Abstract
The effects of oxygen limitation, low redox potential, and high NaCl stress for 7 days in vitro on the rifampin-resistant biocontrol inoculant Pseudomonas fluorescens CHA0-Rif and its subsequent persistence in natural soil for 54 days were investigated. Throughout the experiment, the strain was monitored using total cell counts (immunofluorescence microscopy), Kogure's direct viable counts, and colony counts (on rifampin-containing plates). Under in vitro conditions, viable-but-nonculturable (VBNC) cells of CHA0-Rif were obtained when the strain was exposed to a combination of low redox potential (230 mV) and oxygen limitation. This mimics a situation observed in the field, where VBNC cells of the strain were found in the waterlogged soil layer above the plow pan. Here, VBNC cells were also observed in vitro when CHA0-Rif was subjected to high NaCl levels (i.e., NaCl at 1.5 M but not 0.7 M). In all treatments, cell numbers remained close to the inoculum level for the first 12 days after inoculation of soil, regardless of the cell enumeration method used, but decreased afterwards. At the last two samplings in soil, VBNC cells of CHA0-Rif were found in all treatments except the one in which log-phase cells had been used. In the two treatments that generated high numbers of VBNC cells in vitro, VBNC cells did not display enhanced persistence compared with culturable cells once introduced into soil, which suggests that this VBNC state did not represent a physiological strategy to improve survival under adverse conditions.
The biocontrol bacterium Pseudomonas fluorescens strain CHA0 protects several cultivated plants against soilborne fungal pathogens (13). Since efficient biocontrol entails the release of large cell numbers of the inoculant in the soil environment, the commercial use of biocontrol pseudomonads such as strain CHA0 implies that ecological safety considerations have to be addressed (6, 31). Information on survival, persistence, and physiological states of released biocontrol agents in situ is a key aspect of risk assessment studies.
Monitoring of Pseudomonas inoculants released in the field is usually carried out by colony counts on selective media (5, 8, 23, 37). However, when pseudomonads are introduced into soil, some of the cells may lose their colony-forming ability, which leads to a situation where the strains persist as mixed populations of culturable and nonculturable cells in soil (3, 34, 38). Several methods have been proposed to assess the viability and/or the physiological activity of such cells (3, 17, 27, 42). Kogure's test (17) is based on the assumption that viable cells can engage in a few cycles of cell division, although they are not necessarily capable of forming a colony on plates. A similar assumption is made in the microcolony epifluorescence technique (3). In Kogure's test, the samples are incubated in the presence of small amounts of nutrients (to stimulate cell division) and nalidixic acid. Division of nutrient-responsive cells is prevented by the action of nalidixic acid, and consequently those cells increase in size. Thus, nutrient-responsive cells (viable cells) can be distinguished from small, nonresponsive cells and counted (Kogure's direct viable counts).
Kogure's direct viable counts were used in combination with total immunofluorescence (IF) cell counts and colony counts on selective medium to monitor over time the survival of the spontaneous rifampin-resistant strain P. fluorescens CHA0-Rif in the surface horizons of large outdoor lysimeters (34). The inoculant was found mostly as nonculturable cells several months after inoculation into soil, and a significant proportion of these nonculturable cells responded positively to Kogure's test, indicating that they were not dead (34). Environmental factors hypothesized as playing a part in the occurrence of nonculturable cells of CHA0-Rif in lysimeter soil included abiotic stress (linked to unfavorable conditions of temperature and/or water availability in soil) and nutrient starvation (34), but the importance of nutrient starvation was refuted in a subsequent study (9). Strain CHA0-Rif was also released at the surface of a field plot, and persistence of the pseudomonad was investigated at various depths in the soil profile at 72 days after inoculation. Nonculturable cells of the inoculant were detected at different soil depths (7, 32). Interestingly, cells of CHA0-Rif in a viable-but-nonculturable (VBNC) state were found in significant amounts in the few millimeters of soil located immediately above the plow plan, where decomposing crop residues were apparent and the soil was water logged and blue-gray, with a smell of hydrogen sulfide. These morphological symptoms suggest that oxygen limitation and reducing conditions were likely to be present. When oxygen becomes exhausted in soil, facultative anaerobic bacteria channel respiratory electrons to alternative acceptors and reducing conditions become established (1, 25). It can be hypothesized that abiotic stress resulting from a combination of oxygen limitation and reducing conditions had a role in the formation of VBNC cells of CHA0-Rif in this field experiment.
The first objective of the present study was to demonstrate that a combination of oxygen limitation and reducing conditions could lead to the occurrence of VBNC cells in P. fluorescens CHA0-Rif. The effect of combined oxygen limitation and reducing conditions on strain CHA0-Rif was also achieved using a single stress factor (i.e., high NaCl levels) when intensity of the stress was high enough. The second objective was to assess if VBNC cells of CHA0-Rif obtained by abiotic stress could persist better than culturable cells of the strain once introduced into nonsterile soil.
MATERIALS AND METHODS
Bacterial strain and growth conditions.
The experiments were carried out with strain CHA0-Rif (24), a spontaneous rifampin-resistant mutant of the biocontrol agent P. fluorescens CHA0 (30). Strain CHA0-Rif was kept at −80°C in 44% glycerol and grown routinely at 27°C with shaking (150 rpm) in King's B broth (15) containing 100 μg of rifampin (Sigma, Steinheim, Germany) per ml. The inoculum for the experiments was prepared by growing CHA0-Rif in 500 ml of M9 medium (22 mM KH2PO4, 42 mM Na2HPO4, 19 mM NH4Cl, 9 mM NaCl, 1 mM MgSO4 and 0.09 mM CaCl2, pH 6.8) (21) containing 5.5 mM glucose as a carbon source. The culture was incubated at 27°C with shaking (150 rpm) until mid-log phase (i.e., 108 CFU ml−1, corresponding to an optical density of 0.12 at 600 nm). The cells were washed three times in sterile distilled water (7,000 × g for 12 min) prior to use in the experiments.
Exposure of P. fluorescens CHA0-Rif to low redox potential and oxygen limitation.
In the first experiment, P. fluorescens CHA0-Rif was exposed for 7 days to a low redox potential (230 mV, with O2), oxygen limitation (480 mV, with N2), or a combination of both (230 mV, with N2). The strain was not exposed to those stresses in the control (480 mV, with O2).
CHA0-Rif cells were resuspended in M9 medium or M9 amended with 50 mM potassium hexacyanoferrate(II) [K4Fe(CN)6] (obtained from Fluka AG, Buchs, Switzerland) to achieve a low redox potential. The coordination compound potassium hexacyanoferrate was used previously in a redox buffer system by Unden et al. (36). Potassium hexacyanoferrate at 50 mM in M9 medium was not toxic to CHA0-Rif, as it had no effect on growth of the strain (data not shown). The redox potential (Eh) was measured with a One Stick redox electrode (Inlab 501 REDOX; Mettler-Toledo, Urdorf, Switzerland) containing an Argental reference electrode (electric potential of +207 mV, referred to the standard hydrogen electrode). Prior to measurement, the electrode was conditioned overnight in concentrated nitric acid, rinsed with tap water and then with distilled water, and finally kept for at least 1 h in distilled water. Immediately before measurement, the electrode was calibrated with Mettler-Toledo reference buffers at 220 mV (pH 7). In parallel, the pH was determined. Conventionally, redox potentials are expressed by reference to the standard hydrogen electrode at pH 7, and this was achieved by adding 207 mV (i.e., the potential of the Argental reference electrode) and the term (pH − 7) × 59 mV (i.e., correction for pH), as described by Zausig (41). The redox potential of M9 containing 50 mM potassium hexacyanoferrate was 230 ± 20 mV and remained stable over the 7-day incubation period (data not shown).
The cell suspensions were prepared in 500-ml Erlenmeyer flasks (aerobic conditions) or 200-ml serum flasks (oxygen-limited conditions). To achieve oxygen-limited conditions, the serum flasks were placed in a glass dessicator, the suspension was stirred continuously with a magnetic stirrer, and the air was suctioned out at 0.008 MPa. Nitrogen gas was then added up to normal air pressure (i.e., about 0.105 MPa). This procedure was repeated four times. All flasks were placed on a rotary shaker (150 rpm) at 27°C during the 7-day incubation. In all four treatments, CHA0-Rif cells were used at the rate of 108 CFU ml−1.
Exposure of P. fluorescens CHA0-Rif to high NaCl levels.
In the second experiment, P. fluorescens CHA0-Rif was exposed for 7 days to high NaCl levels by resuspending cells of the strain in M9 medium containing 0.7 or 1.5 M NaCl. CHA0-Rif cells were used at the rate of 108 CFU ml−1, as in the first experiment. The control treatment (i.e., no NaCl added) was the same as the control in the first experiment (i.e., 480 mV with O2). The Erlenmeyer flasks were incubated as described above.
Soil characteristics and preparation of soil microcosms.
The soil was collected from the surface horizon of a loamy cambisol (15% clay, 42% silt, and 43% sand) from Eschikon, near Zürich, Switzerland (24). The soil (noncalcareous) had a neutral pH reaction, 3.5% organic matter, and a cation exchange capacity of 33 cmol kg−1. The soil was air dried at room temperature until friable and sieved through a 5-mm mesh screen prior to use. Stones and roots were removed. Before inoculation, the water potential of the soil was adjusted to about −0.03 MPa by drying to a 22% (wt/wt) water content. Porous plates (26) were used to determine the water content of the soil at a water potential of −0.03 MPa, and then a filter paper method (20) was used routinely to determine the water potential of soil. The water content was determined by oven drying of soil samples at 105°C to a constant weight. Soil microcosms consisted of 10 ± 0.1 g of soil in sterile 25-ml glass vials.
Inoculation of soil microcosms with P. fluorescens CHA0-Rif.
After completion of the 7-day incubation in vitro, the cells of CHA0-Rif from the three replicates of each treatment were placed together in the same flask and washed three times, as described above. The cell densities in the final cell suspensions were increased 100-fold compared with the ones at the end of the 7-day incubation in vitro, and microcosms were inoculated by adding 0.1 ml of cell suspension to the soil in the vials. Uninoculated control samples received the same volume of sterile distilled water.
After inoculation, the microcosms were placed in loosely capped 250-ml plastic containers (three vials per container). In order to minimize water loss during incubation but allow aeration of the samples, the containers were placed together under a loosely closed plastic cover (eight containers under each cover). Incubation took place in the dark in an incubator set at 12°C with a relative humidity of 70%. During the 54-day experiment, the soil water content remained at 22% ± 0.1% (wt/wt), and water was not added.
Sampling and enumeration of P. fluorescens CHA0-Rif.
Cell counts were performed at the start and the end of the 7-day incubation in vitro and during incubation of soil microcosms. When soil was studied, the entire content of each vial (10 g of soil) was transferred into a 500-ml Erlenmeyer flask containing 100 ml of sterile distilled water, and the flask was shaken at 300 rpm for 15 min. A dilution series was then prepared from each soil extract.
Culturable cells of CHA0-Rif were counted by spread plating on solid King's B medium containing rifampin at 100 μg ml−1 and the antifungal compound cycloheximide (Fluka AG) at 190 μg ml−1. The plates were incubated for 2 days at 27°C in the dark, and colonies were counted. Incubation of plates for an additional 7 days did not increase the number of colonies. In the uninoculated control, no rifampin-resistant colonies were found (the detection limit was 102 CFU per g of soil).
The total number of CHA0-Rif cells was determined by IF microscopy as described by Troxler et al. (34). The bacteria were fixed on 0.2-μm-pore-size polycarbonate filters stained with Irgalan Black (Nucleopore; Costar Scientific Corporation, Cambridge, Mass.). The filters were treated successively with the primary antiserum (specific for CHA0) (33) and the secondary antibody (fluorescein isothiocyanate-conjugated goat anti-rabbit immunoglobulins) (Sigma), each time for at least 30 min. After the filters were mounted with DAPCO [1,4-diazobicyclo-(2,2,2)-octan-glycerol] medium to prevent fading, CHA0-Rif cells were counted using a Zeiss Axioskop epifluorescence microscope (450- to 490-nm filters; at least 20 fields and/or 150 bacteria per filter). No cross-reaction was found with uninoculated soil samples in the present work.
The number of viable CHA0-Rif cells was determined by using the technique of Kogure et al. (17), in combination with IF microscopy. Yeast extract (250 μg ml−1) and nalidixic acid (20 μg ml−1) were added, and the samples were incubated for 6 h at room temperature (about 22°C) in the dark, prior to fixation with formaldehyde. Substrate-responsive cells increased their length to 3.5 μm or more, and they were counted as viable cells. At least 20 fields and/or 150 bacteria were studied per filter (when population levels were low, filters were examined until at least 10 elongated cells were found).
Experimental design and statistical analyses.
All treatments were replicated three times, along a randomized block design. Cell counts were log transformed before calculation of means and standard deviations and performance of statistical analyses. A total of four treatments (first experiment) and three treatments (second experiment) were studied in vitro. Both experiments had the same control. All treatments were also studied in soil microcosms, where the control was also compared with a treatment in which log-phase cells of CHA0-Rif (from M9 cultures) were used.
First, the influence of the cell count method was assessed by comparing total IF counts, viable counts, and colony counts of CHA0-Rif within each treatment at each sampling time (i.e., in vitro and in soil). Second, treatments were compared for each cell count method at each sampling time. Data were processed using analysis of variance (version 5 of SYSTATS for Windows; SPSS Inc., Evanston, Ill.). When appropriate, Tukey's honestly significant difference (HSD) test was then used to compare treatments. Regression analysis was employed to study trends over several samplings in soil. All statistical analyses were performed at a P value of 0.05.
RESULTS AND DISCUSSION
Effect of combined stress factors (oxygen limitation and low redox potential) on P. fluorescens CHA0-Rif in vitro.
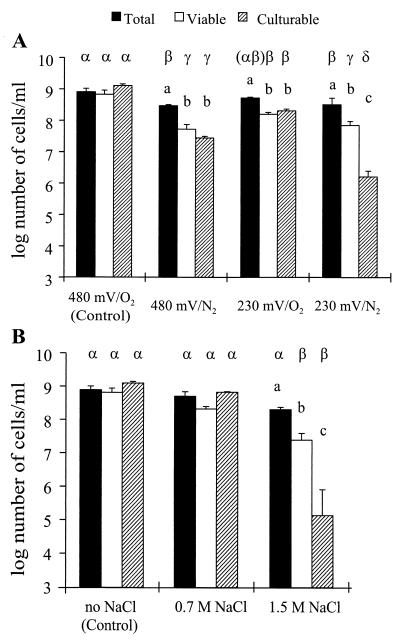
In the control (480 mV, with O2), P. fluorescens CHA0-Rif was found at about 109 cells ml−1 at the end of the 7-day incubation in vitro, regardless of whether cells were enumerated using total IF counts, viable counts, or colony counts (Fig. 1A). In contrast, total IF counts exceeded viable counts and colony counts when the strain had been exposed to oxygen limitation (480 mV, with N2) (Fig. 1A). Therefore, the lack of a suitable electron acceptor (i.e., oxygen) alone did not induce the formation of VBNC cells in the strictly aerobic strain CHA0-Rif. In contrast, the loss in colony-forming ability undergone by the facultative anaerobic strain Pseudomonas aeruginosa PAO303 when incubated in a medium lacking a suitable electron acceptor resulted in significant numbers of nonculturable cells that responded positively in a viability test (2). Oxygen limitation in waterlogged soil causes a rapid drop in soil redox potential, as facultative anaerobic bacteria begin to use alternative compounds as final electron acceptor (1, 25). Subjection of CHA0-Rif to low redox potential under aerobic conditions, in M9 amended with potassium hexacyanoferrate (as proposed by Unden et al. [36]), resulted in nonculturable cells, but here also there was no difference between colony counts and viable counts of the strain (230 mV, with O2) (Fig. 1A). When both types of stress (i.e., oxygen limitation and low redox potential) were combined (230 mV, with N2), the total IF counts of CHA0-Rif were statistically higher than the viable counts (by less than 1 log unit, as in the last two treatments), but the latter exceeded colony counts by almost 2 log units (indicating the presence of VBNC cells). This may account for the predominance of nonculturable cells (including VBNC cells) of CHA0-Rif found in the field in the waterlogged layer above the plow pan (7).
FIG. 1.
Effect of stress on P. fluorescens CHA0-Rif in vitro. The pseudomonad (108 log-phase cells ml−1) was subjected for 7 days to a low redox potential (230 mV) and/or oxygen limitation (N2) (A) or to high NaCl levels (B). Error bars show standard deviations. Statistical differences between the different types of counts (i.e., total IF counts, viable counts, and colony counts of CHA0-Rif) within each treatment are shown with letters a, b and c, whereas those between the four treatments (A) or three treatments (B) within each type of cell count are shown with letters α, β, γ, and δ (A) and α and β (B).
Effect of different intensities of a single stress factor (high NaCl levels) on P. fluorescens CHA0-Rif in vitro.
The fact that VBNC cells of CHA0-Rif were observed when the strain was exposed to a combination of two stress factors that were unable alone to cause the formation of VBNC cells was due either to a particular interaction between these two factors or the fact that the intensity of each stress factor alone was insufficient. To assess whether VBNC cells could be obtained using a single stress factor, CHA0-Rif cells were subjected to different intensities of a single, model stress (high NaCl levels). Exposure of the strain to 0.7 M NaCl had no effect on the colony-forming ability of CHA0-Rif, but the number of cells capable of growing on plate was lower than total IF counts by about 3 log units when NaCl was used at 1.5 M (Fig. 1B). Furthermore, a significant proportion of the nonculturable CHA0-Rif cells were in a VBNC state, as they were still nutrient responsive in Kogure's viability test. High NaCl concentrations do not have the same effect on all gram-negative bacteria. In accordance with our findings, the culturability of cells of P. fluorescens AH9 was reduced after incubation in 1.7 M NaCl but not after incubation in 1 M NaCl (10). In contrast, P. aeruginosa PAO1 (39) and Escherichia coli (29) were already affected at 0.7 and 0.8 M NaCl, respectively. This may, in part, reflect differences in osmotic potential between the habitats from which these bacteria originate. Interestingly, tolerance to high NaCl concentrations was suggested to be an important bacterial property for successful colonization of the root (18, 22). In conclusion, significant subpopulations of VBNC cells of CHA0-Rif were observed at the end of the 7-day incubation in vitro, provided that either (i) two types of stress were combined (Fig. 1A) or (ii) the intensity of a single type of stress was sufficiently high (Fig. 1B).
Persistence in soil of cells of P. fluorescens CHA0-Rif not subjected to stress in vitro.
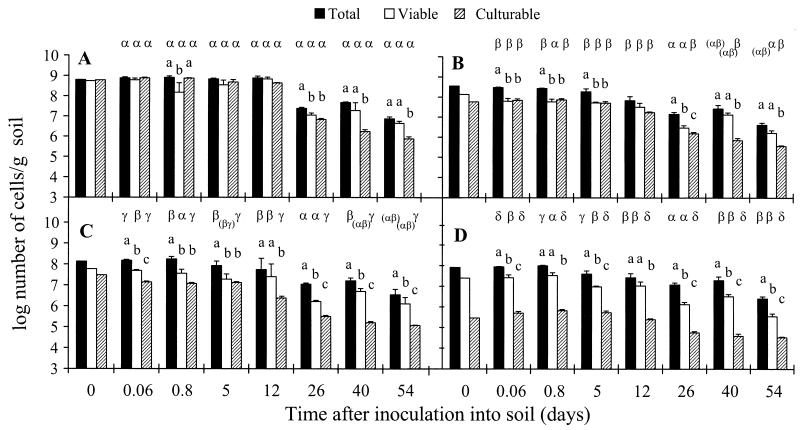
To determine whether aerobic incubation of P. fluorescens CHA0-Rif for 7 days in M9 medium could be considered nonstress conditions, CHA0-Rif cells from the control in vitro were introduced into soil and their persistence was compared with that of log-phase cells of the strain. Cells obtained from the control in vitro persisted in soil at population levels essentially similar to the inoculum level for 12 days regardless of the cell count method (Fig. 2A). Cell numbers were statistically lower at subsequent samplings. At the last two samplings, total IF counts and viable counts were statistically higher than colony counts (by 1 log unit or less), indicating the occurrence of VBNC cells.
FIG. 2.
Persistence in soil of cells of P. fluorescens CHA0-Rif after a 7-day aerobic incubation in M9 (control [i.e., 480 mV with O2]) (A) or subjected for 7 days in vitro to oxygen limitation (480 mV with N2) (B), low redox potential (230 mV with O2) (C), or a combination of both oxygen limitation and low redox potential (230 mV with N2) (D). Error bars show standard deviations. At each sampling time, statistical differences between the different types of counts (i.e., total IF counts, viable counts, and colony counts of CHA0-Rif) within each treatment are shown with a, b, and c, whereas those between the four treatments within each type of cell count are shown with letters α, β, γ, and δ.
The population dynamics of CHA0-Rif were largely similar when log-phase cells of the strain were introduced into soil, except that viable counts and colony counts were statistically identical at the last sampling (data not shown). Nutrient limitation was unlikely to account for this difference, because pseudomonads previously deprived of a single nutrient for several days in vitro displayed colony counts similar to those of log-phase cells once introduced into soil (9, 38), and multiple nutrient starvation for 7 days resulted in lower numbers of culturable cells in soil but not in the formation of VBNC cells (9). Therefore, the 7-day incubation in M9 medium might represent stressful conditions, leading to VBNC cells during subsequent incubation in soil. These findings could be of significance for production and formulation of biocontrol bacteria and other commercial soil inoculants. In conclusion, the behavior in soil of CHA0-Rif cells obtained from the control in vitro was to a large degree comparable to that of cells obtained from a log-phase culture of the strain, except that significant amounts of VBNC cells were found at the last two samplings in soil for the control.
Persistence in soil of cells of P. fluorescens CHA0-Rif subjected to low redox potential and/or oxygen limitation in vitro.
Under in vitro conditions, total IF counts of P. fluorescens CHA0-Rif exceeded both viable counts and colony counts at the end of the 7-day incubation of the strain under oxygen limitation (Fig. 1A). A comparable situation was found at 5 days after the introduction of the cells into soil (Fig. 2B). At the last two samplings in soil, however, there was no difference between total IF counts and viable counts, and both counts were statistically higher than colony counts of CHA0-Rif, a situation similar to that found for the control (Fig. 2A). In contrast, total IF counts of CHA0-Rif exceeded viable counts at six of seven samplings in soil for the treatment where cells had been exposed to a low redox potential in vitro (Fig. 2C). From day 12 on, viable counts of the strain were statistically higher than colony counts, as found at the last two samplings in the previous two treatments. At the end of the 7-day incubation of CHA0-Rif under conditions of low redox potential and oxygen limitation in vitro, total IF counts of the strain were higher than viable counts and each count was higher than colony counts (Fig. 1A). Similar findings were obtained at five of seven samplings once the cells were introduced into soil (Fig. 2D). In conclusion, VBNC cells of CHA0-Rif were found at the last two samplings in soil, and total IF counts of the strain exceeded viable counts at those samplings for the two treatments where cells had been subjected to a low redox potential in vitro.
Persistence in soil of cells of P. fluorescens CHA0-Rif subjected to high NaCl levels in vitro.
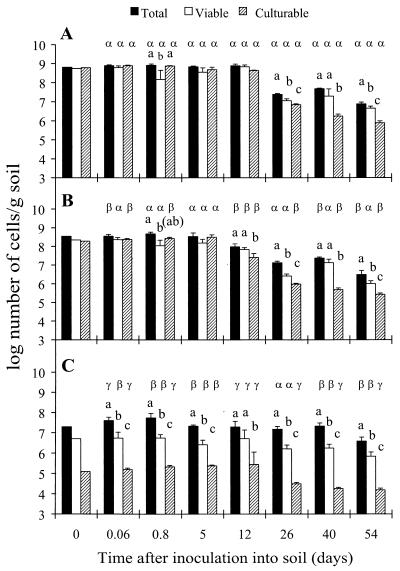
Exposure of P. fluorescens CHA0-Rif to 0.7 M NaCl for 7 days in vitro resulted in cell counts that were statistically identical to those in the control (Fig. 1B). The population dynamics of the strain following the introduction of the cells into soil were also similar to those observed for the control, regardless of whether monitoring was done using total IF counts, viable counts, or colony counts (Fig. 3A and B). At the end of the 7-day incubation of CHA0-Rif in 1.5 M NaCl, total IF counts and viable counts of the strain exceeded colony counts (Fig. 1B). Colony counts remained lower than the other counts for 54 days after inoculation into soil, whereas total IF counts were higher than viable counts at six of seven samplings in soil (Fig. 3C). In conclusion, exposure of CHA0-Rif to 0.7 M NaCl in vitro had no effect on the subsequent persistence of the cells in soil, whereas incubation in presence of 1.5 M NaCl resulted in nonculturable cells (including VBNC cells), both in vitro and subsequently in soil.
FIG. 3.
Persistence in soil of cells of P. fluorescens CHA0-Rif after a 7-day aerobic incubation in M9 (control) (A) or M9 containing 0.7 M NaCl (B) or 1.5 M NaCl (C). Error bars show standard deviations. At each sampling time, statistical differences between the different types of counts (i.e., total IF counts, viable counts, and colony counts of CHA0-Rif) within each treatment are shown with letters a, b, and c, whereas those between the three treatments within each type of cell count are shown with letters α, β, and γ.
Ecological significance of VBNC cells of P. fluorescens CHA0-Rif.
The occurrence of VBNC cells during residence of bacteria in soil is well documented (3, 34, 35). However, the ecological significance of the loss of colony-forming ability displayed by bacterial cells is not clear and remains the subject of considerable debate (4, 14, 19), all the more so because the mechanisms involved in the transition to a VBNC state are still to be ascertained. In some cases, viable cells may fail to grow on nutrient-rich media because they have lost the defense mechanisms to cope with radical oxygen species generated during oxygen respiration (2, 4, 12). In accordance with this, the root colonizer Pseudomonas putida needs catalases for colony-forming ability after exposure to oxidative stress from hydrogen superoxide (16). Alternatively, the VBNC state(s) may represent a survival strategy enabling nonsporulating gram-negative bacteria to overcome adverse environmental conditions (28), which implies the possibility of recovering cell culturability when adverse conditions are replaced with environmental conditions allowing growth. Indeed, “resuscitation” of VBNC cells has been achieved on a few occasions, e.g., with Vibrio vulnificus (40) and Micrococcus luteus (11).
Another implication is that cells in a VBNC state would persist better in the environment than cells in other physiological states. In the present experiment, the number of VBNC cells of P. fluorescens CHA0-Rif obtained using a combination of low redox potential and oxygen limitation in vitro (i.e., 230 mV with N2) (Fig. 1A) decreased from 7.4 to 5.4 log cells per g of soil in 54 days once the strain was introduced into soil (Fig. 2D). The number of culturable cells of CHA0-Rif was reduced from 5.5 to 4.4 log cells per g of soil in the same treatment (Fig. 2D). In parallel, the number of VBNC cells of CHA0-Rif generated using high NaCl levels in vitro (Fig. 1B) decreased from 6.7 to 5.7 log cells per g of soil in 54 days in soil, whereas in the same treatment colony counts in soil went from 5.1 to 4.0 log cells per g of soil (Fig. 3C). Apparently, CHA0-Rif cells in a VBNC state did not persist better than culturable cells in soil, regardless of the stress conditions used to generate them.
In conclusion, exposure of P. fluorescens CHA0-Rif to a combination of two stress factors or to a high intensity of a single type of stress in vitro resulted in mixed populations of culturable and nonculturable cells, and VBNC cells represented a significant proportion of the latter. Once in soil, the VBNC cells thus obtained did not display enhanced persistence compared with culturable cells, suggesting that their physiological state did not represent a successful adaptive response to adverse environmental conditions.
ACKNOWLEDGMENTS
We thank Philipp Wettstein for technical assistance.
This work was supported by the Swiss National Foundation for Scientific Research (Priority Program Biotechnology, project 5002-04502311) and the Swiss Federal Office for Education and Science (EU IMPACT 2, project BIO4-CT96-0027).
REFERENCES
- 1.Bartlett J R, James B R. Redox chemistry of soils. Adv Agron. 1993;50:151–208. [Google Scholar]
- 2.Binnerup S J, Sørensen J. Long-term oxidant deficiency in Pseudomonas aeruginosa PAO303 results in cells which are non-culturable under aerobic conditions. FEMS Microbiol Ecol. 1993;13:79–84. [Google Scholar]
- 3.Binnerup S J, Jensen D F, Thordal-Christensen H, Sørensen J. Detection of viable, but non-culturable Pseudomonas fluorescens DF57 in soil using a microcolony epifluorescence technique. FEMS Microbiol Ecol. 1993;12:97–105. [Google Scholar]
- 4.Bloomfield S F, Steward G S A B, Dodd C E R, Booth I R, Power E G M. The viable but non-culturable phenomenon explained? Microbiology. 1998;144:1–3. doi: 10.1099/00221287-144-1-1. [DOI] [PubMed] [Google Scholar]
- 5.Défago G, Berling C-H, Henggeler S, Hungerbühler W, Kern H, Schleppi P, Stutz E W, Zürrer M. Survie d'un Pseudomonas fluorescens dans le sol et protection du blé contre les maladies d'origine fongique. Schweiz Landw Fo. 1987;26:155–160. [Google Scholar]
- 6.Défago G, Keel C, Moënne-Loccoz Y. Fate of introduced biocontrol agent Pseudomonas fluorescens CHA0 in soil: biosafety considerations. In: Wenhua T, Cook R J, Rovira A, editors. Advances in biological control of plant diseases. Beijing, China: China Agricultural University Press; 1996. pp. 241–245. [Google Scholar]
- 7.Défago G, Keel C, Moënne-Loccoz Y. Fate of released Pseudomonas bacteria in the soil profile: implications for the use of genetically-modified microbial inoculants. In: Zelikoff J T, Lynch J M, Shepers J, editors. Ecotoxicology: responses, biomarkers and risk assessment. Fair Heaven, N.J: SOS Publications; 1997. pp. 403–418. [Google Scholar]
- 8.de Leij F A A M, Sutton E J, Whipps J M, Fenlon J S, Lynch J M. Field release of a genetically modified Pseudomonas fluorescens on wheat: establishment, survival, and dissemination. Bio/Technology. 1995;13:1488–1492. [Google Scholar]
- 9.Hase C, Mascher F, Moënne-Loccoz Y, Défago G. Nutrient deprivation and the subsequent survival of biocontrol Pseudomonas fluorescens CHA0 in soil. Soil Biol Biochem. 1999;31:1181–1188. [Google Scholar]
- 10.Jørgensen F, Nybroe O, Knøchel S. Effects of starvation and osmotic stress on viability and heat resistance of Pseudomonas fluorescens AH9. J Appl Bacteriol. 1994;77:340–347. [Google Scholar]
- 11.Kaprelyants A S, Mukamolova G V, Kell D B. Estimation of dormant Micrococcus luteus cells by penicillin lysis and by resuscitation in cell-free spent medium at high dilution. FEMS Microbiol Lett. 1994;115:347–352. [Google Scholar]
- 12.Katsuwon J, Anderson A J. Catalase and superoxide dismutase of root-colonizing saprophytic fluorescent pseudomonads. Appl Environ Microbiol. 1990;56:3576–3582. doi: 10.1128/aem.56.11.3576-3582.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Keel C, Défago G. Interactions between beneficial soil bacteria and root pathogens: mechanisms and ecological impact. In: Gange A C, Brown V K, editors. Multitrophic interactions in terrestrial systems. London, United Kingdom: Blackwell Scientific Publishers; 1997. pp. 27–46. [Google Scholar]
- 14.Kell D B, Kaprelyants A S, Weichart D H, Harwood C R, Barer M R. Viability and activity in readily culturable bacteria: a review and discussion of the practical issues. Antonie Leeuwenhoek. 1998;73:169–187. doi: 10.1023/a:1000664013047. [DOI] [PubMed] [Google Scholar]
- 15.King E O, Ward M K, Rancy D E. Two simple media for the demonstration of pyocyanin and fluorescein. J Lab Clin Med. 1954;44:301–307. [PubMed] [Google Scholar]
- 16.Klotz M G, Anderson A J. The role of catalase isozymes in the culturability of the root colonizer Pseudomonas putida after exposure to hydrogen peroxide and antibiotics. Can J Microbiol. 1994;40:382–387. [Google Scholar]
- 17.Kogure K, Simidu U, Taga N. A tentative direct microscopic method for counting living marine bacteria. Can J Microbiol. 1979;25:415–420. doi: 10.1139/m79-063. [DOI] [PubMed] [Google Scholar]
- 18.Loper J E, Haack C, Schroth M N. Population dynamics of soil pseudomonads in the rhizosphere of potato (Solanum tuberosum L.) Appl Environ Microbiol. 1985;49:416–422. doi: 10.1128/aem.49.2.416-422.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McDougald D, Rice S A, Weichart D, Kjelleberg S. Nonculturability: adaptation or debilitation? FEMS Microbiol Ecol. 1998;25:1–9. [Google Scholar]
- 20.McInnes K J, Weaver R W, Savage M J. Soil water potential. In: Weaver R W, Angle S, Bottomley P, editors. Methods of soil analysis, part 2. Microbiological and biochemical properties. SSSA Books Series no. 5. Madison, Wis: SSSA; 1994. pp. 53–58. [Google Scholar]
- 21.Miller J H. Experiments in molecular genetics. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1972. pp. 431–432. [Google Scholar]
- 22.Miller K J, Wood J M. Osmoadaptation by rhizosphere bacteria. Annu Rev Microbiol. 1996;50:101–136. doi: 10.1146/annurev.micro.50.1.101. [DOI] [PubMed] [Google Scholar]
- 23.Moënne-Loccoz Y, Powell J, Higgins P, McCarthy J, O'Gara F. An investigation of the impact of biocontrol Pseudomonas fluorescens F113 on the growth of sugarbeet and the performance of subsequent clover-Rhizobium symbiosis. Appl Soil Ecol. 1998;7:225–237. [Google Scholar]
- 24.Natsch A, Keel C, Pfirter H A, Haas D, Défago G. Contribution of the global regulator gene gacA to persistence and dissemination of Pseudomonas fluorescens biocontrol strain CHA0 introduced into soil microcosms. Appl Environ Microbiol. 1994;60:2553–2560. doi: 10.1128/aem.60.7.2553-2560.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ponnamperuma F N. The chemistry of submerged soils. Adv Agron. 1972;24:29–96. [Google Scholar]
- 26.Richard L A. Porous plate apparatuses for measuring moisture retention and transmission by soil. Soil Sci. 1948;66:76–83. [Google Scholar]
- 27.Rodriguez G G, Phipps D, Ishiguro K, Ridgeway H F. Use of a fluorescent redox probe for direct visualization of actively respiring bacteria. Appl Environ Microbiol. 1992;58:1801–1808. doi: 10.1128/aem.58.6.1801-1808.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rollins D M, Colwell R R. Viable but nonculturable stage of Campylobacter jejuni and its role in survival in the natural aquatic environment. Appl Environ Microbiol. 1986;52:531–538. doi: 10.1128/aem.52.3.531-538.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Roth W G, Leckie M P, Dietzler D N. Restoration of colony-forming activity in osmotically stressed Escherichia coli by betaine. Appl Environ Microbiol. 1988;54:3142–3146. doi: 10.1128/aem.54.12.3142-3146.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stutz E W, Défago G, Kern H. Naturally occurring fluorescent pseudomonads involved in suppression of black root rot of tobacco. Phytopathology. 1986;76:181–185. [Google Scholar]
- 31.Tiedje J M, Colwell R R, Grossman Y L, Hodson R E, Lenski R E, Mack R N, Regal P J. The planned introduction of genetically engineered organisms: ecological considerations and recommendations. Ecology. 1989;70:298–315. [Google Scholar]
- 32.Troxler J. Assessing the potential risks of introducing bacteria into soil for sustaining plant health. Ph.D. dissertation. 1997. no. 11986. Swiss Federal Institute of Technology, Zürich, Switzerland. [Google Scholar]
- 33.Troxler J, Berling C-H, Moënne-Loccoz Y, Keel C, Défago G. Interactions between the biocontrol agent Pseudomonas fluorescens CHA0 and Thielaviopsis basicola in tobacco roots observed by immunofluorescence microscopy. Plant Pathol. 1997;46:62–71. [Google Scholar]
- 34.Troxler J, Zala M, Natsch A, Moënne-Loccoz Y, Keel C, Défago G. Predominance of nonculturable cells of the biocontrol strain Pseudomonas fluorescens CHA0 in the surface horizon of large outdoor lysimeters. Appl Environ Microbiol. 1997;63:3776–3782. doi: 10.1128/aem.63.10.3776-3782.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Turpin P E, Maycroft C L, Rowlands C L, Wellington E M H. Viable but non-culturable salmonellas in soil. J Appl Bacteriol. 1993;74:421–427. doi: 10.1111/j.1365-2672.1993.tb05149.x. [DOI] [PubMed] [Google Scholar]
- 36.Unden G, Trageser M, Duchêne A. Effect of positive redox potentials (>+400mV) on the expression of anaerobic respiratory enzymes in Escherichia coli. Mol Microbiol. 1990;4:315–319. doi: 10.1111/j.1365-2958.1990.tb00598.x. [DOI] [PubMed] [Google Scholar]
- 37.van Elsas J D, van Overbeek L S. Bacterial responses to soil stimuli. In: Kjelleberg S, editor. Starvation in bacteria. New York, N.Y: Plenum Press; 1993. pp. 55–79. [Google Scholar]
- 38.van Overbeek L S, Eberl L, Givskov M, Molin S, van Elsas J D. Survival of, and induced stress resistance in, carbon-starved Pseudomonas fluorescens cells residing in soil. Appl Environ Microbiol. 1995;61:4202–4208. doi: 10.1128/aem.61.12.4202-4208.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Velasco R, Burgoa R, Flores E, Hernandez E, Villa A, Vaca S. Osmoregulation in Pseudomonas aeruginosa under hyperosmotic shock. Rev Lat Am Microbiol. 1995;37:209–216. [PubMed] [Google Scholar]
- 40.Whitesides M D, Oliver J D. Resuscitation of Vibrio vulnificus from the viable but nonculturable state. Appl Environ Microbiol. 1997;63:1002–1005. doi: 10.1128/aem.63.3.1002-1005.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zausig J. Redox potential measurement. In: Alef K, Nannipieri P, editors. Methods in applied soil microbiology and biochemistry. London, United Kingdom: Academic Press; 1995. pp. 274–276. [Google Scholar]
- 42.Zimmermann R, Iturriaga R, Becker-Birck J. Simultaneous determination of the total number of aquatic bacteria and the number thereof involved in respiration. Appl Environ Microbiol. 1978;36:926–935. doi: 10.1128/aem.36.6.926-935.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]