and P. bivia have been associated with PTB in previous studies23,33,34. Staphylococcus spp., Escherichia spp., and Sphingomonas spp. are commonly known as opportunistic bacteria35–37. In our study, B. thetaiotaomicron and B. fragilis were found to increase in PTB cases. Prevotella spp. is one of the genera known to be associated with PTB and BV33. The Mollicutes class, which includes Ureaplasma and Mycoplasma, has also been studied for its potential association with PTB22,34, and this is also associated with the cause of a shorter cervical length38. However, there was controversy about colonization in healthy women, and this study revealed that colonization itself was not associated with all infection and pregnancy complications21.
Therefore, our research is meaningful because, in the analysis of the vaginal microbiome, we revealed the importance of interpretation through an understanding of the microbiome relationship. Ureaplasma and Prevotella, previously considered pathogenic, did not cause complications when coexisting with Lactobacillus in our study. Furthermore, while a previous study showed cervical epithelial damage increased PTB, the protective role of Lactobacillus may explain why experimental Ureaplasma infection only resulted in a maximum of 28% induced PTB26. This was consistent with the bacterial risk score model, a study that analyzed the microbiome using machine learning, developed by this research team to predict PTB21. In this study, Ruminococcus and Gemmiger were suggested to play a protective role against pathogenic bacteria. They have been studied as part of a healthy gut microbiome, so we suggest the possibility of an association with the healthy intestinal microbiome and TB39,40.
The results of this study using correlation analysis suggest that Lactobacillus is thought to show a protective effect against Ureaplasma and Prevotella as well as most pathogenic bacteria. However, in this study, only the results using bioinformatics were shown, and no experiments were conducted to reveal the causal relationship. Therefore, for future research, in order to confirm the biological mechanism, it may be necessary to study the analysis of proteomics and metabolomics in addition to genomics. Furthermore, studies such as changes in cytokines or immune activation to determine how this microbiome acts with the host should be conducted together.
To our knowledge, this is the first study to examine the microbiome relationship using pattern search through correlation analysis. Through this study, it was suggested that the relationship through the quantitative analysis of the microbiome, not simply the presence or absence of pathogenic bacteria, would be more important in predicting PTB. Furthermore, after target selection through metagenome analysis, multiplex quantitative PCR (qPCR), which can save relatively cost and time, can be used in clinical practice for predicting PTB21. This study has strengths as a large-scale, multicenter study targeting pregnant Korean women. The limitations of this study are that it was not analyzed, including strain level measurements for U. parvum and G. vaginalis, despite recent studies showing that the pathogenicity of Ureaplasma and Gardnerella differs depending on the serovar or clade levels. In addition, this study may be limited in that it did not analyze the microbiome in amniotic fluid or placenta to confirm intra-amniotic infection. As a limitation of the method itself, 16s rRNA metagenome sequencing can analyze all colonized microbiome of the vagina with high sensitivity, but it is difficult to identify the actual activity and pathogenicity of the microbiome. However, despite these limitations, candidates related to PTB were discovered through various bioinformatics analyses to understand their relationship.
Ureaplasma and Prevotella colonization with Lactobacillus abundance during pregnancy facilitates TB, although Ureaplasma and Prevotella are associated with PTB. Balanced colonization between Lactobacillus and Ureaplasma and Prevotella is important for preventing PTB.
Methods
Study subjects and CVF collection
In this case-cohort study, subjects were recruited from the Ewha Womans University Mokdong Hospital and Yonsei University Severance Hospital from 2018 to 2020. This study was approved by the Ethical Research Committee of Ewha Womans University Mokdong Hospital (no. 2018-07-007) and Yonsei University Severance Hospital (no. 4-2018-0564). The experiments were conducted in accordance with the approved guidelines, and informed consent was obtained from all the subjects.
Subjects included women with a singleton pregnancy and at a gestational age between 15 and 36 weeks, asymptomatic pregnant women who visited the outpatient clinic department regularly, and hospitalized pregnant women with symptoms of PTL or preterm premature rupture of membranes. The CVF sample was collected from the posterior vaginal fornix using sterile cotton swab through the speculum exam before any vaginal examination or clinical treatment, such as antibiotics, steroids, progesterone, and tocolytics.
For all study subjects, baseline demographic data and health-related characteristics including age, pre-pregnancy body mass index, education level, and maternal PTB history were collected. A routine blood test was performed to examine the WBC CL at the time of CVF collection. After delivery, the outcomes of the pregnancy were evaluated, including delivery mode; GAB; neonatal birth weight; APGAR score; and NICU admission. Among the enrolled subjects, those diagnosed with gestational diabetes mellitus, preeclampsia, and insufficient medical records were excluded. The PTB group was categorized as subjects who delivered at < 37 weeks of gestation, whereas the TB group was characterized by subjects who delivered after 37 weeks of gestation.
Metagenome analysis using 16s rRNA gene sequencing
The collected samples were subjected to bacterial DNA extraction for microbiome analysis using the NucleoSpin Tissue Kit (Macherey-Nagel, Düren, Germany) following the manufacturer's instructions. A 16S rRNA sequencing library was constructed according to the 16S metagenomic sequencing library preparation protocol targeting the V3 and V4 hypervariable regions of the 16S rRNA gene41–43. The KAPA HiFi HotStart ReadyMix (KAPA Biosystems, Wilmington, USA) and Agencourt AMPure XP system (Beckman Coulter Genomics, Brea, USA) were used for PCR and purification of the PCR product, respectively. The initial PCR was performed with 12 ng of the template DNA using region-specific primers that were compatible with the Illumina index and sequencing adapters (Supplementary Table 1). After magnetic bead-based purification of PCR products, a second PCR was performed using primers from the Nextera XT Index Kit (Illumina, San Diego, USA) with a limited cycle. Subsequently, purified PCR products were visualized using gel electrophoresis and quantified with a DropSense96 (Trinean, Gentbrugge, Belgium). The pooled samples were run on the Agilent 2100 Bioanalyzer (Agilent, Santa Clara, CA, USA) for quality analysis prior to sequencing. Libraries were quantified by qPCR using the CFX96 Real-Time System. After normalization, sequencing of the prepared library was conducted using the MiSeq system (Illumina, San Diego, USA) with 300 bp paired-end reads.
Sequencing data were processed using QIIME1.9.1 to assemble paired-end reads into tags according to their overlapping relationships. In the pre-processing step, the primer was removed, demultiplexed, and filtered for quality (Phred ≥ 20). USEARCH7 was used to perform denoising and chimera detection/filtering in the operational taxonomic unit (OTU) group. Then, the Silva132 and NCBI databases were used to determine the OTUs with 97% similarity using UCLUST and the close-reference analysis method and to determine the OTU identifiers. Comparative OTU assignment was performed with the database in terms of phylum, class, order, family, genus, and species separately using RDP classifiers. Using QIIME, the α-diversity was analyzed with the Shannon index to understand the local population of the microbiome and the β-diversity was analyzed for estimating the correlation among other factors and microbes by Bray–Curtis.
Basic statistical analyses, such as t-test, Mann–Whitney U test, Chi-square test, Fisher’s exact test, heat map, Krona chart, and the linear discriminant analysis (LDA) effect size (LEfSe) were performed. Multivariate analyses, such as principal coordinate analysis and non-metric multidimensional scaling, were performed. The adjusted P value was calculated by adjusting the false-positive rate using the false discovery rate. Correlations between the taxa and sample groups were analyzed using the Pearson correlation coefficient r as the distance measure. Statistical analyses were performed using R software (version 3.6.2), and microbiome analysis was performed using the MicrobiomeAnalyst (https://www.microbiomeanalyst.ca/) and the Calypso (http://cgenome.net/Calypso/) software.
Supplementary Information
Acknowledgements
We would like to thank Editage (www.editage.co.kr) for English language editing.
Author contributions
S.P. enrolled subjects, wrote and edited the manuscript. Y.-A.Y. developed the extraction of protocols and interpreted analyzed data. Y.-H.K. enrolled subjects and designed the study. E.K. and A.Z.A. developed the extraction of protocols. S.M.K. and G.L. performed the experiments. Y.M.H. and Y.J.J. enrolled subjects. K.K. analyzed the data. Y.J.K. enrolled subjects and designed the study.
Funding
This study was supported by funding from the National Research Foundation of Korea (NRF) grant funded by the Korean government (MSIT) (Grant no. 2020R1A2C3011850) and the BK21 FOUR (Fostering Outstanding Universities for Research) funded by the Ministry of Education (MOE, Korea) and National Research Foundation of Korea (NRF).
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-022-13871-1.
References
- 1.Goldenberg RL, Culhane JF, Iams JD, Romero R. Epidemiology and causes of preterm birth. Lancet. 2008;371:75–84. doi: 10.1016/s0140-6736(08)60074-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lawn JE, Cousens S, Zupan J. 4 million neonatal deaths: when? Where? Why? Lancet. 2005;365:891–900. doi: 10.1016/s0140-6736(05)71048-5. [DOI] [PubMed] [Google Scholar]
- 3.Vogel JP, et al. The global epidemiology of preterm birth. Best Pract. Res. Clin. Obstet. Gynaecol. 2018;52:3–12. doi: 10.1016/j.bpobgyn.2018.04.003. [DOI] [PubMed] [Google Scholar]
- 4.Ananth CV, Friedman AM, Goldenberg RL, Wright JD, Vintzileos AM. Association between temporal changes in neonatal mortality and spontaneous and clinician-initiated deliveries in the United States, 2006–2013. JAMA Pediatr. 2018;172:949–957. doi: 10.1001/jamapediatrics.2018.1792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Newnham JP, et al. Applying precision public health to prevent preterm birth. Front. Public Health. 2017;5:66. doi: 10.3389/fpubh.2017.00066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chan RL. Biochemical markers of spontaneous preterm birth in asymptomatic women. Biomed. Res. Int. 2014;2014:164081. doi: 10.1155/2014/164081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Abele-Horn M, Scholz M, Wolff C, Kolben M. High-density vaginal Ureaplasma urealyticum colonization as a risk factor for chorioamnionitis and preterm delivery. Acta Obstet. Gynecol. Scand. 2000;79:973–978. [PubMed] [Google Scholar]
- 8.Jefferson KK. The bacterial etiology of preterm birth. Adv. Appl. Microbiol. 2012;80:1–22. doi: 10.1016/b978-0-12-394381-1.00001-5. [DOI] [PubMed] [Google Scholar]
- 9.Park S, et al. Cervicovaginal fluid cytokines as predictive markers of preterm birth in symptomatic women. Obstet. Gynecol. Sci. 2020;63:455–463. doi: 10.5468/ogs.19131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Romero R, Dey SK, Fisher SJ. Preterm labor: One syndrome, many causes. Science. 2014;345:760–765. doi: 10.1126/science.1251816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bennett PR, Brown RG, MacIntyre DA. Vaginal microbiome in preterm rupture of membranes. Obstet. Gynecol. Clin. N. Am. 2020;47:503–521. doi: 10.1016/j.ogc.2020.08.001. [DOI] [PubMed] [Google Scholar]
- 12.Martius J, Eschenbach DA. The role of bacterial vaginosis as a cause of amniotic fluid infection, chorioamnionitis and prematurity—A review. Arch. Gynecol. Obstet. 1990;247:1–13. doi: 10.1007/bf02390649. [DOI] [PubMed] [Google Scholar]
- 13.Yoo JY, et al. 16S rRNA gene-based metagenomic analysis reveals differences in bacteria-derived extracellular vesicles in the urine of pregnant and non-pregnant women. Exp. Mol. Med. 2016;48:e208. doi: 10.1038/emm.2015.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Amabebe E, Anumba DOC. The vaginal microenvironment: The physiologic role of Lactobacilli. Front. Med. (Lausanne) 2018;5:181. doi: 10.3389/fmed.2018.00181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dominguez-Bello MG. Gestational shaping of the maternal vaginal microbiome. Nat. Med. 2019;25:882–883. doi: 10.1038/s41591-019-0483-6. [DOI] [PubMed] [Google Scholar]
- 16.DiGiulio DB, et al. Temporal and spatial variation of the human microbiota during pregnancy. Proc. Natl. Acad. Sci. U. S. A. 2015;112:11060–11065. doi: 10.1073/pnas.1502875112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dunlop AL, et al. Vaginal microbiome composition in early pregnancy and risk of spontaneous preterm and early term birth among african american women. Front. Cell Infect. Microbiol. 2021;11:641005. doi: 10.3389/fcimb.2021.641005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fettweis JM, et al. The vaginal microbiome and preterm birth. Nat. Med. 2019;25:1012–1021. doi: 10.1038/s41591-019-0450-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fox C, Eichelberger K. Maternal microbiome and pregnancy outcomes. Fertil. Steril. 2015;104:1358–1363. doi: 10.1016/j.fertnstert.2015.09.037. [DOI] [PubMed] [Google Scholar]
- 20.Vinturache AE, Gyamfi-Bannerman C, Hwang J, Mysorekar IU, Jacobsson B. Maternal microbiome—A pathway to preterm birth. Semin. Fetal Neonatal Med. 2016;21:94–99. doi: 10.1016/j.siny.2016.02.004. [DOI] [PubMed] [Google Scholar]
- 21.Park S, et al. Prediction of preterm birth based on machine learning using bacterial risk score in cervicovaginal fluid. Am. J. Reprod. Immunol. 2021 doi: 10.1111/aji.13435. [DOI] [PubMed] [Google Scholar]
- 22.Sprong KE, Mabenge M, Wright CA, Govender S. Ureaplasma species and preterm birth: Current perspectives. Crit. Rev. Microbiol. 2020;46:169–181. doi: 10.1080/1040841x.2020.1736986. [DOI] [PubMed] [Google Scholar]
- 23.de Freitas AS, et al. Defining microbial biomarkers for risk of preterm labor. Braz. J. Microbiol. 2020;51:151–159. doi: 10.1007/s42770-019-00118-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brown RG, et al. Vaginal dysbiosis increases risk of preterm fetal membrane rupture, neonatal sepsis and is exacerbated by erythromycin. BMC Med. 2018;16:9. doi: 10.1186/s12916-017-0999-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Breugelmans M, Vancutsem E, Naessens A, Laubach M, Foulon W. Association of abnormal vaginal flora and Ureaplasma species as risk factors for preterm birth: A cohort study. Acta Obstet. Gynecol. Scand. 2010;89:256–260. doi: 10.3109/00016340903418769. [DOI] [PubMed] [Google Scholar]
- 26.Pavlidis I, et al. Cervical epithelial damage promotes Ureaplasma parvum ascending infection, intrauterine inflammation and preterm birth induction in mice. Nat. Commun. 2020;11:199. doi: 10.1038/s41467-019-14089-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Romero R, et al. The composition and stability of the vaginal microbiota of normal pregnant women is different from that of non-pregnant women. Microbiome. 2014;2:4. doi: 10.1186/2049-2618-2-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chu DM, Seferovic M, Pace RM, Aagaard KM. The microbiome in preterm birth. Best Pract. Res. Clin. Obstet. Gynaecol. 2018;52:103–113. doi: 10.1016/j.bpobgyn.2018.03.006. [DOI] [PubMed] [Google Scholar]
- 29.Oliver A, et al. Cervicovaginal microbiome composition is associated with metabolic profiles in healthy pregnancy. MBio. 2020 doi: 10.1128/mBio.01851-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Menard JP, et al. High vaginal concentrations of Atopobium vaginae and Gardnerella vaginalis in women undergoing preterm labor. Obstet. Gynecol. 2010;115:134–140. doi: 10.1097/AOG.0b013e3181c391d7. [DOI] [PubMed] [Google Scholar]
- 31.Machado A, Cerca N. Influence of biofilm formation by Gardnerella vaginalis and other anaerobes on bacterial vaginosis. J. Infect. Dis. 2015;212:1856–1861. doi: 10.1093/infdis/jiv338. [DOI] [PubMed] [Google Scholar]
- 32.Janulaitiene M, et al. Prevalence and distribution of Gardnerella vaginalis subgroups in women with and without bacterial vaginosis. BMC Infect. Dis. 2017;17:394. doi: 10.1186/s12879-017-2501-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.You YA, et al. Vaginal microbiome profiles of pregnant women in Korea using a 16S metagenomics approach. Am. J. Reprod. Immunol. 2019;82:e13124. doi: 10.1111/aji.13124. [DOI] [PubMed] [Google Scholar]
- 34.Freitas AC, Bocking A, Hill JE, Money DM. Increased richness and diversity of the vaginal microbiota and spontaneous preterm birth. Microbiome. 2018;6:117. doi: 10.1186/s40168-018-0502-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Paharik AE, Horswill AR. The staphylococcal biofilm: Adhesins, regulation, and host response. Microbiol. Spectr. 2016 doi: 10.1128/microbiolspec.VMBF-0022-2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Agarwal J, Srivastava S, Singh M. Pathogenomics of uropathogenic Escherichia coli. Indian J. Med. Microbiol. 2012;30:141–149. doi: 10.4103/0255-0857.96657. [DOI] [PubMed] [Google Scholar]
- 37.Ryan MP, Adley CC. Sphingomonas paucimobilis: A persistent Gram-negative nosocomial infectious organism. J. Hosp. Infect. 2010;75:153–157. doi: 10.1016/j.jhin.2010.03.007. [DOI] [PubMed] [Google Scholar]
- 38.Hassan S, et al. A sonographic short cervix as the only clinical manifestation of intra-amniotic infection. J. Perinat. Med. 2006;34:13–19. doi: 10.1515/jpm.2006.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yin C, et al. Preterm birth is correlated with increased oral originated microbiome in the gut. Front. Cell Infect. Microbiol. 2021;11:579766. doi: 10.3389/fcimb.2021.579766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sparvoli LG, et al. Women's multisite microbial modulation during pregnancy. Microb. Pathog. 2020;147:104230. doi: 10.1016/j.micpath.2020.104230. [DOI] [PubMed] [Google Scholar]
- 41.Van Der Pol WJ, et al. In silico and experimental evaluation of primer sets for species-level resolution of the vaginal microbiota using 16S ribosomal RNA gene sequencing. J. Infect. Dis. 2019;219:305–314. doi: 10.1093/infdis/jiy508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Abellan-Schneyder I, et al. Primer, pipelines, parameters: Issues in 16S rRNA gene sequencing. mSphere. 2021 doi: 10.1128/mSphere.01202-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bernabeu A, et al. Effect of the vaginal microbiome on the pregnancy rate in women receiving assisted reproductive treatment. J. Assist. Reprod. Genet. 2019;36:2111–2119. doi: 10.1007/s10815-019-01564-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.