Abstract
Background:
The US Preventive Services Task Force (USPSTF) 2021 updated recommendations on lung cancer screening with chest computed tomography to apply to individuals 50–80 years of age (previously 55–80), with a ≥20 pack-year history (previously ≥30), whether currently smoking or quit ≤15 years ago. Despite being at higher risk for lung cancer, persons with HIV (PWH) were not well-represented in the National Lung Screening Trial, which informed the USPSTF 2013 recommendations. It is unknown/unclear how PWH are affected by the 2021 recommendations.
Setting:
This study was a retrospective analysis of PWH with and without lung cancer in the Women’s Interagency HIV Study and the Multicenter AIDS Cohort Study.
Methods:
We identified PWH, ages 40–80, who currently or previously smoked, with (cases) and without lung cancer (non-cases). The sensitivity and specificity of the old, new, and alternative screening criteria were evaluated in each cohort.
Results:
We identified 52 women and 19 men with lung cancer and 1950 women and 1599 men without lung cancer. Only 11 women (22%) and 6 men (32%) with lung cancer met 2013 screening criteria; however, more women (22; 44%) and men (12; 63%) met 2021 criteria. Decreased age and tobacco exposure thresholds in women further increased sensitivity of the 2021 criteria.
Conclusions:
The 2021 USPSTF lung cancer screening recommendations would have resulted in more PWH with lung cancer being eligible for screening at the time of their diagnosis. Further investigation is needed to determine optimal screening criteria for PWH, particularly in women.
Keywords: Lung cancer, HIV, AIDS, Lung cancer screening
INTRODUCTION
Antiretroviral therapy (ART) has resulted in near-normal lifespans for persons with HIV (PWH).1 Effective HIV testing and treatment have also reduced the incidence of AIDS-related malignancies like Kaposi’s sarcoma, and lung cancer is now the most common cause of malignancy-related death in PWH.2–4 Although PWH have higher smoking rates than the general population, HIV infection is an additional, independent risk factor for lung cancer.5,6 Compared to those without HIV, PWH have up to a 3-fold increased risk of developing lung cancer, are diagnosed at a younger age, have lower cumulative smoking history, and have worse survival.6–10 Given increased risk for and worse survival from lung cancer, PWH may benefit from early detection via lung cancer screening (LCS).
The National Lung Screening Trial (NLST) demonstrated a 20% relative reduction in lung cancer mortality with annual screening by low-dose computed tomography (LDCT) in high-risk individuals who currently or previously smoked.11 As a result, in 2013, the US Preventative Services Task Force (USPSTF) recommended screening with LDCT in adults ages 55–80 who have a ≥30 pack-year smoking history and who currently smoke or quit within the last 15 years. In March 2021, the USPSTF released updated screening recommendations based on a systematic review and a collaborative modeling study.12 The 2021 recommendations now include adults ages 50–80 who have a ≥20 pack-year history and currently smoke or quit within the last 15 years. Evidence suggests that the 2021 criteria increase the relative percent eligible for screening in high-risk groups, including Black persons and women, reducing racial and sex disparities in screening and diagnosis.12,13 Despite their uniquely high risk of lung cancer, PWH were not well-represented in the NLST or considered in the USPSTF update.
The objective of this study was to examine the performance characteristics – sensitivity and specificity – of the 2013 and 2021 USPSTF LCS criteria and alternative LCS criteria among PWH from two longitudinal studies of PWH in the United States (US). As there is no existing cohort of LCS in PWH, we took advantage of a convenience cohort of PWH with and without diagnosed lung cancer to examine the performance of screening criteria. We hypothesized that the 2021 USPSTF criteria would perform better than the 2013 version but that there would still be opportunity to improve screening eligibility by further reducing the age and pack-year thresholds or by including markers of severe HIV infection (low nadir CD4 count, prior AIDS diagnosis) to improve early detection of lung cancer in PWH.
METHODS
Study Population
We analyzed data from two US cohort studies of persons with or at high risk for HIV, the Women’s Interagency HIV Study (WIHS) and the Multicenter AIDS Cohort Study (MACS).14,15 WIHS and MACS data collection procedures are described in the supplementary material. Cases were PWH with lung cancer, currently smoking or formerly smoked, and ≥40 years old at the time of cancer diagnosis. The age cutoff of 40 was chosen as there were no lung cancers detected in subjects under 40. To ascertain cases, WIHS and MACS used cancer registries, medical records, death certificates, and the National Death Index, with medical record confirmation (in the MACS only). Participants who reported a history of lung cancer at cohort entry were excluded. Non-cases were PWH ≥40 years old, who currently or formerly smoked without lung cancer. Data were abstracted from the visit preceding the lung cancer confirmation date for cases and non-cases, from a randomly selected person-visit at age ≥40 years with current/former smoking history. All years of cohort follow-up were used.
Data Collection
Participants reported demographic data, smoking status, and intensity, ART adherence, medical comorbidities, and substance use history. All cases and non-cases were included in analyses of demographic and clinical characteristics. Only cases and non-cases with available pack-year history and smoking cessation data were used in the analysis of LCS criteria. Pack-years were calculated by multiplying the self-reported total years smoked and the average number of daily cigarettes smoked in the six months before each visit. Measures of HIV disease, including CD4 count and HIV viral load, were obtained from labs drawn at the selected visit. Nadir CD4 count and history of AIDS were obtained from clinical history. As a sensitivity analysis, we also selected CD4 counts from visits 1 and 5 years before diagnosis to account for effects of lung cancer on CD4 count. Similar lung cancer morphologies indicated by ICD-O-2 or ICD-O-3 codes were categorized together (e.g., papillary adenocarcinoma and adenocarcinoma with mixed subtypes were grouped with adenocarcinoma).16
Statistical Methods
Differences in demographic and clinical factors between cases and non-cases were compared using chi-square, Fisher’s exact, or Wilcoxon rank-sum tests as appropriate, stratified by cohort. To examine the performance of alternative thresholds of LCS criteria, changes in each risk factor (age, smoking pack-years, and time since quitting) were made by 1-year or one pack-year increment while keeping the other two risk factors constant as they are in the 2021 recommendations. Nadir CD4 count (≤200 cells/mL or ≤350 cells/mL) and history of AIDS were separately added as additional criteria. In each cohort, the criteria sensitivity and specificity with each combination of discrete threshold for age (starting with ≥40, then increasing by one year to ≥55), smoking pack-years (starting from ≤30, then decreasing by one pack-year to ≤15), and years since quitting (starting from ≤15, then increasing by one year to ≤30) were calculated.
We selected the maximal Youden’s J value17 to determine the combination for which screening criteria sensitivity and specificity were balanced. This statistic assumes that sensitivity and specificity are of equal clinical importance, which may not be the case, but was chosen to illustrate multiple points on a receiver operating characteristic (ROC) curve. The ROC curve was generated by plotting the highest sensitivity for each unique specificity. In cases where different combinations yielded the same value, the combination that screened the highest age, highest pack-year history, and lowest quit time was chosen, as this represented the most efficient criteria. All analyses were completed with SAS 9.4 (SAS Institute Inc., Cary, NC).
RESULTS
Participant Characteristics
Lung cancer incidence among those who currently/formerly smoked and between 40–80 years of age was 258.1 cases per 100,000 person-years in women (95%CI, 251.2–265.2) and 111.2 cases per 100,000 person-years (95%CI, 106.3–116.4) in men. The overall analytic dataset included 52 women and 19 men with confirmed lung cancer, and 1950 women and 1599 men without lung cancer, all with HIV (Table 1). The median year of diagnosis was 2010 (interquartile range [IQR] 2005–2013) in the WIHS and 2009 (IQR 1997–2015) in the MACS. The median time from the visit at which data was abstracted and the cancer diagnosis was 114 days (IQR 51–176) in the WIHS and 113 days (IQR 70–166) in the MACS.
Table 1.
Characteristics of participants with HIV and lung cancer (age ≥40, current/former smoker at time of diagnosis) and HIV without lung cancer (for each, random selection of one person-visit at age ≥40 with current/former smoking)
| WIHS women | MACS men | |||||
|---|---|---|---|---|---|---|
| Characteristic | With lung cancer (n=52) | Without lung cancer (n=1950) | p-value* | With lung cancer (n=19) | Without lung cancer (n=1599) | p-value* |
| Age, years | 54.9 (49.7–58.9) | 46.8 (42.8–52.0) | <0.001 | 52.4 (48.0–59.5) | 46.2 (42.4–52.4) | 0.002 |
| Black race | 41 (79) | 1276 (66) | 0.10 | 4 (21) | 384 (24) | 0.94 |
| Body mass index, kg/m2 | 25 (22–27) | 27 (23–33) | <0.01 | 24 (20–26) | 24 (22–27) | 0.30 |
| Prior AIDS | 31 (60) | 869 (45) | 0.03 | 6 (32) | 277 (17) | 0.12 |
| CD4 count, cells/µL | ||||||
| Median | 368 (231–655) | 451 (246–703) | 0.28 | 400 (150–833) | 463 (246–680) | 0.94 |
| Nadir | 187 (75–286) | 208 (94–337) | 0.21 | 150 (78–420) | 199 (62–331) | 0.91 |
| HIV RNA, copies/mL† | ||||||
| Not detected** | 20 (38) | 871 (45) | 0.37 | 10 (56) | 608 (40) | 0.19 |
| Median | 262 (48–7573) | 82 (20–12000) | 0.66 | 40 (10–21700) | 735 (40–40177) | 0.33 |
| Antiretroviral therapy use | ||||||
| Ever | 38 (73) | 1502 (77) | 0.50 | 14 (74) | 907 (57) | 0.14 |
| Adherent§** | 15 (56) | 587 (49) | 0.50 | 3 (30) | 297 (41) | 0.80 |
| Smoking history | ||||||
| Current smoker | 41 (79) | 1244 (64) | 0.03 | 14 (74) | 807 (47) | 0.02 |
| ≥20 pack-years smoked** | 29 (56) | 424 (22) | <0.001 | 15 (79) | 775 (53) | 0.03 |
| Quit <15 years ago** | 51 (94) | 491 (77) | 0.08 | 5 (100) | 603 (83) | 0.60 |
| Total years smoked | 31.4 (24.9–40.7) | 24.0 (15.0–30.7) | <0.001 | NA | NA | NA |
| Less than high school education** | 22 (42) | 729 (37) | 0.48 | 1 (5) | 237 (15) | 0.34 |
| Low annual income**†† | 41 (79) | 1453 (75) | 0.48 | 11 (57) | 580 (44) | 0.08 |
| Employed | 6 (12) | 458 (23) | 0.04 | 5 (26) | 929 (58) | 0.005 |
| Currently insured** | 51 (98) | 1808 (93) | 0.18 | 11 (92) | 790 (93) | 0.58 |
| Injection drug use‡ | 14 (27) | 269 (14) | 0.01 | 2 (11) | 236 (15) | 1.00 |
| Marijuana use‡ | 24 (46) | 958 (49) | 0.67 | 16 (84) | 1195 (80) | 0.44 |
| Heavy alcohol use in past 6 months¶ | 4 (8) | 166 (9) | 1.00 | 3 (16) | 149 (9) | 0.41 |
| Comorbidity, at or prior to visit | ||||||
| Asthma | 27 (52) | 797 (41) | 0.11 | 6 (32) | 200 (13) | 0.03 |
| Cardiovascular | 13 (25) | 262 (13) | 0.02 | 2 (11) | 99 (6) | 0.34 |
| Diabetes | 5 (10) | 319 (16) | 0.19 | 0 (0) | 224 (14) | 0.10 |
| Depression** | 46 (88) | 1581 (81) | 0.18 | 12 (71) | 1009 (65) | 0.62 |
| Hypertension | 40 (77) | 1366 (70) | 0.29 | 14 (74) | 996 (62) | 0.31 |
| Tuberculosis | 5 (10) | 152 (5) | 0.63 | 1 (5) | 25 (2) | 0.27 |
| Non-tuberculous mycobacteria | 4 (8) | 46 (2) | 0.02 | 1 (1) | 22 (1) | 0.24 |
| Menopause | 47 (90) | 1036 (53) | <0.001 | NA | NA | NA |
| Pneumonia history | ||||||
| non-Pneumocystis | 32 (62) | 715 (37) | <0.001 | NA | NA | NA |
| Pneumocystis | 21 (40) | 397 (20) | <0.001 | NA | NA | NA |
All values n (%) or median (interquartile range)
NA- Not available
Chi-square test for categorical variables and Wilcoxon rank-sum test for continuous variables
Of subjects with data available or of using appropriate denominator (e.g., for “Quit<15 years ago” out of total number of quitters)
Lower limit of detection for viral load assay has varied over time within each cohort
Defined as 100% adherence in last 6 months
Household income of ≤$18,000 for the WIHS and individual income ≤$20,000 for the MACS
During cohort follow-up
Heavy alcohol use ≥12 drinks/week for the WIHS and ≥13 drinks/week for the MACS
Compared to WIHS non-cases, WIHS cases were older and had a lower BMI (Table 1). A higher proportion of cases were menopausal and reported a history of cardiovascular disease, prior pneumonia, or AIDS. Larger proportions of cases had reported injection drug use, ≥30 pack-year history and current smoking; cases had more median total years smoked than non-cases. Cases and non-cases in the WIHS had similar current CD4 counts, CD4 counts 1 and 5 years before cancer diagnosis, nadir CD4 counts, median HIV viral load, and proportions of ART-adherent participants.
In the MACS, cases were older, and higher proportions reported asthma (Table 1). A greater proportion of cases reported current smoking and a ≥30 pack-year smoking history compared to non-cases. Cases and non-cases had similar non-tobacco substance use, prior AIDS diagnoses, ART adherence, current CD4 counts, CD4 counts 1 and 5 years prior to cancer diagnosis, nadir CD4 counts, and median HIV viral load.
Lung Cancer Histology in the WIHS and MACS
The WIHS and MACS use SEER lung cancer staging data,18 which stages cancer as localized, regional, or distant. Of the 28 WIHS cases with available stage data, 11 (39%) had localized cancer, 8 (29%) had regional disease, and 9 (32%) had distant disease. Of the 52 total WIHS cases, 32 had histologic data from state registries. The most commonly identified cancers were adenocarcinoma (9; 28%), non-small cell lung cancer not otherwise specified (NOS) (8; 25%), and squamous cell carcinoma (7; 22%) (Table 2). Of the six MACS cases with available SEER stage data, two (33%) had localized disease, one (17%) had regional disease, and three (50%) had distant disease. Among MACS cases, adenocarcinoma was the most identified subtype (8; 42%) followed by carcinoma NOS (3; 16%) and squamous cell carcinoma (2; 11%) (Table 2). Across both cohorts, there were no significant differences in demographics or current smoking status between those diagnosed with early and late-stage cancers.
Table 2.
Cancer histology*
| Histology type | WIHS women with lung cancer (n=32) | MACS men with lung cancer (n=19) |
|---|---|---|
| Adenocarcinoma | 9 (28) | 8 (42) |
| Squamous cell carcinoma | 7 (22) | 2 (11) |
| Non-small cell carcinoma, NOS | 8 (25) | 1 (5) |
| Small cell carcinoma | 1 (3) | 1 (5) |
| Carcinoma, NOS | 4 (13) | 3 (16) |
| Broncho-alveolar carcinoma | 2 (6) | 0 |
| Large cell neuroendocrine, carcinoma | 0 | 1 (5) |
| Pleiomorphic carcinoma | 1 (3) | 0 |
| Tumor cells, malignant | 0 | 1 (5) |
| Neoplasm, metastatic | 0 | 1 (5) |
| Pseudosarcomatous carcinoma | 0 | 1 (5) |
Based on data from state cancer registries. Data available on 32 of 52 lung cancers identified in the WIHS and all cancers identified in the MACS.
Lung cancer screening criteria in the WIHS and MACS
2013 USPSTF criteria recommend LCS for individuals ages 55–80 years with pack-year history ≥30 pack-years currently smoking or quit ≤15 years. 2021 USPSTF criteria recommend LCS screening in a younger cohort, ages 50–80, with lower smoking intensity, ≥20 pack-years. Information on all screening criteria was available for 50 of 52 cases and 1880 of 1950 non-cases in the WIHS and all 19 cases and 1424 of 1599 non-cases in the MACS. Only 22% (95%CI, 12%−36%) of women and 32% (95%CI, 13%−57%) of men with lung cancer met 2013 criteria at the time of their diagnosis (Table 3). The overall number of non-cases who would have been screened was low – only 2% (95%CI, 1%−3%) of women and 7% (95%CI, 5%−8%) of men. A greater percentage of PWH with lung cancer would have been eligible for screening with the 2021 USPSTF criteria – 44% (95%CI, 30%−59%) of women and 63% (95%CI, 38%−84%) of men. The number of non-cases eligible for screening would also be higher, 8% (95%CI, 7%−9%) in women and 17% (95%CI, 15%−19%) in men.
Table 3.
Performance characteristics of lung cancer screening criteria using various combinations of three screening metrics, by sex.
| Age | Pack-years | Time since quit | Sensitivity (95% CI) |
Specificity (95% CI) |
Youden’s J | |
|---|---|---|---|---|---|---|
| WIHS | ||||||
| Old USPSTF criteria | ≥55 | ≥30 | ≤15 | 22% (12%–36%) | 98% (97%–99%) | 0.20 |
| New USPSTF criteria | ≥50 | ≥20 | ≤15 | 44% (30%–59%) | 92% (91%–93%) | 0.36 |
| High sensitivity | ≥40 | ≥15 | ≤22 | 74% (60%–85%) | 66% (64%–69%) | 0.40 |
| High specificity | ≥55 | ≥30 | ≤16 | 22% (12%–36%) | 98% (97%–99%) | 0.20 |
| Balanced | ≥49 | ≥16 | ≤24 | 62% (47%–75%) | 86% (85%–88%) | 0.48 |
| MACS | ||||||
| Old USPSTF criteria | ≥55 | ≥30 | ≤15 | 32% (13%–57%) | 93% (92%–95%) | 0.25 |
| New USPSTF criteria | ≥50 | ≥20 | ≤15 | 63% (38%–84%) | 83% (81%–85%) | 0.46 |
| High sensitivity | ≥43 | ≥19 | ≤15 | 84% (60%–97%) | 62% (59%–65%) | 0.46 |
| High specificity | ≥55 | ≥30 | ≤15 | 32% (13%–57%) | 93% (92%–95%) | 0.25 |
| Balanced | ≥47 | ≥30 | ≤15 | 68% (43%–87%) | 82% (80%–84%) | 0.51 |
USPSTF: United States Preventive Services Task Force
Alternative thresholds generated different LCS criteria sensitivities and specificities by sex (Table 3). In women, when the lower limit of age was decreased to 40 years while keeping the other thresholds the same as the 2021 criteria, sensitivity increased to 54% (95%CI, 39%−68%), and specificity decreased to 78% (95%CI, 76%−80%) relative to the 2021 recommendations (Figure 1a). When their smoking history was reduced to ≥15 pack-years keeping age and quit time the same as 2021 criteria, sensitivity and specificity were 50% (95%CI, 36%−64%) and 88% (95%CI, 86%−89%), respectively (Figure 1b). Lastly, when time since quitting was increased to ≤30 years keeping age and pack-year history the same as 2021, sensitivity increased to 46% (95%CI, 32%−61%) while specificity decreased to 91% (95%CI, 90%−93%) (Figure 1c). In women, the greatest sensitivity of 74% (95%CI, 60%−85%) was achieved by screening women ages 40–80, with a ≥15 smoking pack-year history and a quit date ≤22 years before screening; specificity was 66% (95%CI, 64%−69%). The greatest specificity of 98% (95%CI, 97%−99%) was achieved by screening women ages 55–80, with a ≥30 smoking pack-year history, and a quit date ≤16 years before screening, but sensitivity declined to 22% (95%CI, 12%−36%). Sensitivity and specificity were balanced in these women using screening criteria of ages 49–80, ≥16 smoking pack-year history, and a quit date ≤22 years prior, with a sensitivity of 62% (95%CI, 47%−75%) and a specificity of 86% (95%CI, 85%−88%) (Figure 2).
Figure 1. Change in performance characteristics in WIHS women and MACS men when one of the 2021 criteria was altered while keeping other two criteria at 2021 recommendations.
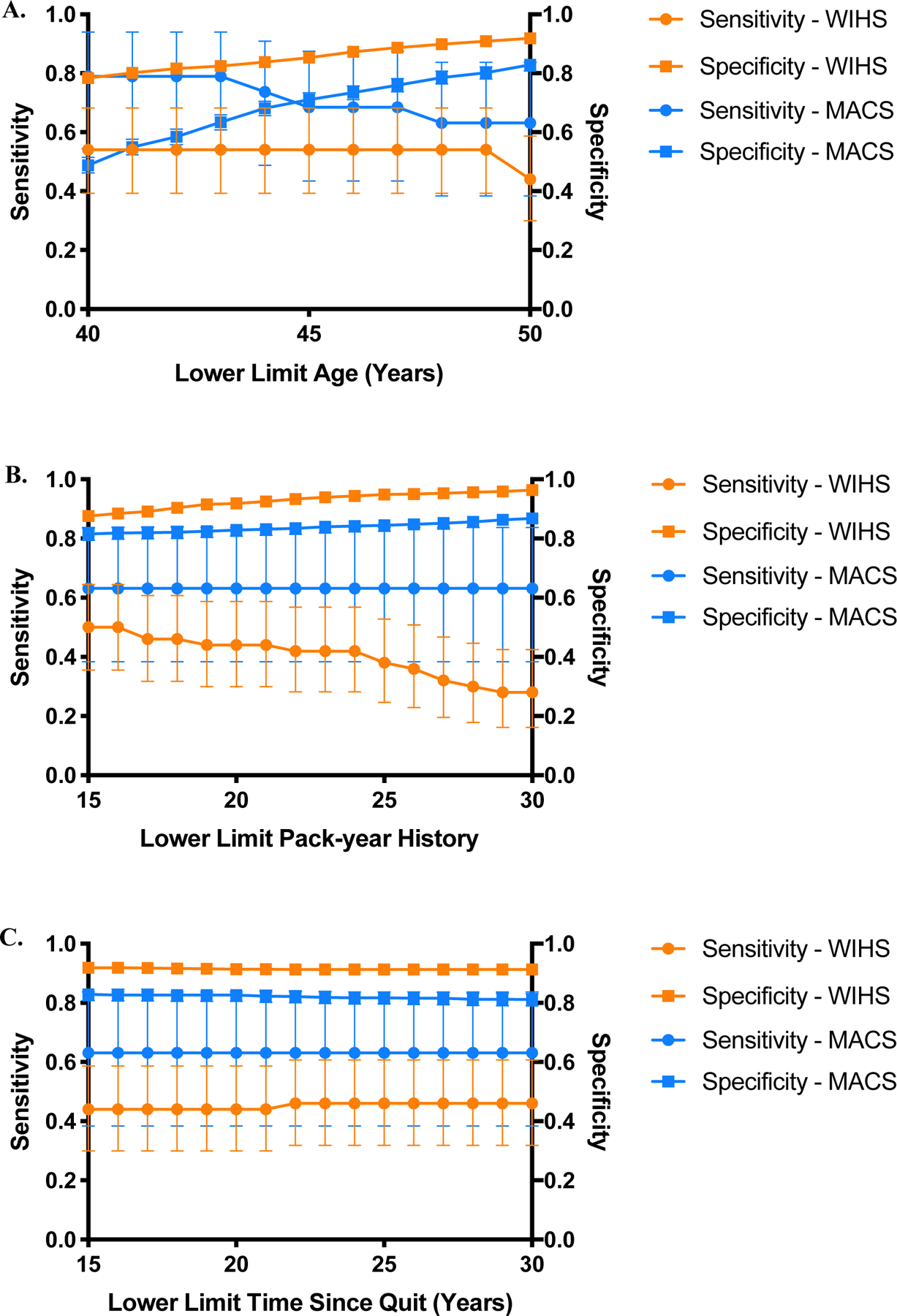
a) In women (orange), when the lower limit of age was independently decreased in 1-year increments from ≥50 to ≥40, sensitivity increased from 44% (95%CI, 20%−59%) to 54% (95%CI, 39%−68%) while specificity decreased from 92% (95%CI, 91%−93%) to 78% (95%CI, 76%−80%). In men (blue), when the lower limit of age was independently decreased in 1-year increments to ≥40, sensitivity increased from 63% (95%CI, 38%−84%) to 79% (95%CI, 54%−94%) while specificity decreased from 83% (95%CI, 81%−85%) to 49% (95%CI, 46%−51%). b) In women (orange), when the pack-year history was decreased from ≥30 to ≥15 in increments of 1, sensitivity and specificity were 50% (95%CI, 36%−64%) and 88% (95%CI, 86%−89%) respectively. In men (blue), when the pack-year history was decreased from ≥30 to ≥15 in increments of 1, there were no changes in sensitivity (63%; 95%CI, 38%−84%) but specificity decreased to 82%; 95%CI, 79%−84%). c) In women (orange), when years since quitting was increased from ≤15 years to ≤30 years, sensitivity increased to 46% (95%CI, 32%−61%) while specificity decreased to 91% (95%CI, 90%−93%) In men (blue), when years since quitting was increased from ≤15 years to ≤30 years, sensitivity remained unchanged (63%; 95%CI, 38%−84%) and specificity decreased to 81% (95%CI, 79%−83%).
Figure 2. Receiver operating characteristic (ROC) curves depicting performance characteristics of all unique specificities in the WIHS and MACS cohorts.
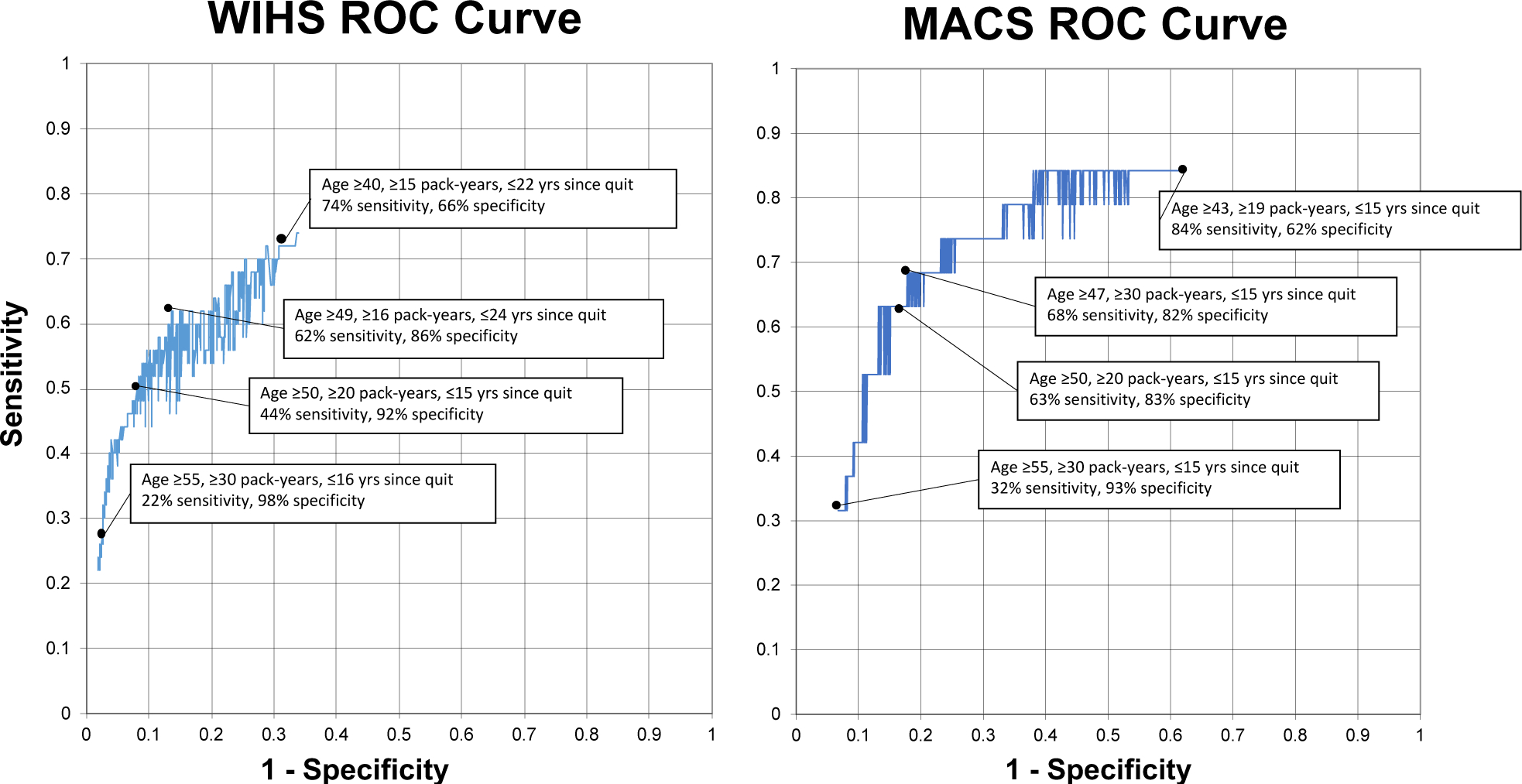
All unique specificities generated when evaluating all possible combinations of the three lung cancer screening criteria were plotted with the highest associated sensitivity. The points with the highest sensitivity, highest specificity, and balance between sensitivity and specificity are marked.
In men, decreasing the screening age to 40 years, without changing the other two thresholds from 2021 recommendations, increased the sensitivity from 63% to 79% (95%CI, 54%−94%) and decreased specificity from 82% to 49% (95%CI, 46%−51%) (Figure 1a). Reducing their smoking pack-year history to ≥15 years, keeping age and quit time the same as 2021, did not change sensitivity but decreased specificity very minimally from 83% to 82% (95%CI, 79%−84%) (Figure 1b). Similarly, when time since quitting was increased to ≤30 years, keeping age and pack-year history the same as 2021, sensitivity remained the same, and specificity decreased to 81% (95%CI, 79%−83%) (Figure 1c). The greatest sensitivity of 84% (95%CI, 60%−97%) was achieved by screening men ages 43–80, with a ≥19 smoking pack-year history, and a quit date ≤15 years before screening (specificity, 62% [95%CI, 59%−65%]). The greatest specificity (93%; 95%CI, 92%−95%) was achieved with the 2013 USPSTF criteria. Sensitivity (68%; 95%CI, 43%−87%) and specificity (82%; 95%CI, 80%−84%) were balanced by screening men ages 47–80, with a ≥30 pack-year history, and a quit date ≤15 years prior.
When prior AIDS diagnosis or nadir CD4 was added as a fourth criterion among women and men, the three criteria cut points that yielded greatest sensitivity and Youden’s J were unchanged.
DISCUSSION
Our analysis demonstrates that 2021 USPSTF LCS recommendations have improved sensitivity compared to 2013 criteria in real-world cohorts of PWH though the 2021 criteria still under-screen women with HIV and lung cancer compared to men. A simulation study of PWH with 100% ART adherence and CD4 counts ≥500 cells/μL projected that 2013 recommendations would reduce lung cancer mortality by 18.9% in PWH.19 Our findings in a real-world population of PWH complement these results. They mirror a recent study examining the performance of the 2013 USPSTF criteria in a large French cohort of PWH.20 Inclusion of HIV markers in our study did not improve the sensitivity of screening criteria in the WIHS or MACS. However, the absolute numbers of those who met the additional low nadir CD4 or AIDS criteria in both cohorts were low. Based on our results in the WIHS, assuming an annual lung cancer incidence of 0.17%, we estimated that for every 2674 women with HIV screened by the 2021 criteria versus the 2013 criteria, one additional lung cancer case would be detected and160 non-cases would be screened.21
Older age is a significant risk factor for lung cancer. However, evidence suggests that PWH are diagnosed with lung cancer at younger ages than the general population,9,10,22 consistent with our analysis where the median age of lung cancer diagnosis was 55 in women and 52 in men, compared to 70 years in the general population.23 However, PWH are more likely to be younger than the general population, which biases this observation. We observed the most impressive impact on the performance of LCS criteria in both cohorts when we lowered the age threshold while maintaining the remaining criteria at current cut points, suggesting this may be a critical adjustment.
One of the most important risk factors for the development of lung cancer remains tobacco exposure. The relationship between HIV and lung cancer in PWH can be partially explained by the high prevalence of smoking in HIV cohorts, with published estimates of current smoking ranging from 31% to 84%, compared to 20% in the general population.24 However, when controlling for smoking, PWH still have an up to 3-fold increased risk of developing lung cancer, suggesting that additional oncogenic pathways may be activated in PWH.6–8 As a result, PWH may be under-screened for lung cancer due to an inappropriately high threshold of pack-year smoking history in LCS criteria, as illustrated in the WIHS cohort, where just over half of cases had a ≥20 pack-year history. Among women, decreasing the pack-year history criterion steadily improved sensitivity and decreased specificity. These findings demonstrate that among women with HIV, the 2013 pack-years threshold would lead to substantial under-screening of women with lung cancer; the 2021 criteria ameliorate this. However, the same was not observed in men, suggesting that the cumulative effect of smoking differs by sex.
Quitting smoking remains the primary strategy to reduce lung cancer mortality, but screening remains an important additional strategy, and the two are complementary. The USPSTF recommends patients who are currently smoking receive smoking cessation interventions with screening. Although evidence suggests that engaging in LCS does not impact tobacco cessation,25 abnormal screening results are associated with abstinence in the general population.26 The relationship between smoking cessation efforts and screening programs in PWH is not yet understood but will be important to elucidate.
The simulations supporting the 2021 criteria demonstrate disproportionate increases in screening eligibility in non-Hispanic Blacks compared to non-Hispanic whites and in women compared to men, theoretically reducing screening disparities by race and gender, though not eradicating them.13,27 Similarly to HIV infection, Black race imparts risk for lung cancer at a lower age and lower cumulative smoking history. Reduction in lung cancer incidence in women has lagged behind men over time.28–31 Critically, Black individuals and Black women account for a disproportionate burden of HIV infection in the US.32,33 In this cohort, we are unable to assess how much of the improvement in sensitivity is due to the overlap between higher-risk profiles in PWH based on race and sex versus the risk imparted by HIV infection.
Even when adjusting the thresholds of each LCS criterion, the maximum sensitivity we achieved in women was only 74%, compared to 84% in men. Data suggest that sex-based risk factors affect lung cancer in the general population. Lung cancer incidence is significantly higher in young women compared to young men despite similar smoking behavior patterns. It is also higher in females who have never smoked compared to males who have never smoked.30,34 Proposed hypotheses for these differences include higher frequencies of genetic mutations, sex differences in nicotine metabolism, and the effects of estrogen.35–37 Although further research is needed to understand differences in lung cancer risk in women, the especially poor performance of the current LCS criteria among women in our study supports the use of risk prediction modeling that incorporates sex.
While evidence demonstrates that HIV is an independent risk factor for lung cancer, the relationship between the degree of immunosuppression and lung cancer risk has not been elucidated.5,6 Several prior studies have identified an association between CD4 count and lung cancer risk.38,39 In contrast, others have failed to demonstrate this link.5,40 Our study did not identify any differences in markers of HIV disease between cases and non-cases. Addition of prior AIDS diagnosis or prior low nadir CD4 count did not improve performance characteristics, suggesting that the traditional risk factors may outweigh markers of HIV disease in identifying PWH with lung cancer.
The use of LCS in PWH has been hampered by concern that high rates of prior pulmonary infections in this population could lead to a high false-positive rate. This concern is unsupported by current published literature in younger PWH.41–43 Since the NLST, in which the false-positive rate was 26.6%, several measures to reduce the false-positive rate have been developed.11 This includes the American College of Radiology’s classification system, Lung-RADS, which, when applied retrospectively to the NLST cohort, reduced the false-positive rate to 12.8%.44 In our study, using the balanced screening criteria identified in the WIHS, only 14% of non-cases would have undergone LCS. In the MACS, balanced screening criteria would have resulted in screening 18% of non-cases. These findings demonstrate that adjusting LCS criteria to capture at-risk PWH might not substantially decrease specificity. For example, for 1000 WIHS women without cancer, the balanced criteria we identified would have resulted in 60 more women undergoing LCS than the 2021 recommendations.
Our study has several limitations. First, although we utilized two large cohorts of PWH, the total number of lung cancer cases was small, limiting our ability to make specific recommendations about criteria cutoffs in similar populations. Second, differences in demographic characteristics between WIHS and MACS participants limited our ability to make sex-based comparisons. The MACS and WIHS may not reflect the larger population of US PWH, which is more racially and socioeconomically heterogeneous– impacting the generalizability of our findings. Third, as the cohorts date back to 1984 and lung cancer screening was not recommended until 2014, most cases, if not all, were detected through approaches other than screening. Finally, using Youden’s J to identify criteria at which sensitivity and specificity are balanced assumes that sensitivity and specificity are equally weighted clinically. We elected to present this information primarily to illustrate multiple points on the ROC curve for the criteria. However, this approach has been used to identify cut-points for other screening measures.45–48 Though our study did not support the inclusion of nadir CD4 count or prior AIDS in addition to LCS criteria, it was not powered to do so – risk prediction modeling may ultimately demonstrate a utility for including markers of HIV disease.
CONCLUSION
The 2021 USPSTF LCS criteria demonstrated improved sensitivity compared to the 2013 recommendations in two longitudinal cohorts of PWH, as 44% of women and 63% of men with lung cancer met the 2021 eligibility criteria at time of diagnosis compared to 22% of women and 32% of men when the 2013 criteria were applied. However, the 2021 criteria still under-selected women with HIV. When alternative thresholds were explored, we found that decreasing the age in both sexes and the pack-years and increasing the time since quitting in women would lead to increased identification of lung cancers but with some loss of specificity. Specifically, the highest sensitivities were achieved if PWH were screened at a younger age cutoff (40–80 for women, 43–80 for men), with a lower pack-year history (≥15 for women, ≥19 for men), and among women, increasing the time since quit to ≤22 years before screening from ≤15 years. This study demonstrates the increased applicability of the 2021 LCS criteria in PWH and highlights the need for further evaluation of risk prediction modeling incorporating sex in identifying PWH with lung cancer who would benefit from LCS.
Supplementary Material
Acknowledgments:
The authors gratefully acknowledge the contributions of the study participants and dedication of the staff at the MWCCS sites. In addition, we would like to acknowledge the National Program of Cancer Registries of the Centers for Disease Control and Prevention (CDC) for the funds that helped support the collection and availability of the cancer registry data and thank the following state cancer registries for their help: AL, CA, FL, GA, IL, MD, MS, NY, NC, PA, and VA. The authors assume full responsibility for analyses and interpretations of these data.
Conflicts of Interest and Sources of Funding:
Data in this manuscript were collected by the MACS/WIHS Combined Cohort Study (MWCCS). The contents of this publication are solely the authors’ responsibility and do not represent the official views of the US Government, National Institutes of Health (NIH) or Department of Veterans Affairs. MWCCS (Principal Investigators): Atlanta CRS (Ighovwerha Ofotokun, Anandi Sheth, and Gina Wingood), U01-HL146241; Baltimore CRS (Todd Brown and Joseph Margolick), U01-HL146201; Bronx CRS (Kathryn Anastos and Anjali Sharma), U01-HL146204; Brooklyn CRS (Deborah Gustafson and Tracey Wilson), U01-HL146202; Data Analysis and Coordination Center (Gypsyamber D’Souza, Stephen Gange and Elizabeth Golub), U01-HL146193; Chicago-Cook County CRS (Mardge Cohen and Audrey French), U01-HL146245; Chicago-Northwestern CRS (Steven Wolinsky), U01-HL146240; Northern California CRS (Bradley Aouizerat, Jennifer Price, and Phyllis Tien), U01-HL146242; Los Angeles CRS (Roger Detels and Matthew Mimiaga), U01-HL146333; Metropolitan Washington CRS (Seble Kassaye and Daniel Merenstein), U01-HL146205; Miami CRS (Maria Alcaide, Margaret Fischl, and Deborah Jones), U01-HL146203; Pittsburgh CRS (Jeremy Martinson and Charles Rinaldo), U01-HL146208; UAB-MS CRS (Mirjam-Colette Kempf, Jodie Dionne-Odom, and Deborah Konkle-Parker), U01-HL146192; UNC CRS (Adaora Adimora), U01-HL146194. The MWCCS is funded primarily by the National Heart, Lung, and Blood Institute (NHLBI), with additional co-funding from the Eunice Kennedy Shriver National Institute Of Child Health & Human Development (NICHD), National Institute On Aging (NIA), National Institute Of Dental & Craniofacial Research (NIDCR), National Institute Of Allergy And Infectious Diseases (NIAID), National Institute Of Neurological Disorders And Stroke (NINDS), National Institute Of Mental Health (NIMH), National Institute On Drug Abuse (NIDA), National Institute Of Nursing Research (NINR), National Cancer Institute (NCI), National Institute on Alcohol Abuse and Alcoholism (NIAAA), National Institute on Deafness and Other Communication Disorders (NIDCD), National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK), National Institute on Minority Health and Health Disparities (NIMHD), and in coordination and alignment with the research priorities of the National Institutes of Health, Office of AIDS Research (OAR). MWCCS data collection is also supported by UL1-TR000004 (UCSF CTSA), UL1-TR003098 (JHU ICTR), UL1-TR001881 (UCLA CTSI), P30-AI-050409 (Atlanta CFAR), P30-AI-073961 (Miami CFAR), P30-AI-050410 (UNC CFAR), P30-AI-027767 (UAB CFAR), and P30-MH-116867 (Miami CHARM).
Support for SAS was provided by Program in Multidisciplinary Training in Pulmonary Diseases at The University of North Carolina at Chapel Hill [T32-HL-007106]. LH was partly supported by the National Institutes of Health [K24 HL087713]. This material is also the result of work supported with resources and the use of facilities at the Minneapolis Veterans Affairs Medical Center.
AAA has received personal funds for consulting from Merck, Gilead, and Viiv; her institution has received funds from Merck and Gilead for her research. KMK reports personal funds from Nuvaira and Allergan outside of the presented work. All other authors report no conflicts of interest.
Footnotes
These results were presented at the Conference of Retroviruses and Opportunistic Infections (CROI) in Seattle, WA, in March 2019.
REFERENCES
- 1.van Sighem AI, Gras LA, et al. Life expectancy of recently diagnosed asymptomatic HIV-infected patients approaches that of uninfected individuals. AIDS. 2010;24:1527–1535. [DOI] [PubMed] [Google Scholar]
- 2.Yanik EL, Katki HA, Engels EA. Cancer risk among the HIV-infected elderly in the United States. AIDS. 2016;30:1663–1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vandenhende MA, Roussillon C, Henard S, et al. Cancer-Related Causes of Death among HIV-Infected Patients in France in 2010: Evolution since 2000. PLoS One. 2015;10:e0129550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Reddy KP, Kong CY, Hyle EP, et al. Lung Cancer Mortality Associated With Smoking and Smoking Cessation Among People Living With HIV in the United States. JAMA Intern Med. 2017;177:1613–1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kirk GD, Merlo C, O’ Driscoll P, et al. HIV infection is associated with an increased risk for lung cancer, independent of smoking. Clin Infect Dis. 2007;45:103–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sigel K, Wisnivesky J, Gordon K, et al. HIV as an independent risk factor for incident lung cancer. AIDS. 2012;26:1017–1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shiels MS, Cole SR, Mehta SH, et al. Lung cancer incidence and mortality among HIV-infected and HIV-uninfected injection drug users. J Acquir Immune Defic Syndr. 2010;55:510–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Marcus JL, Leyden WA, Chao CR, et al. Immunodeficiency, AIDS-related pneumonia, and risk of lung cancer among HIV-infected individuals. AIDS. 2017;31:989–993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.D’Jaen GA, Pantanowitz L, Bower M, et al. Human immunodeficiency virus-associated primary lung cancer in the era of highly active antiretroviral therapy: a multi-institutional collaboration. Clin Lung Cancer. 2010;11:396–404. [DOI] [PubMed] [Google Scholar]
- 10.Shiels MS, Althoff KN, Pfeiffer RM, et al. HIV Infection, Immunosuppression, and Age at Diagnosis of Non-AIDS-Defining Cancers. Clin Infect Dis. 2017;64:468–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.National Lung Screening Trial Research Team, Aberle DR, Adams AM, et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med. 2011;365:395–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Krist AH, Davidson KW, Mangione CM, et al. Screening for lung cancer: US preventive services task force recommendation statement. JAMA. 2021;325:962–970. [DOI] [PubMed] [Google Scholar]
- 13.Meza R, Jeon J, Toumazis I, et al. Evaluation of the Benefits and Harms of Lung Cancer Screening With Low-Dose Computed Tomography: Modeling Study for the US Preventive Services Task Force. JAMA. 2021;325:988–997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Adimora AA, Ramirez C, Benning L, et al. Cohort Profile: The Women’s Interagency HIV Study (WIHS). Int J Epidemiol. 2018;47:393–394i. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kaslow RA, Ostrow DG, Detels R, et al. The Multicenter AIDS Cohort Study: rationale, organization, and selected characteristics of the participants. Am J Epidemiol. 1987;126:310–318. [DOI] [PubMed] [Google Scholar]
- 16.Fritz A, Percy C, Jack A, et al. , eds. International Classification of Diseases for Oncology. Third ed. Geneva: World Health Organization; 2013. [Google Scholar]
- 17.Youden WJ. Index for rating diagnostic tests. Cancer. 1950;3:32–35. [DOI] [PubMed] [Google Scholar]
- 18.Ruhl J, Callaghan C, Hurlbut A, et al. Summary Stage 2018: Codes and Coding Instructions. Bethesda: National Cancer Institute; 2018. [Google Scholar]
- 19.Kong CY, Sigel K, Criss SD, et al. Benefits and harms of lung cancer screening in HIV-infected individuals with CD4+ cell count at least 500 cells/μl. AIDS. 2018;32:1333–1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Makinson A, Tron L, Grabar S, et al. Potential lung cancer screening outcomes using different age and smoking thresholds in the ANRS-CO4 French Hospital Database on HIV cohort. HIV Med. 2020;21:180–188. [DOI] [PubMed] [Google Scholar]
- 21.The American Cancer Society medical and editorial content team. Key Statistics for Lung Cancer. American Cancer Society, January 12, 2021. Available at: https://www.cancer.org/cancer/lung-cancer/about/key-statistics.html. [Google Scholar]
- 22.Lambert AA, Merlo CA, Kirk GD. Human immunodeficiency virus-associated lung malignancies. Clin Chest Med. 2013;34:255–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Howlader N, Noone A, Krapcho M, et al. , eds. SEER Cancer Statistics Review, 1975–2016, National Cancer Institute. Bethesda: National Cancer Institute; 2019. [Google Scholar]
- 24.Kirk GD, Merlo CA, Lung HIV Study. HIV infection in the etiology of lung cancer: confounding, causality, and consequences. Proc Am Thorac Soc. 2011;8:326–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Slatore CG, Baumann C, Pappas M, et al. Smoking behaviors among patients receiving computed tomography for lung cancer screening. Systematic review in support of the U.S. preventive services task force. Ann Am Thorac Soc. 2014;11:619–627. [DOI] [PubMed] [Google Scholar]
- 26.Tammemägi MC, Berg CD, Riley TL, et al. Impact of lung cancer screening results on smoking cessation. J Natl Cancer Inst. 2014;106:dju084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Reese TJ, Schlechter CR, Potter LN, et al. Evaluation of Revised US Preventive Services Task Force Lung Cancer Screening Guideline Among Women and Racial/Ethnic Minority Populations. JAMA Netw Open. 2021;4:e2033769–e2033769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Haiman CA, Stram DO, Wilkens LR, et al. Ethnic and racial differences in the smoking-related risk of lung cancer. New Engl J Med. 2006;354:333–342. [DOI] [PubMed] [Google Scholar]
- 29.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7–30. [DOI] [PubMed] [Google Scholar]
- 30.Jemal A, Miller KD, Ma J, et al. Higher Lung Cancer Incidence in Young Women Than Young Men in the United States. N Engl J Med. 2018;378:1999–2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Haddad DN, Sandler KL, Henderson LM, et al. Disparities in Lung Cancer Screening: A Review. Ann Am Thorac Soc. 2020;17:399–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Centers for Disease Control and Prevention. HIV surveillance report, 2018 (Updated); vol. 31. Centers for Disease Control and Prevention, 2020. Available at: https://www.cdc.gov/hiv/pdf/library/reports/surveillance/cdc-hiv-surveillance-report-2018-updated-vol-31.pdf. [Google Scholar]
- 33.Bradley ELP, Williams AM, Green S, et al. Disparities in Incidence of Human Immunodeficiency Virus Infection Among Black and White Women - United States, 2010–2016. MMWR Morb Mortal Wkly Rep. 2019;68:416–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Freedman ND, Leitzmann MF, Hollenbeck AR, et al. Cigarette smoking and subsequent risk of lung cancer in men and women: analysis of a prospective cohort study. Lancet Oncol. 2008;9:649–656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lewis DR, Check DP, Caporaso NE, et al. US lung cancer trends by histologic type. Cancer. 2014;120:2883–2892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Barrera-Rodriguez R, Morales-Fuentes J. Lung cancer in women. Lung Cancer (Auckl). 2012;3:79–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pauk N, Kubik A, Zatloukal P, et al. Lung cancer in women. Lung Cancer. 2005;48:1–9. [DOI] [PubMed] [Google Scholar]
- 38.Silverberg MJ, Chao C, Leyden WA, et al. HIV infection, immunodeficiency, viral replication, and the risk of cancer. Cancer Epidemiol Biomarkers Prev. 2011;20:2551–2559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Reekie J, Kosa C, Engsig F, et al. Relationship between current level of immunodeficiency and non-acquired immunodeficiency syndrome-defining malignancies. Cancer. 2010;116:5306–5315. [DOI] [PubMed] [Google Scholar]
- 40.Chaturvedi AK, Pfeiffer RM, Chang L, et al. Elevated risk of lung cancer among people with AIDS. AIDS. 2007;21:207–213. [DOI] [PubMed] [Google Scholar]
- 41.Makinson A, Eymard-Duvernay S, Raffi F, et al. Feasibility and efficacy of early lung cancer diagnosis with chest computed tomography in HIV-infected smokers. AIDS. 2016;30:573–582. [DOI] [PubMed] [Google Scholar]
- 42.Hulbert A, Hooker CM, Keruly JC, et al. Prospective CT screening for lung cancer in a high-risk population: HIV-positive smokers. J Thorac Oncol. 2014;9:752–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sigel K, Wisnivesky J, Shahrir S, et al. Findings in asymptomatic HIV-infected patients undergoing chest computed tomography testing: implications for lung cancer screening. AIDS. 2014;28:1007–1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pinsky PF, Gierada DS, Black W, et al. Performance of Lung-RADS in the National Lung Screening Trial: a retrospective assessment. Ann Intern Med. 2015;162:485–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kami-Onaga K, Tateyama M, Kinjo T, et al. Comparison of two screening tests for HIV-Associated Neurocognitive Disorder suspected Japanese patients with respect to cART usage. PLoS One. 2018;13:e0199106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dong B, Wang Z, Wang HJ, et al. Improving Hypertension Screening in Childhood Using Modified Blood Pressure to Height Ratio. J Clin Hypertens (Greenwich). 2016;18:557–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wannhoff A, Hov JR, Folseraas T, et al. FUT2 and FUT3 genotype determines CA19–9 cutoff values for detection of cholangiocarcinoma in patients with primary sclerosing cholangitis. J Hepatol. 2013;59:1278–1284. [DOI] [PubMed] [Google Scholar]
- 48.Makarov SN, Noetscher GM, Arum S, et al. Concept of a Radiofrequency Device for Osteopenia/Osteoporosis Screening. Sci Rep. 2020;10:3540. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


