Abstract
Background:
It is not definitively known if persons with HIV (PWH) are more likely to be SARS-CoV-2 tested or test positive than persons without HIV (PWoH). We describe SARS-CoV-2 testing and positivity in 6 large geographically and demographically diverse cohorts of PWH and PWoH in the United States.
Setting:
The Corona-Infectious-Virus Epidemiology Team (CIVET) comprises five clinical cohorts within a health system (Kaiser Permanente Northern California, Oakland, CA; Kaiser Permanente Mid-Atlantic States, Rockville, MD; University of North Carolina Health, Chapel Hill, NC; Vanderbilt University Medical Center, Nashville, TN; Veterans Aging Cohort Study) and one interval cohort (MACS/WIHS Combined Cohort Study).
Methods:
We calculated the proportion of patients SARS-CoV-2 tested and the test positivity proportion by HIV status from March 1 to December 31, 2020.
Results:
The cohorts ranged in size from 1,675 to 31,304 PWH and 1,430 to 3,742,604 PWoH. The proportion of PWH who were tested for SARS-CoV-2 (19.6%–40.5% across sites) was significantly higher than PWoH (14.8%–29.4%) in the clinical cohorts. However, among those tested, the proportion of patients with positive SARS-CoV-2 tests was comparable by HIV status; the difference in proportion of SARS-CoV-2 positivity ranged from 4.7% lower to 1.4% higher.
Conclusion:
Although PWH had higher testing proportions compared with PWoH, we did not find evidence of increased positivity in 6 large, diverse populations across the United States. Ongoing monitoring of testing, positivity, and COVID-19 related outcomes in PWH are needed given availability, response, and durability of COVID-19 vaccines; emergence of SARS-CoV-2 variants; and latest therapeutic options.
Keywords: COVID-19, SARS-CoV-2, testing, HIV
INTRODUCTION
The US Centers for Disease Control and Prevention classified persons with HIV (PWH) as a population that may “be at an increased risk for severe illness from the virus that causes COVID-19,” 1 given they may be immunocompromised. As the COVID-19 pandemic rapidly evolved in 2020, the clinical and public health communities depended on case studies and limited published evidence that explored the association between HIV and severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection.2–9 To better understand the epidemiology of COVID-19 among PWH, longer observation windows and larger sample sizes representative of the greater heterogenous PWH population are needed. It is not known if PWH in the United States (US) have different access to SARS-CoV-2 reverse transcription-polymerase chain reaction (RT-PCR) testing or whether the proportions of patients who test positive differs from persons without HIV (PWoH). Characterizing SARS-CoV-2 testing and positivity of the novel coronavirus is the first step to explore questions of COVID-19 severity in the context of HIV.
The Corona-Infectious-Virus Epidemiology Team (CIVET) is comprised of five COVID-19 clinical cohorts within health systems and one established HIV interval cohort. The CIVET collaboration leverages the long-standing research consortium of the North American – AIDS Cohort Collaboration on Research and Design (NA-ACCORD)10 to answer urgent COVID-19 questions among PWH and PWoH. In this first analysis from the CIVET collaboration, we describe SARS-CoV-2 testing and positivity proportions in 6 large geographically and demographically diverse cohorts of PWH and PWoH.
METHODS
Study Population
The CIVET collaboration was assembled from NA-ACCORD participating cohorts who independently initiated the establishment of SARS-CoV-2/COVID-19 cohorts within regional health systems (Kaiser Permanente Northern California, Oakland, California; Kaiser Permanente Mid-Atlantic States, Rockville, Maryland, serving the District of Columbia, Maryland, and northern Virginia; University of North Carolina Health, Chapel Hill, North Carolina; Vanderbilt University Medical Center, Nashville, Tennessee), with an existing national large-scale cohort of PWH and PWoH with access to SARS-CoV-2 testing results (Veterans Aging Cohort Study [VACS]), or within an HIV interval cohort study launching COVID-19 surveys (Multicenter AIDS Cohort Study [MACS] Women’s Interagency HIV Study [WIHS] Combined Cohort Study [MWCCS]) as of March 2020. The overall goals of the CIVET collaboration were to 1) leverage the long-standing partnerships of these collaborators and to answer questions pertinent to the COVID-19 US pandemic; 2) translate clinical and epidemiologic expertise conducting HIV research to the study of SARS-CoV-2; 3) identify the selection biases in identifying individuals with COVID-19 through testing or diagnoses or symptoms (due to changing barriers to testing and care); and 4) share challenges and barriers in accessing COVID-19 data in different electronic health record (EHR) systems by researchers experienced in EHR systems for longitudinal research.
The CIVET collaboration maximized the efficiency of a research collaboration by performing parallel analyses and comparing results to specific scientific questions of interest across the cohorts, which allowed for examination of cohort heterogeneity versus pooling individual level data that would have obscured the impact of care delivery systems. This approach also simplified data use agreements across the cohorts and accelerated combined results. The cohorts were assigned a number (1–6), and results are presented with cohort numbers to protect their anonymity. Each participating cohort was restricted to adults (≥18 years) who were alive as of March 1, 2020 and “in-cohort,” which was operationalized differently for each cohort but based on the construct of individuals who had recently interacted with the health system or interval study (Supplemental Table S1).
Primary exposure and outcomes
We calculated the proportion of patients in-cohort who were SARS-CoV-2 RT-PCR tested and, among those tested with RT-PCR, the proportion that tested positive for SARS-CoV-2, by HIV status in each month over the study period (March 1 to December 31, 2020). The one exception was for cohort 6 where data were only available through September 30, 2020. In all cohorts, HIV status was determined from history of: HIV diagnosis, HIV positive laboratory test result, detectable HIV-1 RNA viral load measurement, or prescription for antiretroviral (ARV) therapy (excluding pre-exposure prophylaxis ARV use) based on inclusion criteria and available data per cohort (Supplemental Table S1). SARS-CoV-2 RT-PCR laboratory test dates and results (positive or negative) were extracted from EHR systems in five of the six cohorts; cohort 6 captured self-reported SARS-CoV-2 testing and results. In all cohorts, patients were classified as “tested” if there was a valid positive or negative (not indeterminate) SARS-CoV-2 test result available during the study period. Patients were classified as SARS-CoV-2 positive at the time of their first positive test result, or SARS-CoV-2 negative at their last negative result. If a patient had both positive and negative results, we prioritized the positive test result as the primary outcome.
Covariates
We examined patient demographics: age (as of March 1, 2020), race/ethnicity, and sex or gender in each cohort. Race/ethnicity and sex/gender categories were defined by data availability in each cohort. Five of the 6 cohorts used patient sex, cohort 1 used “sex or gender defined at last patient visit.” For PWH, we summarized CD4 T-lymphocyte (CD4) cell count (cells/μL), HIV-1 RNA viral load, and most recent ARV classes prescribed to patients.
Statistical Analysis
Aggregated estimates were reported from each cohort. No individual-level data were shared between cohorts. We compared the proportion of patients tested and proportion SARS-CoV-2 RT-PCR positive in each cohort by HIV status during the entire study period. SARS-CoV-2 positivity was estimated as the number of patients who tested positive divided by the total number tested. Given the large sample sizes, we determined a priori that a ≥5 percentage point difference was clinically important. We calculated χ2 p-values for differences in proportions. We also examined the SARS-CoV-2 RT-PCR positivity proportion monthly, by HIV status.
Sensitivity analyses were conducted by adding data captured in select cohorts where there was other available evidence of SARS-CoV-2 testing outside of PCR laboratory test results, such as International Classification of Diseases (ICD)-10 diagnosis codes or self-reported results available in the EHR (Supplementary Table S2). The objective of this sensitivity analysis was to determine the robustness of our definition of SARS-CoV-2 positivity using our estimate of internal health system testing in comparison to internal testing plus potential external testing or symptomatic diagnoses.
RESULTS
Across the 6 cohorts, the total study population included 55,349 PWH, with individual cohort sizes ranging from 1,675 to 31,304 PWH and 1,430 to 3,742,604 PWoH. Overall, PWH were older and more likely to be non-Hispanic Black and male (Table 1). Differences in demographics and HIV clinical factors across cohorts were observed reflecting the diversity of cohorts and the geographic locations of patients included. The majority of PWH had high CD4 count ≥500 (50.7%–68.1% among tested, 45.7%–65.7% among untested) and suppressed HIV-RNA viral load (68.3%–85.2% among tested, 67.0%–87.4% among untested).
Table 1.
Demographics and HIV clinical characteristics by HIV and SARS-CoV-2 RT-PCR testing status in 6 cohorts in the United States in 2020
| Cohort 1 | Cohort 2 | Cohort 3 | Cohort 4 | Cohort 5 | Cohort 6* | |||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PWH | PWoH | PWH | PWoH | PWH | PWoH | PWH | PWoH | PWH | PWoH | PWH | PWoH | |||||||||||||||||||
| Tested | Tested | Tested | Tested | Tested | Tested | |||||||||||||||||||||||||
| Yes | No | Yes | No | Yes | No | Yes | No | Yes | No | Yes | No | Yes | No | Yes | No | Yes | No | Yes | No | Yes | No | Yes | No | |||||||
| Age | % | % | % | % | % | % | % | % | % | % | % | % | % | % | % | % | % | % | % | % | % | % | % | % | ||||||
| 18–29 | 8.2 | 6.8 | 19.7 | 21.0 | 1.3 | 1.7 | 0.2 | 0.5 | 10.0 | 9.7 | 19.8 | 22.4 | 10.6 | 10.8 | 18.8 | 14.3 | 5.1 | 7.4 | 13.6 | 14.8 | 0.4 | 0.6 | 0.8 | 1.0 | ||||||
| 30–39 | 17.4 | 14.1 | 20.8 | 19.2 | 7.9 | 9.0 | 4.3 | 6.7 | 19.6 | 20.0 | 19.0 | 18.8 | 17.7 | 20.0 | 15.5 | 11.3 | 13.9 | 17.5 | 13.7 | 13.1 | 8.6 | 8.2 | 7.9 | 7.6 | ||||||
| 40–49 | 19.5 | 19.0 | 17.2 | 16.9 | 10.1 | 11.1 | 5.9 | 9.4 | 21.3 | 21.6 | 16.5 | 16.5 | 20.7 | 19.6 | 12.4 | 10.5 | 20.7 | 18.1 | 14.4 | 14.4 | 19.1 | 19.7 | 15.3 | 15.1 | ||||||
| 50–59 | 30.4 | 31.9 | 16.9 | 16.7 | 26.3 | 28.2 | 23.4 | 25.0 | 27.2 | 27.3 | 18.7 | 16.9 | 29.5 | 27.0 | 12.5 | 11.7 | 33.3 | 31.8 | 18.0 | 17.5 | 38.1 | 38.3 | 29.4 | 24.0 | ||||||
| 60–69 | 18.6 | 20.7 | 13.7 | 14.1 | 35.5 | 32.5 | 41.6 | 35.9 | 17.4 | 17.5 | 15.2 | 14.3 | 14.1 | 13.9 | 11.4 | 11.9 | 22.5 | 20.6 | 18.7 | 18.2 | 28.2 | 27.1 | 29.2 | 32.7 | ||||||
| 70–79 | 5.5 | 6.7 | 7.9 | 8.1 | 17.0 | 15.6 | 22.2 | 19.8 | 4.2 | 3.4 | 8.2 | 8.0 | 4.5 | 3.1 | 7.1 | 8.7 | 3.8 | 4.7 | 14.3 | 14.3 | 5.6 | 5.3 | 15.1 | 17.3 | ||||||
| 80–89 | 0.5 | 0.7 | 3.2 | 3.3 | 1.8 | 1.8 | 2.1 | 2.5 | 0.4 | 0.5 | 2.3 | 2.6 | 0.4 | 0.3 | 2.3 | 3.2 | 0.8 | 0.9 | 6.1 | 6.2 | 0.1 | 0.8 | 2.2 | 2.4 | ||||||
| ≥ 90 | 0.0 | 0.1 | 0.7 | 0.8 | 0.2 | 0.1 | 0.3 | 0.2 | 0.1 | 0.0 | 0.3 | 0.5 | 0.1 | 0.0 | 0.4 | 0.7 | 0.0 | 0.1 | 1.4 | 1.5 | 0.0 | 0.0 | 0.2 | 0.0 | ||||||
| Race/ethnicity | ||||||||||||||||||||||||||||||
| Non-Hispanic white | 45.1 | 44.3 | 43.5 | 40.1 | 31.1 | 35.9 | 30.0 | 36.8 | 13.6 | 13.5 | 30.0 | 24.8 | 52.5 | 43.5 | 64.4 | 57.8 | 32.1 | 29.2 | 67.1 | 63.7 | 27.9 | 27.3 | 45.1 | 49.5 | ||||||
| Non-Hispanic black | 15.6 | 18.6 | 6.4 | 6.4 | 49.0 | 43.7 | 52.0 | 43.7 | 75.6 | 75.8 | 37.8 | 33.3 | 35.6 | 37.1 | 10.0 | 9.9 | 55.8 | 59.6 | 21.0 | 19.9 | 46.6 | 47.0 | 34.0 | 32.7 | ||||||
| Hispanic | 24.8 | 21.4 | 23.6 | 18.6 | 8.7 | 7.4 | 10.0 | 8.4 | 8.0 | 5.6 | 14.7 | 10.5 | 0.0 | 8.8 | 3.2 | 3.0 | ||||||||||||||
| Hispanic white | 2.3 | 2.9 | 2.0 | 2.2 | 1.5 | 0.0 | 1.0 | 0.8 | 4.3 | 3.7 | 3.2 | 2.9 | ||||||||||||||||||
| Hispanic black | 0.2 | 0.4 | 0.1 | 0.1 | 0.0 | 0.0 | 0.0 | 0.1 | 2.0 | 1.5 | 1.0 | 0.9 | ||||||||||||||||||
| Asian Pacific Islander | 7.7 | 8.1 | 17.5 | 19.9 | 0.9 | 1.9 | 10.9 | 12.5 | 1.0 | 1.0 | 1.8 | 1.9 | 0.8 | 0.2 | 1.4 | 1.7 | 0.8 | 0.5 | 0.8 | 1.1 | ||||||||||
| Native American/Alaskan | 0.1 | 0.4 | 0.3 | 0.3 | 2.4 | 1.7 | 2.4 | 1.9 | 0.3 | 1.2 | 0.4 | 0.4 | 2.6 | 2.6 | 2.0 | 0.6 | ||||||||||||||
| Multiracial | 1.4 | 1.0 | 0.9 | 0.8 | 11.2 | 11.2 | 10.5 | 9.2 | ||||||||||||||||||||||
| Other | 3.8 | 4.1 | 3.2 | 2.8 | 11.2 | 13.1 | 8.0 | 11.1 | 1.2 | 1.5 | 2.1 | 2.0 | 0.7 | 2.1 | 3.7 | 5.4 | 9.6 | 2.1 | 6.0 | 3.0 | 4.8 | 6.2 | 3.4 | 3.1 | ||||||
| Unknown/non-response | 3.1 | 3.6 | 5.9 | 12.4 | 0.7 | 1.3 | 4.3 | 16.6 | 3.9 | 10.1 | 14.7 | 20.1 | 0.0 | 0.0 | 0.1 | 7.3 | 0.0 | 0.1 | 0.0 | 0.0 | ||||||||||
| Sex (or gender)† | ||||||||||||||||||||||||||||||
| Female | 9.6 | 10.6 | 56.0 | 50.0 | 3.8 | 3.5 | 3.8 | 3.6 | 31.2 | 31.3 | 58.3 | 52.3 | 24.2 | 25.4 | 55.7 | 56.6 | 31.3 | 29.4 | 60.8 | 59.0 | 58.5 | 62.2 | 41.1 | 39.0 | ||||||
| Male | 90.4 | 89.4 | 44.0 | 50.0 | 96.2 | 96.5 | 96.2 | 96.4 | 68.9 | 68.7 | 41.7 | 47.7 | 75.8 | 74.6 | 44.3 | 43.3 | 68.7 | 70.6 | 39.2 | 40.7 | 41.5 | 37.8 | 59.0 | 61.0 | ||||||
| Unknown/non-response | 0.0 | 0.0 | 0.0 | 0.1 | 0.0 | 1.1 | 0.0 | 0.3 | ||||||||||||||||||||||
| CD4 | ||||||||||||||||||||||||||||||
| ≥500 | 58.2 | 55.3 | 50.5 | 45.7 | 68.1 | 56.0 | 50.7 | 57.6 | 59.3 | 65.7 | 62.5 | 60.5 | ||||||||||||||||||
| 350–499 | 13.2 | 12.9 | 14.9 | 11.9 | 14.7 | 14.1 | 10.8 | 12.1 | 14.9 | 15.5 | 11.7 | 13.3 | ||||||||||||||||||
| 200–349 | 7.4 | 7.7 | 10.6 | 7.8 | 7.2 | 9.8 | 8.4 | 7.4 | 10.1 | 8.5 | 7.0 | 7.6 | ||||||||||||||||||
| <200 | 3.2 | 3.1 | 10.8 | 8.8 | 3.7 | 4.3 | 6.8 | 5.2 | 11.1 | 3.9 | 4.0 | 3.0 | ||||||||||||||||||
| Unknown | 18.0 | 21.0 | 13.3 | 25.8 | 6.3 | 15.8 | 23.3 | 17.7 | 4.5 | 7.5 | 14.8 | 15.6 | ||||||||||||||||||
| CD4%† | ||||||||||||||||||||||||||||||
| >65% | 0.1 | 0.1 | 0.0 | 0.1 | 0.3 | 0.1 | 0.0 | 0.2 | 0.1 | 0.1 | ||||||||||||||||||||
| 31%-65% | 46.5 | 41.7 | 53.8 | 45.2 | 42.1 | 48.6 | 47.0 | 51.3 | 55.5 | 57.1 | ||||||||||||||||||||
| 16%-30% | 31.3 | 26.2 | 33.4 | 30.9 | 25.0 | 26.7 | 32.1 | 34.9 | 25.4 | 22.8 | ||||||||||||||||||||
| ≤ 15% | 8.5 | 6.0 | 6.5 | 8.0 | 9.4 | 6.5 | 7.6 | 5.9 | 3.9 | 4.2 | ||||||||||||||||||||
| unknown | 13.5 | 26.1 | 6.3 | 15.8 | 23.2 | 18.1 | 13.4 | 8.7 | 15.1 | 15.9 | ||||||||||||||||||||
| HIV-RNA† | ||||||||||||||||||||||||||||||
| <40 | 82.7 | 78.3 | 74.2 | 67.9 | 85.2 | 75.3 | 68.3 | 67.0 | 83.6 | 87.4 | 71.0 | 70.6 | ||||||||||||||||||
| ≥40 | 8.8 | 8.7 | 15.9 | 12.3 | 10.0 | 10.6 | 11.1 | 11.0 | 14.9 | 11.6 | 14.4 | 13.7 | ||||||||||||||||||
| Unknown | 8.5 | 13.1 | 9.9 | 19.8 | 4.8 | 14.1 | 20.6 | 22.1 | 1.5 | 2.1 | 14.7 | 15.7 | ||||||||||||||||||
| Antiretroviral therapy (ARV) | ||||||||||||||||||||||||||||||
| Yes | 94.7 | 89.8 | 81.4 | 73.1 | 95.7 | 90.3 | 74.4 | 64.8 | 99.0 | 99.2 | 88.0 | 85.4 | ||||||||||||||||||
| No | 5.3 | 10.2 | 18.6 | 26.9 | 4.4 | 9.7 | 25.6 | 35.2 | 1.0 | 1.9 | 5.5 | 6.0 | ||||||||||||||||||
| Unknown | 6.5 | 8.6 | ||||||||||||||||||||||||||||
| ARV class | ||||||||||||||||||||||||||||||
| NRTI | 90.0 | 85.7 | 93.3 | 94.4 | 92.0 | 92.1 | 46.8 | 44.4 | 9.1 | 5.1 | 83.0 | 82.4 | ||||||||||||||||||
| NNRTI | 22.5 | 26.3 | 18.1 | 20.1 | 22.3 | 21.4 | 7.6 | 8.7 | 11.4 | 10.9 | 20.4 | 23.1 | ||||||||||||||||||
| Protease inhibitors (PI) | 15.6 | 15.9 | 15.4 | 14.0 | 12.1 | 10.7 | 18.8 | 16.7 | 6.3 | 4.7 | 20.4 | 18.9 | ||||||||||||||||||
| Fusion inhibitors | 0.1 | 0.0 | 0.7 | 0.8 | 0.0 | 0.1 | 0.4 | 0.2 | 0.0 | 0.0 | 1.7 | 1.1 | ||||||||||||||||||
| Integrase inhibitors | 74.6 | 66.5 | 83.4 | 79.8 | 78.6 | 76.0 | 53.9 | 39.9 | 72.2 | 78.6 | 69.8 | 64.9 | ||||||||||||||||||
| CCR5 | 0.7 | 0.8 | 0.7 | 0.8 | 0.9 | 0.4 | ||||||||||||||||||||||||
Cohort 6 used a validated self-reported cohort survey to determine testing status
Cohort 1: Used a definition of sex or gender; CD4% not available; HIV-RNA used a threshold <48, ≥48 for HIV-RNA (all other cohorts used <40, ≥40)
Abbreviations: PWH= Persons with HIV; PWoH= Persons without HIV
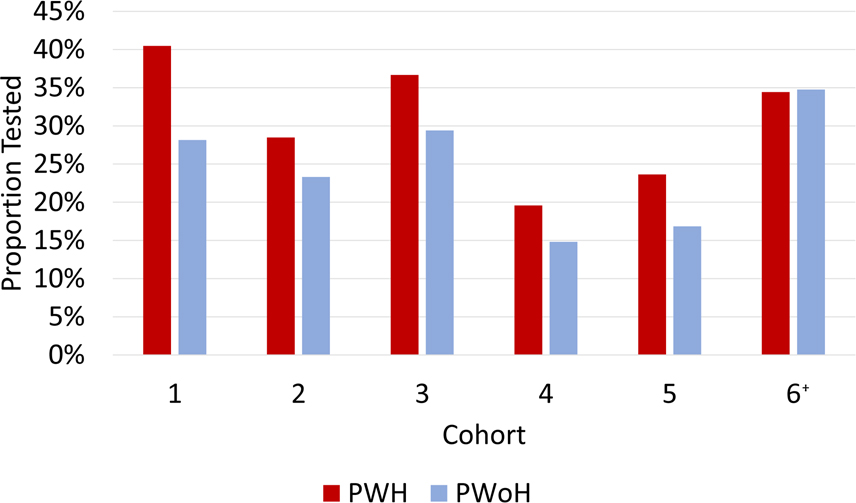
From March 1 to December 31, 2020, the proportion of PWH in cohorts 1–5 who were tested for SARS-CoV-2 (19.6%–40.5%) was higher than PWoH within the same cohort (14.8%–29.4%). The difference in proportions ranged from 4.8% in cohort 4 to 12.3% in cohort 1 (p<0.001 for cohorts 1–5, Figure 1, Table 2). The one cohort (cohort 6) that used a validated self-reported cohort survey to obtain testing status showed similar proportions tested by HIV status (PWH 34.4% vs. PWoH 34.8%, p=0.835).
Figure 1. Proportion SARS CoV-2 RT-PCR tested at least once from March 1-December 31, 2020 by HIV status*.
Bars represent the proportion tested across the 6 cohorts by HIV status. Red bars represent persons with HIV (PWH), and blue bars represent persons without HIV (PWoH).
*χ2 p-values <0.001 for differences in proportions for cohorts 1-5, p=0.835 for cohort 6.
† Cohort 6 used a validated self-reported cohort survey to determine testing status and reported results through September 30, 2020.
Abbreviations: PWH= Persons with HIV; PWoH= Persons without HIV
Table 2.
Proportion SARS-CoV-2 tested and proportion positive comparing laboratory tests only to laboratory tests with reported diagnoses
| % SARS CoV-2 tested | ||||||||
| Labs only | Labs + reported diagnoses | |||||||
| PWH | PWoH | PWH | PWoH | |||||
| % | % | Difference | p-value * | % | % | Difference | p-value * | |
| Cohort 1 | 40.5% | 28.2% | 12.3% | <0.001 | ||||
| Cohort 2 | 28.5% | 23.3% | 5.2% | <0.001 | 30.5% | 25.5% | 5.0% | <0.001 |
| Cohort 3 | 36.7% | 29.4% | 7.3% | <0.001 | 37.1% | 29.7% | 7.4% | <0.001 |
| Cohort 4 | 19.6% | 14.8% | 4.8% | <0.001 | 21.8% | 16.2% | 5.6% | <0.001 |
| Cohort 5 | 23.6% | 16.8% | 6.8% | <0.001 | 24.3% | 18.2% | 6.2% | <0.001 |
| Cohort 6 | 34.4% | 34.8% | −0.3% | 0.835 | ||||
| % SARS CoV-2 positive | ||||||||
| Labs only | Labs + reported diagnoses | |||||||
| PWH | PWoH | PWH | PWoH | |||||
| % | % | Difference | p-value * | % | % | Difference | p-value * | |
| Cohort 1 | 9.1% | 11.6% | −2.4% | <0.001 | ||||
| Cohort 2 | 10.0% | 9.8% | 0.1% | 0.737 | 12.9% | 13.9% | −0.9% | 0.026 |
| Cohort 3 | 17.7% | 16.3% | 1.4% | 0.143 | 19.0% | 17.4% | 1.5% | 0.118 |
| Cohort 4 | 3.6% | 8.2% | −4.7% | <0.001 | 10.5% | 14.9% | −4.4% | <0.001 |
| Cohort 5 | 9.1% | 10.0% | −0.9% | 0.543 | 12.4% | 17.5% | −5.1% | 0.007 |
| Cohort 6 | 8.2% | 4.4% | 3.7% | 0.009 | ||||
χ2 p-values for differences in proportions
Abbreviations: PWH= Persons with HIV; PWoH= Persons without HIV
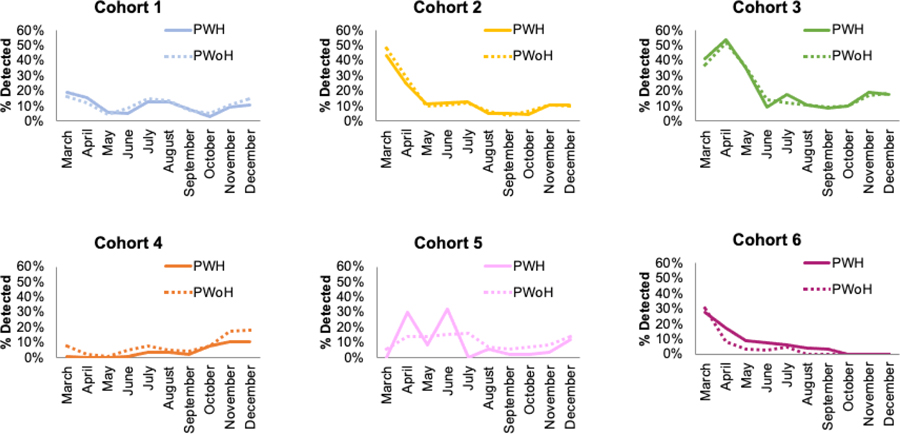
Among those tested, the proportion of patients with detectable SARS-CoV-2 was similar, regardless of HIV status (Table 2). Distributions of age, race/ethnicity, and sex for patients who tested positive were similar to those for patients who tested negative, in both PWH and PWoH (Supplemental Table S2). In two cohorts, of those who tested positive, a substantially higher proportion were Hispanic compared to those who tested negative, in both PWH and PWoH. The difference in SARS-CoV-2 positivity proportions ranged from 4.7% lower in cohort 4 to 1.4% higher in cohort 1 for PWH compared to PWoH. While some of the differences by HIV status were statistically significant, the difference in positivity proportion was <5% across all of the cohorts and therefore (by our a priori cutoff) not clinically significant. Early months of positivity proportion estimates fluctuated; however, for most cohorts, positivity proportions declined over time. While there was a modest increase towards the end of the study period, positivity proportions remained low and tracked similarly by HIV status (Figure 2). For the cohorts where COVID-19 diagnosis data or self-reported test results were available, sensitivity analyses generated similar conclusions (Table 2 & Supplemental Figure S1).
Figure 2. Proportion SARS CoV-2 RT-PCR positive from March 1-December 31, 2020 in 6 cohorts in the United States.
Lines represent the proportion positive monthly across the 6 cohorts by HIV status. Solid lines represent persons with HIV (PWH), and dashed lines represent persons without HIV (PWoH). Proportion positive is the proportion of RT-PCR results with detectable SARS-CoV-2 among those tested by SARS-CoV2 RT-PCR. Cohort 6 used a validated self-reported cohort survey to determine testing status and reported results through September 30, 2020.
Abbreviations: PWH= Persons with HIV; PWoH= Persons without HIV
DISCUSSION
In a large nationally diverse sample, we found that among individuals in care, PWH were more likely to be tested for SARS-CoV-2 than PWoH, but there was no evidence of clinically important differences in positivity by HIV status among those tested. Among four regional health systems, one national health system, and one interval cohort study, we found similar overall trends in SARS-CoV-2 testing and positivity for PWH and PWoH. Differences in the proportions tested and positive between the cohorts reflect the heterogeneity of our collaboration. The variation in the proportion tested among PWH (19.6%–40.5%) across the 6 cohorts may be due to differences in trends by geographic region (Northern California, Mid-Atlantic, North Carolina, and Tennessee, compared with national samples) or by the underlying patient population (privately/publicly insured, Veteran, health systems, recruited study participants). Three of the cohorts required enrollment based on membership (publicly or privately insured), which may allow for more comprehensive coverage of testing in their populations. In addition, we included one cohort in our primary results which used a validated self-reported cohort survey to obtain testing status.
A greater proportion of PWH were tested for SARS-CoV-2 compared to PWoH during the study period in five of the 6 cohorts. Clinicians may be more likely to recommend testing for PWH due to concerns of higher risk for poorer outcomes, although these concerns have yet to be supported with comprehensive evidence. PWH in these cohorts may also be more connected to their health systems due to regular care they receive for HIV. In one cohort, there was no difference in proportion tested by HIV status, and the proportion PWoH tested was substantially higher compared to the other cohorts. This study actively recruited PWH and seronegative persons who are at risk for HIV from 14 urban sites across the US. The recruitment methods and geographic distribution of this cohort may explain the differences between these results from the five other cohorts.
In the early months of the pandemic, there was concern that PWH would be disproportionately impacted by COVID-19. Early estimates of testing and positivity in PWH were reflective of surges in specific geographic regions in the US.11 Any potential signal of higher risk of testing positive in the early weeks and months of the pandemic diminished over time in our cohorts. Fluctuations in estimates were likely influenced by the rollout in testing availability and heterogeneity in testing guidelines by state and health system and by relatively smaller sample sizes for cohorts where there were fewer PWH who were tested. Nearly a year into the pandemic, our crude estimates show that the proportion SARS-CoV-2 positive was similar for PWH and PWoH. Our findings were consistent with a population cohort study in Western Cape, South Africa, which found similar SARS-CoV-2 positivity proportions among PWH and PWoH.4
Our descriptive study had limitations. We relied on available EHR data for the majority of the cohorts and therefore elected to use laboratory test results as our primary measure. While three of the included health systems provide comprehensive care, there was evidence of outside testing. Therefore, our calculations are an underestimate of testing as a function of the proportion of tests that occurred outside of the health systems. In a sensitivity analysis including additional sources of SARS-CoV-2 testing data, we found similar increases in testing for PWH and PWoH, such that the testing proportion remained similar in the two groups. While four of the 6 cohorts were distinctive in the geographic regions and patients included, the VACS and MACS/WIHS national cohorts may include a small number of patients participating in the other cohorts as well. VACS participants have reported that they typically receive the majority of their HIV care in the VA. For both cohorts, based on prior research collaborations, we estimate the overlap is small and would not influence the results and conclusions of overall testing and positivity proportions in this study.
We aimed to execute a large-scale descriptive analysis on testing and positivity comparing PWH and PWoH, and therefore did not examine HIV-specific factors, such as CD4 cell count or HIV-1 RNA, and whether there was a causal relationship with positivity rates. We did not have data to examine facilitators of testing that resulted in PWH having higher testing proportions than PWoH nor did we measure COVID-19 severity and outcomes after positivity. While this work demonstrates that PWH and PWoH had comparable SARS-CoV-2 positivity, we cannot infer that the risk of infection for the two groups was similar and acknolwedge the limitation of using test positivity as a proxy for risk of infection. Although PWoH were from the same geographic regions as PWH, our data did not include information on structural factors that would impact a person’s exposure to SARS-CoV-2, such as employment type and location (namely, the proportion who were first responders or front-line workers, or generally individuals without work-from-home options) and housing (stability and crowding) that differentially impact positivity rates. Differences in individual behavior, such as adherence to masking, hand washing, and social distancing, could have also impacted the risk of SARS-CoV-2 acquisition.
In this first analysis from the CIVET collaboration, we found that PWH had higher testing rates compared with PWoH, with no evidence of increased positivity among those tested. Moreover, results were robust among six cohorts with large diverse populations across the US while there were different barriers to accessing testing that changed over time. These results are encouraging. However, given the availability, response, and durability of COVID-19 vaccines; emergence of SARS-CoV-2 variants; and latest therapeutic options, we will continue to monitor testing, positivity, and COVID-19 related health outcomes in PWH and PWoH using our multiple data sources and leveraging the expertise of established longitudinal cohort studies in the CIVET collaboration. Future work will also explore whether clinical outcomes differ among PWH and PWoH after COVID-19.
Supplementary Material
Sources of Support:
This work was supported by a COVID-19 focused supplement to the NA-ACCORD from the NIH National Institute of Allergy and Infectious Diseases (U01-AI069918), as well as other NIH institutes (UNC: P30-AI050410, UL1-TR002489; VACS: U24-AA020794, U01-AA020790, U24-AA022001, U10 AA013566-completed; MACS/WIHS Full Acknowledgement may be found here https://statepi.jhsph.edu/mwccs/acknowledgements/), and in kind by the US Department of Veterans Affairs. The views and opinions expressed in this manuscript are those of the authors and do not necessarily represent those of the Department of Veterans Affairs or the United States Government.
We would like to acknowledge the members of the CIVET collaboration, including Rosemary McKaig, Lucas Gerace, and Aimee Freeman for their input and advice on this work.
Conflicts of Interest and Source of Funding:
M.S. has received research funding from Gilead Sciences, Inc. For the remaining authors none were declared.
Footnotes
These findings have been previously presented as a “science spotlight” at the 2020 virtual Conference on Retroviruses and Opportunistic Infections (CROI).
Contributor Information
Lesley S. Park, Center for Population Health Sciences, Department of Epidemiology & Population Health, Stanford University School of Medicine, Stanford, CA, USA
Kathleen A. McGinnis, Department of Internal Medicine, VA Connecticut Healthcare, West Haven, CT, USA
Kirsha S. Gordon, VA Connecticut Healthcare System, West Haven; Yale University School of Medicine, New Haven, CT, USA
Amy C. Justice, VA Connecticut Healthcare System, West Haven; Yale University Schools of Medicine and Public Health, New Haven, CT, USA
Wendy Leyden, Division of Research, Kaiser Permanente Northern California, Oakland, CA, USA.
Michael J. Silverberg, Division of Research, Kaiser Permanente Northern California, Oakland, CA, USA
Jacek Skarbinski, Division of Research, Kaiser Permanente Northern California, Oakland, CA, USA.
Celeena Jefferson, Kaiser Permanente Mid-Atlantic Permanente Research Institute, Rockville, MD, USA.
Michael Horberg, Kaiser Permanente Mid-Atlantic Permanente Research Institute, Rockville, MD, USA.
Julia Certa, Kaiser Permanente Mid-Atlantic Permanente Research Institute, Rockville, MD, USA.
Sonia Napravnik, Department of Epidemiology, University of North Carolina at Chapel Hill, Chapel Hill, NC, USA.
Jessie K. Edwards, Department of Epidemiology, University of North Carolina at Chapel Hill, Chapel Hill, NC, USA
Daniel Westreich, Department of Epidemiology, University of North Carolina at Chapel Hill, Chapel Hill, NC, USA.
Lisa Bastarache, Department of Biomedical Informatics, Vanderbilt University Medical Center, TN, USA.
Srushti Gangireddy, Department of Biomedical Informatics, Vanderbilt University Medical Center, TN, USA.
Lorie Benning, Department of Epidemiology, Johns Hopkins Bloomberg School of Public Health, Baltimore, MD, USA.
Gypsyamber D’Souza, Department of Epidemiology, Johns Hopkins Bloomberg School of Public Health, Baltimore, MD, USA.
Carolyn Williams, Epidemiology Branch, Division of AIDS, National Institute of Allergy and Infectious Diseases, Bethesda, MD, USA.
Keri N. Althoff, Department of Epidemiology, Johns Hopkins Bloomberg School of Public Health, Baltimore, MD, USA
References
- 1.Centers for Disease Control and Prevention. COVID-19: People with Certain Medical Conditions. https://www.cdc.gov/coronavirus/2019-ncov/need-extra-precautions/people-with-medical-conditions.html#immunocompromised-state. Published 2021. Updated May 13, 2021. Accessed June 9, 2021.
- 2.Altuntas Aydin O, Kumbasar Karaosmanoglu H, Kart Yasar K. HIV/SARS-CoV-2 coinfected patients in Istanbul, Turkey. J Med Virol. 2020;92(11):2288–2290. [DOI] [PubMed] [Google Scholar]
- 3.Bhaskaran K, Rentsch CT, MacKenna B, et al. HIV infection and COVID-19 death: a population-based cohort analysis of UK primary care data and linked national death registrations within the OpenSAFELY platform. Lancet HIV. 2021;8(1):e24–e32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Western Cape Department of Health in collaboration with the National Institute for Communicable Diseases, South Africa, Erratum to: Risk Factors for Coronavirus Disease 2019 (COVID-19) Death in a Population Cohort Study from the Western Cape Province, South Africa, Clin Infect Dis, 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hadi YB, Naqvi SFZ, Kupec JT, Sarwari AR. Characteristics and outcomes of COVID-19 in patients with HIV: a multicentre research network study. AIDS. 2020;34(13):F3–F8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Harter G, Spinner CD, Roider J, et al. COVID-19 in people living with human immunodeficiency virus: a case series of 33 patients. Infection. 2020;48(5):681–686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Miyashita H, Kuno T. Prognosis of coronavirus disease 2019 (COVID-19) in patients with HIV infection in New York City. HIV Med. 2021;22(1):e1–e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tesoriero JM, Swain CE, Pierce JL, et al. COVID-19 Outcomes Among Persons Living With or Without Diagnosed HIV Infection in New York State. JAMA Netw Open. 2021;4(2):e2037069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.D’Souza G, Springer G, Gustafson D, et al. COVID-19 symptoms and SARS-CoV-2 infection among people living with HIV in the US: the MACS/WIHS combined cohort study. HIV Res Clin Pract. 2020;21(5):130–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gange SJ, Kitahata MM, Saag MS, et al. Cohort profile: the North American AIDS Cohort Collaboration on Research and Design (NA-ACCORD). Int J Epidemiol. 2007;36(2):294–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rentsch CT, Kidwai-Khan F, Tate JP, et al. Patterns of COVID-19 testing and mortality by race and ethnicity among United States veterans: A nationwide cohort study. PLoS Med. 2020;17(9):e1003379. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.