Abstract
Intravenous thrombolysis with recombinant tissue plasminogen activator (rtPA) within 3 h of symptom onset is currently approved for treatment of acute ischemic stroke. Those who present within 3 h and have a vascular occlusion and a good CT scan are the ideal candidates for thrombolysis. Clinical trials and phase IV data has shed substantial light on the factors associated with more favorable outcomes with tPA. In the 3-6 h time window, cerebral perfusion information can be used for selection of patients for thrombolytic therapy. In many special circumstances, such as seizure at stroke onset, stroke on awakening, age more than 80 years, and patients with rapidly improving symptoms, the decision to treat depends on expert judgment. Due to the narrow time window, the fear of bleeding complications, and doubts regarding its effectiveness, tPA is still underused. Constant efforts are required to educate the public on the fact that stroke is a treatable emergency.
Keywords: Acute ischemic stroke, thrombolysis
Intravenous thrombolysis with recombinant tissue plasminogen activator (rtPA) within 3 h of symptom onset is currently approved for treatment of acute ischemic stroke. It improves the rate of favorable outcomes despite the risk of hemorrhagic transformation. The number needed to treat to prevent one death or disability in this time window is 8.[1] Patients with mild to moderate strokes, younger persons, and those treated very early have the best chance for a favorable outcome.[2,3] Unfortunately, this life-saving, disability-reducing drug is still underused, the important reasons being the narrow time window, the fear of bleeding complications, doubts regarding its effectiveness, and economic constraints. Constant efforts are required to educate the public that stroke is a treatable emergency.
Randomized controlled trials for intravenous thrombolysis
There have been seven randomized controlled trials evaluating the role of intravenous thrombolytic agents in acute ischemic stroke.[1,4,5,6,7,8,9] The major trials investigating streptokinase used 1.5 MU of streptokinase up to 6 h after stroke onset.[4,6,7] All the three streptokinase studies were terminated early due to increased mortality and significant increase in the rate of symptomatic intracerebral hemorrhage (SICH), thus ending the hopes for the use of streptokinase as a treatment for stroke. The high SICH rate in the streptokinase trials is due to the large doses of streptokinase used, the large strokes, and the delay in starting treatment. The first reported trial of rtPA was the European Cooperative Acute Stroke Study (ECASS I), which revealed a significant increase in SICH in the tPA-treated group (19.8%)vscontrols (6.5%); there was also an increase in the 30-day mortality in the tPA-treated group.[5] The importance of early ischemic changes on CT were brought to light in this study, which led to improvement in safety standards in subsequent studies. The relatively high dose of tPA (1.1 mg/kg) that was used in ECASS 1 as compared to the other studies (which used 0.9 mg/kg) and the time window of 6 h (mean time to treatment was 4.5 h) might also have contributed to the increase in the SICH rate. In the ECASS II, more stringent adherence to rigid blood pressure control and CT criteria resulted in lower rates of SICH (8.8% in the tPA-treated group vs 3.4% in the control group). Although the primary outcome did not demonstrate significant benefit from tPA, other important clinical outcomes [modified Rankin Scale (mRS) 0-2] suggested some effect.[8] The landmark trial for FDA approval for tPA was based on the National Institute of Neurological Disorders and Stroke Study Group (NINDS) trials.[1] The NINDS trial (partsA and B)was the first and only major trial to demonstrate the clear benefit of using intravenous tPA in acute ischemic stroke. The global statistic simultaneously tested for effect in all four outcome measures: National Institute of Health Stroke Scale Score (NIHSS), Barthel Index (BI), Glasgow Coma Scale (GOS), and mRS. Despite a SICH rate of 6.4%, there was a 12% absolute increase in the number of patients with minimal or no disability in the rtPA-treated group compared to placebo. Approximately 1 in 5 (20%) patients also demonstrated dramatic neurologic improvement within 24 h (NIHSS 14-point improvement) compared to 4% of patients receiving placebo. The most important finding in the NINDS trial was the relationship between time-to-treatment and efficacy. It was found that patients treated within 0-90 min had more benefit than those treated within 90-180 min.[10] Later, in an effort to extend the time window for treatment, the Alteplase Thrombolysis for Acute Noninterventional Therapy in Acute Ischemic Stroke (ATLANTIS) trial investigated the efficacy of tPA used within 3-5 h of onset of stroke.[9] Unfortunately, statistically significant benefit was not demonstrated, with high rates of SICH in the tPA group (7%) compared to the controls (1.1%). However, a meta-analysis of all four tPA stroke trials suggests that this drug is beneficial not only within the standard 3-h time window, but up to 4.5 h after stroke onset.[3]
Phase IV experience
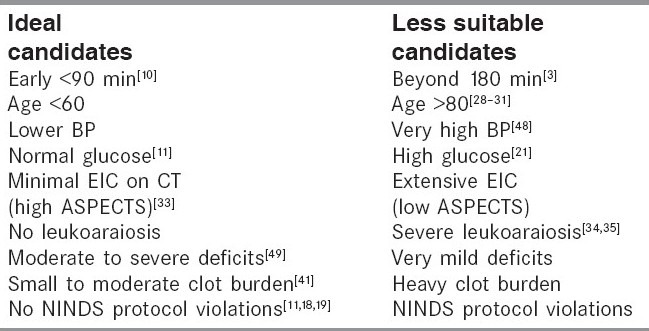
There have been numerous phase IV studies since the publications of the randomized trials. These trials have provided insights about who is ideal and who is less suitable for tPA treatment [Table 1]. The Canadian Alteplase for Stroke Effectiveness Study (CASES), which was a prospective study to assess the effectiveness of alteplase in routine clinical practice, showed outcomes similar to the NINDS study.[11] In the multivariate analysis, an elevated serum glucose level before treatment and increased time from stroke onset to treatment were independent predictors of SICH. Similarly the Safe Implementation of Thrombolysis in Stroke-Monitoring Study (SITS-MOST) confirmed that alteplase is safe and effective in routine clinical settings when used within 3 h of symptom onset. SITS-MOST demonstrated low SICH rates (1.7-7.3%, depending on definition) in over 6400 tPA-treated patients from 285 stroke centers in 14 countries. Functional independence was achieved by 55%.The low SICH rate and the better functional outcome is probably related to the rigorous protocol, which excluded patients with severe strokes (NIHSS > 24), patients more than 80 years, and those with disability. Importantly, SICH rates were not higher in centers new to thrombolytic therapy.[12]
Table 1.
Selection of ideal candidates for tPA
The risk of SICH
Although thrombolysis clearly has the potential to benefit individual patients, there is always the question of the safety of rtPA in view of the risk of SICH. In the NINDS trial, SICH was defined as a CT-documented hemorrhage temporally related to clinical deterioration as judged by the treating physician. The recent definitions are more specific and describe a hemorrhage as symptomatic if the patient has a clinical deterioration causing an increase in the NIHSS score of ≥ 4 points. In the ECASS study, intracerebral hemorrhage was divided by CT appearance into hemorrhagic infarction (HI) or parenchymal hematoma (PH). HI was defined as petechial hemorrhage within the infarct without a space-occupying effect and PH was defined as a confluent, hyperdense hematoma with a space-occupying effect. In the NINDS rtPA stroke trial, the risk factors for SICH were severity of neurological deficit at baseline and the presence of early ischemic changes in the pretreatment scan.[13] However, further analysis of the early ischemic changes in the CT scans showed that this was not an independent predictor of SICH, although NIHSS was an independent predictor.[47] In the ECASS I and II, risk factors for SICH and HI were severity of neurological deficits at baseline, presence of early ischemic changes on the pretreatment scan, congestive heart failure, increasing age, and treatment with aspirin before thrombolysis. Similar to randomized controlled trials, data from real-life clinical practice of 1205 patients found that history of diabetes, cardiac disease, increasing stroke severity, advancing age, use of antiplatelet drugs other than aspirin before stroke onset, elevated pretreatment mean blood pressure, elevated serum glucose, and early ischemic CT changes were associated with tPA-related ICH.[14] It is likely that HI carries a much better prognosis for patients than PH. A trend towards good neurological recovery at 24 h was seen in patients with HI, suggesting that HI may be a marker of early successful reperfusion.[15,16] All the previous studies evaluating SICH focused on clinical and CT-based variables for assessing the hemorrhagic risk after thrombolysis. A recent study on MRI variables showed that a large baseline Diffusion Weighted Imaging (DWI) lesion which achieves early reperfusion is at the greatest risk of SICH after tPA therapy.[17]
NINDS protocol violation is high risk
Careful selection of patients for thrombolytic therapy is very important for a favorable outcome. There is evidence to suggest that when the NINDS protocol is violated, the rate of SICH increases. In a study of data from 29 hospitals in Cleveland, there was 15.7% SICH when the protocol violations were 50%. With decrease in the protocol violations to 19%, the SICH rate dropped to 6.4%.[18,19] In a study from Indianapolis by Yunez et al. the rate of SICH was 38% in patients with protocol violations and 5% when the NINDS protocol was followed.[20]
Hyperglycemia and tPA
A clear association has been shown between baseline serum glucose and / or diabetes with ICH after tPA.[13,20] Marked hyperglycemia produces damaging effects on the microvasculature with increased edema and hemorrhagic transformation after reperfusion. With every 5.5 mmol/l increase of serum glucose, SICH increased significantly (OR: 2.26; 95% CI 1.05 to 4.83) A serum glucose of > 11.1 mmol/l was associated with a 25% symptomatic hemorrhage rate.[21]
Stroke severity and tPA
Initial stroke severity assessed by NIHSS score was consistently found to be an independent predictor of ICH after tPA,.[5,8,13,14] but these patients should not be denied thrombolytic therapy as they tend to benefit the most. The ideal NIHSS for tPA benefit was NIHSS 6-15.[11] Prognosis of patients with mild neurological deficit is not necessarily favorable. About one-third of acute stroke patients who had rapid improvement of neurological deficits after arrival in hospital had severe neurological deterioration later.[22,23] Thrombolysis can improve the outcome in these patients.[24]
Intravenous thrombolysis in the elderly
Although patients more than 80 years of age account for 30% of patients with strokes, the role of intravenous thrombolysis in this age-group is less well defined. There are no randomized trials that focus specifically on elderly patients with acute ischemic stroke. Recent cohort studies comparing intravenous rtPA-treated stroke patients aged > 80 years with those younger than 80 years showed inconsistent findings. In some studies, the risk of SICH in the elderly was higher than in younger patients,[26,27] whereas in other studies this was not so.[25,28,29,30] A systematic review showed that intravenous rtPA-treated patients aged > 80 years have a less favorable outcome than younger patients, but the risk of SICH is comparable between the two age-groups.[31] To get more reliable evidence, we need to include more such patients in randomized controlled studies. A stroke physician who sees an elderly patient with an acute ischemic stroke should carefully assess the riskvsthe benefit in that patient and the exclusion criteria should be rigidly applied before the decision to treat. Therefore, an elderly patient who presents within 3 h of stroke onset and has good prestroke functioning and quality of life should still be considered for thrombolytic treatment, although the expectations for full recovery should be tempered.
Ischemic changes on imaging and tPA
One imaging parameter consistently associated with increased risk for SICH is the presence of early infarct signs on CT.[5,8,13] The ECASS investigators found that if more than one-third of the Middle Cerebral Artery (MCA) MCA territory showed signs of ischemia, there was a higher risk of SICH. An alternative approach for grading early signs of ischemia on the baseline CT scan is the Alberta Stroke Program Early CT Score (ASPECTS). It is a 10-point scale that rates absence or presence of ischemia in 10 regions of the brain.[46] The entire MCA territory is evaluated with this scale for the extent of early ischemic changes. The scale provides a systematic approach to evaluating subtle early ischemic changes. The ASPECTS score found a strong association with independent outcome after thrombolysis when ASPECTS was dichotomized 0 through 7 (moderate to severe early ischemic change)vs8 through 10 (mild / no early ischemic change). [32] Review of the CT scans from the NINDS trial revealed greatest benefit from tPA in high ASPECTS score patients (8-10), with more modest benefit seen with moderate MCA territory involvement (ASPECTS 3-7).[33] Severe leukoaraiosis is also an independent risk factor for SICH after thrombolysis[34]; however, in the study by Palumbo et al., though there was an increase in the risk of SICH, the clinical outcome at 3 months was not affected.[35]
Future role of neuroimaging beyond 3 hours
Studies have shown that selection of patients for thrombolysis should not only depend on time but on imaging information as well, which helps to decide the risk and benefit of thrombolysis. The Desmoteplase in Acute Ischemic Stroke (DIAS) trial, which examined the efficacy and safety of desmoteplase administered 3-9 h after ischemic stroke, showed that in selected patients with a diffusion-perfusion mismatch, there was increased reperfusion with better clinical outcome.[36] A recent study, Diffusion and Perfusion Imaging Evaluation for Understanding Stroke Evolution (DEFUSE), showed that patients with diffusion-perfusion mismatch on MR were more likely to have early recanalization and better outcome with intravenous tPA given within 3-6 h after stroke onset. This was further supported by the recently published EPITHET trial:[37] those without a diffusion-perfusion mismatch did not benefit from tPA. Those with large DWI lesions who reperfused were at the maximum risk of clinical worsening and ICH.[17] However, the use of tPA beyond 3 h should only be as part of institutional protocol or clinical trial.
Imaging before thrombolysis
Intravenous treatment with rtPA is approved in patients with acute ischemic stroke within 3 h after CT-based exclusion of ICH. Recent prospective open and nonrandomized analysis suggested that MRI-based treatment yields comparable or higher safety with regard to symptomatic intracerebral bleeds and is as effective as CT-based thrombolysis.[38,39] This is based on the perfusion imaging / diffusion-weighted imaging (PI/DWI) mismatch concept, where the area of decreased diffusion represents the ischemic core of the infarct and the PWI/DWI mismatch is a marker of critically hypoperfused, yet potentially salvageable, brain tissue (ischemic penumbra). In a recent study, MRI-based thrombolysis appeared to be safer and more effective than standard CT-based thrombolytic therapy.[40] But in a hyperacute setting, noncontrast CT combined with CT angiography (CTA) appears ideal and provides all the information for decision on thrombolysis. Recent work suggests CTA may be ideal to evaluate the extent of thrombus burden, which may be critical to successful recanalization and outcome.[41] In the 3-h time window, whether selection of patients for thrombolysis could be improved by evaluation of brain perfusion is not known, but during this period cerebral perfusion information can be used for selection of patients for thrombolytic therapy. However, comparative studies have shown that perfusion CT and DWI/PWI are equivalent.[42] With perfusion CT, identification of the final infarct and the tissue at risk were well correlated with the DWI/PWIMR results. With the available evidence, within the 3-h time window, the choice of imaging modality may be at the discretion of the treating physician and may depend on the institutional protocols, but beyond the 3-h time window, more patients can be safely selected for thrombolytic treatment on the basis of the MRI penumbral pattern.
What we do
In our hospital, if the patient comes within the 3-h time window, a CT head is done first followed by a CTA. The CTA gives information on the arterial status and the mechanism of stroke. This helps us to decide on the need for intraarterial therapy. Irrespective of whether they have occlusion or not, patients receive IV thrombolysis with alteplase at a dose of 0.9 mg/kg, with 10% of the dose given as a bolus and rest as an infusion over the next 1 h. In case there is a major arterial occlusion of the internal carotid, middle cerebral, or basilar artery, IV therapy is followed by intraarterial therapy with alteplase if there is no improvement within 30 min of IV tPA. In situations with no vessel occlusion, within the 3-h time window patients still receive IV tPA since distal occlusions can be missed in CTA and lacunar strokes still benefit from tPA. If, however, the patient is improving clinically and no occlusion is visible, tPA may be withheld because major spontaneous recovery can be anticipated. In patients who come after 3 h, there is no consensus regarding tPA administration. We often have patients undergo additional vascular imaging (CT, CTA, CT perfusion, or multimodal MRI with DWI/PWI) and the decision to treat depends on the ischemic penumbra. In patients who come after 3 h, selection for tPA treatment is on a case-by-case basis and needs additional penumbral imaging data. A large North American trial (the Interventional Management of Stroke-IMS III study) is ongoing, comparing IV tPA with combined IV and intraarterial tPA therapy; this may give more information regarding selection of patients for intraarterial therapy.
Problems for thrombolysis
The major hindrance to thrombolytic therapy, both in developed and developing countries is the delay in patients reaching the hospital. There are many other issues of importance in India which needs to be looked into. There is a lack of an organized health care system; an Emergency medical services (EMS) system is almost nonexistent, which further increases the delay.[43] About 80% of the population live in rural areas where the health care infrastructure is poor and there are many cultural beliefs and traditional medicines.[44] The cost of treatment has to be borne by the patient as health insurance is not very prevalent in the country; most of the patients cannot afford the cost of treatment.
Present status of thrombolytic therapy
Although thrombolysis is the only effective treatment in patients with acute ischemic stroke, < 10% of the patients receive thrombolysis. Those who present within 3 h and have a vascular occlusion and a good CT scan are ideal candidates for thrombolysis. Present guidelines exclude patients who wake up with symptoms, patients with seizure at stroke onset, and those with mild and improving symptoms. A recent study showed that CTA was a useful modality in differentiating Todd's paralysis from early seizure with ischemia by detection of intracranial occlusion and may contribute to decision making for thrombolysis.[45]
Conclusion
Acute ischemic stroke is a medical emergency and requires urgent treatment. Clinical trial and phase IV data has shed substantial light on the factors associated with more favorable outcome following treatment with tPA [Table 1]. In many special circumstances, like seizure at stroke onset, stroke on awakening, age more than 80 years, and those with rapidly improving symptoms, the decision to treat depends on expert judgment. Physicians should use modern brain and vascular imaging to aid decision making for thrombolytic therapy. Intracranial arterial occlusion is the target of treatment and early recanalization the goal. Successful use of intravenous thrombolysis depends on an understanding of the inclusion and exclusion criteria, good organization of the treating team who strives for early treatment initiation, and strict adherence to the protocol. Intravenous rtPA within 3 h of onset should now be standard treatment for acute disabling ischemic stroke throughout the world.48,49]
Footnotes
Source of Support: Nil
Conflict of Interest: Nil.
References
- 1.NINDS The National Institute of Neurological Disorders and Stroke rtPA Stroke Study Group. Tissue plasminogen activator for acute ischemic stroke. N Engl J Med. 1995;333:1581–7. doi: 10.1056/NEJM199512143332401. [DOI] [PubMed] [Google Scholar]
- 2.The NINDS t-PA Stroke Study Group. Generalized efficacy of t-PA for acute stroke: Subgroup analysis of the NINDS t-PA Stroke Trial. Stroke. 1997;28:2119–25. doi: 10.1161/01.str.28.11.2119. [DOI] [PubMed] [Google Scholar]
- 3.Hacke W, Donnan G, Fieschi C, Kaste M, vonKummer R, Broderick JP, et al. Association of outcome with early stroke treatment: Pooled analysis of ATLANTIS, ECASS and NINDS rt-PA stroke trials. Lancet. 2004;363:768–74. doi: 10.1016/S0140-6736(04)15692-4. [DOI] [PubMed] [Google Scholar]
- 4.Multicentre Acute Stroke Trial-Italy Group. Randomized controlled trial of streptokinase, aspirin and combination of both in treatment of acute ischemic stroke. Lancet. 1995;346:1509–14. [PubMed] [Google Scholar]
- 5.Hacke W, Kaste M, Fieschi C, Toni D, Lesaffre E, vonKummer R, et al. Intravenous thrombolysis with recombinant tissue plasminogen activator for acute hemispheric stroke. The European Co-operative Acute Stroke Study (ECASS) JAMA. 1995;274:1017–25. [PubMed] [Google Scholar]
- 6.Europe Study Group. Thrombolytic therapy with streptokinase in acute ischemic stroke: The Multicentre Acute Stroke Trial. N Engl J Med. 1995;335:145–50. doi: 10.1056/NEJM199607183350301. [DOI] [PubMed] [Google Scholar]
- 7.Donnan GA, Davis SM, Chambers BR, Gates PC, Hankey GJ, McNeil JJ, et al. Streptokinase for acute ischemic stroke with relationship to time of administration. JAMA. 1996;276:961–6. [PubMed] [Google Scholar]
- 8.Hacke W, Kaste M, Fieschi C, vonKummer R, Davalos A, Meier D, et al. Randomized double blind placebo controlled trial of thrombolytic therapy with intravenous alteplase in acute ischemic stroke (ECASS II), Second European- Australian Acute Stroke Study Investigators. Lancet. 1998;352:1241–5. doi: 10.1016/s0140-6736(98)08020-9. [DOI] [PubMed] [Google Scholar]
- 9.Clark WM, Wissman S, Albers GW, Jhamandas JH, Madden KP, Hamilton S, et al. Recombinant tissue type plasminogen activator (alteplase) for ischemic stroke 3 to 5 h after symptom onset The ATLANTIS Study: A randomized controlled trial Alteplase Thrombolysis for Acute Noninterventional Therapy in Ischemic Stroke. JAMA. 1999;282:2019–26. doi: 10.1001/jama.282.21.2019. [DOI] [PubMed] [Google Scholar]
- 10.Marler JR, Tilley BC, Lu M, Brott TG, Lyden PC, Grotta JC, et al. Early stroke treatment associated with better outcome: The NINDS rt-PA stroke study. Neurology. 2000;55:1649–55. doi: 10.1212/wnl.55.11.1649. [DOI] [PubMed] [Google Scholar]
- 11.Hill MD, Buchan AM for the Canadian Alteplase for Stroke Effectiveness Study (CASES) Investigators. Thrombolysis for acute ischemic stroke: Results of the Canadian Alteplase for Stroke Effectiveness Study. CMAJ. 2005;172:1307–12. doi: 10.1503/cmaj.1041561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wahlgren N, Ahmed N, Davalos A, Ford GA, Grond M, Hacke W, et al. Thrombolysis with alteplase for acute ischemic stroke in Safe Implementation of Thrombolysis in Stroke -Monitoring Study (SITS-MOST): An observational study. Lancet. 2007;369:275–82. doi: 10.1016/S0140-6736(07)60149-4. [DOI] [PubMed] [Google Scholar]
- 13.The NINDS t-PA Stroke Study Group. Intracerebral hemorrhage after intravenous t-PA therapy for ischemic stroke. Stroke. 1997;28:2109–18. doi: 10.1161/01.str.28.11.2109. [DOI] [PubMed] [Google Scholar]
- 14.Tanne D, Kasner SE, Demchuk AM, Koren-Morag N, Hanson S, Grond M, et al. Markers of increased risk of intracerebral hemorrhage after intravenous recombinant tissue plasminogen activator therapy for acute ischemic stroke in clinical practice: The Multicenter rt-PA Acute Stroke Survey. Circulation. 2002;105:1679–85. doi: 10.1161/01.cir.0000012747.53592.6a. [DOI] [PubMed] [Google Scholar]
- 15.Fiorelli M, Bastianello S, vonKummer R, del Zoppo GJ, Larrue V, Lesaffre E, et al. Hemorrhagic transformation within 36 hours of a cerebral infarct: Relationship with early clinical deterioration and 3-month outcome in the European Co-operative Acute Stroke Study I(ECASS I)cohort. Stroke. 1999;30:2280–4. doi: 10.1161/01.str.30.11.2280. [DOI] [PubMed] [Google Scholar]
- 16.Molina CA, Alvarez-Sabin J, Montaner J, Abilleira S, Arenillas JF, Coscojuela P, et al. Thrombolysis-related hemorrhagic infarction: A marker of early reperfusion, reduced infarct size and improved outcome in patients with proximal middle cerebral artery occlusion. Stroke. 2002;33:1551–6. doi: 10.1161/01.str.0000016323.13456.e5. [DOI] [PubMed] [Google Scholar]
- 17.Lansberg MG, Thijs VN, Bammer R, Kemp S, Wijman CA, Marks MP, et al. Risk factors of symptomatic intracerebral hemorrhage after tPA therapy for acute stroke. Stroke. 2007;38:2275–8. doi: 10.1161/STROKEAHA.106.480475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Katzan IL, Furlan AJ, Lioyd LE, Frank JI, Harper DL, Hinchey JA, et al. Use of tissue -type plasminogen activator for acute ischemic stroke: The Cleveland area experience. JAMA. 2000;283:1151–8. doi: 10.1001/jama.283.9.1151. [DOI] [PubMed] [Google Scholar]
- 19.Katzan IL, Hammer MD, Furlan AJ, Hixson ED, Nadzam DM Cleveland Clinic Health System Stroke Quality Improvement Team. Quality improvement and tissue type plasminogen activator for acute ischemic stroke: Cleveland update. Stroke. 2003;34:799–800. doi: 10.1161/01.STR.0000056944.42686.1E. [DOI] [PubMed] [Google Scholar]
- 20.Lopes Yunez AM, Bruno A, Williams LS, Yilmaz E, Zurru C, Biller J. Protocol violations in community -based rtPA stroke treatment are associated with symptomatic intracerebral hemorrhage. Stroke. 2001;32:12–6. doi: 10.1161/01.str.32.1.12. [DOI] [PubMed] [Google Scholar]
- 21.Demchuk AM, Morgenstern LB, Kreiger DW, Linda Chi T, Hu W, Wein TH, et al. Serum glucose level and diabetes predict tissue plasminogen activator-related intra cerebral hemorrhage in acute ischemic stroke. Stroke. 1999;30:34–9. doi: 10.1161/01.str.30.1.34. [DOI] [PubMed] [Google Scholar]
- 22.Smith EE, Abdullah AR, Petkovska I, Rosenthal E, Koroshetz WJ, Schwamm LH. Poor outcomes in patients who do not receive intravenous tissue plasminogen activator because of mild or improving ischemic stroke. Stroke. 2005;36:2497–9. doi: 10.1161/01.STR.0000185798.78817.f3. [DOI] [PubMed] [Google Scholar]
- 23.Barber PA, Zhang J, Demchuk AM, Hill MD, Buchan AM. Why are stroke patients excluded from tPA therapy? An analysis of patient eligibility. Neurology. 2001;56:1015–20. doi: 10.1212/wnl.56.8.1015. [DOI] [PubMed] [Google Scholar]
- 24.Baumann CR, Baumgartner RW, Gandjour J, von Budingen HC, Siegel AM, Georgiadia D. Good outcomes in ischemic stroke patients treated with intravenous thrombolysis despite regressing neurological symptoms. Stroke. 2006;37:1332–3. doi: 10.1161/01.STR.0000217272.38455.a2. [DOI] [PubMed] [Google Scholar]
- 25.Sylaja PN, Dong W, Grotta JC, Miller MK, Tomita K, Hamilton S, et al. Safety outcomes of alteplase among acute ischemic stroke patients with special characteristics. Neurocrit Care. 2007;6:181–5. doi: 10.1007/s12028-007-0018-8. [DOI] [PubMed] [Google Scholar]
- 26.Engelter ST, Reichhart M, Sekoranja L, Georgiadis D, Baumann A, Weder B, et al. Thrombolysis in stroke patients aged 80 years and older: Swiss survey of IV thrombolysis. Neurology. 2005;65:1795–8. doi: 10.1212/01.wnl.0000183702.04080.27. [DOI] [PubMed] [Google Scholar]
- 27.Heushmann PU, Kolominsky-Rabas PL, Roether J, Misselwitz B, Lowitzsch K, Heidrich J, et al. Predictors of in-hospital mortality in patients with acute ischemic stroke treated with thrombolytic therapy. JAMA. 2004;292:1831–8. doi: 10.1001/jama.292.15.1831. [DOI] [PubMed] [Google Scholar]
- 28.Sylaja PN, Cote R, Buchan AM, Hill MD. Thrombolysis for acute ischemic stroke patients aged 80 years and older: Canadian Alteplase for Stroke Effectiveness Study. J Neurol Neurosurg Psychiatry. 2006;77:826–9. doi: 10.1136/jnnp.2005.086595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vatankhah B, Dittmar MS, Fehm NP. Thrombolysis for stroke in the elderly. T Thromb Thrombolysis. 2005;20:5–10. doi: 10.1007/s11239-005-2477-0. [DOI] [PubMed] [Google Scholar]
- 30.Chen CI, Iguchi Y, Grotta JC, Garami Z, Uchino K, Shaltoni H, et al. Intravenous TPA for very old patients. Eur Neurol. 2005;54:140–4. doi: 10.1159/000089086. [DOI] [PubMed] [Google Scholar]
- 31.Engelter ST, Bonati LH, Lyrer PA. Intravenous thrombolysis in stroke patients ≥ 80 versus et al. Leukoaraiosis is a risk factor for symptomatic intracerebral hemorrhage after thrombolysis for acute stroke. Stroke. 2006;37:2463–6. doi: 10.1161/01.STR.0000239321.53203.ea. [DOI] [PubMed] [Google Scholar]
- 32.Barber PA, Demchuk AM, Zhang J, Buchan AM for the ASPECTS Study Group. Validity and reliability of a quantitative computed tomographic score in predicting outcome of hyperacute stroke before thrombolytic therapy. Lancet. 2000;355:167–74. doi: 10.1016/s0140-6736(00)02237-6. [DOI] [PubMed] [Google Scholar]
- 33.Demchuk AM, Hill MD, Barber PA, Silver B, Patel S, Levine SR. Importance of CT early ischemic change using ASPECTS in NINDS rt-PA study. Stroke. 2005;36:2110–5. doi: 10.1161/01.STR.0000181116.15426.58. [DOI] [PubMed] [Google Scholar]
- 34.Neumann-Haefelin T, Hoelig S, Berkefeld J, Fiehler J, Gass A, Humpich M, et al. Leukoaraiosis is a risk factor for symptomatic intracerebral hemorrhage after thrombolysis for acute stroke. Stroke. 2006;37:2463–6. doi: 10.1161/01.STR.0000239321.53203.ea. [DOI] [PubMed] [Google Scholar]
- 35.Palumbo V, Boulanger JH, Hill MD, Inzitari D, Buchan AM. Leukoaraiosis and intracerebral hemorrhage after thrombolysis in acute stroke. Neurology. 2007;68:1020–4. doi: 10.1212/01.wnl.0000257817.29883.48. [DOI] [PubMed] [Google Scholar]
- 36.Hacke W, Albers G, Al-Rawi Y, Bogousslavsky J, Davalos A, Eliasziw M, et al. The desmoteplase in acute ischemic stroke trial (DIAS) Stroke. 2005;36:66–73. doi: 10.1161/01.STR.0000149938.08731.2c. [DOI] [PubMed] [Google Scholar]
- 37.Davis SM, Donnan GA, Parsons MW, Levi C, Butcher KS, Peeters A, et al. Effects of alteplase beyond 3 h after stroke in the Echoplanar Imaging Thrombolytic Evaluation Trial (EPITHET): A placebo-controlled randomized trial. Lancet Neurol. 2008;7:299–309. doi: 10.1016/S1474-4422(08)70044-9. [DOI] [PubMed] [Google Scholar]
- 38.Thomalla G, Schwark C, Sobesky J, Bluhmki E, Fiebach JB, Fiehler J, et al. Outcome and symptomatic bleeding complications of intravenous thrombolysis within 6 hours in MRI selected stroke patients: Comparison of a German multicenter study with the pooled data of ATLANTIS, ECASS and NINDS tPA trials. Stroke. 2006;37:852–8. doi: 10.1161/01.STR.0000204120.79399.72. [DOI] [PubMed] [Google Scholar]
- 39.Kohrmann M, Juttler E, Fiebach JB, Huttner HB, Siebert S, Schwark C, et al. MRI versus CT-based thrombolysis treatment within and beyond the 3 h time window after stroke onset: A cohort study. Lancet Neurol. 2006;5:661–7. doi: 10.1016/S1474-4422(06)70499-9. [DOI] [PubMed] [Google Scholar]
- 40.Schellinger PD, Thomalla G, Fiehler J, Kohrmann M, Molina CA, Neumann-Haefelin T, et al. MRI-based and CT-based thrombolytic therapy in acute ischemic stroke within and beyond established time windows: An analysis of 1210 patients. Stroke. 2007;38:2640–5. doi: 10.1161/STROKEAHA.107.483255. [DOI] [PubMed] [Google Scholar]
- 41.Puetz V, Dzialowski I, Hill MD, Subramaniam S, Sylaja PN, Krol A, et al. Intracranial thrombus extent predicts clinical outcome, final infarct size and hemorrhagic transformation in ischemic stroke: The Clot Burden Score. Int J Stroke. 2008 doi: 10.1111/j.1747-4949.2008.00221.x. in press. [DOI] [PubMed] [Google Scholar]
- 42.Wintermark M, Reichhart M, Cuisenaire O, Thiran JP, Schnyder P, Bogousslavsky J, et al. Comparison of admission perfusion computed tomography and qualitative diffusion- and perfusion-weighted magnetic resonance imaging in acute stroke patients. Stroke. 2002;33:2025–31. doi: 10.1161/01.str.0000023579.61630.ac. [DOI] [PubMed] [Google Scholar]
- 43.Pandian JD, Kalra G, Jaison A, Deepak SS, Shamsher S, Padala S, et al. Factors delaying admission to a hospital based stroke unit in India. J Stroke Cerebrovasc Dis. 2006;15:81–7. doi: 10.1016/j.jstrokecerebrovasdis.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 44.Pandian JD, Jaison A, Deepak SS, Kara G, Shamsher S, Lincoln DJ, et al. Public awareness of warning symptoms, risk factors and treatment of stroke in Northwest India. Stroke. 2005;36:644–8. doi: 10.1161/01.STR.0000154876.08468.a0. [DOI] [PubMed] [Google Scholar]
- 45.Sylaja PN, Dzialowski I, Krol A, Roy J, Federico P, Demchuk AM. Role of CT angiography in thrombolysis decision making for patients with presumed seizure at stroke onset. Stroke. 2006;37:915–7. doi: 10.1161/01.STR.0000202678.86234.84. [DOI] [PubMed] [Google Scholar]
- 46.Pexman JH, Barber PA, Hill MD, Sevick RJ, Demchuk AM, Hudon ME, et al. Use of the Alberta Stroke Program Early CT score (ASPECTS) for assessing CT scans in patients with acute stroke. AJNR Am J Neuroradiol. 2001;22:1532–42. [PMC free article] [PubMed] [Google Scholar]
- 47.Patel SC, Levine SR, Tilley BC, Grotta JC, Lu M, Frankel M, et al. Lack of clinical significance of early ischemic changes on computed tomography in acute stroke. JAMA. 2001;286:2830–8. doi: 10.1001/jama.286.22.2830. [DOI] [PubMed] [Google Scholar]
- 48.Gilligan AK, Markus R, Read S, Srikanth V, Hirano T, Fitt G, et al. Baseline blood pressure but not early computed tomography changes predicts major hemorrhage after streptokinase in acute ischemic stroke. Stroke. 2002;33:2236–42. doi: 10.1161/01.str.0000027859.59415.66. [DOI] [PubMed] [Google Scholar]
- 49.Gladstone DJ, Hill MD, Black S. tPA for acute stroke: Balancing baseline imbalances. CMAJ. 2002;25:166. [PMC free article] [PubMed] [Google Scholar]


