Abstract
Objectives:
To evaluate post-discharge health resource use in pediatric survivors of septic shock and determine patient and hospitalization factors associated with health resource use.
Design:
Secondary analyses of a multicenter prospective observational cohort study.
Setting:
Twelve academic pediatric intensive care units.
Patients:
Children ≥1 month and <18 years old hospitalized for community-acquired septic shock who survived to 1-year.
Interventions:
None
Measurements and Main Results:
For 308/338 (91%) patients with baseline and ≥ 1 post-discharge survey, we evaluated readmission, emergency department (ED) visits, new medication class, and new device class use during the year after sepsis. Using negative binomial regression with bidirectional stepwise selection, we identified factors associated with each outcome. Median age was 7 years (IQR 2, 13), 157 (51%) had a chronic condition, and nearly all patients had insurance (private [n=135; 44%] or government [n=157; 51%]). During the year after sepsis, 128 (42%) patients were readmitted, 145 (47%) had an ED visit, 156 (51%) started a new medication class, and 102 (33%) instituted a new device class. Having a complex chronic condition was independently associated with readmission and ED visit. Documented infection and higher sum of PELOD-2 hematologic score were associated with readmission whereas younger age and having a non-complex chronic condition were associated with ED visit. Factors associated with new medication class use were private insurance, neurologic insult, and longer PICU stays. Factors associated with new device class use were pre-admission chemotherapy or radiotherapy, pre-sepsis Functional Status Scale score, and ventilation duration ≥10 days. Of patients who had a new medication or device class, most had a readmission (56% and 61%) or ED visit (62% and 67%).
Conclusions:
Children with septic shock represent a high-risk cohort with high resource needs after discharge. Interventions and targeted outcomes to mitigate post-discharge resource use may differ based on patients’ pre-existing conditions.
Keywords: critical care outcome, intensive care units, pediatric, child, septic shock, medical device, hospital readmission
Introduction
In the United States, more than 72,000 children are hospitalized with severe sepsis annually and hospitalization rates are increasing (1). Although survival rates have improved to 75-95%, retrospective studies using administrative databases suggest high rates of morbidities, hospital readmission, outpatient health resource use, and device acquisition (2–9). However, administrative data lack granular clinical information related to risk factors and outcomes, which may be enhanced by prospective clinical studies.
The prospective cohort study, Life After Pediatric Sepsis Evaluation (LAPSE), was originally conducted to characterize mortality, health-related quality of life morbidity, and risk factors for poor outcomes in children with community-acquired septic shock (10, 11). In this study, we leveraged the rich clinical data from the survivors in the LAPSE cohort to 1) characterize post-discharge health resource use including hospital readmission, emergency department (ED) visits, new devices, and new medications and 2) identify patient and hospitalization risk factors associated with these outcomes. We hypothesized risk factors for readmission would be related to patient (e.g., chronic conditions) and hospitalization (e.g., duration of organ failure) factors.
Methods
The LAPSE study was conducted at twelve academic Pediatric Intensive Care Units (PICU) across the United States and a central or site-specific Institutional Review Board approved the study (Supplemental Digital Content (SDC), eText 1). Parents provided written permission for participation and patients with appropriate developmental capacity provided assent.
The LAPSE protocol has been previously published (10, 11). Briefly, PICU patients (2013-2017) were continuously screened by research coordinators, and patients with evidence of septic shock were eligible for participation. Septic shock was defined as infection (documented or suspected) with onset within 48 hours of hospital admission, at least 2 systemic inflammatory response syndrome criteria (necessitated inclusion of either abnormal leukocyte count or differential or abnormal body temperature), need for fluid resuscitation and vasoactive-inotropic support within 72 hours of hospital admission and 48 hours of PICU admission. Sepsis care was not protocolized. Patients were categorized as having pre-existing complex chronic condition(s), non-complex chronic condition(s), or no chronic conditions based on the Pediatric Medical Complexity Algorithm using data collected within the pediatric health information system during the 3 years prior to index PICU admission (12). Baseline functional status was assessed using the Functional Status Scale (FSS) (13). Organ dysfunction was quantified by the Pediatric Logistic Organ Dysfunction-2 (PELOD-2) score (14). Neurologic insult was collected through chart review by trained research staff and defined as pathologic breathing pattern, stereotypic or flaccid posturing, seizure activity or abnormal electroencephalogram, new anoxic-ischemic injury on brain imaging, treatment for increased intracranial pressure, neurologic injury suspected by care provider, autonomic storming, or cardiopulmonary arrest or chest compressions. Health resource use data were collected at admission reflective of pre-admission baseline, Day 7, and Months 1, 3, 6, and 12 after enrollment by parent/guardian survey conducted centrally by the Seattle Children’s Research Institute.
For the current study, we included only participants who survived to 1 year after enrollment and had completed the baseline and at least one post-discharge health resource use survey. We evaluated surveys reflective of post-discharge status only. The primary outcomes were evaluated separately and included number of hospital readmissions, ED visits, new medication classes and new device classes during the year after discharge. Outpatient medications were classified based on Anatomic Therapeutic Category level 2 and individually reviewed (AM) to ensure classifications reflected most frequent use in children. When available data were insufficient to determine classification, the medication was excluded (SDC, eTable 1). New devices were individually reviewed (AM) and classified based on morbidity domain: respiratory, activity of daily living assistance, feeding, infusion/access, urinary catheters, neurologic, communication, dialysis, or other. We identified the Area Deprivation Index (ADI), a validated measure of national percentile ranking of neighborhoods by socioeconomic disadvantage based on patients’ zip codes (15, 16). Percentile rankings are block grouped from 1-100 with higher numbers indicating higher disadvantage.
Statistical Analysis
Missing survey data were not imputed. Categorical data are reported as counts and percentages and continuous measures summarized using medians and interquartile ranges (IQR). To assess for potential bias, we compared the characteristics between children included in this investigation and those eligible for follow-up but excluded due to missing data. Negative binomial regression was performed to evaluate associations between patient and hospitalization characteristics with each of the four outcomes, with estimates reported as rate ratios (RR) and 95% confidence intervals (CI). Univariable analyses were performed to identify candidate variables for multivariable analyses. Variables were selected if they were available for >90% of the cohort and demonstrated potential associations with the outcome of interest, defined as p<0.20. Four outcome-specific multivariable models were developed using bi-directional stepwise selection with entry and exit criteria set at p≤0.20 and p≤0.05, respectively. We also compared the overlap of new medication class or device class with readmission and ED visit. All analyses were completed using SAS v9.4 (SAS Institute Inc., Cary, NC).
Results
The clinical cohort in the LAPSE study was 389 patients of whom 35 (9%) died during the hospitalization and 16 (4%) died during the one-year follow-up (SDC, eFigure 1). Of the 338 survivors, 308 (91%) completed a baseline survey and at least one post-discharge survey and were included in this analysis (SDC, eTable 2). Children (n=30) who were excluded from the analysis due to missing data had a higher ADI representing higher socioeconomic disadvantage (85 [IQR 66, 94] versus 32 [IQR 15, 68], p=.006) and higher initial illness severity (day 1 PELOD score 10 [8, 11] versus 8 [6, 11], p=0.04) (SDC, eTable 3).
The median age of our cohort was 7 years (IQR 2, 13) (Table 1). One hundred forty (45%) patients had a complex chronic condition, 17 (6%) had a non-complex chronic condition, and 150 (49%) did not have a chronic condition. Fifty patients (16%) had an immune-related comorbidity, most often chemotherapy or radiation within the prior 3 months, malignancy, steroid use, or neutropenia (SDC, eTable 4). Nearly all patients had insurance, 135 (44%) had private insurance and 157 (51%) had government insurance. The range of disadvantage varied (median ADI 32 [IQR 15, 68]).
Table 1.
Baseline characteristics by hospital readmission and emergency department visits
| Hospital Readmissions |
Emergency Department Visits |
||||||
|---|---|---|---|---|---|---|---|
| Patient Characteristics | Overall (N = 308) |
0 (N = 180) |
1-2 (N = 74) |
3+ (N = 54) |
0 (N = 163) |
1-2 (N = 84) |
3+ (N = 61) |
| Age (years) | 7 [2, 13] | 8 [2, 13] | 6 [2, 13] | 6 [2, 12] | 8 [3, 14] | 6 [2, 13] | 4 [1, 11] |
| Male Sex | 169 (55%) | 96 (53%) | 40 (54%) | 33 (61%) | 89 (55%) | 41 (49%) | 39 (64%) |
| Area Deprivation Index (national rank)1 | 32 [15, 68] | 32 [17, 68] | 36 [15, 77] | 21 [12, 51] | 31 [13, 65] | 31 [15, 68] | 32 [15, 71] |
| Health insurance status | |||||||
| Private insurance | 135 (44%) | 77 (43%) | 31 (42%) | 27 (50%) | 71 (44%) | 35 (42%) | 29 (48%) |
| State Medicaid | 157 (51%) | 93 (52%) | 40 (54%) | 24 (44%) | 82 (50%) | 46 (55%) | 29 (48%) |
| Other2/Unknown | 16 (5%) | 10 (6%) | 3 (4%) | 3 (6%) | 10 (6%) | 3 (4%) | 3 (5%) |
| Pre-sepsis Functional Status Scale score | 6 [6, 11] | 6 [6, 10] | 8 [6, 12] | 8 [6, 12] | 6 [6, 11] | 7 [6, 12] | 7 [6, 12] |
| Pediatric Medical Complexity Algorithm2 | |||||||
| No chronic conditions | 150 (49%) | 113 (63%) | 22 (30%) | 15 (28%) | 93 (57%) | 36 (43%) | 21 (34%) |
| Non-complex chronic condition(s) | 17 (6%) | 8 (4%) | 6 (8%) | 3 (6%) | 8 (5%) | 4 (5%) | 5 (8%) |
| Complex chronic condition(s) | 140 (45%) | 59 (33%) | 46 (62%) | 35 (65%) | 62 (38%) | 44 (52%) | 34 (56%) |
| Day 1 PELOD3 | 8 [6, 11] | 8 [6, 10] | 8 [7, 10] | 8 [6, 11] | 9 [6, 11] | 8 [6, 10] | 7 [6, 11] |
| PRISM III4 | 11 [6, 16] | 10 [5, 16] | 13 [8, 18] | 10 [6, 14] | 11 [6, 17] | 12 [7, 18] | 10 [6, 14] |
| Infectious disease status5 | |||||||
| Suspected | 180 (58%) | 116 (64%) | 40 (54%) | 24 (44%) | 104 (64%) | 42 (50%) | 34 (56%) |
| Documented | 128 (42%) | 64 (36%) | 34 (46%) | 30 (56%) | 59 (36%) | 42 (50%) | 27 (44%) |
| Any immune-related comorbid conditions6 | 50 (16%) | 23 (13%) | 14 (19%) | 13 (24%) | 24 (15%) | 16 (19%) | 10 (16%) |
| Hospitalization Characteristics | |||||||
|
| |||||||
| ΔFSS Day 77 | 3 [0, 9] | 3 [0, 9] | 4 [0, 9] | 2 [0, 7] | 2 [0, 10] | 4 [0, 8] | 3 [0, 8] |
| Neurologic insult(s) during PICU stay | 118 (38%) | 63 (35%) | 35 (47%) | 20 (37%) | 60 (37%) | 35 (42%) | 23 (38%) |
| Cardiopulmonary resuscitation | 12 (4%) | 6 (3%) | 5 (7%) | 1 (2%) | 6 (4%) | 3 (4%) | 3 (5%) |
| Cumulative 28-day PELOD-2 | 50 [29, 81] | 48 [28, 76] | 64 [31, 115] | 51 [24, 66] | 50 [27, 82] | 46 [30, 91] | 55 [31, 80] |
| PICU length of stay (days) | 9 [5, 15] | 8 [5, 14] | 10 [6, 19] | 8 [4, 14] | 8 [5, 15] | 9 [6, 14] | 9 [6, 13] |
| Ventilation (days) | |||||||
| 9 days or less | 195 (63%) | 115 (64%) | 43 (58%) | 37 (69%) | 101 (62%) | 53 (63%) | 41 (67%) |
| 10+ days | 113 (37%) | 65 (36%) | 31 (42%) | 17 (31%) | 62 (38%) | 31 (37%) | 20 (33%) |
| Hospital length of stay (days) | 16 [9, 24] | 14 [9, 22] | 19 [12, 31] | 18 [11, 25] | 15 [9, 24] | 15 [10, 26] | 17 [11, 24] |
Area deprivation index (ADI) was unavailable for 58 patients.
Patient categorized based on data collected within the pediatric health information system (PHIS) for the 3 years prior to LAPSE index ICU admission. PHIS data was unavailable for 1 patient.
Day 1 Pediatric Logistic Organ Dysfunction 2 (PELOD-2) refers to the calculated score on the calendar day of PICU admission if subject was admitted before noon. If admitted after 12 pm noon, it refers to the following day.
Pediatric Risk of Mortality III (PRISM III) score uses the worst physiologic values obtained during the 6-hour period from 2 hours prior to PICU admission through four hours post PICU admission.
Documented infections were collected from PICU admission until Day 28 or ICU discharge, whichever occurred first.
Immune-related comorbid conditions were collected daily from PICU admission until Day 28 or PICU discharge, whichever occurred first.
Relative to pre-sepsis baseline.
During the year after sepsis, 128 (42%) patients required hospital readmission including 54 (18%) who had ≥ 3 readmissions. The median number of readmissions for readmitted patients was 2.0 [IQR 1.0, 5.0]. Most patients (n=87, 68%) who experienced a readmission did so within 3 months (Figure 1). Similarly, 145 (47%) children had an ED visit during the year after discharge including 61 (20%) who had ≥ 3 visits. The median number of visits for patients who had an ED visit was 2.0 [IQR 1.0, 4.0]. Most patients (n=100, 69%) who had an ED visit did so within 3 months.
Figure 1.
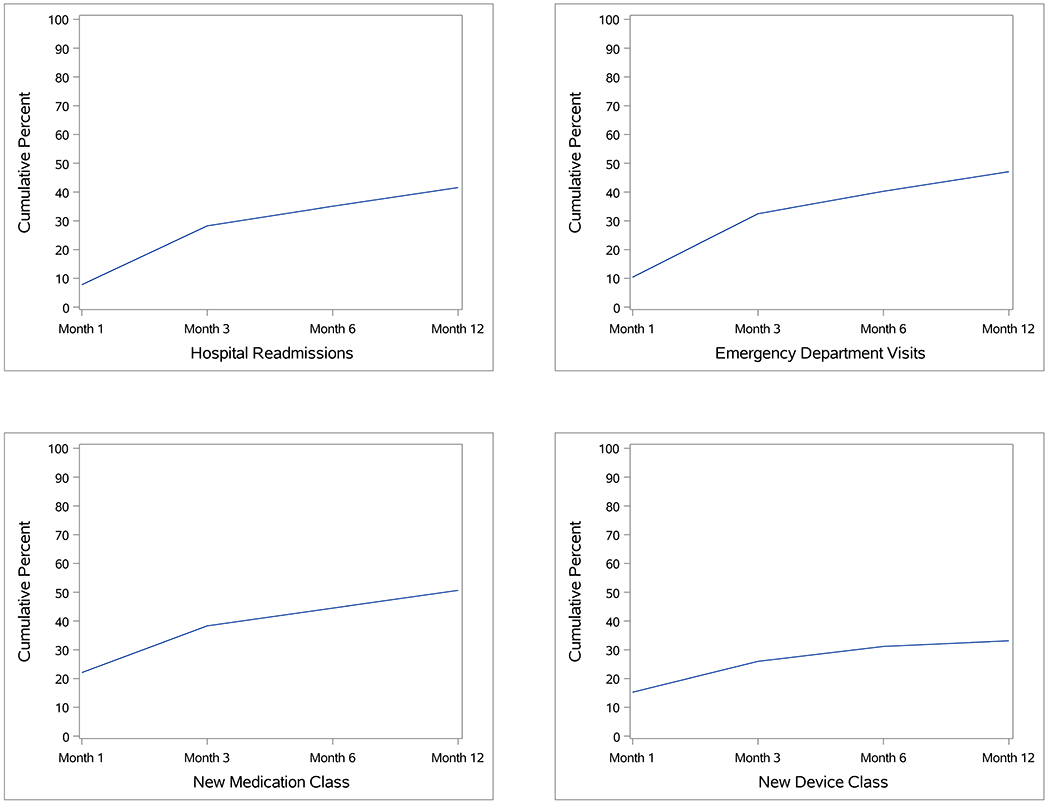
Cumulative proportion of patients who had a hospital readmission, emergency department visit, new medication class, or new device class at each survey timepoint during the post-discharge year.
Factors associated with hospital readmission or ED visit in univariable analyses are reported in SDC, eTable 5. In multivariable analysis, having a complex chronic condition was independently associated with both hospital readmission (RR 1.96 [1.20, 3.21]) and ED visit (RR 1.86 [1.28. 2.70]) (Table 2). Whereas having a documented infection (RR 1.75 [1.09, 2.84]) and higher sum of PELOD-2 hematologic score (RR 1.03 [95% CI: 1.00, 1.06]) were associated with readmission, younger age (RR 0.95 [0.92, 0.98]) and having a non-complex chronic condition (RR 2.29 [95% CI 1.11, 5.13] were associated with ED visits.
Table 2:
Multivariable models for factors associated with number of post-discharge encounters
| Predictor | Rate ratio (95% Confidence Interval) | p-value |
|---|---|---|
| Number of hospital readmissions | ||
| Pediatric Medical Complexity Algorithm 1 | 0.017 | |
| No chronic conditions | Reference | |
| Non-complex chronic condition(s) | 2.35 (0.92, 7.58) | |
| Complex chronic condition(s) | 1.96 (1.20, 3.21) | |
| Sum of PELOD-2 Hematologic Score | 1.03 (1.00, 1.06) | 0.026 |
| Infection at eligibility | 0.021 | |
| Documented | 1.75 (1.09, 2.84) | |
| Suspected | Reference | |
|
| ||
| Number of emergency department visits | ||
|
| ||
| Pediatric Medical Complexity Algorithm 1 | 0.002 | |
| No chronic conditions | Reference | |
| Non-complex chronic condition(s) | 2.29 (1.11, 5.13) | |
| Complex chronic condition(s) | 1.86 (1.28, 2.70) | |
| Age (years) | 0.95 (0.92, 0.98) | 0.003 |
Patient diagnoses data collected within the pediatric health information system (PHIS) for the 3 years prior to the LAPSE index Intensive Care Unit admission was used. PHIS data was not available for 1 patient.
PELOD: Pediatric Logistic Organ Dysfunction score.
During the post-discharge year, 156 (51%) patients reported a new medication class with a median of 2.0 (IQR 1.0, 3.0) new medication classes amongst these patients (Table 3). Most frequently, new medications were over the counter drugs such as vitamins (n=36, 12%) and those used to treat gastric acid-related disorders (n=24, 8%) and constipation (n=21, 7%) (SDC, eTable 1). New prescription medications during the year following admission were most commonly antiepileptics (n=29, 9%), systemic antibiotics (n=27, 9%), and antithrombotic medications (n=17, 6%). Most patients (n=118, 76%) reporting a new medication class did so within 3 months (Figure 1). Use of a new device class after discharge was reported in 102 (33%) patients with median 1.0 (IQR 1.0, 2.0) new device classes reported amongst these patients. Most commonly, new device classes were devices related to respiratory (n=41, 13%), activities of daily living (n=40, 13%), and feeding (n=25, 8%) impairments (Table 4 and SDC, eTable 6). Most patients (n=80, 78%) reporting a new device class did so within 3 months.
Table 3.
Baseline characteristics by new medication and new device classes
| New Medication Classes |
New Device Classes |
|||||
|---|---|---|---|---|---|---|
| Patient Characteristics | 0 (N = 152) |
1-2 (N = 106) |
3+ (N = 50) |
0 (N = 206) |
1-2 (N = 92) |
3+ (N = 10) |
| Age (years) | 8 [3, 13] | 4 [1, 10] | 7 [2, 14] | 7 [2, 13] | 6 [2, 12] | 10 [7, 12] |
| Sex: Male | 79 (52%) | 61 (58%) | 29 (58%) | 115 (56%) | 50 (54%) | 4 (40%) |
| ADI (national rank)1 | 31 [18, 65] | 30 [13, 65] | 39 [12, 77] | 31 [15, 61] | 37 [15, 77] | 20 [15, 51] |
| Health insurance status2 | ||||||
| Private insurance | 63 (41%) | 45 (42%) | 27 (54%) | 88 (43%) | 41 (45%) | 6 (60%) |
| State Medicaid | 82 (54%) | 55 (52%) | 20 (40%) | 106 (51%) | 47 (51%) | 4 (40%) |
| Other/Unknown | 7 (5%) | 6 (6%) | 3 (6%) | 12 (6%) | 4 (4%) | 0 (0%) |
| Pre-sepsis FSS | 6 [6, 13] | 6 [6, 10] | 7 [6, 11] | 6 [6, 11] | 7 [6, 12] | 11 [8, 15] |
| PMCA3 | ||||||
| No chronic conditions | 81 (53%) | 47 (44%) | 22 (44%) | 106 (51%) | 42 (46%) | 2 (20%) |
| Chronic conditions (non-complex) | 7 (5%) | 6 (6%) | 4 (8%) | 12 (6%) | 5 (5%) | 0 (0%) |
| Chronic conditions (complex) | 63 (41%) | 53 (50%) | 24 (48%) | 87 (42%) | 45 (49%) | 8 (80%) |
| Day 1 PELOD4 | 8 [6, 10] | 7 [6, 10] | 9 [7, 12] | 8 [6, 10] | 9 [7, 12] | 8 [5, 10] |
| PRISM III5 | 10 [5, 15] | 10 [6, 17] | 12 [8, 19] | 11 [6, 16] | 10 [6, 16] | 12 [9, 17] |
| Infectious disease status6 | ||||||
| Suspected | 96 (63%) | 58 (55%) | 26 (52%) | 124 (60%) | 51 (55%) | 5 (50%) |
| Documented | 56 (37%) | 48 (45%) | 24 (48%) | 82 (40%) | 41 (45%) | 5 (50%) |
| Any immune-related comorbid conditions7 | 17 (11%) | 23 (22%) | 10 (20%) | 29 (14%) | 19 (21%) | 2 (20%) |
| Hospitalization Characteristics | ||||||
|
| ||||||
| Day 7 FSS8 | 14 [7, 18] | 14 [9, 18] | 15 [12, 18] | 13 [8, 18] | 15 [10, 20] | 17 [16, 27] |
| ΔFSS Day 7: Total score | 1 [0, 7] | 5 [1, 10] | 6 [2, 9] | 2 [0, 8] | 5 [0, 9] | 4 [0, 9] |
| Neurologic insult(s) during PICU stay | 46 (30%) | 42 (40%) | 30 (60%) | 73 (35%) | 39 (42%) | 6 (60%) |
| Cardiopulmonary resuscitation | 3 (2%) | 6 (6%) | 3 (6%) | 8 (4%) | 4 (4%) | 0 (0%) |
| Cumulative 28-day PELOD | 44 [26, 75] | 53 [31, 82] | 56 [38, 102] | 45 [27, 74] | 59 [35, 94] | 60 [45, 103] |
| Hospital LOS (days) | 13 [8, 23] | 18 [12, 25] | 19 [13, 31] | 14 [9, 22] | 20 [12, 28] | 24 [13, 55] |
| PICU LOS (days) | 8 [5, 13] | 10 [5, 15] | 11 [6, 16] | 8 [5, 13] | 11 [5, 16] | 11 [6, 22] |
| Ventilation (days) | ||||||
| 9 days or less | 104 (68%) | 63 (59%) | 28 (56%) | 138 (67%) | 52 (57%) | 5 (50%) |
| 10+ days | 48 (32%) | 43 (41%) | 22 (44%) | 68 (33%) | 40 (43%) | 5 (50%) |
Area deprivation index (ADI) was unavailable for 58 patients.
Other insurance selections included: do not know, no insurance, and self-pay.
Patients categorized based on data collected within the pediatric health information system (PHIS) for the 3 years prior to LAPSE index ICU admission. PHIS data was unavailable for 1 patient.
Day 1 PELOD refers to the calculated PELOD 2 score on the calendar day of PICU admission if subject was admitted before noon. If admitted after noon, it refers to the following day.
PRISM III score uses the worst physiologic values obtained during the 6-hour period from 2 hours prior to PICU admission through four hours post PICU admission.
Documented infections were collected from PICU admission until day 28 or ICU discharge, whichever occurred first.
Immune-related comorbid conditions were collected daily from PICU admission until Day 28 or PICU discharge, whichever occurred first.
Relative to pre-sepsis baseline.
Table 4.
Device classes at baseline and new during follow up
| Device class | Baseline | New at follow-up |
|---|---|---|
| Respiratory | 85 (27%) | 41 (13%) |
| ADL-Assistance | 59 (19%) | 40 (13%) |
| Feeding | 103 (33%) | 25 (8%) |
| Infusion/Access | 58 (19%) | 19 (6%) |
| Urinary Catheters | 28 (9%) | 5 (2%) |
| Neurologic | 20 (7%) | 3 (1%) |
| Communication | 12 (4%) | 3 (1%) |
| Dialysis | 6 (2%) | 0 (0%) |
| Other | 42 (14%) | 10 (3%) |
The table includes 12-month survivors with at least one post-discharge survey, N=308.
Factors associated with use of a new medication class or device class after discharge are reported in SDC, eTable 7. Factors independently associated with use of a new medication class were private insurance (RR 1.65 [95% CI 1.18, 2.31]), experiencing a neurologic insult during the PICU stay (RR 1.47 [95% CI: 1.24, 2.46]), and longer PICU stays (RR 1.01 [95% CI: 1.00, 1.02]) (Table 5). Factors independently associated with use of a new device class were chemotherapy or radiotherapy within the 3 months prior to admission (RR 2.48 [95% CI: 1.34, 4.54]), pre-sepsis FSS (1.05 [95% CI: 1.01, 1.09]), and duration of ventilation ≥10 days (1.52 [95% CI: 1.04, 2.22]).
Table 5.
Multivariable model of factors associated with number of new medication classes or new device classes
| Predictor | Rate ratio (95% Confidence Interval) |
p-value |
|---|---|---|
| Number of new medication classes | ||
|
| ||
| Private insurance (Ref: government insurance) | 1.65 (1.18, 2.31) | 0.004 |
| PICU length of stay (days) | 1.01 (1.00, 1.02) | 0.049 |
| Neurologic insult(s) during PICU stay 1 | 1.74 (1.24, 2.46) | 0.001 |
|
| ||
| Number of new device classes | ||
|
| ||
| Pre-sepsis Functional Status Scale score | 1.05 (1.01, 1.09) | 0.007 |
| Chemotherapy or radiotherapy within last 3 months | 2.48 (1.34, 4.54) | 0.005 |
| Ventilation Duration (days) | 0.030 | |
| 9 days or less | Reference | |
| 10+ days | 1.52 (1.04, 2.22) | |
Neurologic insult was defined as pathologic breathing pattern, stereotypic posturing or flaccid posture, seizure activity and or abnormal electroencephalogram, new anoxic-ischemic injury on brain imaging, treatment for increased intracranial pressure, neurologic injury suspected by care provider, autonomic storming, or cardiopulmonary arrest or chest compressions.
Children who required either a new medication class or new device were more likely to also require hospital readmission or ED visit (SDC, eTable 8). Of patients who had a new medication class after discharge, the majority required hospital readmission (n=87, 56%) or an ED visit (n=96, 62%). Of patients who had a new device class after discharge, the majority required hospital readmission (n=62, 61%) or an ED visit (n=68, 67%).
Discussion
Using prospectively collected data, we determined that children who survive septic shock have high health resource needs after discharge. Approximately half of our cohort experienced a hospitalization or ED visit during the post-discharge year, half reported use of a new medication class, and one-third reported use of a new device class. Nearly all new health resource use was reported during the initial three months following discharge, identifying this period as particularly resource intensive. Patients who experienced post-discharge encounters (hospitalizations or ED visits) were primarily characterized by pre-existing factors such as chronic conditions and younger age. As such, these outcomes may be less likely to be modifiable or interventions may need to be tailored based on the patients’ pre-existing conditions. In contrast, having a new medication class was associated with PICU length of stay which may be a modifiable factor. Factors associated with a new device class were related to both patient and hospitalization characteristics. Finally, our data suggest a connection between new therapeutic needs (new medications and devices) and post-discharge encounters (hospital readmissions and ED visits). The interconnectedness of these outcomes suggests that there exists an identifiable post-discharge phenotype of patients who may benefit from close follow-up and novel interventions during the immediate post-discharge period.
Critical illness represents a period of extreme health vulnerability that can extend beyond the patient’s hospitalization (17, 18). In a study evaluating more than 4,000 hospitalizations for pediatric sepsis derived from an administrative database, nearly one in five patients required readmission within 30 days (5). Similar to our data, patients with chronic conditions suffered higher rates of readmission. The most common reasons for readmission within 30 days were maintenance chemotherapy, complications of a medical device, and sepsis. In a separate study, it is reported that 5% of children with severe sepsis acquired a new device (7). Accordingly, children experiencing sepsis with a pre-existing chronic condition, particularly those who require a new medical device after discharge are at high risk for rehospitalization. This finding is important as improved education or augmentation of post-discharge care may decrease readmission rates in this vulnerable cohort. Providing this post-ICU care in an equitable and family-centered manner may be facilitated by a model overlaid on the patient’s prior medical home (19).
The four health resource use outcomes in our study occurred predominantly during the months immediately after discharge. Readmissions during this period are often considered to be potentially preventable through improved care provision and coordination (20, 21). The high proportion of patients who required new medications/devices represent an opportunity for care coordination and optimization. Adult intensivists are testing care coordination interventions that span the post-ICU inpatient and immediate post-discharge periods to ensure patients are receiving adequate treatment and therapies to enhance recovery and decrease hospital readmission (22–24). In a randomized pilot trial targeting patients at increased risk of readmission, intensivists tested a 10-component strategy initiated at the time of ICU discharge through the 30 days after discharge versus usual care (22). This multidisciplinary strategy involving inpatient education targeting post ICU recovery, outpatient support providing families with direct access to medical advice, and a comprehensive post-discharge clinic evaluation resulted in a significant reduction in hospital readmissions and a longer time to readmission. Inpatient interventions appeared most effective as <15% of patients in the intervention group accessed post-discharge resources. Pediatric sepsis patients may benefit from a similar approach.
Alternatively, intervention strategies could target prevention during the PICU stay. Our data suggest that new devices were most frequently related to feeding or respiratory morbidities or impairments in activities of daily living (e.g., mobility). Risk factors associated with acquiring a new device class after discharge were pre-existing functional impairments, treatment with chemotherapy or radiotherapy and mechanical ventilation durations longer than 9 days. Trials of PICU-specific interventions (e.g., rehabilitation or ventilation strategies) targeting a high-risk cohort identified by these factors may be effective in decreasing morbidities requiring new device acquisition, an important and patient-centered long-term outcome (17, 25–27).
A key component to designing an effective interventional study is prognostic enrichment which requires identifying the population at highest risk of experiencing the study’s primary outcome (28). As suggested by our study, patients with pre-existing medical complexity are a targetable cohort due to their increased risk of experiencing a post-discharge encounter. While this is relevant from a clinical perspective as these patients may benefit from closer post-ICU follow-up care, this finding does not identify a pathway for intervention as the pre-illness state is not modifiable. Additionally, previous research suggests that less than 15% of readmissions after pediatric sepsis were likely to be preventable through appropriate outpatient care, suggesting that hospital readmission may not be a modifiable long-term outcome for pediatric sepsis trials (5).
In contrast, our data demonstrate that several ICU-specific factors including duration of mechanical ventilation, neurologic insults such as delirium or ICU length of stay were associated with the post-discharge outcomes of new medication and device classes. As we develop a more comprehensive understanding of the biological mechanisms linking these risk factors to post-discharge outcomes, these associations may allow for predictive enrichment of interventional trials (28). For example, treatments targeting prevention of neurologic injury (e.g., delirium prevention) or liberation from mechanical ventilation or the ICU may decrease post-discharge health resource needs related to new medications or devices (29, 30). Conversely, our data also suggest that post-discharge medication use may be a less objective outcome due to its association with private insurance and the potential confounder that patients with private insurance may have enhanced access to healthcare.
Our study has several key limitations. We relied on parental report to obtain pre-admission and post-discharge health resource use data. As such, there is risk for recall bias or misrepresentation of medications or devices. We are limited in our ability to characterize the unplanned nature of the post-discharge encounters as we did not collect the reason for hospital readmission. Similarly, we did not collect post-discharge follow-up appointments, thus, we are unable to evaluate the relationship between post-discharge care and our study’s outcomes. Importantly, half of the children in our cohort had pre-existing conditions and post-discharge health resource use may have been related to their underlying diagnosis and not directly related to sepsis. Additionally, children who were excluded from this analysis were more likely to live in more disadvantaged neighborhoods, limiting the generalizability of our findings. Finally, some patients did not have survey data from all post-discharge timepoints. By including patients with at least one post-discharge survey, we identified a minimum event rate, although we may not have accurately identified the total event rate.
Conclusions
Children experiencing septic shock are at high risk of hospital readmission, ED encounters, and need for new medications and devices, especially in the months following hospitalization. Our study provides further evidence that pediatric septic shock patients represent a high-risk cohort even after discharge from the hospital and identifies particularly high-risk sub-populations. Additional work is needed to understand which interventions during or following hospitalization reduce healthcare use with special attention to children with chronic conditions.
Supplementary Material
At the Bedside Box.
This study augments growing literature that identifies children who survive sepsis as a cohort at high risk of post-discharge health resource needs including post-discharge encounters, new medications, and device classes.
Post-ICU care should consider targeting patients based on pre-existing medical conditions and duration of ICU-level support and recognize that the months immediately following discharge are particularly resource intensive time periods for these patients and their families.
Patients who require treatment with an additional class of medications or new device class constitute a cohort at high risk of post-discharge encounters and likely represent a post-discharge phenotype appropriate for targeted post-ICU care.
Funding:
This investigation was funded by the Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, Department of Health and Human Services, R01HD073362 (Zimmerman), and was supported, in part, by the following cooperative agreements associated with the Collaborative Pediatric Critical Care Research Network: UG1HD050096 (Meert), UG1HD049981, UG1HD049983, UG1HD063108, UG1HD083171 (Mourani), UG1HD083166, UG1HD083170, U10HD050012, U10HD063106, and U01HD049934. Additional funding was provided by R03HD104001 (Reeder), K23HD096018 (Maddux), and the Francis Family Foundation (Maddux). No additional conflicts of interest to report.
Copyright Form Disclosure:
Dr. Maddux’s institution received funding from the National Institute of Child Health and Human Development (NICHD) (K23HD096018) and the Francis Family Foundation. Drs. Maddux, Zimmerman, Banks, Reeder, Meert, Berg, Sapru, Carcillo, Newth, and Mourani received support for article research from the National Institutes of Health (NIH). Drs. Zimmerman, Banks, and Carcillo’s institution received funding from the NICHD. Dr. Zimmerman’s institution received funding from Immunexpress; he received funding from Elsevier Publishing. Dr. Banks disclosed government work. Drs. Reeder, Meert, Berg, Sapru, Newth, and Mourani’s institutions received funding from the NIH. Dr. Czaja disclosed that she is a member of the critical care sub-board for the American Board of Pediatrics. Dr. Carcillo’s institution received funding from the National Institute of General Medical Sciences. Dr. Newth received funding from Philips Research North America and Nihon Kohden. Dr. Mourani received funding from the NICHD (UG1HD083171). Dr. Quasney has disclosed that he does not have any potential conflicts of interest.
References
- 1.Carlton EF, Barbaro RP, Iwashyna TJ, et al. Cost of Pediatric Severe Sepsis Hospitalizations. JAMA Pediatr. 2019. Aug 12;173(10):986–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Balamuth F, Weiss SL, Neuman MI, et al. Pediatric severe sepsis in U.S. children’s hospitals. Pediatr Crit Care Med. 2014. Nov;15(9):798–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ruth A, McCracken CE, Fortenberry JD, et al. Pediatric severe sepsis: current trends and outcomes from the Pediatric Health Information Systems database. Pediatr Crit Care Med. 2014. Nov;15(9):828–38. [DOI] [PubMed] [Google Scholar]
- 4.Weiss SL, Fitzgerald JC, Pappachan J, et al. Global epidemiology of pediatric severe sepsis: the sepsis prevalence, outcomes, and therapies study. Am J Respir Crit Care Med. 2015. May 15;191(10):1147–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carlton EF, Kohne JG, Shankar-Hari M, et al. Readmission Diagnoses After Pediatric Severe Sepsis Hospitalization. Crit Care Med. 2019. Apr;47(4):583–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carlton EF, Kohne JG, Hensley MK, et al. Comparison of Outpatient Health Care Use Before and After Pediatric Severe Sepsis. JAMA Netw Open. 2020. Sep 1;3(9):e2015214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carlton EF, Donnelly JP, Hensley MK, et al. New Medical Device Acquisition During Pediatric Severe Sepsis Hospitalizations. Crit Care Med. 2020. May;48(5):725–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Prout AJ, Talisa VB, Carcillo JA, et al. Epidemiology of Readmissions After Sepsis Hospitalization in Children. Hosp Pediatr. 2019. Apr;9(4):249–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Prout AJ, Talisa VB, Carcillo JA, et al. Children with Chronic Disease Bear the Highest Burden of Pediatric Sepsis. J Pediatr. 2018. Aug;199:194–9.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zimmerman JJ, Banks R, Berg RA, et al. Trajectory of Mortality and Health-Related Quality of Life Morbidity Following Community-Acquired Pediatric Septic Shock. Crit Care Med. 2020. Mar;48(3):329–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zimmerman JJ, Banks R, Berg RA, et al. Critical Illness Factors Associated With Long-Term Mortality and Health-Related Quality of Life Morbidity Following Community-Acquired Pediatric Septic Shock. Crit Care Med. 2020. Mar;48(3):319–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Simon TD, Cawthon ML, Stanford S, et al. Pediatric medical complexity algorithm: a new method to stratify children by medical complexity. Pediatrics. 2014. Jun;133(6):e1647–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pollack MM, Holubkov R, Glass P, et al. Functional Status Scale: new pediatric outcome measure. Pediatrics. 2009. Jul;124(1):e18–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Leteurtre S, Duhamel A, Salleron J, et al. PELOD-2: an update of the PEdiatric logistic organ dysfunction score. Crit Care Med. 2013. Jul;41(7):1761–73. [DOI] [PubMed] [Google Scholar]
- 15.2015 Area Deprivation Index version 2.0 [database on the Internet]. University of Wisconsin School of Medicine and Public Health. [cited December 5, 2020]. Available from: https://www.neighborhoodatlas.medicine.wisc.edu/. [Google Scholar]
- 16.Kind AJH, Buckingham WR. Making Neighborhood-Disadvantage Metrics Accessible - The Neighborhood Atlas. N Engl J Med. 2018. Jun 28;378(26):2456–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fink EL, Maddux AB, Pinto N, et al. A Core Outcome Set for Pediatric Critical Care. Crit Care Med. 2020. Oct 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Manning JC, Pinto NP, Rennick JE, et al. Conceptualizing Post Intensive Care Syndrome in Children-The PICS-p Framework. Pediatr Crit Care Med. 2018. Apr;19(4):298–300. [DOI] [PubMed] [Google Scholar]
- 19.Fitzgerald JC, Kelly NA, Hickey C, et al. Implementation of a Follow-Up System for Pediatric Sepsis Survivors in a Large Academic Pediatric Intensive Care Unit. Front Pediatr. 2021;9:691692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Coller RJ, Klitzner TS, Lerner CF, et al. Complex Care Hospital Use and Postdischarge Coaching: A Randomized Controlled Trial. Pediatrics. 2018. Aug;142(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Coller RJ, Klitzner TS, Saenz AA, et al. The Medical Home and Hospital Readmissions. Pediatrics. 2015. Dec;136(6):e1550–60. [DOI] [PubMed] [Google Scholar]
- 22.Bloom SL, Stollings JL, Kirkpatrick O, et al. Randomized Clinical Trial of an ICU Recovery Pilot Program for Survivors of Critical Illness. Crit Care Med. 2019. Oct;47(10):1337–45. [DOI] [PubMed] [Google Scholar]
- 23.Needham DM, Davidson J, Cohen H, et al. Improving long-term outcomes after discharge from intensive care unit: report from a stakeholders’ conference. Crit Care Med. 2012. Feb;40(2):502–9. [DOI] [PubMed] [Google Scholar]
- 24.Sevin CM, Bloom SL, Jackson JC, et al. Comprehensive care of ICU survivors: Development and implementation of an ICU recovery center. J Crit Care. 2018. Aug;46:141–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Carlton EF, Pinto N, Smith M, et al. Overall Health Following Pediatric Critical Illness: A Scoping Review of Instruments and Methodology. Pediatr Crit Care Med. 2021. Jul 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fayed N, Cameron S, Fraser D, et al. Priority Outcomes in Critically Ill Children: A Patient and Parent Perspective. Am J Crit Care. 2020. Sep 1;29(5):e94–e103. [DOI] [PubMed] [Google Scholar]
- 27.Merritt C, Menon K, Agus MSD, et al. Beyond Survival: Pediatric Critical Care Interventional Trial Outcome Measure Preferences of Families and Healthcare Professionals. Pediatr Crit Care Med. 2018. Feb;19(2):e105–e11. [DOI] [PubMed] [Google Scholar]
- 28.Stanski NL, Wong HR. Prognostic and predictive enrichment in sepsis. Nature Reviews Nephrology. 2020. 2020/01/01;16(1):20–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Girard TD, Jackson JC, Pandharipande PP, et al. Delirium as a predictor of long-term cognitive impairment in survivors of critical illness. Crit Care Med. 2010. Jul;38(7):1513–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Girard TD, Kress JP, Fuchs BD, et al. Efficacy and safety of a paired sedation and ventilator weaning protocol for mechanically ventilated patients in intensive care (Awakening and Breathing Controlled trial): a randomised controlled trial. Lancet. 2008. Jan 12;371(9607):126–34. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


