Abstract
Objective:
This randomized controlled trial examined whether an interactive, risk-focused educational program was associated with higher risk perceptions and decreased prescription opioid use/misuse among emerging adults.
Methods:
503 participants aged 15–24 years scheduled for ambulatory surgery were randomized to routine prescription education with or without our Scenario-Tailored Opioid Messaging Program (STOMP) provided prior to receipt of a prescribed opioid. Surveys were completed preoperatively, and at days 7&14, months 1&3 postoperatively. Outcomes included analgesic risk perceptions, opioid use, and misuse intentions/behavior.
Results:
Compared to Controls, STOMP was associated with stable but higher risk perceptions on day 14 (β = 1.76 [95% CI 0.53, 2.99], p = .005) and month 3 (β = 2.13 [95% CI 0.86, 3.40], p = .001). There was no effect of STOMP or analgesic misuse risk perceptions on days of opioid use or subsequent misuse intentions/behavior. The degree to which participants valued pain relief over analgesic risk (trade-off preference) was, however, associated with prolonged postoperative opioid use and later misuse.
Conclusion:
Education emphasizing the risks of opioids was insufficient in reducing opioid use and misuse in youth who were prescribed these analgesics for acute pain relief.
Practice implications:
Education may need to better address analgesic expectations to shorten opioid use and mitigate misuse.
Keywords: Emerging adults, Youth, Acute pain, Prescription opioid misuse, Risk perception, Prescription opioid use, Analgesic preference
1. Introduction
Exposure to legitimately prescribed opioids during adolescence has been associated with subsequent opioid misuse and opioid use disorder symptoms [1–5]. Despite purported decreases in prescription opioid misuse (POM) recently [6,7], the lifetime prevalence of this risky behavior remains worrisome with the most recent self-reported rates ranging from 5% to 15% among teens and 8–21% among emerging adults in the United States [1,8–11]. Policies meant to decrease opioid-related adverse outcomes have led to decreased prescribing rates, and, thus, reduced overall exposure to legitimate prescriptions across the US [12,13]. Yet, one in five teens and nearly one in three young adults recently reported using a prescribed opioid in the previous year [10]. Such exposure presents ongoing risks for misuse and other adverse outcomes in this vulnerable population, making early, primary prevention a priority.
POM behavior and intentions have been associated with lower perceptions of opioid risks among youth [14,15]. Thus, efforts to enhance risk perception and prevent the initiation of misuse behavior may be needed prior to the onset of this risky behavior. On the other hand, since the need to relieve physical pain is the most often self-reported motivation for POM and POM intention across age groups [16–19], education at the time of prescribing must balance risk with information about safe and effective use to manage pain.
To date, education to prevent opioid misuse has mostly targeted relatively healthy teens in schools or community settings or parents whose children receive a prescribed opioid [8,20–24]. While such efforts have been associated with strengthening resilience against misuse, it remains unclear whether similar education presented to youth at the time of prescribing effectively impact their subsequent perceptions and behaviors.
1.1. Study purpose
The purpose of this prospective, randomized, clinical trial was to examine whether a theoretically derived, interactive computer-based educational program administered prior to the receipt of a prescribed opioid would lead to decreased POM behavior and intentions after surgery. We tested the hypotheses that, compared to a control group, youth who received our educational program would:
Have higher analgesic misuse risk perceptions across the study period.
Use prescription opioids for fewer days after surgery.
Report lower misuse behavior and intentions in the first three months after surgery.
2. Methods
2.1. Procedure
With approval from the Institutional Review Board at the University of Michigan (IRBMED HUM00147378) we recruited, by phone, older teens (aged 15–17 years) and emerging adults (aged 18–24) who were scheduled to have an ambulatory or short stay surgical procedure in our tertiary care setting, and who were expected to receive a prescription opioid for short-term postoperative pain management. Written consent was obtained from all participants aged 18 and older and from parents of those aged 15–17 years, with the teen’s written assent. We excluded patients who did not sign the consent/assent document, who could not comprehend written English, did not receive an opioid prescription, required a prolonged hospital stay, and those with a chronic pain diagnosis.
Once consented, participants completed baseline surveys using an institutional Qualtrics link that was provided via text message or email per personal preference. To remain eligible, all baseline surveys had to be completed prior to surgery and prescription dispensing/education. Participants were randomized via an a priori computer-generated randomization list to the Control Group (i.e., receipt of routine prescription education provided after surgery and prior to discharge) vs. the Intervention Group (i.e., receipt of routine education plus the intervention described below). Routine care throughout the study period included provision of a standardized discharge education sheet that, for adult patients (i.e., 18 years of age and older), included a one-page opioid consent document briefly describing opioid risks as mandated by Michigan legislation (see appendix).
Participants were sent follow-up surveys after surgery via text link or email on Day 7, Day 14, Months 1 and 3. We chose these timeframes to promote accurate report of pain and medication use during the early postoperative period and to capture ongoing pain, medication use and intentions (Months 1 and 3). Additionally, we wanted to assess risk perceptions early after intervention and over time as pain lessened. Text reminders were sent to minimize attrition. Participants self-entered their pre-assigned study identification numbers to protect privacy and to facilitate merging of survey data. Participants were paid up to $50 if they completed the baseline and at least one of the long-term follow-up surveys (month 1–3).
2.2. Educational intervention
The interactive Scenario-Tailored Opioid Messaging Program (STOMP) was designed to provide brief, gist-based (i.e., bottom-line meaning) prescription opioid information that emphasized important opioid risks and safe opioid use [24]. We grounded our educational intervention in the tenets of Fuzzy Trace Theory which supports the notion that people rely on “gist” representations (i.e., essential, or “bottom-line” meaning) when making risk-related decisions - even when more precise or verbatim data (e.g., numerical rates) are provided [25,26]. Gist-based reasoning has been associated with better judgement, risk avoidance and risk reduction behavior compared to details-based reasoning [25]. Gist-based messaging is not meant to exaggerate risks. However, fuzzy trace posits that when the absolute rates of risk are low, as in the case of opioid misuse, overdose, and death, verbatim or details-based reasoning can inadvertently promote risky behavior [25].
The STOMP program used herein was modified from our previously described parental intervention to address youth who receive a prescription and make their own analgesic decisions. The interactive program includes several clinically relevant scenarios wherein participants are asked to imagine themselves in the situation and to select from a dropdown list the medication actions they would take if presented with a similar real-life situation. This study focused primarily on the two scenarios describing situations where the subject has run out of the prescription opioid but is experiencing ongoing and significant pain that interferes with return to usual activities. Decision options for these scenarios included, take a left-over opioid from the medicine cabinet, take a prescription opioid offered from a friend, take only over-the-counter analgesics, or do something else. Open-ended decision options were also included. Each participant decision prompts immediate feedback including a written message with a link to a brief, animated video emphasizing the risks of taking the opioid together with advice about how to avoid risk while managing pain in that situation. Other scenarios addressed routine use of the prescribed opioid posing a decision option, “take a higher dose of the prescribed opioid”. Scenario feedback included the statement, “NEVER take a dose of a prescribed opioid that is higher than ordered or sooner than the dose is due. This is dangerous and has led to oversedation and overdose. ” A description of the opioid use and misuse feedback is provided in the supplement.
2.3. Primary outcome measures
2.3.1. Analgesic misuse risk perceptions
Analgesic misuse risk perceptions were assessed at baseline, day 14, months 1 and 3. Four items, each scored from 0 (not risky) to 10 (extremely risky), were included in this instrument (i.e., taking a higher dose of a prescribed opioid, taking an analgesic more frequently than recommended, taking an opioid without a doctor’s prescription, taking someone else’s prescribed opioid). Internal consistency was supported with Cronbach’s Alpha = .826 [95% CI.799,.851], p < .001) and scores range from 0 to 40. Participants’ postoperative risk perception scores were averaged for inclusion in our regression models.
2.3.2. Prescription opioid use
Participant responses from the postoperative surveys on days 7, 14, months 1 and 3 were tallied to assess the outcome, Days Opioid Used after surgery. To test our second hypothesis, we coded days of use into no use, short-term use (1–4 days), moderate use (5–9 days) and prolonged postoperative use (≥ 10 days).
2.3.3. Prescription opioid misuse (POM) behavior and intentions
At months 1 and 3, we assessed POM behavior using two survey items, i.e., “Since surgery, have you ever (even once) taken an opioid that was not prescribed by your surgeon for this procedure?” and “How often have you used any of the following medicines on your own since surgery (without a doctor prescribing it to you - e.g., from family or friend) or in a manner not prescribed (higher dose or more frequently)?” Responses were collapsed and coded as POM behavior Yes/No. As with our previous work [15], POM intentions were assessed by combining responses from the STOMP decisional scenarios surveyed at months 1 and 3. Participant responses were collapsed and coded as POM intentions (i.e., would take a higher dose, would use a left-over prescription opioid in the family medicine cabinet or from a friend) or no intent. Given anticipated low rates of postoperative POM behavior, we combined months 1 and 3 reported behavior and intentions into the binary outcome, “POM behavior/intention” to test our 3rd hypothesis.
2.4. Main covariates
2.4.1. Analgesic trade-off preferences
We measured Trade-off Preferences at baseline, day 14, months 1 & 3, and averaged the later postoperative scores for analyses. A six-item survey assessed the relative degree to which individuals value analgesic benefit (i.e., preference for pain relief) versus the preference to avoid analgesic risk. This instrument includes alternating statements such as, “Reducing side effects is more important to me than getting rid of my pain”, “Pain relief is more important to me than the side effects of prescription pain drugs.” Each item is rated from −2 (strongly disagree) to +2 (strongly agree), to create a total score range −12 to +12. Lower scores indicate a relative preference for risk avoidance and higher numbers a preference for analgesic benefit, or pain relief. Scores around 0 represent relative ambivalence between the two valuations. Internal consistency and predictive validity of this measure has been supported in large samples of parents [24,27]. Internal consistency of the six-item scale was supported in the present sample with Cronbach’s alpha = 0.713 [95% CI 0.671, 0.752], p < .001. Postoperative preference scores were averaged for analyses.
2.4.2. Substance use
To assess past year substance use/misuse at baseline, we used the National Institute on Drug Addiction (NIDA) Modified Alcohol, Smoking, and Substance Involvement Screening Test (ASSIST Version 1) [28]. This measure provides a quick screen for risky substance use behavior by asking respondents to indicate the frequency of their past year use of substances including, alcohol, tobacco, prescription drugs for non-medical reasons and illegal drugs. For each substance, participants indicate whether they used the substance, Never, Once or Twice, Monthly, Weekly, Almost Daily or Daily. The resultant measure included 14 items with total scores that could range from 0 to 56.
2.4.3. Psychologic and somatic symptoms
We used 11 items from the American Psychologic Association’s DSM-5 Self-Rated Level 1 Cross-Cutting Symptom Measure - Adult, to assess baseline past year comorbid symptomology [29,30]. Each item on the symptom assessment is rated based on frequency of the symptom, from 0 (none or not at all) to 4 (severe or nearly every day). For our purposes, we included only items associated with pain and/or prescription drug misuse, including screeners for depression, anxiety, suicidal ideation, sleep problems, somatic symptoms, and dissociation. We excluded the domains of psychosis and mania, as well as the substance use items which were measured as described above. Total scores on the baseline symptom measure could range from 0 to 44.
2.4.4. Postoperative pain
On each of the follow-up surveys (Day 7, 14, Months 1 and 3), participants recorded their highest pain intensity in the past week using the 0–10 Numeric Rating Scale (NRS) where 0 = no pain to 10 = worst possible pain [31]. All postoperative scores were averaged for the Aim 2 analysis. We assessed pain interference with functioning at months 1 and 3 using the Patient Reported Outcomes Measurement Information System (PROMIS) Adult Pain Interference Short Form (8 Items) [32,33]. This instalment scores each of 8 pain interference items from 0, not at all, to 4, very much, to yield scores ranging from 0 to 32. Scores from the assessments were averaged for Aim 3 analysis.
2.4.5. Other measures
Age, sex, race, ethnicity were self-reported on the baseline surveys as were past year pain frequency and analgesic use. Surgical procedure, duration and details of the postoperative opioid prescriptions were recorded from the electronic medical record.
2.5. Statistical analyses
2.5.1. General analyses
All data were analyzed using ST AT A, version 17.0 (College Station, TX, USA). Descriptive statistics are presented using frequencies (i.e., n [%]) or mean (Median), standard deviation or interquartile range, as appropriate. Univariate comparisons between the control and STOMP groups were made with chi square or independent t tests for nominal or interval level data, respectively. Levene’s tests for equality of variances were applied. Beta coefficients, odds ratios (OR) or mean differences (MD) with 95% confidence intervals (CI) are reported, adjusted for covariates where applicable. P values < .05 were accepted as statistically significant.
2.5.2. Sample size determination
We determined, a priori, that a sample of 164/group would sufficiently detect with 95% confidence and 80% power a 30% decrease in participant’s opioid misuse intentions from a baseline rate of 30% [15]. This sample size was deemed sufficient to detect a mean difference in risk perceptions of 0.56, SD 1.21 (sample needed = 122/group) and to test for a modest effect of the interventions in a factorial design (estimated eta squared 0.14; sample needed = 147). To account for expected attrition and missing data, we approached 700 patients and discontinued recruitment when our follow-up sample was achieved (i.e., minimum of 164 in each group).
2.5.3. Aim 1 Hypothesis Test
We fitted ordinary least squares regression models using the generalized estimating equations (GEE) methodology [34,35] with an exchangeable correlation structure to account for the longitudinal design. Models were estimated to determine; 1) whether the STOMP group had higher analgesic misuse risk perception across the study period when compared to the control group, and (2) whether the trajectory of misuse risk perception was significantly higher for the STOMP group compared to the control group. Models included key variables (Group, Time and Group by Time interaction) and relevant baseline covariates (age, sex, race/culture, past year opioid use, baseline symptom and ASSIST scores). Time was treated as a categorical variable using baseline as the reference group. Unstandardized beta coefficients with 95% CIs are reported.
2.5.4. Aim 2 Hypothesis Test
We used a multinomial model to regress the coded outcome, Days Opioid Used onto STOMP and the relevant baseline and postoperative covariates (i.e., age, sex, race/ethnicity, procedure, doses dispensed, past year opioid use, baseline symptom and ASSIST scores, as well as average postoperative pain intensity, analgesic trade-off preference, and analgesic misuse risk perception scores). Given our interest in the secondary pain-related outcomes, opioid refills and unplanned pain-related clinic visit/call, we used logistic regression to model the impact of STOMP on these binary outcomes when controlled for the same covariates
2.5.5. Aim 3 hypothesis Test
Logistic regression was used to test the hypothesis that STOMP would lower the rate of opioid misuse behavior/intention postoperatively. We regressed the binary outcome, postoperative opioid misuse behavior/intention, onto group, relevant baseline characteristics, doses dispensed, and the postoperative variables, days opioid used, average postoperative scores for PROMIS pain interference, analgesic trade-off preference, and misuse risk perception.
3. Results
3.1. Resultant sample
Fig. 1 depicts results of our recruitment and analyses samples. Withdrawals occurred primarily due to incomplete baseline surveys prior to surgery, and lack of postoperative opioid prescription. All recruitment and follow-up procedures were completed between November 2019 and May 2021. Nearly all prescriptions were written for oxycodone to be given every 4–6 h as needed and most included a dose range (e.g., 5–10 mg).
Fig. 1.
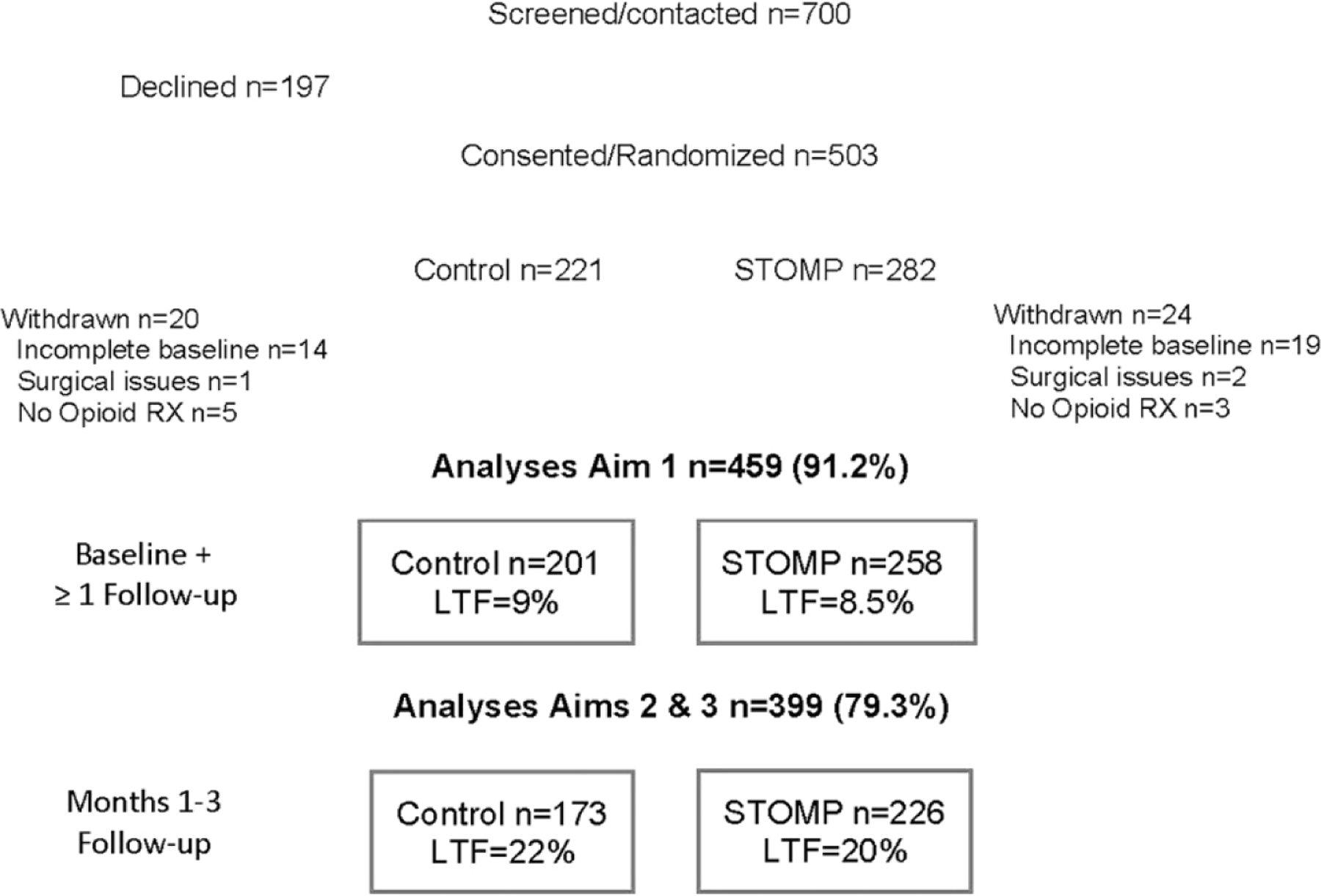
Consort diagram showing recruitment, allocation, and analyses samples. STOMP = Scenario-Tailored Opioid Messaging Program; RX = Prescription; LTF = Loss to Follow-up.
Table 1 depicts the baseline characteristics of the sample showing no significant differences between groups. Nearly all participants reported treating pain in the past year; 98% used a non-pharmacologic method (e. g., massage, physical therapy, meditation, etc.); 90% of Controls and 95% of the STOMP group used an over-the-counter analgesic (i.e., acetaminophen, ibuprofen, or naproxen), while 39 (19.4%) Control participants and 41 (15.9%; p = NS) in the STOMP group had taken a prescribed opioid. Baseline Analgesic Trade-off Preferences leaned toward risk avoidance at baseline and were not significantly different between STOMP and Control groups (see Table 1; p = .115). Analgesic Misuse Risk Perceptions were not significantly different between groups at baseline (see Table 1; p = .546).
Table 1.
Baseline and Perioperative Characteristics of the Study Groups.
| Characteristics | Control (n = 201) | STOMP (n = 258) |
|---|---|---|
| Age | 19.1 ± 2.8 | 19.1 ± 2.7 |
| Sex - Female | 104 (51.7%) | 134 (52.1%) |
| Race/Ethnicity | ||
| White/Non-Hispanic | 158 (78.6%) | 194 (75.2%) |
| Hispanic/LatinX | 7 (3.5%) | 16 (6.2%) |
| Black | 13 (6.5%) | 22 (8.5%) |
| Asian | 15 (7.5%) | 16 (6.2%) |
| Middle Eastern/North African | 7 (3.5%) | 11 (4.3%) |
| Native American | 3 (1.5%) | 2 (0.8%) |
| Modified DSM-5 symptom score (range 0–44) | 11.5 ± 8.0 (M 10) | 11.9 ± 8.3 (M 11) |
| R 0–42 | (R 0–42) | |
| Depression score | 2.6 ± 2.1 (M 2) | 2.7 ± 2.1 (M 2) |
| Antidepressant use | 30 (14.9%) | 48 (18.7%) |
| Anxiety score | 2.9 ± 2.0 (M 3) | 2.8 ± 2.1 (M 3) |
| Anxiolytic use | 10 (5%) | 14 (5.4%) |
| Somatic symptom | 1.1 ± 1.5 (M 1) | 1.4 ± 1.7 (M 1) |
| Previous surgery | 140 (69.7%) | 171 (66.3%) |
| Frequent past year paina | 113 (57.4%) | 128 (50.0%) |
| Headache | 40 (19.9%) | 55 (21.3%) |
| Abdominal | 27 (13.6%) | 22 (8.4%) |
| Musculoskeletal | 57 (28.4%) | 63 (24.4%) |
| Past year prescription opioid use | 39 (19.4%) | 41 (15.9%) |
| Modified ASSIST score (range 0–56) | 3.6 ± 4.2 (M 3) | 3.7 ± 4.6 (M 2) |
| R 0–30 | R 0–24 | |
| Past prescription drug misuse | 44 (21.9%) | 66 (25.6%) |
| Opioid | 18 (9%) | 24 (9.3%) |
| Stimulant | 30 (14.9%) | 39 (15.1%) |
| Anxiolytic | 18 (9.0%) | 27 (10.5% |
| Surgical Procedure | ||
| Sports medicine | 138 (68.7%) | 167 (64.7%) |
| Orthopedic | 30 (14.9%) | 23 (8.9%) |
| Oral Surgery | 24 (11.9%) | 52 (20.2%) |
| Other General surgery | 9 (4.5%) | 16 (6.2%) |
| Perioperative hours | 2.7 ± 1.2 | 2.6 ± 1.1 |
| Perioperative opioid (MME per kg) | 0.20 ± 0.13 | 0.21 ± 0.13 |
| Opioid Doses dispensed | 21.4 ± 7.7 (M 20) | 21.1 ± 7.8 (M 20) |
| Baseline analgesic trade-off preference (range −12 to +12) | −2.2 ± 3.78 | −2.79 ± 3.91 |
| R ‒ 12–7.5 | R – 12–5.5 | |
| Baseline analgesic misuse perception (range 0–40) | 31.12 ±7.2 (M 30.5) | 31.52 ± 7.10 (M 32) |
| R 0–40 | R 15–40 | |
Abbreviations: DSM = Diagnostic and Statistical Manual of Mental Disorders; ASSIST=Alcohol, Smoking and Substance Involvement Screening Test; MME = morphine milliequivalents
Frequent pain = 1–2 days/month or more frequent
Data presented as mean ± standard deviation, n (%), ranges (R) for scores and medians (M) where data were skewed
Table 2 presents pain and analgesic outcomes showing that postoperative pain intensity decreased over time for both groups. Days of opioid use was not different between groups with a majority in both groups using their prescribed opioid for only 1–4 days. Few participants in the STOMP and Control groups reported ongoing, but infrequent (i.e., less than weekly) opioid use at 1–3 months (3.1% and 2.3%, respectively; OR 1.34 [95% CI 0.39, 4.68], p = .637).
Table 2.
Postoperative Pain and Analgesic Outcomes in the Groups (sample = 399, or those with long-term follow-up data).
| Control (n = 173) | STOMP (n = 226) | OR/MD [95% CI], p value | |
|---|---|---|---|
| Worst pain intensity (NRS 0–10) | |||
| Average postoperatively | 4.3 ± 2.1 | 4.3 ± 2.0 | MD 0.04 [−0.37, 0.45],.847 |
| Day 7 | 7.0 ± 2.3 | 7.3 ± 2.1 | |
| Day 14 | 4.2 ± 2.6 | 4.3 ± 2.6 | |
| Month 1–3 | 2.9 ± 2.4 | 2.7 ± 2.5 | |
| PROMIS Pain Interference (0–32) | 8.1 ± 7.7 | 6.2 ± 6.9 | MD 1.83 [0.38, 3.27],.013 |
| Days non-opioid used | M 14 IQR 7–30 | M 15 IQR 8–30 | p = .366 * |
| Days opioid used | M 4 IQR 3–7 | M 4 IQR 2–7 | p = .714 * |
| No use (vs. any use) | 20 (11.6%) | 22 (9.8%) | 1.21 [0.64, 2.29],.563 |
| Minimal use (1–4 days) | 106 (61.6%) | 140 (62.5%) | |
| Moderate use (5–9 days) | 20 (11.6%) | 29 (12.9%) | 0.97 [0.56, 1.69],.915 |
| Prolonged use (≥ 10 days, vs less) | 26 (15.1%) | 33 (14.7%) | |
| Postoperative opioid misuse behavior | 12 (7.0%) | 18 (8.0%) | 1.15 [0.54, 2.47],.712 |
| Postoperative opioid misuse intention | 38 (22%) | 47 (20.8%) | 0.93 [0.58, 1.51],.777 |
Abbreviations: NRS = Numeric rating scale; PROMIS = Patient-reported outcome measures information system; Data are presented as mean ± standard deviation; n (%); median (M) with interquartile range (IQR); odds ratio (OR) or mean difference (MD) with 95% confidence intervals (CI)
compared with Mann-Whitney U test
3.2. Aim 1 analysis
Fig. 2 depicts the Analgesic Misuse Risk Perceptions which decreased over time for the Control Group (MD = −2.82 [95% CI −4.02, −1.64], p < .001), but remained significantly unchanged for the STOMP participants (MD = 0.66 [95% CI −0.35, 1.67], p = .200). Results of our first regression model supported our first hypothesis with a significant interaction effect of STOMP and Time on participants’ Analgesic Misuse Risk Perceptions (see Table 3). Specifically, compared to controls, risk perceptions were modestly higher on day 14 and month 3 for the STOMP group controlled for participant baseline factors.
Fig. 2.
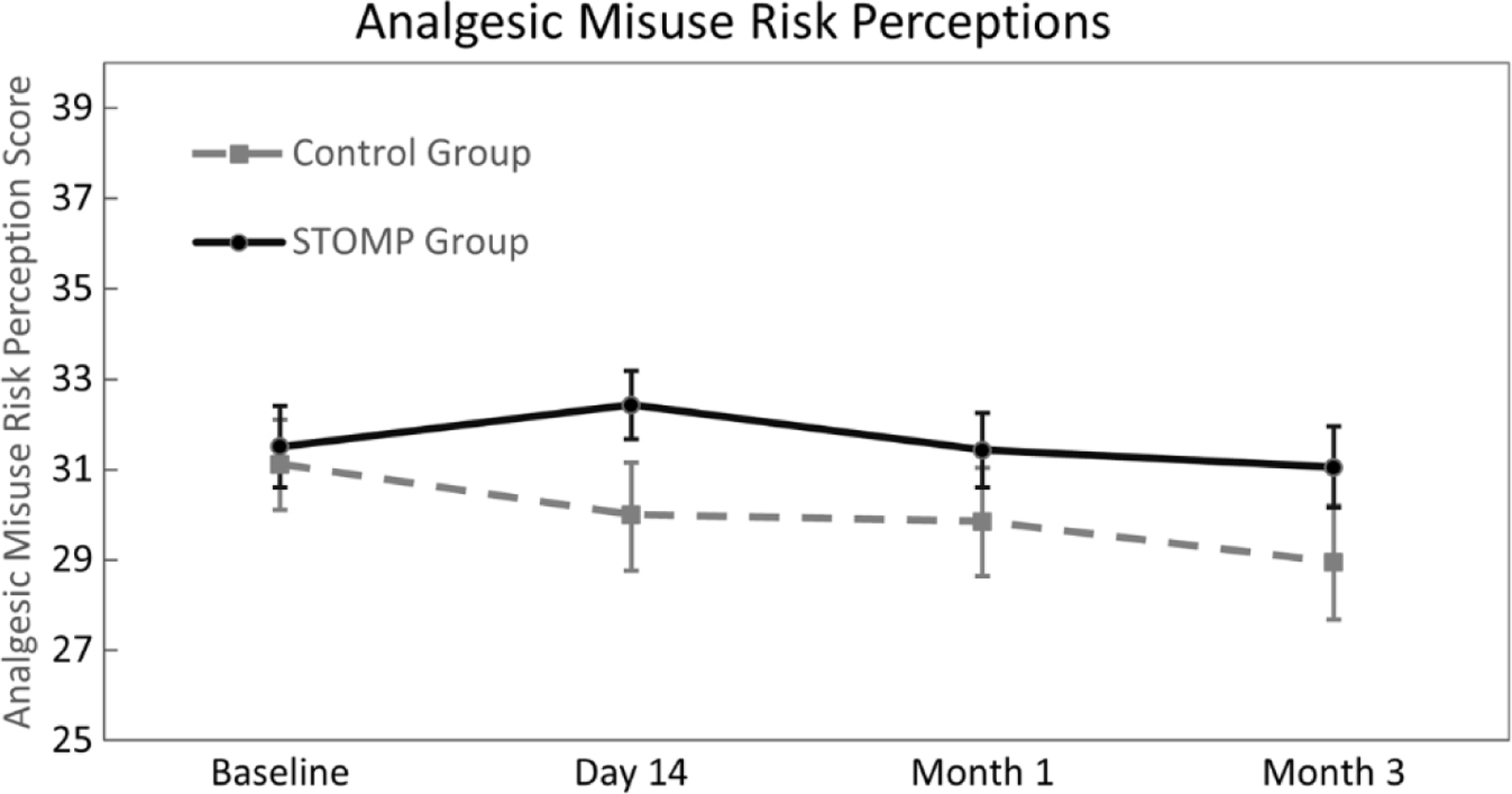
Risk perceptions between groups over time. Repeated measures analysis of variance demonstrated that perceptions decreased from baseline through Month 3 for the Control group (MD = –2.82 [95% CI –4.02, –1.64], p < .001), but remained significantly unchanged from baseline for the STOMP group (MD = 0.66 [95% CI –0.35, 1.67], p = .200).
Table 3.
Effect of STOMP and Time on Analgesic Misuse Risk Perceptions.
| Factor (Reference) | β coefficient [95% CI], p-value |
|---|---|
| STOMP (Control) | 0.39 [−0.86, 1.64],.546 |
| Time (Baseline) | |
| Day 14 | −0.95 [−1.87, −0.03],.043 |
| Month 1 | −1.41 [−2.34, −0.48],.003 |
| Month 3 | −2.68 [−3.64, −1.73], < .001 |
| STOMP*Time (Control) | |
| Day 14 | 1.76 [0.53, 2.99],.005 |
| Month 1 | 1.07 [−0.17, 2.31],.092 |
| Month 3 | 2.13 [0.86, 3.40],.001 |
| Age | 0.11 [−0.10, 0.32],.314 |
| Female sex (Male) | 2.51 [1.41, 3.61], < .001 |
| Non-Hispanic White (Other race/ethnicity) | 0.83 [−0.42, 2.09],.192 |
| Baseline past year opioid use (None) | −1.24 [−2.67, 0.19],.090 |
| Baseline DSM-5 symptom score | −0.05 [−0.12, 0.02],.187 |
| Baseline ASSIST score | −0.22 [−0.36, −0.08],.002 |
Generalized estimating equation (GEE) repeated measures model statistics: Wald χ2 = 92.90 (obs. 1503, obs per group 437); p < .001; time centered at baseline. STOMP = Scenario-Tailored Opioid Messaging Program; CI = Confidence Interval; DSM = Diagnostic and Statistical Manual of Mental Disorders; ASSIST = Alcohol, Smoking and Substance Involvement Screening Test
3.3. Aim 2 analysis
Multinomial regression modeling showed that coded days of use was not associated with group assignment, thus, rejecting our second hypothesis (see Table 4). Instead, compared to no postoperative use, higher pain intensity, analgesic trade-off preferences (i.e., preference for pain relief), and ASSIST scores explained moderate (i.e., 5–9 days) and prolonged postoperative use (≥ 10 days).
Table 4.
Effect of STOMP on Postoperative Days of Opioid Use.
| Factor (reference) | 1–4 days |
5–9 days |
10 or more days |
|---|---|---|---|
| RRR [95% CI], p value | RRR [95% CI], p value | RRR [95% CI], p-value | |
| STOMP (Control) | 1.07 [0.51, 2.27],.852 | 1.13 [0.44, 2.90],.804 | 1.08 [0.43, 2.74],.868 |
| Doses dispensed | 1.03 [0.98, 1.08],.293 | 1.03 [0.96, 1.09],.406 | 1.02 [0.96, 1.09],.459 |
| Age | 1.06 [0.90, 1.25],.466 | 1.01 [0.82, 1.23],.938 | 0.94 [0.77, 1.15],.542 |
| Female sex (Male) | 1.18 [0.53, 2.64],.686 | 0.56 [0.20, 1.53],.256 | 1.20 [0.44, 3.24],.726 |
| Non-Hispanic White (Other race/ethnicity) | 0.88 [0.34, 2.32],.801 | 0.31 [0.10, 0.96],.041 | 0.32 [0.11, 0.98],.045 |
| Procedure type (Sports Medicine) | |||
| Orthopedic | 0.72 [0.25, 2.02],.528 | 0.39 [0.09, 1.73].215 | 0.37 [0.08, 1.69],.199 |
| Oral surgery | 0.67 [0.23, 1.94],.459 | 1.07 [0.28, 4.02].923 | 0.88 [0.24, 3.25],.852 |
| General surgery | 0.64 [0.11, 3.75],.617 | 0.42 [0.03, 5.99].520 | 1.63 [0.21, 12.37],.638 |
| Baseline past year opioid use (None) | 0.94 [0.31, 2.84],.912 | 1.86 [0.50, 6.87],.353 | 1.14 [0.30, 4.34],.847 |
| Baseline DSM-5 symptom score | 1.03 [0.97, 1.09],.322 | 1.01 [0.94, 1.08],.795 | 1.01 [0.95, 1.09],.682 |
| Baseline ASSIST score | 1.19 [1.00, 1.42],.056 | 1.25 [1.03, 1.52],.022 | 1.31 [1.08, 1.58],.005 |
| Postoperative pain intensitya | 1.39 [1.11, 1.74],.004 | 1.68 [1.28, 2.19], < .001 | 1.84 [1.41, 2.39], < .001 |
| Analgesic trade-off preferencea | 1.12 [0.99, 1.26],.072 | 1.18 [1.02, 1.38],.027 | 1.22 [1.05, 1.41],.009 |
| Analgesic misuse risk perceptiona | 0.99 [0.92, 1.06],.755 | 1.02 [0.93, 1.11],.720 | 0.99 [0.91, 1.07],.783 |
Multinomial model statistics: χ2 = 99.06 (df 42); p < .001; r2 = 0.12; reference category “No postoperative opioid use”
Scores averaged after surgery for the analysis
STOMP = Scenario-Tailored Opioid Messaging Program; RRR = Relative Risk Ratio; CI = Confidence Interval; DSM = Diagnostic and Statistical Manual of Mental Disorders; ASSIST = Alcohol, Smoking and Substance Involvement Screening Test
3.4. Aim 3 analysis
Our Aim 3 hypothesis was also rejected as shown in Table 5 where STOMP had no effect on postoperative opioid misuse behavior/intention. Rather, prolonged opioid use after surgery (≥ 10 days) and higher analgesic trade-off preference scores were associated with higher odds of this outcome.
Table 5.
Effect of STOMP on Month 1–3 Opioid Misuse Behavior/Intention.
| Factor (Reference) | OR [95% CI], p-value |
|---|---|
| STOMP (Control) | 1.17 [0.72, 1.90],.534 |
| Age | 0.97 [0.88, 1.07],.569 |
| Female sex (Male) | 1.15 [0.70, 1.91],.580 |
| Non-Hispanic White (Other race/ethnicity) | 0.75 [0.43, 1.31],.307 |
| DSM-5 Symptom Score | 0.97 [0.94, 1.00],.060 |
| ASSIST Score | 1.03 [0.96, 1.10],.438 |
| Doses dispensed | 1.00 [0.97, 1.03],.812 |
| Days opioid used postoperatively (No use) | |
| 1–4 days | 2.20 [0.85, 5.73],.106 |
| 5–9 days | 2.21 [0.70, 7.02],.177 |
| 10 or more days | 3.31 [1.11, 9.87],.032 |
| PROMIS pain interference scorea | 0.96 [0.93, 1.00],.044 |
| Analgesic misuse risk perceptiona | 0.96 [0.93, 1.01],.056 |
| Analgesic trade-off preferencea | 1.09 [1.02, 1.17],.015 |
Logistic regression model statistics: χ2 = 29.70 (df 13); p = .005; r2 = 0.07; reference categoiy “No postoperative opioid use”
Scores averaged after surgery for the analysis
STOMP = Scenario-Tailored Opioid Messaging Program; RRR = Relative Risk Ratio; CI = Confidence Interval; DSM = Diagnostic and Statistical Manual of Mental Disorders; ASSIST = Alcohol, Smoking and Substance Involvement Screening Test
4. Discussion and conclusion
4.1. Discussion
Although we found a significant but modest effect of our STOMP preoperative educational intervention on analgesic misuse risk perceptions in this clinical sample of teens and young adults, there was no effect on opioid use or misuse behavior/intentions during the study period. Moderate to prolonged opioid use after surgery was, instead, explained by participants’ self-reported pain intensity, a trade-off preference for analgesic benefit, and other patient-related factors while misuse behavior/intention was largely explained by longer postoperative days of opioid use and higher trade-off preference scores. These findings suggest that to reduce prescription opioid use and misuse in adolescents and young adults who are prescribed these analgesics, educational interventions may need to focus less on opioid riskiness and, perhaps, more on the potential benefits of non-opioid methods of managing pain.
Previous studies have suggested that higher risk perceptions and negative attitudes about POM may protect against this risky behavior as well as misuse intentions [8,14,15,36]. However, such findings were derived front large, community-based samples of healthy youth that likely had varied motivations for misusing prescription opioids. Our study included only youth who were prescribed opioids to manage postoperative pain. In this clinical sample, risk perceptions were somewhat high at baseline for both groups and remained relatively stable over the study period for the STOMP group but decreased and were lower at follow-up for the control group. Previous data show that risk perceptions can be shaped by risk education but are also strongly influenced by experience [15,24]. Thus, as control participants were exposed to analgesic benefit front their prescribed opioids, their perceptions of risk went down. Our educational intervention successfully stabilized perceptions in the study group; however, misuse risk perceptions were not associated with participants’ use or misuse behavior/intentions. Thus, educational interventions like STOMP that emphasize opioid risks may fall short of reducing use and misuse behaviors among teens and emerging adults, particularly if there is a perceived and experiential benefit to using the opioid analgesic for pain management.
Like our previous studies [24,27,37], higher analgesic trade-off preference scores which indicate a preference for pain relief benefit (versus analgesic risk avoidance) were strongly associated with misuse behavior/intention in our sample. The need to relieve pain remains a common motivation for POM across samples of youth and adults [18, 38]. A recent ecological momentary four-week study of college students demonstrated how day-to-day prescription opioid misuse intentions were strongly associated with higher reported pain [17]. Thus, higher pain combined with greater preferences for analgesic benefit (vs. risk avoidance) may remain significant motivators of POM among youth. This may be particularly germane for those with a painful condition, like recent surgery.
Importantly, longer duration of opioid use after surgery (i.e., ≥ 10 days) was associated with POM behavior/intention in our sample supporting the need to decrease days of use. Previous studies have similarly described associations between longer use, subsequent misuse, and opioid use disorder [5,13,39]. Given such data, policy makers have, in some states like ours, restricted opioid prescribing to 7 days or less. However, patients can extend usage by using a lower prescribed dose over a longer period or by obtaining a refill. In our sample, prolonged use was associated with higher analgesic trade-off preferences and not impacted by our educational intervention. Reshaping pain and analgesic expectations by emphasizing the benefits of non-opioid strategies may, therefore, be a better way to reduce opioid consumption given recent research showing associations between analgesic expectations, earlier opioid tapering, and discontinuation [40].
Although our findings are strengthened by the randomized, controlled study design, the nature of our study sample and methodology pose several limitations to generalizing findings beyond our setting. Firstly, a selection bias may have been introduced since 28% of those eligible approached declined participation and 21% of those who consented were lost to follow-up. Non-participants may have differed from those included in the analyses. For instance, our resultant sample had lower self-reported substance use and opioid misuse compared to rates in community samples of emerging adults [1,2]. Additionally, our sample was more analgesic risk avoidant compared to an earlier sample of community-based teens and young adults [15]. Our intervention may have a larger effect in a population-based sample compared to our clinical that included patients with postoperative pain. Secondly, although explicit verbal and written instructions were given to all STOMP participants to encourage attention to the educational feedback, we could not establish fidelity given the electronic and remote implementation of the intervention. It is therefore possible that some or many participants ignored the STOMP feedback pages partially or entirely. Similarly, although institutional policy had largely standardized postoperative discharge prescription education and start-talking opioid consent practices prior to the study onset, verbal instruction from various clinicians could have differed between participants. Finally, a vast majority of participants were recruited remotely during the Covid-19 pandemic which may have had an unknown impact on symptoms and our findings.
4.2. Conclusion
In summary, although our intervention resulted in stable analgesic misuse risk perceptions that were higher compared to a control sample, we found no impact on prescription opioid use and misuse behavior/intentions during the first three months after surgery.
4.3. Practice implications
It is critically important to consider the potential for medication misuse when educating teens and young adults about their prescription analgesics. Providing information using scenario-tailored feedback may promote risk understanding but may be insufficient to reduce this risky behavior. This may be particularly so when pain is present and not well-managed since pain and the desire to relieve it remain potent motivators for analgesic misuse. Thus, in conjunction with providing risk information, there must be an educational emphasis on use of non-opioid strategies that can reduce opioid use and, perhaps, subsequent misuse. Such emphasis may better shape patient preferences around non-opioid pain-relieving strategies. Educational approaches that flip the balance of information away from opioid risk to the benefits of non-opioid strategies may ultimately be a better approach to reduce opioid use and misuse.
Supplementary Material
Funding source
This work was supported by the National Institutes of Health, National Institute on Drug Abuse, USA, grant #R01DA044245.
Footnotes
CRediT authorship contribution statement
Terri Voepel-Lewis: Conceptualization, Funding acquisition, Methodology, Supervision, Writing - original draft, Writing - review & editing, Administration. Phillip Veliz: Data curation, Formal analysis, Writing - review & editing. Justin Heinze: Methodology, Writing - review & editing. Carol J. Boyd: Conceptualization, Writing - review & editing. Brian Zikmund-Fisher: Conceptualization, Methodology, Writing - review & editing. Rachel Lenko: Investigation, Project administration, Writing - review & editing. John Grant: Conceptualization, Resources, Writing - review & editing. Harrison Bromberg: Investigation, Writing - review & editing. Alyssa Kelly: Investigation, Writing - review & editing. Alan Tait: Conceptualization, Methodology, Writing - review & editing.
Clinical Trial Registration
NCT03863353, Posted March 5, 2019.
Conflict of interest statement
The authors have no conflicts to declare.
Appendix A. Supporting information
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.pec.2022.01.015.
References
- [1].McCabe SE, Schulenberg J, McCabe W, Veliz PT. Medical use and misuse of prescription opioids in US 12th-grade youth: school-level correlates. Pediatrics 2020;146:e20200387. 10.1542/peds.2020-0387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].McCabe SE, West BT, Veliz P, McCabe W, Stoddard SA, Boyd CJ. Trends in medical and nonmedical use of prescription opioids among US adolescents: 1976–2015. Pediatrics 2017;139:e20162387. 10.1542/peds.2016-2387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Miech R, Johnston L, O’Malley PM, Keyes KM, Heard K. Prescription opioids in adolescence and future opioid misuse. Pediatrics 2015;136:ell69–1177. 10.1542/peds.2015-1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Schroeder AR, Dehghan M, Newman TB, Bentley JP, Park KT. Association of opioid prescriptions from dental clinicians for US adolescents and young adults with subsequent opioid use and abuse. JAMA Intern Med 2019;179:145–52. 10.1001/jamaintemmed.2018.5419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Wilson JD, Abebe KZ, Kraemer K, Liebschutz J, Merlin J, Miller E, Kelley D, Donohue J. Trajectories of opioid use following first opioid prescription in opioid-naive youths and young adults. JAMA Netw Open 2021;4:e214552. 10.1001/jamanetworkopen.2021.4552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Johnston LD, Miech RA, O’Malley PM, Bachman JG, Schulenberg JE, Patrick ME. Monitoring the Future national survey results on drug use, 1975–2017: Overview, key findings on adolescent drug use 2018.
- [7].Muench J, Fankhauser K, Voss RW, Huguet N, Hartung DM, O’Malley J, Bailey SR, Cowburn S, Wright D, Barker G, Ukhanova M, Chamine I. Assessment of opioid prescribing patterns in a large network of US community health centers, 2009 to 2018. JAMA Netw Open 2020;3:e2013431. 10.1001/jamanetworkopen.2020.13431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Bonar EE, Coughlin L, Roche JS, Philyaw-Kotov ML, Bixler EA, Sinelnikov S, et al. Prescription opioid misuse among adolescents and emerging adults in the United States: a scoping review. Prev Med 2020;132:105972. 10.1016/j.ypmed.2019.105972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Carmona J, Maxwell JC, Park J-Y, Wu L-T. Prevalence and health characteristics of prescription opioid use, misuse, and use disorders among U.S. adolescents. J Adolesc Health Publ Soc Adolesc Med 2020;66:536–44. 10.1016/j.jadohealth.2019.11.306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Hudgins JD, Porter JJ, Monuteaux MC, Bourgeois FT. Prescription opioid use and misuse among adolescents and young adults in the United States: a national survey study. PLoS Med 2019;16:el002922. 10.1371/journal.pmed.1002922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Jones CM, Clayton HB, Deputy NP, Roehler DR, Ko JY, Esser MB, et al. Prescription opioid misuse and use of alcohol and other substances among high school students - youth risk behavior survey, United States, 2019. MMWR Suppl 2020;69:38–46. https://doi.org/l0.15585/mmwr.su6901a5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Hudgins JD, Porter JJ, Monuteaux MC, Bourgeois FT. Trends in opioid prescribing for adolescents and young adults in ambulatory care settings. Pediatrics 2019;143: e20181578. 10.1542/peds.2018-1578. [DOI] [PubMed] [Google Scholar]
- [13].Pielech M, Kruger E, Rivers WE, Snow HE, Vowles KE. Receipt of multiple outpatient opioid prescriptions is associated with increased risk of adverse outcomes in youth: opioid prescribing trends, individual characteristics, and outcomes from 2005 to 2016. Pain 2020;161:1297–310. 10.1097/j.pain.0000000000001812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Ford JA, Rigg KK. Racial/Ethnic differences in factors that place adolescents at risk for prescription opioid misuse. Prev Sci J Soc Prev Res 2015;16:633–41. 10.1007/s11121-014-0514-y. [DOI] [PubMed] [Google Scholar]
- [15].Voepel-Lewis T, Boyd CJ, McCabe SE, Zikmund-Fisher BJ, Malviya S, Grant J, et al. Deliberative prescription opioid misuse among adolescents and emerging adults: opportunities for targeted Interventions. J Adolesc Health 2018;63:594–600. https://doi.org/l0.1016/j.jadohealth.2018.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].McCabe SE, West BT, Boyd CJ. Medical use, medical misuse, and nonmedical use of prescription opioids: results from a longitudinal study. Pain 2013;154:708–13. 10.1016/j.pain.2013.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Papp LM, Kouros CD, Curtin JJ. Real-time associations between young adults’ momentary pain and prescription opioid misuse intentions in daily life. Am Psychol 2020;75:761–71. 10.1037/amp0000648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Schuler MS, Dick AW, Stein BD. Heterogeneity in prescription opioid pain reliever misuse across age groups: 2015–2017 National Survey on Drug Use and Health. J Gen Intern Med 2020;35:792–9. 10.1007/s11606-019-05559-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Young A, McCabe SE, Cranford JA, Ross-Durow P, Boyd CJ. Nonmedical use of prescription opioids among adolescents: subtypes based on motivation for use. J Addict Dis 2012;31:332–41. 10.1080/10550887.2012.735564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Abraham O, Thakur T, Brown R. Prescription opioid misuse and the need to promote medication safety among adolescents. Res Soc Adm Pharm 2019;15: 841–4. 10.1016/j.sapharm.2019.01.003. [DOI] [PubMed] [Google Scholar]
- [21].Crowley DM, Jones DE, Coffman DL, Greenberg MT. Can we build an efficient response to the prescription drug abuse epidemic? assessing the cost effectiveness of universal prevention in the PROSPER trial. Prev Med 2014;62:71–7. 10.1016/j.ypmed.2014.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Hodder RK, Freund M, Wolfenden L, Bowman J, Nepal S, Dray J, et al. Systematic review of universal school-based “resilience” interventions targeting adolescent tobacco, alcohol or illicit substance use: a meta-analysis. Prev Med 2017;100: 248–68. 10.1016/j.ypmed.2017.04.003. [DOI] [PubMed] [Google Scholar]
- [23].Spoth R, Trudeau L, Shin C, Ralston E, Redmond C, Greenberg M, Feinberg M. Longitudinal effects of universal preventive intervention on prescription drug misuse: three randomized controlled trials with late adolescents and young adults. Am J Public Health 2013;103:665–72. 10.2105/AJPH.2012.301209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Voepel-Lewis T, Malviya S, Grant JA, Dwyer S, Becher A, Schwartz JH, Tait AR. Effect of a brief scenario-tailored educational program on parents’ risk knowledge, perceptions, and decisions to administer prescribed opioids: a randomized controlled trial. Pain 2021;162:976–85. 10.1097/j.pain.0000000000002095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Blalock SJ, Reyna VF. Using fuzzy-trace theory to understand and improve health judgments, decisions, and behaviors: a literature review. Health Psychol 2016;35: 781–92. https://doi.org/10.1037Aea0000384. J Div Health Psychol Am Psychol Assoc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Reyna VF. Risk perception and communication in vaccination decisions: a fuzzy-trace theory approach. Vaccine 2012;30:3790–7. 10.1016/j.vaccine.2011.11.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Voepel-Lewis T, Zikmund-Fisher BJ, Smith EL, Zyzanski S, Tait AR. Parents’ preferences strongly influence their decisions to withhold prescribed opioids when faced with analgesic trade-off dilemmas for children: a prospective observational study. Int J Nurs Stud 2015;52:1343–53. 10.10l6/j.ijnurstu.2015.05.003. [DOI] [PubMed] [Google Scholar]
- [28].NIDA, NIDA-Modified ASSIST VI .0, Natl Inst Health US Dep Health Hum Serv (n. d.) (https://www.drugabuse.gov/sites/default/files/pdf/nmassist.pdf).
- [29].American Psychiatric Association, DSM-5 Self-Rated Level 1 Cross-Cutting Symptom Measure - Adult, 2014. (https://www.psychiatry.org/psychiatrists/practice/dsm/educational-resources/assessment-measures) (accessed 11 June 2021).
- [30].Meaklim H, Swieca J, Junge M, Laska I, Kelly D, Joyce R, et al. The DSM-5 self-rated Level 1 cross-cutting symptom measure identifies high levels of coexistent psychiatric symptomatology in patients referred for insomnia treatment. Nat Sci Sleep 2018;10:377–83. 10.2147/NSS.S173381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Voepel-Lewis T, Burke CN, Jeffreys N, Malviya S, Tait AR. Do 0–10 numeric rating scores translate into clinically meaningful pain measures for children? Anesth Analg 2011;112:415–21. 10.1213/ANE.0b013e318203f495. [DOI] [PubMed] [Google Scholar]
- [32].Amtmann D, Cook KF, Jensen MP, Chen W-H, Choi S, Revicki D, Celia D, Rothrock N, Keefe F, Callahan L, Lai J-S. Development of a PROMIS item bank to measure pain interference. Pain 2010;150:173–82. 10.1016/j.pain.2010.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Cook KF, Bamer AM, Amtmann D, Molton IR, Jensen MP. Six patient-reported outcome measurement information system short form measures have negligible age- or diagnosis-related differential item functioning in individuals with disabilities. Arch Phys Med Rehabil 2012;93:289–1291. 10.1016/j.apmr.2011.11.022. [DOI] [PubMed] [Google Scholar]
- [34].Hanley JA, Negassa A, Edwardes MD, Forrester JE. Statistical analysis of correlated data using generalized estimating equations: an orientation. Am J Epidemiol 2003; 157:364–75. 10.1093/aje/kwf215. [DOI] [PubMed] [Google Scholar]
- [35].Zeger SL, Liang KY, Albert PS. Models for longitudinal data: a generalized estimating equation approach. Biometrics 1988;44:1049–60. [PubMed] [Google Scholar]
- [36].Romberg AR, Rath JM, Miller Lo EJ, Mayo A, Liu M, Vallone DM, Hair EC. Young adults’ opioid prescription history and opioid misuse perceptions. Am J Health Behav 2019;43:361–72. 10.5993/AJHB.43.2.12. [DOI] [PubMed] [Google Scholar]
- [37].Lenko R, Voepel-Lewis T. To relieve pain or avoid opioid-related risk? a comparison of parents’ analgesic trade-off preferences and decision-making in 2019 versus 2013 in a single U.S. pediatric hospital. Paediatr Anaesth 2021;31: 878–84. 10.1111/pan.14209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Han B, Compton WM, Blanco C, Crane E, Lee J, Jones CM. Prescription opioid use, misuse, and disorders in U.S. adults: 2015 National Survey on Drug Use and Health. Ann Intern Med 2017;167:293–301. 10.7326/M17-0865. [DOI] [PubMed] [Google Scholar]
- [39].Brat GA, Agniel D, Beam A, Yorkgitis B, Bicket M, Homer M, et al. Postsurgical prescriptions for opioid naive patients and association with overdose and misuse: retrospective cohort study. BMJ 2018;360:j5790. https://doi.org/10.1136Amj.j5790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Darnall BD, Fields HL. Clinical and neuroscience evidence supports the critical importance of patient expectations and agency in opioid tapering. Pain 2021. 10.1097/j.pain.0000000000002443. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


