Abstract
Rationale
Despite high prevalence of e-cigarette use (vaping), little is currently known regarding the health effects of secondhand nicotine vape exposure.
Objective
To investigate whether exposure to secondhand nicotine vape exposure is associated with adverse respiratory health symptoms among young adults.
Method
We investigated the effect of secondhand nicotine vape exposure on annually reported wheeze, bronchitic symptoms and shortness of breath in the prospective Southern California Children Health Study cohort. Data were collected from study participants (n=2097) with repeated annual surveys from 2014 (average age: 17.3 years) to 2019 (average age: 21.9). We used mixed effect logistic regression to evaluate the association between secondhand nicotine vape and respiratory symptoms after controlling for relevant confounders.
Results
Prevalence of secondhand nicotine vape increased from 11.7% to 15.6% during the study period in this population. Prevalence of wheeze, bronchitic symptoms and shortness of breath ranged from 12.3% to 14.9%, 19.4% to 26.0% and 16.5% to 18.1%, respectively, during the study period. Associations of secondhand nicotine vape exposure with bronchitic symptoms (OR 1.40, 95% CI 1.06 to 1.84) and shortness of breath (OR 1.53, 95% CI 1.06 to 2.21) were observed after controlling for vaping, active and passive exposure to tobacco or cannabis, and demographic characteristics (age, gender, race/ethnicity and parental education). Stronger associations were observed when analysis was restricted to participants who were neither smokers nor vapers. There were no associations with wheezing after adjustment for confounders.
Conclusion
Secondhand nicotine vape exposure was associated with increased risk of bronchitic symptoms and shortness of breath among young adults.
Keywords: tobacco and the lung
Key message.
What is the key question?
Is secondhand vape exposure detrimental to respiratory health in young adults?
What is the bottom line?
Secondhand vape exposure was associated with increased risk of bronchitic symptoms and shortness of breath in young adults, even after accounting for active smoking and vaping.
Why read on?
Primary and secondhand exposure to vaping is highly prevalent among the adolescents and young adults but there has been little research investigating the effects of secondhand vape exposure on respiratory health.
Introduction
The prevalence of e-cigarette use (vaping, largely with nicotine) increased from 11% to 25.4% among 12th graders in the USA between 2017 and 2019.1 Therefore, secondhand nicotine vape exposure is likely to be common. Although there is increasing evidence that e-cigarette use is associated with adverse health outcomes, there has been little study of health effects of secondhand nicotine vape exposure.2 A recent cross-sectional study reported an association between asthma exacerbation and secondhand nicotine vape exposure3; however, to our knowledge, there has been no longitudinal study investigating the chronic effect of secondhand nicotine vape exposure on respiratory health outcomes. This is an important question because, unlike active smoking, secondhand nicotine vape exposure is not a voluntary exposure. Evidence of adverse health effects of secondhand nicotine vape would provide a compelling rationale for reducing home exposure to secondhand nicotine vape, and for restricting vaping in public places, just as adverse health effects of secondhand smoking resulted in regulation of smoking in public places.
Parents often have more relaxed ‘vape-free’ policies at home than ‘smoke-free’ policies.4 5 In a recent survey, smoking inside the home was allowed in 37.4% of homes of current and former smokers, while vaping inside the home was allowed in 60.4% of homes of vapers, as secondhand nicotine vape exposure was perceived to be less harmful than secondhand smoking.6 Although secondhand exposure to particulate matter from e-cigarettes is lower than from combustible cigarettes,7 the ultrafine particle concentration in e-cigarette aerosol can be higher than in cigarette smoke, potentially delivering toxins to the distal airways and alveoli.8 E-cigarette aerosol also contains volatile aldehydes and oxidant metals,9–14 compounds that are known to cause lung toxicity.
Based on the known toxicity of vaping aerosols, we hypothesised that secondhand nicotine vape exposure would be associated with adverse respiratory symptoms such as wheezing, bronchitic symptoms and shortness of breath. We addressed this question in the Southern California Children’s Health Study,15 a cohort with detailed annual information on self-reported active vaping and secondhand nicotine vape exposure, combusted tobacco and cannabis smoke exposure, and self-reported respiratory health outcomes.
Methods
Study design
A baseline (wave 1) population of 2097, 11–12th grade Children Health Study participants (mean age 17.3 years, SD 0.6 years), were enrolled in this study from a cohort from schools throughout Southern California.15 Detailed exposure and health data were collected using self-completed paper questionnaires in classrooms for wave 1 and online using research electronic data capture (REDCap)16 tools hosted at the University of Southern California across three additional waves of follow-up through 2019. The current analysis was limited to 2090 participants who provided data on secondhand nicotine vape exposure in at least one of the four waves. The total number of participants was 2090 in wave 1 (year 2014), 1609 in wave 2 (year 2015), 1502 in wave 3 (year 2017) and 1637 (78.3% of the original sample) in wave 4 (year 2018). An initial loss of 481 participants (23%) in wave 2 occurred during the transition from classroom to online collection after completing high school, some of whom participated in later follow-up. Those who were lost to follow-up at wave- 2 were more likely to be male, Hispanic whites and parents not having any college education, compared with those who remained in the study (online supplemental table S1). The prevalence of secondhand nicotine vape exposure, bronchitic symptoms and wheeze did not differ substantially between those who were lost to follow-up in wave 2 and those who remained at wave 2. Additional description of the cohort has been reported previously.15 17
thoraxjnl-2021-217041supp001.pdf (73.9KB, pdf)
Measures
Respiratory symptoms
Respiratory symptoms assessed, based on prior reports of effects of vaping,15 18 were bronchitic symptoms, wheeze and shortness of breath during the previous year. Bronchitic symptoms (bronchitis, cough or phlegm bronchitic symptoms) were based on reported (1) bronchitis in the previous 12 months, (2) daily cough in the morning for 3 months in a row, (3) daily cough at other times of the day for 3 months in a row or (4) congestion or phlegm other than when accompanied by a cold. Participants were considered to have bronchitic symptoms if they responded yes to any of the four symptoms.15 18 Wheeze was based on a report of wheezing or whistling in the chest during the previous 12 months. Shortness of breath was based on a report of having been troubled by shortness of breath when hurrying on level ground or walking up a slight hill. Information on shortness of breath was collected starting in wave 2 of the study.
Secondhand vaping and smoking exposure
Detailed information on secondhand exposure to e-cigarette aerosol and cigarette smoke was collected at each wave. Secondhand nicotine vape exposure at each wave was defined based on participants’ reports that anyone in the household (other than the participant) used ‘e-cigarette or other electronic nicotine delivery devices’. Secondhand smoking exposure at each wave was defined based on reports of secondhand exposure to combustible tobacco. Secondhand cannabis exposure at each wave was defined based on participants’ responses that anyone in the household ‘smoked or vaped cannabis’. Information on secondhand cannabis was available only in waves 3 and 4.
Primary vaping or smoking was defined based on participants’ reports of any active vaping or smoking of nicotine or cannabis in the previous 30 days (yes/no) at each wave. Use of cannabis was available only in waves 3 and 4.
Sociodemographic information
Information was available on participants’ gender, ethnicity (Hispanic white, non-Hispanic white, Asian, African American and other (ie, Pacific Islander, native American/Alaska Native, multiracial or not defined)), and highest parental educational level, as previously described.15
Statistical analysis
Mixed effect logistic regression models were used to evaluate the association between secondhand nicotine vape exposure in each wave and each respiratory symptom (bronchitic symptoms, wheeze and shortness of breath) in that wave. All models included a participant-level intercept (random effect) to account for multiple observations over time, with adjustment (fixed effect) for sex, race/ethnicity, baseline age and parental education. All models were additionally adjusted for primary vaping or smoking and secondhand smoking/secondhand cannabis exposure. To assess the impact of the timing of exposure, we also fitted a model containing a four-level categorical exposure variable based on the joint distribution of 1-year lagged and current secondhand nicotine vape exposure: no secondhand nicotine vape exposure in the previous and current year (reference group), secondhand nicotine vape exposure in previous year only, secondhand nicotine vape exposure in current year only and secondhand nicotine vape exposure in both previous and current year. As sensitivity analyses, we additionally restricted the sample to participants (1) without primary vaping or smoking, (2) who were not lost to follow-up in wave 2 and (3) who reported no history of physician diagnosed asthma at wave 1. Statistical analyses were conducted using SAS V.9.4 (SAS Institute).
Results
Study population
Among the 2090 study participants, about half were Hispanic white (51.8%) and male (50.4%; table 1). The distribution of the demographic factors of participants with respiratory symptoms was largely similar to that of the overall population, except that participants with shortness of breath were more likely to be female (shortness of breath participants: 70.2% female versus non-shortness of breath participants: 43.4% female, p<0.01).
Table 1.
Demographic characteristics of participants and those who reported wheeze, bronchitic symptoms, shortness of breath or secondhand nicotine vape exposure during the study period*
| Total | Wheeze† | Bronchitic symptoms† | Shortness of breath† | Secondhand nicotine vape exposure† | |
| N=2090 | N=510 | N=811 | N=480 | N=542 | |
| Race | |||||
| Asian | 72 (3.4) | 16 (3.1) | 27 (3.3) | 19 (4.0) | 17 (3.1) |
| Black | 31 (1.5) | 9 (1.8) | 12 (1.5) | 2 (0.4) | 6 (1.1) |
| Hispanic white | 1081 (51.8) | 214 (42.0) | 378 (46.7) | 254 (53.0) | 241 (44.5) |
| Non-hispanic white | 732 (35.1) | 222 (43.5) | 320 (39.5) | 168 (35.1) | 237 (43.7) |
| Others | 172 (8.2) | 49 (9.6) | 73 (9.0) | 36 (7.5) | 41 (7.6) |
| Parental education at baseline | |||||
| Completed grade 12 or less | 574 (27.5) | 113 (22.2) | 185 (22.8) | 142 (29.6) | 128 (23.6) |
| Some college | 648 (31.0) | 164 (32.2) | 268 (33.0) | 154 (32.1) | 208 (38.4) |
| Completed college or more | 622 (29.8) | 183 (35.9) | 279 (34.4) | 136 (28.3) | 153 (28.2) |
| Missing | 246 (11.8) | 50 (9.8) | 79 (9.7) | 48 (10.0) | 53 (9.8) |
| Sex | |||||
| Female | 1036 (49.6) | 258 (50.6) | 419 (51.7) | 337 (70.2) | 264 (48.7) |
| Male | 1054 (50.4) | 252 (49.4) | 392 (48.3) | 143 (29.8) | 278 (51.3) |
*χ2 tests were performed comparing the demographic characteristics of participants with wheeze, bronchitic symptoms, shortness of breath, secondhand nicotine vape exposure to participants without the specific respiratory outcome or secondhand nicotine vape. N (%) is shown in the table. Significant differences (p<0.05) are shown in bold.
†Total number of participants reporting wheeze, bronchitic symptoms, shortness of breath or secondhand nicotine vape exposure, at least once, over the study period (waves 1–4).
Prevalence of vaping and smoking exposure
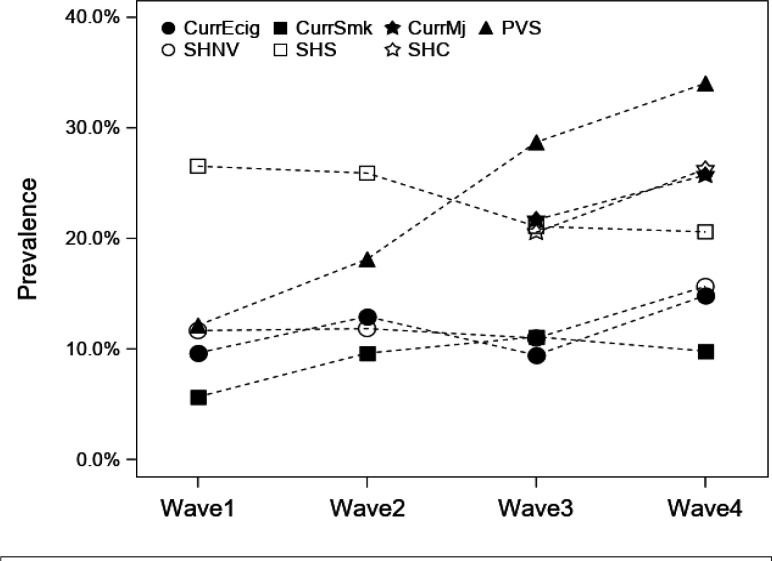
The prevalence of secondhand nicotine vape exposure increased from 11.7% to 15.6% from wave 1 to wave 4 in the study population (figure 1), while the prevalence of secondhand smoking decreased from 26.6% to 20.6%. Past 30-day use of cigarettes, e-cigarettes and cannabis (wave 3 to wave 4) increased over the study period. None of those changes were statistically significant except for the observed increase of primary vaping or smoking from 12.1% in wave 1 to 34.0% in wave 4 (p<0.001).
Figure 1.
Prevalence of personal and secondhand tobacco and cannabis smoking and nicotine vaping during the study period (waves 1–4). CurrEcig, e-cigarette use in past 30 days; CurrMj, smoking or vaping cannabis in past 30 days; CurrSmk, smoking cigarette, cigar or cigarillos in past 30 days; PVS, personal vaping or smoking of nicotine or cannabis products in the previous 30 days; SHNV, secondhand vape exposure (nicotine); SHS, secondhand smoke exposure (tobacco); SHC-secondhand cannabis (vape or smoke) exposure.
A majority of the participants (76.0%–93.1%) who had secondhand nicotine vape exposure during any of the study waves was also likely to have personal use of tobacco or cannabis products or secondhand exposure to combustible products (online supplemental table S2). For example, during wave 4, out of the participants who reported secondhand nicotine vape (n=223), only 6.9% had no other vaping or smoking related exposures, while 11.5% had primary vaping or smoking, but no secondhand smoking exposure, 33.2% had secondhand smoking, but no primary vaping or smoking exposure, and 48.4% had both secondhand smoking and primary vaping or smoking exposure.
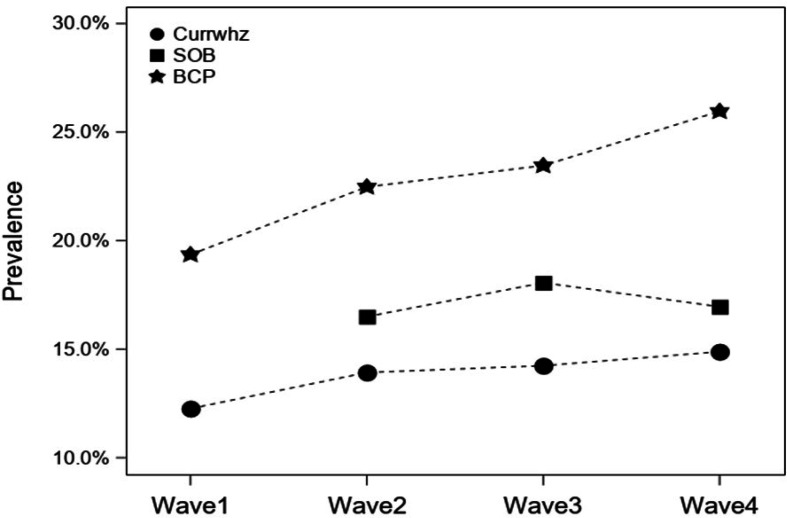
Prevalence of respiratory symptoms
Over the study period, an increase in the prevalence of wheeze and bronchitic symptoms was observed in the study population (not statistically significant). The prevalence increased from 12.3% to 14.9% for wheeze and 19.4% to 26.0% for bronchitic symptoms (figure 2). The prevalence of shortness of breath did not show any clear trend over time, with prevalence of 16.5% in wave 2, 18.1% in wave 3 and 17% in wave 4.
Figure 2.
Prevalence of wheeze, bronchitic symptoms (BCP) and shortness of breath (SOB) during the study period (waves 1–4). BCP, Bronchitic symptoms reported in previous 12 months; Currwhz, wheeze reported in 12 months; SOB, shortness of breath reported in previous 12 months.
Effect of secondhand nicotine vape exposure on respiratory symptoms
Compared with unexposed participants, participants with secondhand nicotine vape exposure had increased odds of reporting wheeze during the same year (OR 1.56, 95% CI 1.12 to 2.17), bronchitic symptoms (OR 1.90, 95% CI 1.48 to 2.44) and shortness of breath (OR 1.87, 95% CI 1.33 to 2.63), after controlling for age, sex, race/ethnicity and parental education (table 2: model 1). After additionally adjusting for secondhand smoking, secondhand cannabis, and primary vaping or smoking, the secondhand nicotine vape exposure associations with bronchitic symptoms (OR 1.40, 95% CI1.06 to 1.84) and shortness of breath (OR 1.53, 95% CI 1.06 to 2.21) were attenuated, and the association between secondhand nicotine vape exposure and wheeze was not statistically significant (table 2: model 2). We observed no statistically significant modifying effect of race/ethnicity, sex, and age on the association between secondhand nicotine vape exposure and respiratory outcomes.
Table 2.
Effect of past 30-day secondhand nicotine vape exposure on wheeze, bronchitic symptoms and shortness of breath; N=2090*
| Wheeze | Bronchitic symptoms | Shortness of breath | |
| OR (95% CI) | OR (95% CI) | OR (95% CI) | |
| Model 1† | 1.56 (1.12 to 2.17) | 1.90 (1.48 to 2.44) | 1.87 (1.33 to 2.63) |
| Model 2‡ | 1.21 (0.84 to 1.72) | 1.40 (1.06 to 1.84) | 1.53 (1.06 to 2.21) |
*Model 1: OR and 95% CI is based on mixed-effect logistic regression with person-specific random intercept and adjustment (fixed effect) for wave, age, sex, race and parental education.
†Model 2: OR and 95% CI is based on mixed-effect logistic regression similar to model 1 with additional adjustment (fixed effect) for primary smoking or vaping of nicotine or cannabis, and secondhand smoking or cannabis smoking or vaping exposure.
‡The OR and 95% CI for wheeze, bronchitic symptoms and shortness of breath is based on mixed-effect logistic regression models.
Sensitivity analysis
We restricted the analysis to the participants who reported no primary vaping or smoking in past 30 days (n=1181) in order to reduce potential for confounding by personal use. A stronger association was observed between secondhand nicotine vape exposure and respiratory symptoms in this sub-population (table 3: sensitivity analysis 1). The odds of reporting wheeze, bronchitic symptoms and shortness of breath were 2.27 (95% CI 0.74 to 6.95), 3.10 (95% CI 1.52 to 6.35) and 2.05 (95% CI 1.01 to 4.19), respectively, among those who reported past 30-day exposure to secondhand nicotine vape, compared with those not reporting current secondhand nicotine vape exposure, after adjusting for demographic factors and secondhand smoking/secondhand cannabis. To assess the potential influence of lost to follow-up in wave 2, we removed those participants from the analysis (table 3: sensitivity analysis 2). The estimates of association between secondhand nicotine vape exposure and respiratory symptoms were similar to those in the corresponding table 2: model 2. To address the possibility that the effect of secondhand nicotine vape exposure was largely among participants with asthma, we restricted the analysis to participants without any history of physician diagnosed asthma at wave 1 (table 3: sensitivity analysis 3). The effect estimate remained largely unchanged from table 2: model 2.
Table 3.
Effect of past 30-day secondhand nicotine vape exposure on wheeze, bronchitic symptoms and shortness of breath in sensitivity analyses restricted to participants with no past 30-day primary vaping or smoking of nicotine or cannabis§
| Wheeze | Bronchitic symptoms | Shortness of breath | |
| OR (95% CI) | OR (95% CI) | OR (95% CI) | |
| Sensitivity analysis 1* | 2.27 (0.74 to 6.95) | 3.10 (1.52 to 6.35) | 2.05 (1.01 to 4.19) |
| Sensitivity analysis 2† | 1.30 (0.90 to 1.87) | 1.39 (1.05 to 1.84) | 1.56 (1.08 to 2.26) |
| Sensitivity analysis 3‡ | 1.15 (0.74 to 1.80) | 1.63 (1.16 to 2.31) | 1.47 (0.93 to 2.32) |
*Sensitivity analysis 1: Analysis was restricted to participants without any reported primary vaping or smoking of nicotine or cannabis in the past 30 days (primary vaping or smoking) (n=1181).
†Sensitivity analysis 2: Participants were included if they participated in both wave 1 and wave 2 (n=1609), adjusted additionally for primary vaping or smoking.
‡Sensitivity analysis 3: Participants who reported no history of physician diagnosed asthma at wave 1 (n=1463), adjusted additionally for primary vaping or smoking.
§OR and 95% CI are based on mixed-effect logistic regression model with person-specific random intercept and adjustment (fixed effect) for wave, age, sex, race, parental education and secondhand smoking or cannabis (smoking or vaping secondhand cannabis) exposure.
Among ever reporters of secondhand nicotine vape exposure, 75.5% reported secondhand nicotine vape in at least two consecutive waves (eg, waves 1 and 2; waves 2 and 3 or waves 3 and 4). Therefore, we investigated whether the timing and consistency of exposure (previous, current and both) had different effects on respiratory symptoms in a subpopulation (n=1609) who had exposure and respiratory symptom information in at least two consecutive waves (online supplemental table S3). After adjusting for demographic factors, primary vaping or smoking, secondhand smoking and secondhand cannabis, no substantial difference was observed in the strength of association of secondhand nicotine vape exposure, based on timing and consistency of exposure, with respiratory symptoms (online supplemental table S3).
Discussion
To our knowledge, this is the first report of effects of secondhand nicotine vape exposure on respiratory symptoms among adolescents and young adults from a prospective study. We show associations of exposure to secondhand nicotine vape with bronchitic symptoms and shortness of breath, after controlling for sociodemographic characteristics, past 30-day active vaping and smoking of tobacco or cannabis products, and secondhand cigarette or cannabis smoke exposure. These results have important public health implications for the population exposed to secondhand nicotine vape. Unlike active vaping and smoking, secondhand nicotine vape exposure is not a voluntary exposure. Therefore, if the associations we observed are causal, prohibiting e-cigarette use in public places is warranted, just as secondhand tobacco smoke exposure has been prohibited in public spaces for decades, and reducing use indoors would reduce the burden of respiratory illness in the households of e-cigarette users. Further epidemiological study is needed to assess the replicability and generalisability of these findings to other age groups. Experimental studies are needed to help determine whether the effects are causal.
secondhand nicotine vape exposure reported at the time of symptom reporting was associated with statistically significant 40% greater odds of bronchitic symptoms and 53% greater odds of shortness of breath, but not with wheeze, compared with individuals with no currently reported secondhand nicotine vape exposure, after controlling for confounders and coexposures. Because this is the first study of effects of secondhand nicotine vape exposure on wheeze, bronchitic symptoms and shortness of breath, we cannot directly compare our results to previous findings. The observed association between secondhand nicotine vape exposure and wheeze (OR 1.21, 95% CI 0.84 to 1.72) in the current study was similar to the association between secondhand nicotine vape exposure and asthma exacerbation in a recent cross-sectional study (OR 1.27, 95% CI 1.11 to 2.47).3 The estimated effects of secondhand nicotine vape exposure on bronchitic symptoms in this study were similar in magnitude to those of secondhand smoking. For example, in a recent study of bronchitic symptoms among Chinese adolescents, the OR for secondhand smoking, compared with adolescents with no secondhand smoking exposure, was 1.53 (95% CI 1.25 to 1.87).19 Bronchitic symptoms are a response to irritant exposures such as secondhand smoking, active smoking and ambient particulate matter, among others.
Increased risk of shortness of breath has also been associated with secondhand smoking.20 The interpretation of shortness of breath associated with secondhand nicotine vape exposure is not clear, but shortness of breath is a central feature of bronchiolitis obliterans that has been reported in workers heavily exposed to diacetyl and 2, 3-pentanedione, which are flavourings that have also been measured in e-cigarette liquids.9 13 21–24 There is a growing body of evidence that vaping is associated with a number of pulmonary diseases, including bronchiolitis obliterans,25 acute respiratory distress syndrome26 and acute eosinophilic pneumonia.27 A recently published report described a case of hypersensitivity pneumonitis with secondhand nicotine vape exposure.28 While only 3 years of data were collected in the current analysis to assess the effect of secondhand nicotine vape exposure on shortness of breath, the observed associations were both moderately strong and robust to confounders. Further study is warranted to determine potential causes of shortness of breath associated with secondhand nicotine vape exposure.
Many study participants with secondhand nicotine vape exposure were also exposed to a variety of other tobacco and cannabis products (online supplemental table S2). Disentangling the effect of secondhand nicotine vape exposure on respiratory symptoms from effects of other products was challenging. However, the models provided stable estimates of secondhand nicotine vape effects after adjustment for coexposures. We also observed stronger effects of secondhand nicotine vape exposure on respiratory symptoms among study participants who reported no past 30-day use of e-cigarette or combustible products (comparing table 3, sensitivity analysis 1 with table 2, model 2).
A potential explanation for the observed association between secondhand nicotine vape exposure and respiratory symptoms is selection bias due to the lost to follow-up of 481 participants following high-school graduation (at wave 2). However, those who remained in the study at wave 2 and those who were lost to follow-up had similar secondhand nicotine vape exposure and respiratory symptoms (online supplemental table S1). There were some modest differences in sociodemographic characteristics between those retained and those lost to follow-up, but all analyses were adjusted for those factors. Furthermore, secondhand nicotine vape exposure effect estimates in analyses restricted to participants not lost to follow-up at wave-2 (table 3, model 2) were similar to the estimates in the entire population (table 2). Therefore, selection bias is not likely to explain the associations with secondhand nicotine vape exposure we observed.
We also assessed whether the associations between secondhand nicotine vape exposure and respiratory symptoms were due to secondhand nicotine vape exposure induced asthma exacerbation.3 However, when we restricted the analysis to participants without any history of asthma at study entry, the secondhand nicotine vape exposure effect estimates (table 3, sensitivity analysis 3) were similar to effects in the entire sample (table 2, model 2). Asthma status of the subjects did not substantially affect the effect estimates observed between secondhand nicotine vape exposure and the respiratory outcomes (online supplemental table S4).
Measurement error due to self-reported exposure and respiratory outcomes could have occurred. Because respiratory health outcomes were reported for last 12 months, it is possible that the participants under-reported symptoms occurring many months back. Exposure misclassification was likely to be small as exposure was assessed for the previous 30 days and seems unlikely to have been substantially differential with respect to outcome that could have biased the estimated effects of exposure.
Conclusion
We observed associations of secondhand exposure to e-cigarettes in the home with bronchitic symptoms and shortness of breath, after accounting for coexposures to other tobacco and cannabis products. The study satisfies several criteria of causation, including prospective assessment of the temporal relationship, relatively strong associations and biological plausibility. However, these findings need to be replicated in other study populations and in study designs including secondhand nicotine vape exposure challenge studies, and merit study in animal models, to establish a causal association between secondhand nicotine vape exposure and respiratory symptoms. If causal, reduction of secondhand e-cigarette exposure in the home would reduce the burden of respiratory symptoms and would provide a compelling rationale for regulation of e-cigarette use in public places.
Footnotes
Twitter: @meradfor
Contributors: TSI was involved in the planning, conducting, drafting and reporting the study and is accountable for findings reported in the manuscript. JB was involved in the drafting and revising. SPE was involved in the data analysis and drafting. FL was involved in data analysis. APT was involved in interpretation and revising. MR was involved in interpretation and revising. JB-T was involved in the acquisition, interpretation and revising, and RM was involved in acquisition, revising, interpretation and final approval of the study.
Funding: The study was supported by National Institutes of Health (grants U54CA180905, R21HD084812, P30ES007048 and 5P30CA014089).
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
No data are available, however, data might be requested through USC TCORS.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
This study was approved by the institutional review board of the University of Southern California (HS-18-00706). Written informed consent was obtained for all participants prior to data collection.
References
- 1. Miech R, Johnston L, O’Malley PM, et al. Trends in adolescent vaping, 2017–2019. N Engl J Med Overseas Ed 2019;381:1490–1. 10.1056/NEJMc1910739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. SHEER . Scientific committee on health EaER. scientific opinion on electronic cigarettes, 2020. Available: https://ec.europa.eu/health/sites/health/files/scientific_committees/scheer/docs/scheer_o_017.pdf
- 3. Bayly JE, Bernat D, Porter L, et al. Secondhand exposure to aerosols from electronic nicotine delivery systems and asthma exacerbations among youth with asthma. Chest 2019;155:88–93. 10.1016/j.chest.2018.10.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Drehmer JE, Nabi-Burza E, Hipple Walters B, et al. Parental smoking and e-cigarette use in homes and cars. Pediatrics 2019;143:e20183249. 10.1542/peds.2018-3249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Li D, Shi H, Xie Z, et al. Home smoking and vaping policies among US adults: results from the population assessment of tobacco and health (path) study, wave 3. Prev Med 2020;139:106215. 10.1016/j.ypmed.2020.106215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Nahhas GJ, Braak D, Cummings KM, et al. Rules about smoking and vaping in the home: findings from the 2016 international tobacco control four country smoking and vaping survey. Addiction 2019;114 Suppl 1:107–14. 10.1111/add.14579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. National Academies of Sciences E, Medicine, Health . Eaton DL, Kwan LY, Stratton K, eds. Public health consequences of e-cigarettes. Washington (DC): National Academies Press (US), 2018. [PubMed] [Google Scholar]
- 8. Palmisani J, Di Gilio A, Palmieri L, et al. Evaluation of second-hand exposure to electronic cigarette vaping under a real scenario: measurements of ultrafine particle number concentration and size distribution and comparison with traditional tobacco smoke. Toxics 2019;7:59. 10.3390/toxics7040059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Barrington-Trimis JL, Samet JM, McConnell R. Flavorings in electronic cigarettes: an unrecognized respiratory health hazard? JAMA 2014;312:2493–4. 10.1001/jama.2014.14830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Talih S, Balhas Z, Salman R, et al. "Direct dripping": a high-temperature, high-formaldehyde emission electronic cigarette use method. Nicotine Tob Res 2016;18:453–9. 10.1093/ntr/ntv080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Jensen RP, Luo W, Pankow JF, et al. Hidden formaldehyde in e-cigarette aerosols. N Engl J Med Overseas Ed 2015;372:392–4. 10.1056/NEJMc1413069 [DOI] [PubMed] [Google Scholar]
- 12. Saffari A, Daher N, Ruprecht A, et al. Particulate metals and organic compounds from electronic and tobacco-containing cigarettes: comparison of emission rates and secondhand exposure. Environ Sci Process Impacts 2014;16:2259–67. 10.1039/C4EM00415A [DOI] [PubMed] [Google Scholar]
- 13. Allen JG, Flanigan SS, LeBlanc M, et al. Flavoring chemicals in e-cigarettes: diacetyl, 2,3-pentanedione, and acetoin in a sample of 51 products, including fruit-, candy-, and cocktail-flavored e-cigarettes. Environ Health Perspect 2016;124:733–9. 10.1289/ehp.1510185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lee M-S, LeBouf RF, Son Y-S, et al. Nicotine, aerosol particles, carbonyls and volatile organic compounds in tobacco-and menthol-flavored e-cigarettes. Environ Health 2017;16:42. 10.1186/s12940-017-0249-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. McConnell R, Barrington-Trimis JL, Wang K, et al. Electronic cigarette use and respiratory symptoms in adolescents. Am J Respir Crit Care Med 2017;195:1043–9. 10.1164/rccm.201604-0804OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Harris PA, Taylor R, Thielke R, et al. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009;42:377–81. 10.1016/j.jbi.2008.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. McConnell R, Berhane K, Yao L, et al. Traffic, susceptibility, and childhood asthma. Environ Health Perspect 2006;114:766–72. 10.1289/ehp.8594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gotts JE, Jordt S-E, McConnell R, et al. What are the respiratory effects of e-cigarettes? BMJ 2019;1:l5275. 10.1136/bmj.l5275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Chen Z, Liu G, Chen J, et al. Frequency–risk relationships between second-hand smoke exposure and respiratory symptoms among adolescents: a cross-sectional study in South China. BMJ Open 2018;8:e019875. 10.1136/bmjopen-2017-019875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. LC A, Berg CJ, Klatt CM. Symptoms of cough and shortness of breath among occasional young adult smokers. Nicotine Tob Res 2009;11:126–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Klager S, Vallarino J, MacNaughton P, et al. Flavoring chemicals and aldehydes in e-cigarette emissions. Environ Sci Technol 2017;51:10806–13. 10.1021/acs.est.7b02205 [DOI] [PubMed] [Google Scholar]
- 22. Kreiss K, Gomaa A, Kullman G, et al. Clinical bronchiolitis obliterans in workers at a microwave-popcorn plant. N Engl J Med Overseas Ed 2002;347:330–8. 10.1056/NEJMoa020300 [DOI] [PubMed] [Google Scholar]
- 23. Kreiss K. Work-related spirometric restriction in flavoring manufacturing workers. Am J Ind Med 2014;57:129–37. 10.1002/ajim.22282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Cummings KJ, Boylstein RJ, Stanton ML, et al. Respiratory symptoms and lung function abnormalities related to work at a flavouring manufacturing facility. Occup Environ Med 2014;71:549–54. 10.1136/oemed-2013-101927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Landman ST, Dhaliwal I, Mackenzie CA, et al. Life-threatening bronchiolitis related to electronic cigarette use in a Canadian youth. Can Med Assoc J 2019;191:E1321–31. 10.1503/cmaj.191402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lilly CM, Khan S, Waksmundzki-Silva K, et al. Vaping-associated respiratory distress syndrome: case classification and clinical guidance. Crit Care Explor 2020;2:e0081. 10.1097/CCE.0000000000000081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Chaaban T. Acute eosinophilic pneumonia associated with non-cigarette smoking products: a systematic review. Adv Respir Med 2020;88:142–6. 10.5603/ARM.2020.0088 [DOI] [PubMed] [Google Scholar]
- 28. Galiatsatos P, Gomez E, Lin CT, et al. Secondhand smoke from electronic cigarette resulting in hypersensitivity pneumonitis. BMJ Case Rep 2020;13:e233381. 10.1136/bcr-2019-233381 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
thoraxjnl-2021-217041supp001.pdf (73.9KB, pdf)
Data Availability Statement
No data are available, however, data might be requested through USC TCORS.