Abstract
Objective:
To assess recent trends in the monitoring of antiretroviral therapy (ART) and detection of ART failure in adult and pediatric HIV clinics.
Methods:
We used data collected from 21 adult and 17 pediatric sites (across 13 and 6 countries/territories, respectively) in the International Epidemiology Databases to Evaluate AIDS – Asia-Pacific cohort. ART failure was defined as viral, immune, or clinical consistent with WHO guidelines.
Results:
A total of 8,567 adults and 6,149 children contributed data. Frequency of CD4 count monitoring declined between 2010-2019 among adult sites (from 1.93 to 1.06 tests/person/year, a 45.1% decline) and pediatric sites (from 2.16 to 0.86 tests/person/year, a 60.2% decline) while rates of viral load monitoring remained relatively stable. The proportion of adult and pediatric treatment failure detected as immune failure declined (from 73.4% to 50.0% and from 45.8% to 23.1%, respectively) while the proportion of failure detected as viral failure increased (from 7.8% to 25.0% and from 45.8% to 76.9%, respectively). The proportion of ART failure detected as clinical failure remained stable among adult and pediatric sites. The largest shifts in ART monitoring and failure type occurred in lower middle-income countries.
Conclusion:
Although viral failure in our Asian cohort now comprises a larger portion of ART failure than in prior years, the diagnostic characteristics of immune and clinical failure, and recommendations on their management, remain important inclusions for regional ART guidelines.
Keywords: HIV, Antiretroviral therapy, Viral load, CD4, Treatment failure, Asia
INTRODUCTION
An important goal of antiretroviral therapy (ART) is the sustained suppression of HIV. Low viral burden substantially reduces the risk of immune and clinical failure,1,2 both advanced states of treatment failure that tend to occur after viral non-suppression and lead to a poor clinical prognosis.3–8 Early detection of viral proliferation allows ART adherence issues to be addressed promptly thereby reducing the likelihood of antiretroviral resistance and retaining second and third line options.4,9–11
Despite providing superior health outcomes, viral load monitoring is more expensive than monitoring for immune or clinical ART failure. CD4 cell count monitoring for detection of immune failure has historically been favoured. However, global access to viral load testing has increased substantially over the past decade due to changes in HIV policy and guidelines, including recommendations to reduce the frequency of CD4 testing, and the decreasing price of viral load monitoring.12 Although this does not appear to have reduced rates of viral non-suppression over time,12 rates of ART failure have declined,13,14 indicating viral failure may comprise a larger portion of treatment failure than in past years. This has important implications for future policy and guidance on the management of ART failure.
The aim of this analysis was to assess recent trends in the monitoring of ART and detection of ART failure in adult and pediatric HIV clinics in Asia.
METHODS
Study population
We used adult and pediatric data from the International Epidemiology Databases to Evaluate AIDS – Asia Pacific (IeDEA-AP) cohort. Both the adult and pediatric IeDEA-AP cohorts have been described elsewhere.15–17 Briefly, the adult IeDEA-AP cohort is a prospective observational database involving 21 sites which began enrolling individuals receiving HIV care at major urban referral centres in 2003. The pediatric IeDEA-AP cohort is also a prospective HIV observational database. It involves 17 major pediatric centres that have been contributing data since 2008. There are seven clinics in the IeDEA-AP network that contribute to both adult and pediatric databases. Adult and pediatric clinics in the IeDEA-AP network generally do not have a strict age restriction. Nevertheless, pediatric sites tend to care for patients <18-years-old and adult clinics tend to care for patients ≥18-years-old. Data collected in the adult and pediatric cohorts includes information on participant demographics, laboratory results, clinical diagnoses, and ART use. Both cohorts perform six-monthly data transfers from clinic sites to the regional data centre at the Kirby Institute. Our study population included participants in the March 2020 IeDEA-AP cohort data transfers who had been using ART for 6 months
Ethical approval
Adult and pediatric IeDEA-AP cohorts have received approval from the human research ethics committees at participating sites, the Kirby Institute, and the co-ordination center at TREAT Asia/amfAR. Participating clinics follow local guidelines and regulations regarding patient consent.
Outcomes and definitions
Rates of viral load and CD4 monitoring were calculated for each participant as the number of measurements in a calendar year over the total follow up time accumulated in that year.
ART failure was defined as viral, immune, or clinical failure in the 6 months leading up to an antiretroviral drug class change or death. First, second and third episodes of ART failure were included in our analyses and individuals could contribute up to one failure for each.
Our definitions of viral, immune, and clinical failure followed recommendations described in recent World Health Organization (WHO) guidelines.18,19 Table S1 describes our definitions in detail. Where an individual had evidence of multiple types of failure during a single episode of failure (i.e., involving one episode of a drug class change or death), priority was given to clinical, immune, then viral failure, in that order. This is because clinical failure typically follows immune failure which typically follows viral failure.3 We also evaluated viral load measurements taken within 6 months of individuals developing immune or clinical failure, and CD4 measurements taken within 6 months of individuals developing clinical failure.
Statistical analysis
We calculated the mean per patient viral load and CD4 monitoring frequency each calendar year, by adult/pediatric site and by country income status. Annual proportions of viral, immune, and clinical ART failure were calculated as the number of viral, immune, or clinical failures divided by the total number of failures in the year. Annual proportions of first, second, and third ART failure were calculated as the number of first, second, and third failures divided by the total number of failures in the year. We evaluated calendar year trends in ART failure proportion, by adult/pediatric site and by country income status. Bivariate linear regression was used to estimate, and calculate the statistical significance of, annual changes in mean viral load and CD4 monitoring frequency, proportion of total failures detected as viral, immune, and clinical failure, and proportion of total failures that were a first, second, and third episode. All statistical analyses were conducted using Stata 16 (Stata Corp., College Station, Texas).
RESULTS
Adult site characteristics
Table S1 shows that among the 21 adult sites, 7 (33.3%), 9 (42.9%) and 5 (23.8%) were situated in lower middle, upper middle and high-income countries, respectively. Eighteen (85.7%) were public facilities and 17 (81.9%) had an academic affiliation. Most (42.9%) sites served an adult patient population of between 1,000-5,000. A total of 8,567 individuals contributed data to our adult analyses.
CD4 and viral load monitoring in adults
Figure 1A and Table S2 show that, among adult sites, the mean rate of viral load monitoring declined from 1.46 tests/adult/year in 2010 to 1.22 tests/adult/year in 2019, a 16.4% decline at 0.02 tests/adult/calendar year (p=0.05). Over the same period, the mean rate of CD4 monitoring declined from 1.93 tests/adult/year in 2010 to 1.06 tests/adult/year in 2019, a 45.1% decline at 0.10 tests/adult/calendar year (p<0.01).
Figure 1.
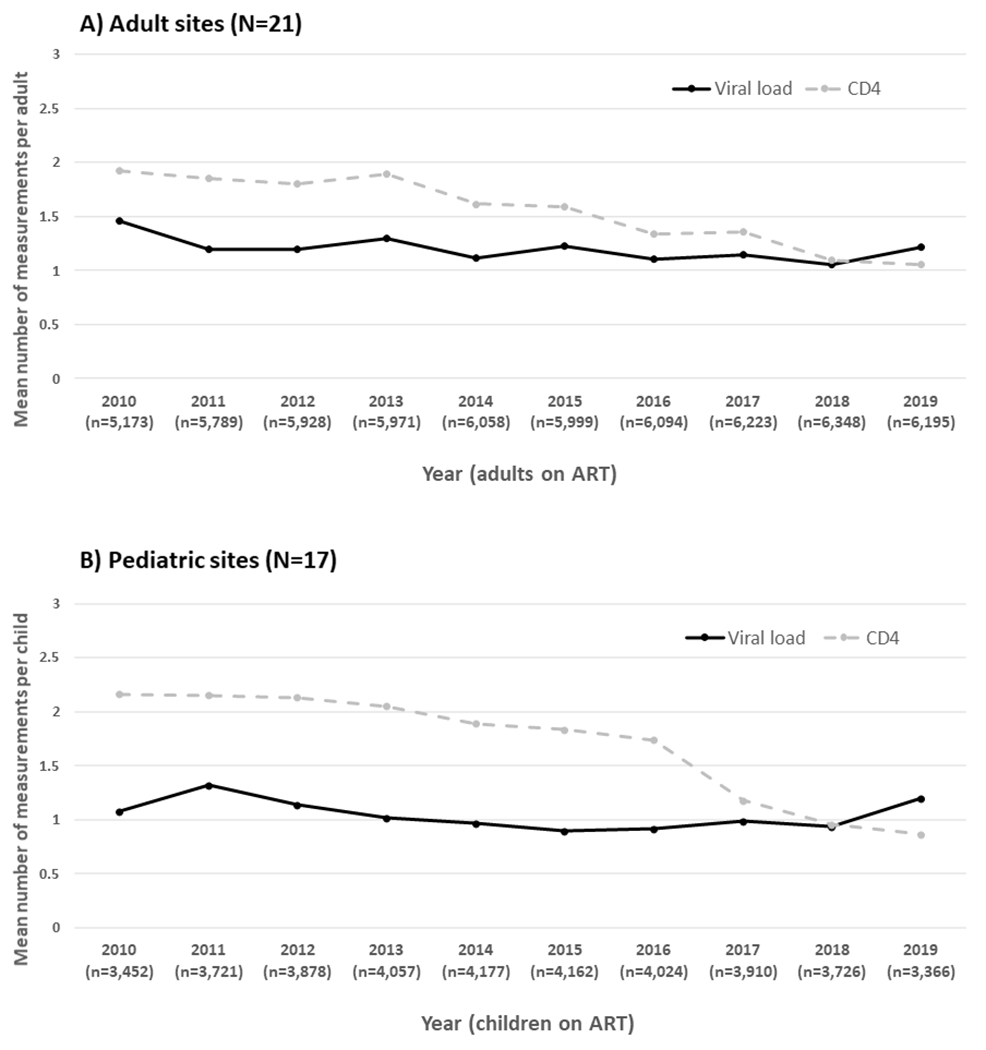
Calendar year trends in HIV viral load and CD4 cell count monitoring
Figure 2A–C and Table S2 show the decline in viral load monitoring rate was driven by a reduction in monitoring among sites situated in upper middle-income countries (18.4% decline at 0.04 tests/adult/calendar year, p=0.01). Declines in CD4 monitoring were significant in sites from each country income status (lower middle, 75.9% decline at 0.18 tests/adult/calendar year, p<0.01; upper middle, 33.3% decline at 0.08 tests/adult/calendar year, p<0.01; high, 17.4% decline at 0.06 tests/adult/calendar year, p=0.01).
Figure 2.
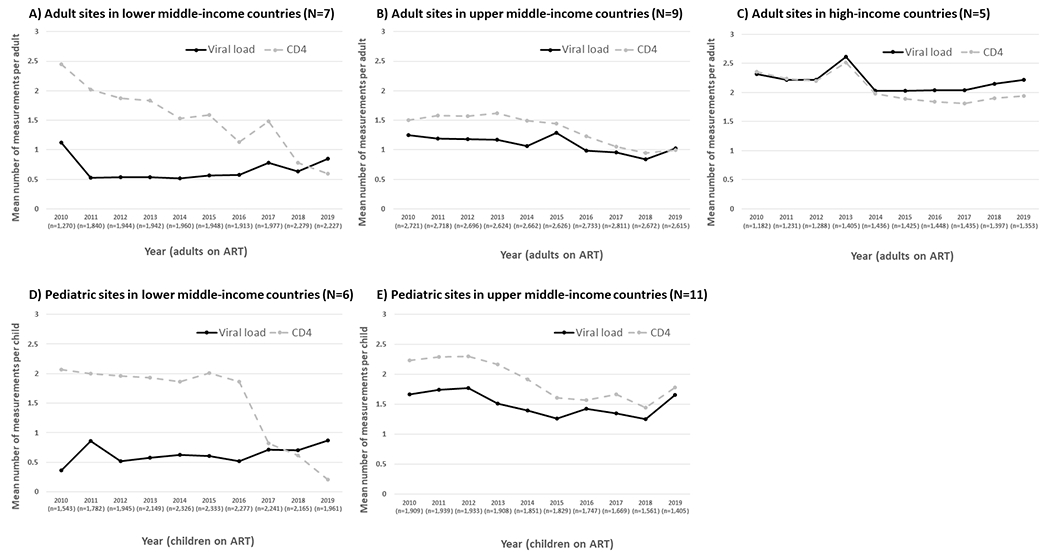
Calendar year trends in HIV viral load and CD4 cell count monitoring by country income status
Antiretroviral treatment failure in adults
Figure 3A and Table S3 show that the proportion of ART failure detected as viral failure among adults increased from 7.8% in 2010 to 25.0% in 2019 at a rate of 1.9% per calendar year (p=0.04). Over the same period, the proportion of ART failure detected as immune failure dropped from 73.4% to 50.0% at a mean decline of 2.32% per calendar year (p=0.04) and the proportion of ART failure detected as clinical failure remained stable between approximately 10-20% each year (p=0.62).
Figure 3.
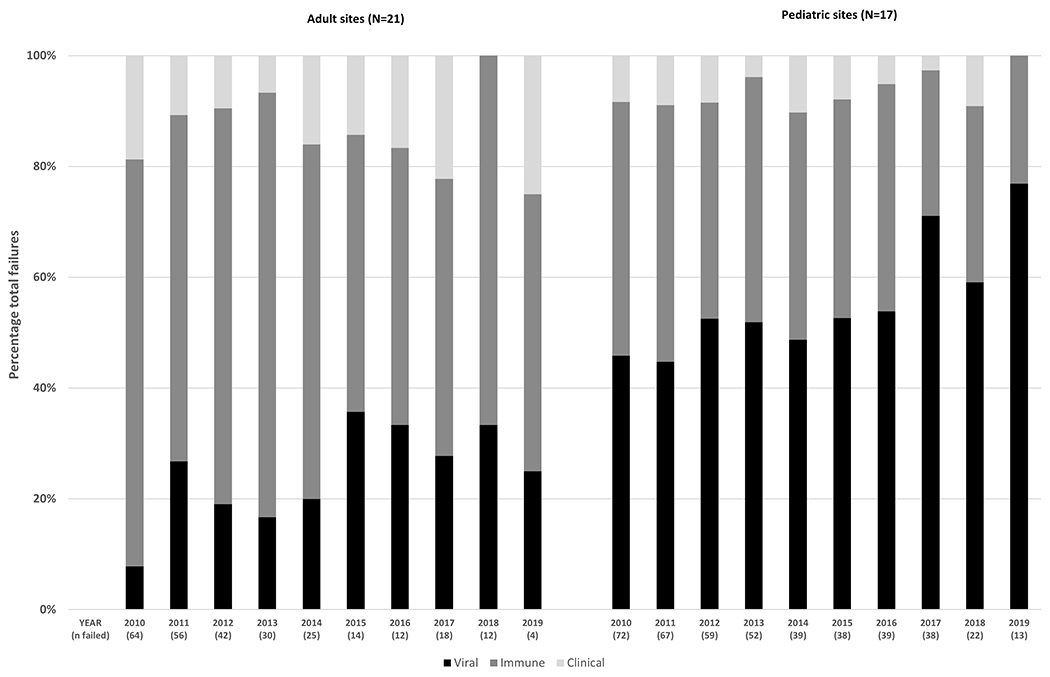
Calendar year trends in the type of antiretroviral therapy failure
Figure 4A–C and Table S3 show the only significant changes in the proportion of ART failure attributable to viral or immune failure occurred among sites situated in lower middle-income countries. Among these sites, the proportion of ART failure detected as viral failure increased from 11.1% in 2010 to 33.3% in 2019 at a mean increase of 3.00% per calendar year (p<0.01). Over the same period, the proportion of ART failure detected as immune failure decreased from 55.6% to 33.3% at a mean decline of 2.72% per calendar year (p=0.06).
Figure 4.
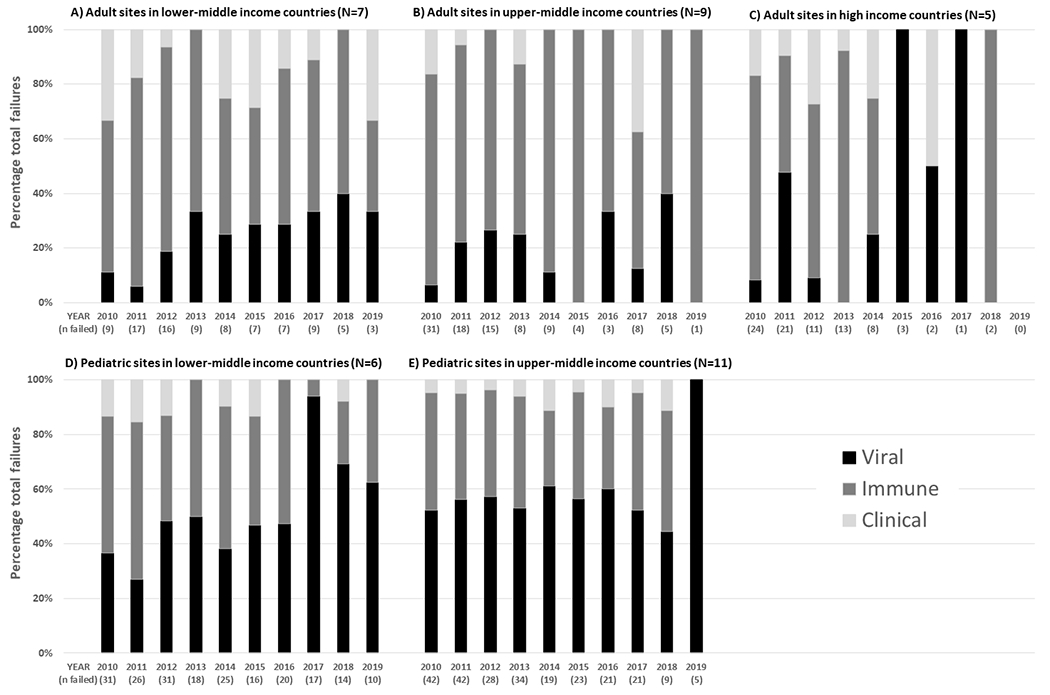
Calendar year trends in the type of antiretroviral therapy failure by country income status
Among 108 adults who developed immune failure and had a recent viral load, 63 (58.3%) had a viral load >1000 copies/ml. In 25 adults who developed clinical failure and had a recent viral load, 7 (28.0%) had a viral load >1000 copies/ml. Among 33 adults who developed clinical failure and had a recent CD4 count, 8 (24.2%) had a CD4 <100 cells/mm3.
Over the duration of the study period, the proportions of first, second, and third ART failure among adults were 80.1%, 16.6%, and 3.3%, respectively. These proportions were similar from year to year and across sites from lower middle, upper middle, and high-income countries (Figure S1, Figure S2, and Table S4). Although, the proportion of second ART failure among sites from high income countries declined by a mean of 3.44% per calendar year between 2010 and 2018 (p<0.01).
Pediatric site characteristics
Table S1 shows that among the 17 pediatric sites, 6 (35.3%) and 11 (64.7%) were situated in lower middle and upper middle-income countries, respectively. No pediatric sites were based in high-income countries. Sixteen (94.1%) sites were public facilities, and all had an academic affiliation. The vast majority (14; 82.4%) of sites served a pediatric patient population of <1,000. A total of 6,149 individuals contributed data to our pediatric analyses.
CD4 and viral load monitoring in children
Figure 1B and Table S2 show that, among paediatric sites, the mean rate of viral load monitoring remained stable at approximately 1 test/child/year between 2010-2019 (mean decline of 0.02 tests/child/calendar year, p=0.24). Over the same period, the mean rate of CD4 monitoring declined from 2.16 tests/child/year in 2010 to 0.86 tests/child/year in 2019, a 60.2% decline at 0.16 tests/child/calendar year (p<0.01). The mean decline in CD4 monitoring was significant among sites from both lower middle and upper middle-income countries (a 90.3% decline at 0.20 tests/child/calendar year, p<0.01 and a 20.2% decline at 0.09 tests/child/calendar year, p<0.01, respectively; Figure 2D–E and Table S2).
Antiretroviral treatment failure in children
Figure 3B and Table S3 show that, between 2010-2019 among pediatric sites, the proportion of ART failure detected as viral failure increased from 45.8% to 76.9% at a mean increase of 2.92% per calendar year (p<0.01). Over the same period, the proportion of ART failure detected as immune failure dropped from 45.8% to 23.1% at a mean decline of 2.31% per calendar year (p<0.01) and the proportion of ART failure detected as clinical failure remained stable between 0-10% (p=0.10).
Figure 4D–E and Table S3 show the largest changes in the proportion of ART failure attributable to viral, immune and clinical failure occurred at sites situated in lower middle-income countries. Among these sites, the proportion of ART failure detected as viral failure increased from 36.7% in 2010 to 62.5% in 2019 at a mean increase of 4.59% per calendar year (p=0.02). Over the same period, the proportion of ART failure detected as immune failure decreased from 50.0% to 37.5% at a mean decline of 3.17% per calendar year (p=0.06) and the proportion of ART failure detected as clinical failure decreased from 13.3% to 0.0% at a mean decline of 1.42% per calendar year (p=0.04).
Among 98 children who developed immune failure and had a recent viral load, 85 (86.7%) had a viral load >1000 copies/ml. In 15 children who developed clinical failure and had a recent viral load, 12 (80.0%) had a viral load >1000 copies/ml. Among 27 children who developed clinical failure and had a recent CD4 measurement, 12 (44.4%) had a CD4 level consistent with immune failure.
Over the duration of the study period, the proportions of first, second, and third ART failure among children were 77.9%, 17.8%, and 4.3%, respectively. These proportions were similar from year to year and across sites from lower middle and upper middle-income countries (Figure S1, Figure S2, and Table S4).
DISCUSSION
Rates of CD4 monitoring declined considerably after 2010 among adult and pediatric sites in our Asia-Pacific HIV cohort, while rates of viral load monitoring remained stable. In line with these changes, and consistent with viral load testing being the most sensitive method of diagnosing ART failure, the proportions of adult and pediatric treatment failure detected as immune failure declined substantially while the proportions of treatment failure detected as viral failure increased. The proportion of ART failure detected as clinical failure remained stable among adult and pediatric sites. The largest shifts in ART monitoring and failure detection occurred among sites situated in lower middle-income countries.
HIV guidelines now firmly recommend initiation of ART regardless of CD4 cell count, and prioritisation of viral load monitoring over CD4 or clinical monitoring.18,19Early ART leads to better health outcomes than delayed ART.20 Strong evidence from the Asia-Pacific region shows that regular CD4 monitoring is unnecessary among adults and children with viral suppression on ART due to their very low risk of immune or clinical treatment failure.1,2 It was expected that we would see a decline in CD4 monitoring over time in our analysis. However, we also expected an increase in viral load monitoring frequency. Instead, rates remained stable over time among both adult and pediatric sites. In fact, among adult sites in upper middle-income countries viral load monitoring rates declined slightly. This could be representative of more effective ART regimens,19 and a maturing HIV patient population21 that has become comfortable with ART overtime resulting in less frequent laboratory monitoring. Although, difficulty accessing affordable viral load testing materials and technology remains a significant issue in some settings.22
Despite adult and pediatric sites having an overall decrease in the proportion of ART failure detected as immune failure and an increase in the proportion of ART failure detected as viral failure, both mainly attributable to changes in lower middle-income countries, the overall proportion of ART failure detected as clinical failure remained relatively stable. Further investigation of this finding may be warranted to ensure HIV treatment centres in the region are not oversimplifying ART laboratory monitoring in response to recent guideline changes encouraging greater efficiency.18,19,23
There are several limitations to this analysis. IeDEA-AP represents only a sample of HIV treatment clinics in the Asia-Pacific. We acknowledge that there is substantial intra-regional and inter-ethnic variation across the region which may limit the generalizability of our findings. Our data are from HIV referral centres where patient characteristics may not be representative of the national HIV programmes within which the referral centres operate. The declining rate of ART failure over time among the IeDEA-AP cohort13 meant our proportion of failure calculations in later years of the study period tended to be impeded by a low denominator.
Ideally, all ART failures would be detected at the stage of viral failure so that the adverse prognoses associated with immune and clinical could be avoided. This study indicates progress is being made toward that vision in the Asia-Pacific region, particularly among the pediatric HIV population. Nevertheless, the diagnostic characteristics of immune and clinical failure, and recommendations on their management, remain key inclusions for regional ART guidelines.
Supplementary Material
Table 1.
Site characteristics(Adult N=21, Pediatric N=17)
| Characteristic | Adult n (%N) | Pediatric n (%N) | |
|---|---|---|---|
| Country income status* | Lower-middle | 7 (33.3) | 6 (35.3) |
| Upper-middle | 9 (42.9) | 11 (64.7) | |
| High | 5 (23.8) | 0 (0.0) | |
| Public/Private | Public | 18 (85.7) | 16 (94.1) |
| Private | 3 (14.3) | 1 (5.9) | |
| Academic affiliation | Yes | 17 (81.0) | 17 (100.0) |
| No | 4 (19.0) | 0 (0.0) | |
| Patient load | <1,000 | 5 (23.8) | 14 (82.4) |
| 1,000-5,000 | 9 (42.9) | 3 (17.6) | |
| >5,000 | 7 (33.3) | 0 (0.0) | |
| Country | Cambodia | 1 (4.8) | 1 (5.9) |
| China | 1 (4.8) | 0 (0.0) | |
| Hong Kong | 1 (4.8) | 0 (0.0) | |
| India | 3 (14.3) | 2 (11.8) | |
| Indonesia | 2 (9.5) | 2 (11.8) | |
| Japan | 1 (4.8) | 0 (0.0) | |
| Malaysia | 2 (9.5) | 4 (23.5) | |
| Philippines | 1 (4.8) | 0 (0.0) | |
| Singapore | 1 (4.8) | 0 (0.0) | |
| South Korea | 1 (4.8) | 0 (0.0) | |
| Taiwan | 1 (4.8) | 0 (0.0) | |
| Thailand | 4 (19.0) | 5 (29.4) | |
| Vietnam | 2 (9.5) | 3 (17.6) | |
As defined in 2020 by The World Bank24
Funding
The International Epidemiology Databases to Evaluate AIDS – Asia-Pacific adult and pediatric cohorts are initiatives of TREAT Asia, a program of amfAR, The Foundation for AIDS Research, with support from the U.S. National Institutes of Health’s National Institute of Allergy and Infectious Diseases, the Eunice Kennedy Shriver National Institute of Child Health and Human Development, the National Cancer Institute, the National Institute of Mental Health, the National Institute on Drug Abuse, the National Heart, Lung, and Blood Institute, the National Institute on Alcohol Abuse and Alcoholism, the National Institute of Diabetes and Digestive and Kidney Diseases, and the Fogarty International Center, as part of the International Epidemiology Databases to Evaluate AIDS (IeDEA; U01AI069907). The Kirby Institute is funded by the Australian Government Department of Health and Ageing, and is affiliated with the Faculty of Medicine, UNSW Sydney. The content of this publication is solely the responsibility of the authors and does not necessarily represent the official views of any of the governments or institutions mentioned above.
Competing interests
DCB has received research funding from Gilead Sciences and is supported by a National Health and Medical Research Council Early Career Fellowship (APP1140503); All other authors report no potential competing interests.
Site investigators and study teams:
The International Epidemiology Databases to Evaluate AIDS – Asia-Pacific adult cohort:
PS Ly, V Khol, National Center for HIV/AIDS, Dermatology & STDs, Phnom Penh, Cambodia; FJ Zhang, HX Zhao, N Han, Beijing Ditan Hospital, Capital Medical University, Beijing, China; MP Lee, PCK Li, TS Kwong, TH Li, Queen Elizabeth Hospital, Hong Kong SAR, China; N Kumarasamy, C Ezhilarasi, Chennai Antiviral Research and Treatment Clinical Research Site (CART CRS), VHS-Infectious Diseases Medical Centre, VHS, Chennai, India; S Pujari, K Joshi, S Gaikwad, A Chitalikar, Institute of Infectious Diseases, Pune, India; IKA Somia, TP Merati, AAS Sawitri, F Yuliana, Faculty of Medicine Udayana University & Sanglah Hospital, Bali, Indonesia; E Yunihastuti, A Widhani, S Maria, TH Karjadi, Faculty of Medicine Universitas Indonesia - Dr. Cipto Mangunkusumo General Hospital, Jakarta, Indonesia; J Tanuma, S Oka, T Nishijima, National Center for Global Health and Medicine, Tokyo, Japan; JY Choi, Na S, JM Kim, Division of Infectious Diseases, Department of Internal Medicine, Yonsei University College of Medicine, Seoul, South Korea; YM Gani, NB Rudi, Hospital Sungai Buloh, Sungai Buloh, Malaysia; I Azwa, A Kamarulzaman, SF Syed Omar, S Ponnampalavanar, University Malaya Medical Centre, Kuala Lumpur, Malaysia; R Ditangco, MK Pasayan, ML Mationg, Research Institute for Tropical Medicine, Muntinlupa City, Philippines; YJ Chan, WW Ku, E Ke, PC Wu, Taipei Veterans General Hospital, Taipei, Taiwan; OT Ng, PL Lim, LS Lee, T Yap, Tan Tock Seng Hospital, Singapore; A Avihingsanon, S Gatechompol, P Phanuphak, C Phadungphon, HIV-NAT/Thai Red Cross AIDS Research Centre, Bangkok, Thailand; S Kiertiburanakul, A Phuphuakrat, L Chumla, N Sanmeema, Faculty of Medicine Ramathibodi Hospital, Mahidol University, Bangkok, Thailand; R Chaiwarith, T Sirisanthana, J Praparattanapan, K Nuket, Research Institute for Health Sciences, Chiang Mai, Thailand; S Khuwuwan, P Payoong, P Kantipong, P Kambua, Chiangrai Prachanukroh Hospital, Chiang Rai, Thailand; TN Pham, KV Nguyen, DTH Nguyen, DT Nguyen, National Hospital for Tropical Diseases, Hanoi, Vietnam; CD Do, AV Ngo, LT Nguyen, Bach Mai Hospital, Hanoi, Vietnam; AH Sohn, JL Ross, B Petersen, TREAT Asia, amfAR - The Foundation for AIDS Research, Bangkok, Thailand; MG Law, A Jiamsakul, D Rupasinghe, The Kirby Institute, UNSW Sydney, NSW, Australia.
The International Epidemiology Databases to Evaluate AIDS – Asia-Pacific pediatric cohort:
PS Ly, V Khol, National Centre for HIV/AIDS, Dermatology and STDs, Phnom Penh, Cambodia; J Tucker, New Hope for Cambodian Children, Phnom Penh, Cambodia; N Kumarasamy, E Chandrasekaran, Chennai Antiviral Research and Treatment Clinical Research Site (CART CRS), VHS-Infectious Diseases Medical Centre, VHS, Chennai, India; A Kinikar, V Mave, S Nimkar, I Marbaniang, BJ Medical College and Sassoon General Hospitals, Maharashtra, India; DK Wati, D Vedaswari, IB Ramajaya, Sanglah Hospital, Udayana University, Bali, Indonesia; N Kurniati, D Muktiarti, Cipto Mangunkusumo – Faculty of Medicine Universitas Indonesia, Jakarta, Indonesia; SM Fong, M Lim, F Daut, Hospital Likas, Kota Kinabalu, Malaysia; NK Nik Yusoff, P Mohamad, Hospital Raja Perempuan Zainab II, Kelantan, Malaysia; TJ Mohamed, MR Drawis, Department of Pediatrics, Women and Children Hospital Kuala Lumpur, Kuala Lumpur, Malaysia; R Nallusamy, KC Chan, Penang Hospital, Penang, Malaysia; T Sudjaritruk, V Sirisanthana, L Aurpibul, Department of Pediatrics, Faculty of Medicine, and Research Institute for Health Sciences, Chiang Mai University, Chiang Mai, Thailand; P Ounchanum, R Hansudewechakul, S Denjanta, A Kongphonoi, Chiangrai Prachanukroh Hospital, Chiang Rai, Thailand; P Lumbiganon, P Kosalaraksa, P Tharnprisan, T Udomphanit, Division of Infectious Diseases, Department of Pediatrics, Faculty of Medicine, Khon Kaen University, Khon Kaen, Thailand; G Jourdain, PHPT-IRD UMI 174 (Institut de recherche pour le développement and Chiang Mai University), Chiang Mai, Thailand; T Puthanakit, S Anugulruengkit, W Jantarabenjakul, R Nadsasarn, Department of Pediatrics and Center of Excellence for Pediatric Infectious Diseases and Vaccines, Faculty of Medicine, Chulalongkorn University, Bangkok, Thailand; K Chokephaibulkit, K Lapphra, W Phongsamart, S Sricharoenchai, Department of Pediatrics, Faculty of Medicine Siriraj Hospital, Mahidol University, Bangkok, Thailand; QT Du, KH Truong, CH Nguyen, Children’s Hospital 1, Ho Chi Minh City, Vietnam; VC Do, TM Ha, VT An Children’s Hospital 2, Ho Chi Minh City, Vietnam; LV Nguyen, DM Tran, HTT Tran, TTT Giang, National Hospital of Pediatrics, Hanoi, Vietnam; ON Le, Worldwide Orphans Foundation, Ho Chi Minh City, Vietnam; AH Sohn, JL Ross, T Suwanlerk, TREAT Asia/amfAR - The Foundation for AIDS Research, Bangkok, Thailand; MG Law, A Kariminia, The Kirby Institute, UNSW Sydney, NSW, Australia.
Data availability
Data were collected as part of a regional cohort collaboration. The cohort collaboration has data-sharing policies that were approved by the corresponding IRB and specify that both internal and external investigators are subject to a formal process to request access to the data through submission of a concept sheet that adheres to these policies. This study was conducted under these policies, and data will only be available upon request for researchers who meet the criteria for access to confidential data. Interested individuals should contact Boondarika Petersen (tor.nakornsri@treatasia.org).
References
- 1.Ahn JY, Boettiger D, Law M, et al. Implementation and Operational Research: Effects of CD4 Monitoring Frequency on Clinical End Points in Clinically Stable HIV-Infected Patients With Viral Suppression. Journal of acquired immune deficiency syndromes. 2015;69(3):e85–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kosalaraksa P, Boettiger DC, Bunupuradah T, et al. Low Risk of CD4 Decline After Immune Recovery in Human Immunodeficiency Virus-Infected Children With Viral Suppression. J Pediatric Infect Dis Soc. 2017;6(2):173–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fauci AS, Pantaleo G, Stanley S, Weissman D. Immunopathogenic mechanisms of HIV infection. Ann Intern Med. 1996;124(7):654–663. [DOI] [PubMed] [Google Scholar]
- 4.Hosseinipour MC, van Oosterhout JJ, Weigel R, et al. The public health approach to identify antiretroviral therapy failure: high-level nucleoside reverse transcriptase inhibitor resistance among Malawians failing first-line antiretroviral therapy. Aids. 2009;23(9):1127–1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Keiser O, MacPhail P, Boulle A, et al. Accuracy of WHO CD4 cell count criteria for virological failure of antiretroviral therapy. Tropical medicine & international health : TM & IH. 2009;14(10):1220–1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rawizza HE, Chaplin B, Meloni ST, et al. Immunologic criteria are poor predictors of virologic outcome: implications for HIV treatment monitoring in resource-limited settings. Clin Infect Dis. 2011;53(12):1283–1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Reynolds SJ, Nakigozi G, Newell K, et al. Failure of immunologic criteria to appropriately identify antiretroviral treatment failure in Uganda. Aids. 2009;23(6):697–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.van Oosterhout JJ, Brown L, Weigel R, et al. Diagnosis of antiretroviral therapy failure in Malawi: poor performance of clinical and immunological WHO criteria. Tropical medicine & international health : TM & IH. 2009;14(8):856–861. [DOI] [PubMed] [Google Scholar]
- 9.Kumarasamy N, Madhavan V, Venkatesh KK, et al. High frequency of clinically significant mutations after first-line generic highly active antiretroviral therapy failure: implications for second-line options in resource-limited settings. Clin Infect Dis. 2009;49(2):306–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Marconi VC, Sunpath H, Lu Z, et al. Prevalence of HIV-1 drug resistance after failure of a first highly active antiretroviral therapy regimen in KwaZulu Natal, South Africa. Clin Infect Dis. 2008;46(10):1589–1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wallis CL, Mellors JW, Venter WD, Sanne I, Stevens W. Varied patterns of HIV-1 drug resistance on failing first-line antiretroviral therapy in South Africa. Journal of acquired immune deficiency syndromes. 2010;53(4):480–484. [DOI] [PubMed] [Google Scholar]
- 12.Zaniewski E, Dao Ostinelli CH, Chammartin F, et al. Trends in CD4 and viral load testing 2005 to 2018: multi-cohort study of people living with HIV in Southern Africa. Journal of the International AIDS Society. 2020;23(7):e25546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Boettiger DC, Kerr S, Ditangco R, et al. Trends in First-Line Antiretroviral Therapy in Asia: Results from the TREAT Asia HIV Observational Database. PloS one. 2014;9(9):e106525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.EuroCoord. Time to Switch to Second-line Antiretroviral Therapy in Children With Human Immunodeficiency Virus in Europe and Thailand. Clin Infect Dis. 2018;66(4):594–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.TREAT Asia HIV Observational Database Collaborators. A Decade of Combination Antiretroviral Treatment in Asia: The TREAT Asia HIV Observational Database Cohort. AIDS Res Hum Retroviruses. 2016;32(8):772–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhou J, Kumarasamy N, Ditangco R, et al. The TREAT Asia HIV Observational Database: baseline and retrospective data. Journal of acquired immune deficiency syndromes. 2005;38(2):174–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kariminia A, Chokephaibulkit K, Pang J, et al. Cohort profile: the TREAT Asia pediatric HIV observational database. Int J Epidemiol. 2011;40(1):15–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.WHO ART Guidelines Committee. Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection: recommendations for a public health approach – June 2013. http://apps.who.int/iris/bitstream/10665/85321/1/9789241505727_eng.pdf?ua=1. Accessed 03 Jan 2014. [PubMed]
- 19.WHO ART Guidelines Committee. Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection: recommendations for a public health approach – June 2016. https://apps.who.int/iris/bitstream/handle/10665/208825/9789241549684_eng.pdf?sequence=1. Accessed 16 August 2020.
- 20.Group ISS, Lundgren JD, Babiker AG, et al. Initiation of Antiretroviral Therapy in Early Asymptomatic HIV Infection. N Engl J Med. 2015;373(9):795–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Puhr R, Kumarasamy N, Ly PS, et al. HIV and Aging: Demographic Change in the Asia-Pacific Region. Journal of acquired immune deficiency syndromes. 2017;74(5):e146–e148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lecher SL, Fonjungo P, Ellenberger D, et al. HIV Viral Load Monitoring Among Patients Receiving Antiretroviral Therapy - Eight Sub-Saharan Africa Countries, 2013-2018. MMWR Morb Mortal Wkly Rep. 2021;70(21):775–778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.WHO. Antiretroviral therapy of HIV infection in infants and children: towards universal access: recommendations for a public health approach - 2010 revision. http://whqlibdoc.who.int/publications/2010/9789241599801_eng.pdf. Accessed 19 Feb 2014, 2014. [PubMed]
- 24.The World Bank. World Bank Country and Lending Groups - Country Classification. https://datahelpdesk.worldbank.org/knowledgebase/articles/906519. Accessed 15 Dec 2020.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data were collected as part of a regional cohort collaboration. The cohort collaboration has data-sharing policies that were approved by the corresponding IRB and specify that both internal and external investigators are subject to a formal process to request access to the data through submission of a concept sheet that adheres to these policies. This study was conducted under these policies, and data will only be available upon request for researchers who meet the criteria for access to confidential data. Interested individuals should contact Boondarika Petersen (tor.nakornsri@treatasia.org).


