Abstract
Background and Objectives:
Red blood cell (RBC) units in hypothermic storage degrade over time, commonly known as the red blood cell (RBC) storage lesion. These older RBC units can cause adverse clinical effects when transfused, as older RBCs in the unit lyse and release cell-free hemoglobin (Hb), a potent vasodilator which can elicit vasoconstriction, systemic hypertension and oxidative tissue injury post transfusion. In this study, we examined a novel method of washing ex vivo stored single RBC units to remove accumulated cellular waste, specifically cell-free Hb, using tangential flow filtration (TFF) driven by a centrifugal pump.
Materials and Methods:
The TFF RBC washing system was run under hypothermic conditions at 4°C, at a constant system volume with 0.9 wt% saline as the wash solution. The RBC washing process was conducted on 10 separate RBC units. For this proof-of-concept study, RBC units were expired at the time of washing (60-70 days old). Cell-free Hb was quantified by UV-visible absorbance spectroscopy and analyzed via the Winterbourn equations. Pre and post wash RBC samples were analyzed by Hemox Analyzer, Coulter Counter, and Brookfield rheometer. The RBC volume fraction in solution was measured throughout the wash process by standard hematocrit (HCT) analysis.
Results:
No substantial decrease in the HCT was observed during the TFF RBC washing process. However, there was a significant decrease in RBC concentration in the first half of the TFF RBC wash process, with no significant change in RBC concentration during the second half of the TFF cell wash process with an 87% overall cell recovery compared to the total number of cells before initiation of cell washing. Utilization of the extinction coefficients and characteristic peaks of each Hb species potentially present in solution was quantified by Winterbourn analysis on retentate and permeate samples for each diacycle to quantify Hb concentration during the washing process. Significant cell-free Hb reduction was observed within the first 4 diacycles with a starting cell-free Hb concentration in the RBC unit of 0.105 mM, which plateaus to a constant Hb concentration of 0.01 mM or a total extracellular Hb mass of 0.2 g in the resultant washed unit. The oxygen equilibrium curve showed a significant decrease in P50 between the initial and final RBC sample cell wash with an initial P50 of 15.6± 1.8 mm Hg and a final P50 of 14 ± 1.62 mm Hg. Cooperativity increased after washing from an initial Hill coefficient of 2.37 ± 0.19 compared to a final value of 2.52 ± 0.12.
Conclusion:
Overall, this study investigated the proof-of concept use of TFF for washing single RBC units with an emphasis on the removal of cell-free Hb from the unit. Compared to traditional cell washing procedures, the designed system was able to more efficiently remove extracellular Hb but resulted in longer wash times. For a more complete investigation of the TFF RBC washing process, further work should be done to investigate the effects of RBC unit storage after washing. The designed system is light weight and transportable with the ability to maintain sterility between uses, providing a potential option for bedside ex vivo transfusion in clinical applications.
Keywords: RBC washing, red blood cell, hemolysis, hemoglobin, tangential flow filtration, diafiltration
2. Introduction
Red blood cells (RBCs) degrade during ex vivo storage, and lead to the accumulation of toxic hemolysis byproducts in the unit such as hemoglobin (Hb) during the maximum 42 day storage period set by the US FDA.[1] [2] Upon transfusion, cell-free Hb in the stored RBC unit can extravasate from the blood volume into the tissue space, where it scavenges nitric oxide (NO), a potent vasodilator, and elicits vasoconstriction and systemic hypertension within the patient.[3] Additionally, tissue extravasation of cell-free Hb leads to tissue deposition of iron, and inevitably leads to oxidative tissue injury.[4]
Therefore, in light of the accumulation of hemolysis byproducts during ex vivo RBC storage, RBC washing is often employed to remove accumulated waste products within an RBC unit prior to transfusion to mitigate any potential side-effects.[5,6] Many commercially available technologies are clinically employed to wash stored RBC units prior to transfusion.[7,8]
Manual washing of single RBC units is an attractive approach due to its’ low cost, but is laborious, limited in processing volume by the available centrifuge cup size, and exposes the unit to a high risk of bacterial contamination.[5,9] In contrast, automated RBC unit washing systems are most commonly used in clinical settings to remove toxic byproducts, one example is the COBE 2991 cell processor (Terumo, Somerset, NJ).[7,10] The COBE 2991 is an open cell processing system that utilizes centrifugation to facilitate separation based on differences in blood component density and can effectively reduce proinflammatory markers, restoring overall RBC quality near the end of the unit’s ex vivo shelf life.[9,10] Unfortunately, levels of hemolysis have been shown to rapidly increase after washing with the COBE 2991, and often surpass prewashed levels before the 24 hr transfusion window is reached.[9] Additional work investigating the ability of the COBE 2991 to wash 40 to 42 day stored RBC units showed that after washing, the COBE 2991 is unable to provide significant reduction in total cell-free Hb after the washing process, Hb being a toxic byproduct of the storage lesion.[11] Regarding this limitation, it is clear that there is an urgent need for an innovative, easy to use RBC washing system that addresses the current pitfalls of both manual and automated washing systems.
Considering the plethora of centrifugation-based RBC washing systems in existence, there has been substantially less research into the use of tangential flow filtration (TFF) for RBC unit washing, with no commercially available system on the market. TFF utilizes a porous hollow fiber or flat sheet membrane to enable continuous flow purification. Molecules larger than the pore size cutoff of the membrane are retained on the membrane and in the system, while molecules smaller than the pore size cutoff permeate through the membrane and are removed from the system. The use of TFF techniques on whole blood currently utilize gravity-driven separation, and are primarily focused on the separation of whole blood into plasma and RBC fractions.[12,13] Compared to centrifugal separation, TFF systems can process a wide range of RBC concentrate volumes, allows for easy storage solution exchange, and has the ability to maintain sterility via the use of autoclavable materials and a closed loop system. Additionally, the currently designed TFF system is light weight and easily transportable, with the system as a whole (system vessel, pump, tubing, and hollow fiber filter) weighing less than 2 kg. One study used TFF to wash RBCs in diafiltration mode, and concentrated a cryopreserved RBC unit, but resulted in significant RBC lysis (likely due to use of a peristaltic pump), and focused on investigating the rheological properties of the washed unit.[14] Alternatively, in this study we explored implementation of a novel TFF system using a low shear stress inducing centrifugal pump to separate stored RBCs from their primary hemolysis byproduct Hb. This proof-of-concept study developed a system that effectively washes RBC units as demonstrated by the successful removal of cell-free Hb and shows negligible process-induced hemolysis, providing a viable alternative to current manual and automated RBC washing systems. Because this is a proof-of-concept study, we evaluated TFF-facilitated RBC washing effectiveness using outdated stored human RBCs and saline as the wash solution to investigate the absolute worst case scenario of RBC unit quality. Future studies will focus on washing unexpired stored RBC units, using FDA approved storage solutions as the wash solution, and expanding the analysis of analytes beyond cell-free Hb.
3. Materials & Methods
3.1. Materials
Sodium chloride (NaCl), sodium hydroxide (NaOH), and 0.2 μm Titan3 sterile filters were purchased from Fisher Scientific (Waltham, MA). Hollow fiber TFF modules (S02-E65U-07N, modified polyethersulfone membrane, 0.65 μm pore size, composed of 110 individual hollow fibers, 0.75 mm internal diameter, 520 cm2 total surface area) were purchased from Repligen (Rancho Dominguez, CA). A biocompatible centrifugal pump (PuraLev i30SU) that exposes cells to low shear stresses was purchased from Levitronix (Framingham, MA). A minicentrifuge (50-090-100, working speed 6,000 rpm, max speed 6,600 rpm) from Fisher Scientific (Waltham, MA) was used to separate RBCs from the wash solution. Expired leuko-reduced packed human RBCs (RBC units, 60-70 days old, stored in AS-1) were generously donated by the Transfusion Services of the Wexner Medical Center at The Ohio State University, Columbus, Ohio. The RBC units used in this study were expired and deidentified and thus required no Ethics Committee approval.
3.2. RBC Washing
The TFF-facilitated RBC washing process was performed on individual stored RBC units expired past the FDA regulated 42-day storage period. All RBC units were stored and washed at 4°C in a chromatography refrigerator. A single RBC unit was transferred to a 1 L Nalgene container by opening and transferring the RBC unit in a sterile biosafety cabinet. The hematocrit (HCT) in the total system volume (which includes the combined fluid volume in the TFF filter, lines, and retentate vessel) was standardized to 45% with 0.9 wt% saline to decrease variability in the starting HCT between RBC units and was selected as an appropriate average between male and female HCT values. Prior to washing, single RBC units were mixed by gentle inversion to yield a homogenous cell suspension. An initial sample of the RBC unit was taken to establish baseline conditions prior to washing. Figure 1 shows the general schematic of the TFF-facilitated RBC washing system. 0.9 wt% saline solution was diafiltered into the retentate vessel to maintain a constant system volume. The sample port in the RBC retentate loop was used to take retentate samples. The retentate line was connected to a centrifugal pump from the reservoir, which operated at a constant flow rate of 1000 ml/min and directed RBCs through the bottom of the TFF filter against gravity, with RBCs being retained in the retentate, while cell debris, proteins and other molecules smaller than 0.65 μm passing into the permeate. The permeate line enters a cell waste container with samples collected directly from the permeate line.
Figure 1:
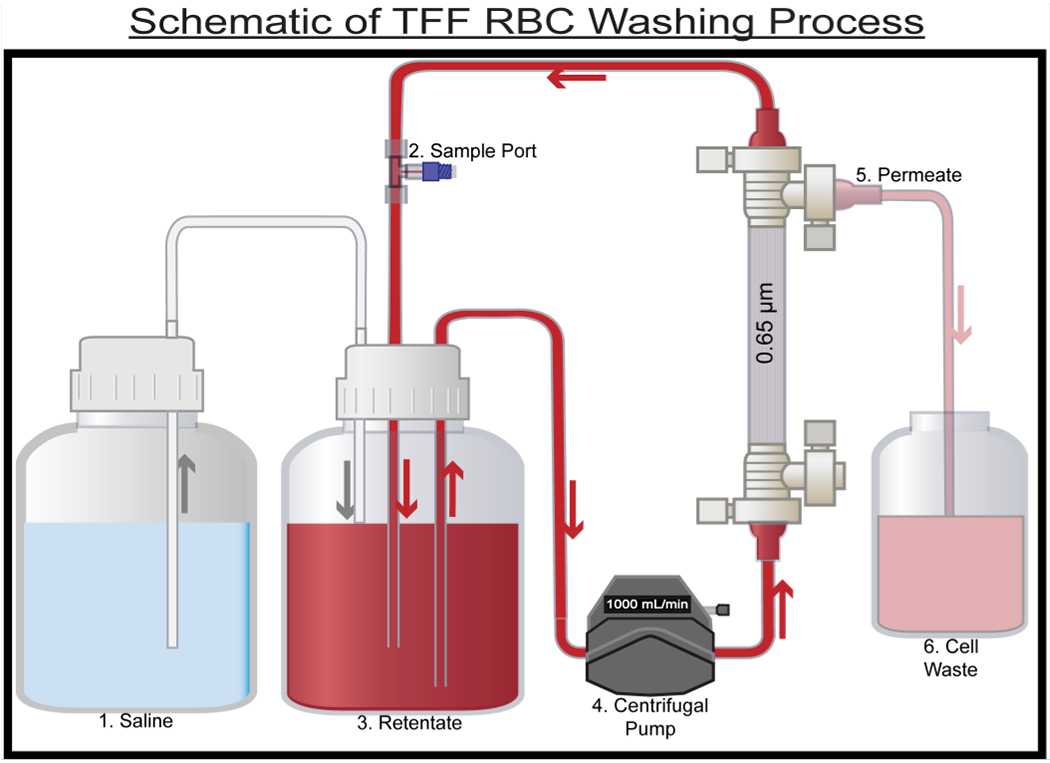
Process flow diagram for the RBC washing process. 10 single RBC units were processed using the TFF RBC washing system.(1) Reservoir containing 0.9 wt% saline.(2) Sample port used for retentate sampling. (3) Retentate vessel, 0.65 μm TFF filter used to wash RBCs. (4) Centrifugal pump. (5) Permeate waste from the process (contains species < 0.65 μm in size). (6) Cell waste. Arrows indicate the direction of flow.
RBCs in the retentate vessel were first acclimated to the system components via circulation for 2 minutes with the permeate line closed. This ensured proper mixing of the RBCs in the system before starting the constant volume diafiltration cell washing process. During the acclimatization period, an initial 0× diacycle sample was taken to confirm the HCT of 45 % was successfully achieved before initiating the diafiltration process. The total system volume was used to determine the volume per diacycle (i.e. one complete system exchange volume) and was measured by collecting permeate leaving the system. Retentate and permeate samples were taken at the end of each diacycle and stored at 4°C for analysis. RBC units were washed with standard 0.9 wt% saline washing solution for the entirety of the process and were not stored ex vivo after the washing process was completed. Instead, newly washed RBCs were utilized for hemoglobin purification based on published procedure. A total of ten diacycles were completed per RBC wash for each individual RBC unit, with a total of ten individual RBC units being subjected to the TFF RBC washing process. The scope of this work focuses primarily on establishing the feasibility of washing expired RBC units as a proof-of-concept of the TFF-facilitated RBC washing approach, however preliminary results from a limited study of TFF-facilitated RBC washing of unexpired units is included in the Supplemental Information section.
3.3. Hematocrit Analysis
The HCT was determined by injecting 65 μL of each retentate sample, including an initial sample from the RBC unit, into a mylar wrapped 75 mm capillary tube (Drummond, Broomall, PA) followed by centrifugation in a Sorvall Legend micro 17 microcentrifuge (Fisher Scientific, Waltham, MA) for 5 minutes to pellet the RBCs. Post centrifugation, the capillary tubes were quantified using a standardized HCT graph to obtain the HCT of RBCs in the retentate.
3.4. Total Hb Quantification
The cell-free Hb concentration was quantified via UV-visible absorbance spectrometry on a diode array spectrophotometer HP 8452A (Olis, Bogart, GA). Retentate supernatants were isolated via centrifugation using a minicentrifuge (Fisher Scientific, Waltham, MA) at 6000 RPM for 2 minutes to pellet the RBCs and analyzed after separation. Processed retentate and permeate samples were sterile filtered through a 0.2 μm Titan3 filter (Fisher Scientific, Waltham, MA) for UV-visible spectral analysis. Sterile filtration was employed to reduce light scattering during optical measurements to only quantify cell-free Hb. The Winterbourn equations were used to determine the total concentration of cell-free Hb in the permeate and retentate samples and further used for the cell-free Hb mass balance.[15] Quantification using UV-visible spectral analysis examines the absorbance of the various Hb oxidation species that could be present in a sample. Using the characteristic absorbance peaks: oxyhemoglobin at 577 nm, methemoglobin at 630 nm, and hemichrome at 560 nm and the defined extinction coefficients of each species at each wavelength previously described by Winterbourn, the quantity of each different species can be found and used to find the total Hb of the sample. The equations used for each species quantification are found below.
| (1) |
| (2) |
| (3) |
3.5. Oxygen Equilibrium of RBCs
Oxygen equilibrium curves (OEC) for RBCs pre and post wash were measured using a Hemox Analyzer (TCS Scientific Corp., New Hope, PA) operated at 37 ± 0.1°C. RBC samples were diluted into 5 mL of Hemox buffer (pH 7.4) with 20 μL additive A, 20 μL additive B, and 20 μL anti-foaming agent (TCS Scientific). Data obtained from the Hemox Analyzer was fit to the Hill equation using an Igor (Wavemetrics, Portland, OR) script to regress the oxygen affinity (P50, pO2 at which half of the Hb is saturated with oxygen), and Hill coefficient (n, cooperativity of O2 binding to Hb).[16]
3.6. RBC Viscosity
A Brookfield DV3T rheometer with a CP-40 spindle (Brookfield, Middleboro, MA) was used to measure the viscosity of RBC samples at 37°C and a shear rate of 160 s−1.[17,18]
3.7. RBC Cell Count
Cell counts for retentate samples were measured using a Multisizer 4e Coulter Counter (Beckman Life Sciences, Indianapolis, IN). RBC samples were diluted 100× prior to addition of 100 μL of the diluted cells into 20 mL of filtered Isoton solution (Beckman Life Sciences) prior to Coulter Counter analysis.
3.9. Data Analysis
Results are reported as the mean ± standard deviation. RStudio (version 1.3.1093, RStudio Inc., Boston, MA) was used to analyze all data. A one-way ANOVA was utilized along with TukeyHSD posttest for data analysis. T-tests were used for P50 and n initial and final comparisons. A two-tailed p-value < 0.05 was considered statistically significant.
4. Results
4.1. Time for Each Diacycle Remained Constant
The average time per diacycle remained constant at ~10 minutes throughout the RBC washing process for a total of 10 diacycles (Figure 2A). The entire process takes 100 minutes to wash one RBC unit (10 diacycles) or 40 minutes to remove the majority of cell-free Hb (4 diacycles). The lesser washing time (40 minutes) necessary to remove the majority of cell-free Hb in the unit is triple the average 14 minutes washing time for processing a single unexpired RBC unit using the COBE 2991.[8] There was no significant difference in the time per diacycle during the washing process (p = 0.999, NS) (Figure 2A). The residence time of RBCs in the retentate reservoir varied slightly due to the variance in the volume of each RBC unit, but on average, the system volume was ~ 350 ml. Based on the system volume and the pump volumetric flow rate, the residence time was calculated to be 0.4 min (i.e. time for the system volume to complete one circuit in the TFF system).
Figure 2:
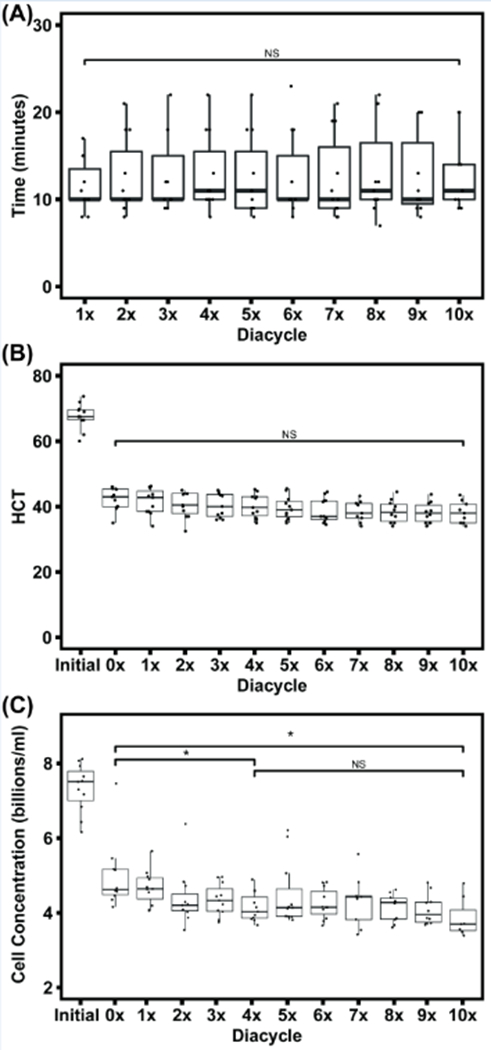
Time per diacycle during the RBC washing process did not vary significantly between diacycles (p = 0.999, NS) (A). HCT was standardized to 45% at the 0× diacycle and did not decrease significantly over the course of washing (p = 0.124, NS) (B). Cell count within the TFF system was measured across diacycles (C). An ANOVA analysis for cell count change from the 0× diacycle to 10× diacycle were significant (p = 0.0107, *), with additional significance found between the 0× diacycle to the 4× diacycle (p = 0.031, *). 10 single RBC units were processed using the TFF RBC washing system.
4.2. Hematocrit Remains Constant Throughout TFF Processing
RBCs from a single RBC unit (initial) were standardized to 45% HCT (0×) in the system from an initial HCT of ~65% (Figure 2B). The effect of each diacycle on the HCT in the system was analyzed using a one-way ANOVA from 0× to 10× diacycle. The HCT did not change significantly throughout the course of the RBC washing process (p = 0.124, NS).
4.3. RBC Concentration Remains Constant Across Diacycles
The concentration of RBCs in the retentate vessel was measured throughout the RBC washing process (Figure 2C). The RBCs were measured at a diameter of 4.4 μm, which corresponds to the approximate spherical diameter of RBCs measured via Coulter Counter analysis. The initial RBC concentration is significantly higher than the 0× diacycle, due to standardization to 45% HCT. The initial RBC concentration measured directly from RBC units was ~ 7.510 ± 0.37 billion cells/ml and decreased to 4.625 ± 0.35 billion cells/ml at the 0× diacycle after standardizing the HCT to 45%. These RBC concentration values are similar to values in the literature.[19] RBC concentration differences between diacycles were analyzed using one-way ANOVA comparing the entire RBC wash process from 0× to 10× diacycle and were found to show a significant decrease in cell concentration over the entire TFF process (p = 0.0107, *). The RBC concentration decreased significantly from 4.625 ± 0.35 billion cells/ml at 0× to 4.030 ± 0.27 billion cells/ml at the 4 × diacycle (p = 0.031, *) corresponding to 87% cell recovery at the end of the wash process, with no significant cell loss after the 4× diacycle (p = 0.356, NS). This suggests hemolysis of cells with significantly compromised cell membranes occurs early in the TFF washing process, and then tapers off as the process continues.
4.4. Hb Concentration in the Retentate and Permeate Decreases Over Multiple Washing Diacycles
The retentate cell-free Hb concentration is shown (Figure 3A). There is, on average, 0.105 mM of cell-free Hb within the RBC unit before processing. Post wash, the cell-free Hb concentration decreases to ~ 0.0157 mM (at the end of the 10× diacycle). The TFF RBC washing process significantly reduces the cell-free Hb concentration after 4 diacycles (p = 2.8E-7, ***) and remains constant from 4× to 10× (p = 0.458, NS) diacycles. A Tukey honestly significant difference (HSD) test was performed within the 0× to 4× diacycles and found significance between the 0× to 1×, 2×, 3×, and 4× diacycles (p = 0.001, 2.9E-5, 1.6E-6, 7E-7 respectively). No significance was observed between 1× to 4× diacycles. This suggests that the majority of cell-free Hb was removed at the beginning of the TFF wash process.
Figure 3:
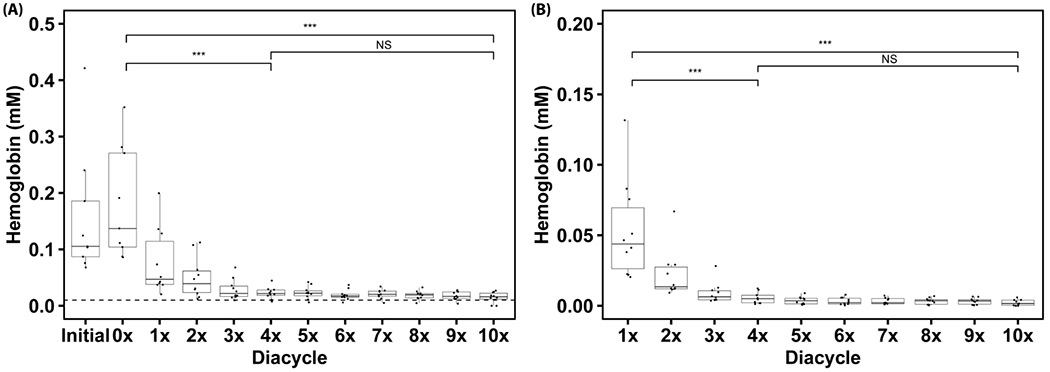
Retentate cell-free Hb concentration (A) over 10 diacycles of RBC washing using TFF. 10 single RBC units were processed using the TFF RBC washing system. An ANOVA test was performed on the data subsets 0× to 10× diacycles, 0× to 4× diacycles, and 4× to 10× diacycles (α = 0.05, Ho = Hb concentration is independent of the diacycle). Significance was found within the 0× to 10× diacycles (p = 8E-16, ***), and 0× to 4× diacycles (p = 2.8E-7, ***) subgroups. Significance was found between the 0× diacycle and 1×, 2×, 3×, and 4× diacycles using TukeyHSD (p = 0.001, 2.9E-5, 1.6E-6, and 7E-7 respectively). No significance was found within the 4× to 10× diacycles subgroup (p = 0.458, NS). The dotted line indicates 1% hemolysis. Permeate cell-free Hb concentration (B) over 10 diacycles of RBC washing using TFF. An ANOVA test was performed on the data subsets 1× to 10× diacycles, 1× to 4× diacycles, and 4× to 10× diacycles (α = 0.05, Ho = Hb concentration is independent of the diacycle). There was significance within the data for 1× to 10× diacycles (p = 6.2E-15, ***), and for 1× to 4× diacycles (p = 1.6E-5, ***). Within the 1× to 4× diacycle subgroup, significant differences were found between the 1× diacycle and 2×, 3×, 4× diacycles using TukeyHSD (p = 0.006, 0.0001, and 3.2E-5 respectively). No significance was found within the 4× to 10× diacycle (p = 0.151, NS).
The permeate cell-free Hb concentration is shown (Figure 3B). Significance was found within the 1× to 10× diacycle dataset (p = 6.2E-15, ***). The overall dataset was then split into two subsets from 1× to 4× diacycle and from 4× to 10× diacycle with significance found within the 1× to 4× diacycle dataset (p = 1.6E-5, ***). A Tukey HSD test was performed within the dataset from the 1× to 4× diacycle, and found that there was a significant difference between the 1× diacycle and the 2×, 3×, and 4× diacycles (p = 0.006, 0.0001, and 3.2E-5, respectively). No significance was found between the other diacycles. The US FDA considers stored RBC units with a hemolysis level less than 1% to be safe for transfusion. 1% hemolysis is roughly equivalent to a cell-free Hb concentration of 0.01 mM in the unit, which is lower than the final cell-free Hb concentration achieved in this study of ~ 0.0157 mM observed post wash.[20] One must, however, remember that the RBC units in this study were outdated (60-70 days old), which likely contributed to the higher final cell-free Hb concentration compared to literature values for washing non-expired RBC units.[21] Again, expired units were used in this proof-of-concept study, as a worst case scenario for RBC unit quality, to demonstrate the feasibility of washing RBCs using this novel TFF RBC washing process. Future studies will examine the effectiveness of washing unexpired RBC units via TFF.
4.5. Total Cell-Free Hb Mass Balance
The total cell-free Hb for each diacycle was quantified in order to perform an overall cell-free Hb mass balance and provides a complete understanding of the fate of the cell-free Hb removed via the TFF washing process. The mass of cell-free Hb for retentate and permeate samples was averaged for all 10 replicates (Table 1). The initial mass of cell-free Hb in individual RBC units is on average, 2.06 g with a Hb concentration of 0.105 mM, which corresponds to a hemolysis level of ~ 10 %. After the completion of the first diacycle, the cell-free Hb in the retentate is ~ 1.05 g, indicating that ~50% of the extracellular Hb has been removed at this stage. Cell-free Hb continues to be removed from the retentate for all subsequent diacycles. The system reached peak performance at ~ 4 diacycles, reaching a hemolysis level of 2.2 ± 1%, and decreased slightly with the additional 6 diacycles. The percent hemolysis at 4 diacycles for unexpired RBC units was decreased to 1.3 ± 0.6 % from an initial value of 4.2 ± 2.1 % hemolysis (Supplemental Information). Therefore, the TFF system is effective at removing cell-free Hb but more optimization is necessary to increase the washing speed and facilitate greater removal of cell-free Hb to match current accepted practices in RBC washing.
Table 1:
Cell-free Hb Overall Mass Balance.
| Retentate Cell-Free Hb | Permeate Cell-Free Hb | |
|---|---|---|
| Diacycle | Free Hb (g) | Free Hb (g) |
| Initial | 2.060 ± 1.539 | |
| 0X | 2.196 ± 1.191 | |
| 1X | 1.052 ± 0.834 | 1.648 ± 1.100 |
| 2X | 0.618 ± 0.347 | 0.678 ± 0.547 |
| 3X | 0.363 ± 0.187 | 0.273 ± 0.227 |
| 4X | 0.296 ± 0.106 | 0.173 ± 0.130 |
| 5X | 0.291 ± 0.106 | 0.114 ± 0.089 |
| 6X | 0.245 ± 0.084 | 0.097 ± 0.081 |
| 7X | 0.256 ± 0.070 | 0.100 ± 0.071 |
| 8X | 0.229 ± 0.066 | 0.099 ± 0.069 |
| 9X | 0.218 ± 0.056 | 0.099 ± 0.068 |
| 10X | 0.211 ± 0.043 | 0.094 ± 0.069 |
| Total Cell-Free Hb Removed: 1.849 g | ||
Additionally, a cell-free Hb mass balance analysis on the permeate samples show significant Hb removal at the start of the diafiltration process (Table 1). The 1× diacycle is the first diacycle with permeate flow, and removes the majority of cell-free Hb. The total mass of cell-free Hb continually decreases in the permeate as washing proceeds, supporting the theory that the TFF system is not inducing additional shear stress on the RBCs to cause lysis beyond what is needed to enable separation of cell-free Hb from the remaining RBCs in the retentate.
4.6. RBC Mechanical Quantification
The viscosity of RBCs in unprocessed RBC units and final post wash RBCs (10× diacycle) were measured to be 9.252 ± 1.477 cP, and 3.928 ± 1.766 cP, respectively. A significant change in RBC viscosity was observed due to the initial dilution of the RBC unit to 45% HCT, followed by removal of cell debris, proteins, and smaller molecules. The final washed RBC concentrate viscosity was higher than fresh RBCs (2.9 cP at 160 s−1 and 37°C) and is indicative of the advanced age of the RBC units used in this current study.[18] This viscosity is, however, a significant improvement from the aged RBC unit’s initial viscosity of 9.252 cP.
At low shear rates, blood behaves as a Casson fluid and is shear thinning, whereas at shear rates above 100 s−1, it behaves as a Newtonian fluid.[22] The following equation was used to calculate the shear stress on the inner wall of the TFF hollow fiber lumen with the assumption that the RBC suspension behaves as a Newtonian fluid above a shear rate of 100 s−1 , and does not require additional analysis based on the Casson fluid model. In equation (4), Po is the pressure at the inlet and PL is pressure at the outlet of an individual hollow fiber in the TFF cartridge. R is the inner radius of the hollow fiber and L is the effective length of each hollow fiber.
| (4) |
The shear rate value was extrapolated to 3670 s−1 from manufacturer provided values of 4000 s−1 at a flow rate of 1.09 L/min. The pressure drop within the TFF system from the inlet to the outlet of the hollow fiber cartridge was measured at an average value of 2 psig over 10 diacycles. From this value, we calculated the shear stress to be 12.9 Pa, which is not significantly higher than physiological conditions, and significantly lower than hemolytic shear stress levels of ~400 Pa.[23,24] By exposing the aged RBC unit to significant shear stress prior to transfusion, RBCs with weakened cell membranes are lysed and removed from the system. From the applied shear stress, we obtained the theoretical viscosity of 3.5 cP for the washed RBC suspension, which corroborates our experimentally measured viscosity using rheometry.
4.7. Oxygen Equilibrium Measurements
To confirm that TFF-facilitated RBC washing does not negatively affect the oxygen delivery characteristics of RBCs, the oxygen equilibrium curve (OEC) of RBCs pre and post wash were measured. The OEC of RBCs directly from the unprocessed RBC unit (initial) and after the 10× diacycle (final) is shown in Figure 4A. The left shift of the curve after washing is indicative of the higher oxygen affinity of washed RBCs versus unwashed RBCs.
Figure 4:
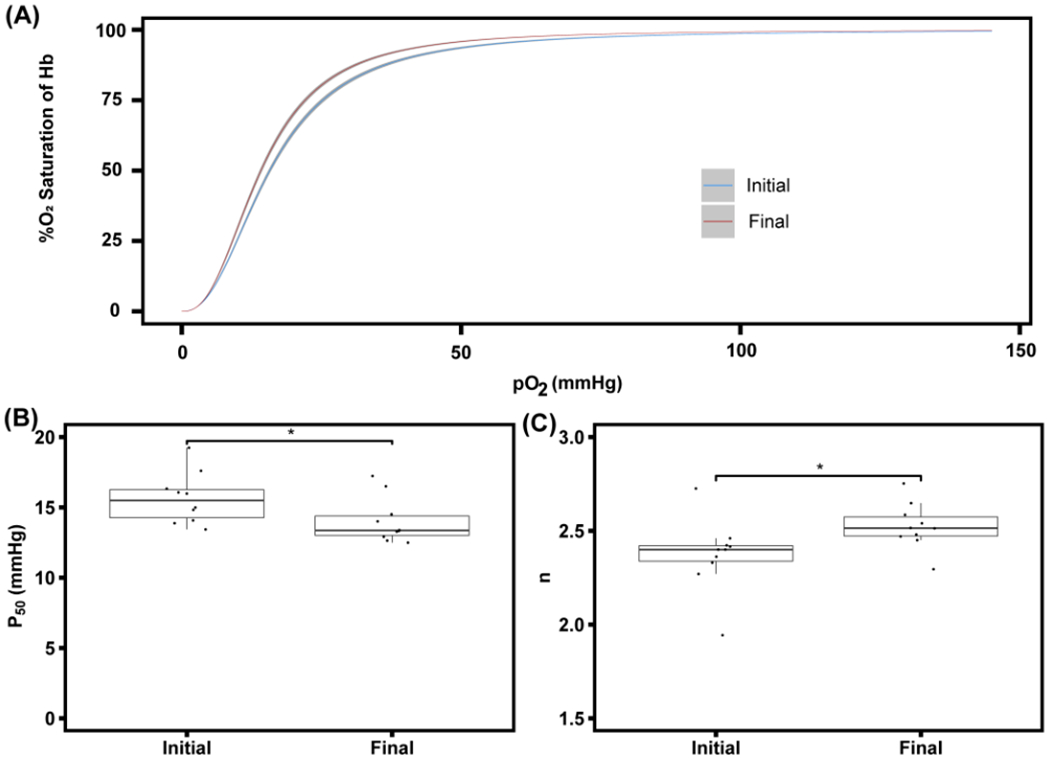
Oxygen equilibrium curve (OEC) of both the initial RBC unit and 10× dicycle sample (A). 10 single RBC units were processed using the TFF RBC washing system. The OEC of the initial RBC unit is shown in blue with a dark grey 95% CI. The OEC of the final 10× sample is shown in red with a dark grey 95% CI. P50 values (B) of the initial sample from the RBC unit and the final sample (10× diacycle) post TFF wash process (p = 0.0493, *). Hill coefficient (n) (C) of the initial sample from the RBC unit and the final sample (10× diacycle) after the TFF wash process (p = 0.0493, *).
The OEC provides key details about the ability of the Hb encapsulated in the RBC to bind and release oxygen, which is represented by the regressed P50 and n. A direct comparison between P50 values of the initial unwashed RBCs and the final washed RBCs shows that the P50 decreased from an initial value of 15.6 ± 1.8 mm Hg to 14 ± 1.62 mm Hg post wash (p = 0.0493, *) (Figure 4B). The Hill coefficient (n) comparison between the initial unwashed RBCs and final washed RBCs shows an increase (p = 0.0497, *) from 2.37 ± 0.19 to 2.52 ± 0.12 (Figure 4C). 2,3-bisphosphoglycerate, the allosteric effector that decreases Hb binding affinity to oxygen by stabilizing the tense quaternary state of Hb is significantly depleted during ex vivo RBC storage, which shifts the oxygen equilibrium curve to the left, increasing the oxygen affinity of the RBCs.[25] A left shift in the OEC suggests increased oxygen affinity and tighter binding of oxygen by Hb.[26]
5. Discussion
In conclusion, a novel RBC washing technique utilizing TFF for removing hemolysis byproducts in a single RBC unit was proposed, with the primary goal of this study to determine the effectiveness of the system in removing extracellular cell-free Hb without inducing further cellular damage. Quantification and characterization of pre and post wash RBC units was centered around the presence of cell-free Hb due to equipment availability at The Ohio State University and does not include the full extent of analytical methods or analytes that could be used to characterize RBC washing effectiveness. While there was no direct comparison between TFF and manual or automated RBC washing systems, comparisons could be drawn between the results from this study to results in the literature. A recent review of RBC washing technology focusing on manual washing and open and closed automated washing systems compares the removal of immunogenic components within RBC units and the resulting long-term storage impacts.[27] Compared to manual and automatic systems, the proposed TFF system uses more wash solution volume, but shows improved removal of Hb and comparable RBC recovery. The wash time is longer for the TFF process in part due to the increased wash volume. Results show that successful removal of free Hb plateaus after 4 diacycles, allowing for the potential for optimization of the number of diacycles beyond the standard 10 that was utilized. A major shortcoming of the current study is the limited information about long term storage after the TFF wash process. This proof-of-concept study was focused on an immediate comparison between the RBC unit pre and post wash and aimed to only verify that the TFF process can remove the majority of cell-free Hb from expired RBC units. Preliminary data, presented in the Supplemental Information section, shows the effectiveness of the TFF RBC washing system in removing cell-free Hb from unexpired units and presents similar results. To provide a more complete comparison between RBC washing by the TFF system and traditional washing procedures, further work characterizing more analytes post wash is necessary along with optimization of the pump flow rate and backpressure regulation for potential process time improvements. Despite the need for an expanded investigation into post-wash storage of RBC units, the designed system has the potential to revolutionize RBC washing systems in part due to its small physical footprint and sterilizable components. This allows the system to easily be transported for potential bedside ex vivo RBC washing and with the implementation of sterile conditions, could allow for direct transfusion of the post-wash RBCs to mitigate increases in potentially immunogenic byproducts derived from ex vivo storage.
Supplementary Material
Acknowledgements:
Authors S.L. and M.A. contributed equally to this project. Both authors ran red blood cell washing processes and analyzed process data. Authors S.L. and M.A. both wrote and edited the manuscript. Author M.W. provided instrumentation resources, and helped in meeting project goals. Authors A.F.P. and J.J.C. provided lab resources and funding, designed experiments, analyzed data, advised S.L., M.A. and M.W., and edited the manuscript.
Funding from grant numbers:
NIH R01HL126945, R01HL131720, R01HL138116, R01HL156526, and R01EB021926 to A.F.P.
Footnotes
There are no conflicts of interest.
References
- 1.Food and Drug Administration (FDA). CFR - Code of Federal Regulations Title 21. Vol. 21, Www.Fda.Gov. 2019. [Google Scholar]
- 2.Hess JR, Sparrow RL, Van Der Meer PF, Acker JP, Cardigan RA, Devine DV. Red blood cell hemolysis during blood bank storage: Using national quality management data to answer basic scientific questions. Transfusion. 2009;49(12):2599–603. [DOI] [PubMed] [Google Scholar]
- 3.Buehler PW, Alayash AI. Toxicities of hemoglobin solutions: In search of in-vitro and in-vivo model systems. Transfusion. 2004;44(10):1516–30. [DOI] [PubMed] [Google Scholar]
- 4.Smith A, McCulloh RJ. Hemopexin and haptoglobin: Allies against heme toxicity from hemoglobin not contenders [Internet]. Vol. 6, Frontiers in Physiology. Frontiers Media S.A.; 2015. [cited 2021 Jun 2]. p. 187. Available from: /pmc/articles/PMC4485156/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cholette JM, Henrichs KF, Alfieris GM, Powers KS, Phipps R, Spinelli SL, et al. Washing red blood cells and platelets transfused in cardiac surgery reduces postoperative inflammation and number of transfusions. Pediatr Crit Care Med. 2012. May;13(3):290–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stapley R, Rodriguez C, Oh JY, Honavar J, Brandon A, Wagener BM, et al. Red blood cell washing, nitrite therapy, and antiheme therapies prevent stored red blood cell toxicity after trauma-hemorrhage. Free Radic Biol Med. 2015;85:207–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hansen A, Yi QL, Acker JP. Quality of red blood cells washed using the ACP 215 cell processor: Assessment of optimal pre- and postwash storage times and conditions. Transfusion. 2013;53(8):1772–9. [DOI] [PubMed] [Google Scholar]
- 8.Bennett-Guerrero E, Kirby BS, Zhu H, Herman AE, Bandarenko N, McMahon TJ. Randomized study of washing 40-to 42-day-stored red blood cells. Transfusion. 2014;54(10):2544–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lu M, Lezzar DL, Vörös E, Shevkoplyas SS. Traditional and emerging technologies for washing and volume reducing blood products. J Blood Med [Internet]. 2019. [cited 2021 Jun 2];10:37–46. Available from: /pmc/articles/PMC6322496/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.O’Leary MF, Szklarski P, Klein TM, Young PP. Hemolysis of red blood cells after cell washing with different automated technologies: Clinical implications in a neonatal cardiac surgery population. Transfusion. 2011;51(5):955–60. [DOI] [PubMed] [Google Scholar]
- 11.Bennett-Guerrero E, Kirby BS, Zhu H, Herman AE, Bandarenko N, McMahon TJ. Randomized study of washing 40- to 42-day-stored red blood cells. Transfusion [Internet]. 2014. Oct 1 [cited 2021 Nov 8];54(10):2544–52. Available from: 10.1111/trf.12660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Johnson L, Kwok M, Marks DC. Preparation of red blood cell concentrates and plasma units from whole blood held overnight using a hollow-fibre separation system. Transfus Med. 2015;25(1):13–9. [DOI] [PubMed] [Google Scholar]
- 13.Brune T, Hannemann-Pohl K, Nißle K, Ecker N, Garritsen H. Quality, stability, and safety data of packed red cells and plasma processed by gravity separation using a new fully integrated hollow-fibre filter device. Adv Hematol. 2009;2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Castino F, Wickramasinghe SR. Washing frozen red blood cell concentrates using hollow fibres. J Memb Sci. 1996;110(2):169–80. [Google Scholar]
- 15.Winterbourn CC. Oxidative reactions of hemoglobin. Methods Enzymol. 1990;186(C):265–72. [DOI] [PubMed] [Google Scholar]
- 16.Hill AV. The possible effects of the aggregation of the molecules of hæmoglobin on its dissociation curves. J Physiol. 1910. Jan;40:i--vii. [Google Scholar]
- 17.Cabrales P, Martini J, Intaglietta M, Tsai AG. Blood viscosity maintains microvascular conditions during normovolemic anemia independent of blood oxygen-carrying capacity. Am J Physiol - Hear Circ Physiol. 2006;291(2):581–90. [DOI] [PubMed] [Google Scholar]
- 18.Cabrales P, Tsai AG, Intaglietta M. Is resuscitation from hemorrhagic shock limited by blood oxygen-carrying capacity or blood viscosity? Shock. 2007;27(4):380–9. [DOI] [PubMed] [Google Scholar]
- 19.Yoon C, Noh S, Lee JC, Ko SH, Ahn W, Kim HC. Influence of the washing program on the blood processing performance of a continuous autotransfusion device. J Artif Organs. 2014;17(1):118–22. [DOI] [PubMed] [Google Scholar]
- 20.Ni J, Zhu W, Wang Y, Wei X, Li J, Peng L, et al. A Reference chart for clinical biochemical tests of hemolyzed serum samples. J Clin Lab Anal. 2021;35(1):1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sowemimo-Coker SO. Red blood cell hemolysis during processing. Transfus Med Rev. 2002. Jan;16(1):46–60. [DOI] [PubMed] [Google Scholar]
- 22.Fournier RL. Basic transport phenomena in biomedical engineering. 2nd ed. New York: Taylor & Francis; 2007. 450 p. [Google Scholar]
- 23.Koutsiaris AG, Tachmitzi SV, Batis N. Wall shear stress quantification in the human conjunctival pre-capillary arterioles in vivo. Microvasc Res. 2013;85(1):34–9. [DOI] [PubMed] [Google Scholar]
- 24.Paul R, Apel J, Klaus S, Schügner F, Schwindke P, Reul H. Shear stress related blood damage in laminar Couette flow. Artif Organs. 2003;27(6):517–29. [DOI] [PubMed] [Google Scholar]
- 25.Valtis DJ, Kennedy AC. DEFECTIVE GAS-TRANSPORT FUNCTION OF STORED RED BLOOD-CELLS. Lancet. 1954. Jan;263(6803):119–25. [DOI] [PubMed] [Google Scholar]
- 26.Hamilton C, Steinlechner B, Gruber E, Simon P, Wollenek G. The oxygen dissociation curve: Quantifying the shift. Perfusion. 2004;19(3):141–4. [DOI] [PubMed] [Google Scholar]
- 27.Cardigan R, New HV, Tinegate H, Thomas S. Washed red cells: theory and practice. Vox Sang. 2020. Nov 1;115(8):606–16. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


