Abstract
Dysphagia and communication impairment are common consequences of stroke. Stroke survivors with either or both of these impairments are likely to have poorer long-term outcomes than those who do not have them. Speech-language pathologists (SLP) play a significant role in the screening, formal assessment, management, and rehabilitation of stroke survivors who present with dysphagia and/or communication impairment. Early diagnosis and referral is critical, as is intensive intervention as soon as the patient is able to participate. The SLP is also responsible for educating carers and staff in strategies that can support the patient and for making appropriate environmental modifications (e.g. altering diet consistencies or providing information in an aphasia-friendly format) to optimize the stroke survivor's participation, initially, in the rehabilitation program and, subsequently, within the community.
Keywords: Communication, dysphagia, speech pathologist, stroke
Stroke unit care is internationally recognized as the gold standard for the acute management and early rehabilitation of stroke patients.[1] A key element of comprehensive stroke unit care is the involvement of a multidisciplinary team. Speech-language pathologists (SLPs), along with medical, nursing, occupational therapy, physiotherapy, and social work staff are considered as core members of the multidisciplinary stroke team as defined in the Australian,[2] New Zealand,[3] United Kingdom,[4] United States,[5] and European[6] guidelines. SLPs working with stroke survivors have particular skills in the assessment and management of dysphagia and will be directly involved in the assessment and management of communication disorders associated with stroke, including aphasia, motor speech disorders, and cognitive communication disorders.
Dysphagia
There are three levels of care for stroke survivors with dysphagia. Stroke patients should receive a swallow screening test within 24 h of admission; those patients with evidence of dysphagia should have a formal clinical and/or instrumental assessment, which is generally undertaken by the SLP; and following this, a swallowing management and/or rehabilitation program should be developed with the involvement of the multidisciplinary team, the patient, and their significant others.
Dysphagia Screening
Up to 50% of acute stroke patients are likely to experience dysphagia.[7,8] If it is not recognized early, dysphagia may be responsible for aspiration, aspiration pneumonia, and/or undernutrition and dehydration.[9] Stroke patients with dysphagia are likely to have poorer outcomes.[9] Therefore, dysphagia screening should be undertaken as soon as possible after the patient has been admitted to hospital and before starting oral intake of food, fluids, or medication.
A number of dysphagia screening tools exist,[10] many of which combine an evaluation of the clinical predictors of dysphagia (i.e., dysphonia, dysarthria, abnormal volitional cough, abnormal gag reflex, cough after swallow, and voice change after swallow)[11] with a water swallowing test. While this combination appears comprehensive, it may prove time consuming, both because of the amount of education required to train nursing or emergency department staff and because of the actual time taken to screen. A combination of the water swallow test with pulse oximetry has also been advocated,[12] and this may prove to be an equally effective and more efficient tool. SLPs may or may not be responsible for the dysphagia screening; however, in countries where they are the lead dysphagia clinician, they should be involved in the selection of a suitable screening tool for their hospital and in providing training to the staff responsible for screening.
Dysphagia Assessment
If the patient fails dysphagia screening, referral to an SLP should occur for more comprehensive assessment. Alternative means for hydration and nutrition should be considered and implemented if a full assessment is not available within 24 h and if such intervention is deemed medically appropriate. [2,3,4,5,6] Formal dysphagia assessment includes an initial clinical bedside evaluation. If the clinical examination is inconclusive, the patient develops signs consistent with aspiration despite adherence to a dysphagia management plan, or dysphagia continues without improvement for longer than 7 days, instrumental evaluation such as a videofluoroscopic modified barium swallow (VMBS) or fiberendoscopic examination of swallowing (FEES) is indicated. Either of these tests (or both) can be used in conjunction with clinical examination to inform the swallowing management plan or to develop a swallowing rehabilitation plan.
Clinical Bedside Evaluation
Prior to undertaking the clinical bedside evaluation of swallowing, the SLP should review the patient's medical chart regarding the admission and make a note of comorbidities and medications which may impact on swallowing. The first part of the bedside swallowing evaluation involves screening for communication involvement, particularly with regard to the presence of dysarthria and dysphonia, the patient's ability to understand instructions (aphasia), and whether or not they are able to perform simple motor tasks in response to commands (apraxia). An oral examination is undertaken to note issues such as poor dentition, mucosal lesions, oral thrush, excessive or inadequate saliva, and halitosis. In addition, the SLP looks for facial (in particular, cheek and lip), tongue, soft palate, jaw, and laryngeal weakness. The SLP will examine for asymmetry at rest and in movement and, where possible, use resistance techniques to determine weakness. Subsequently, the patient's ability to perform a voluntary cough and saliva swallow will be checked. In combination, this provides information about the risk factors associated with oral health[13] and the motor function of the key cranial nerves for swallowing (i.e., V, VII, IX [small motor component to stylopharyngeus], X, XI, and XII). The SLP may also screen for oropharyngeal sensory involvement by testing taste recognition and perception (VII, IX, and X),[14] light and deep touch, and perception of hot and cold (V).[15] In some countries, dysphagia assessment and management is undertaken by other suitably trained health professionals; however, the SLP has particular skill in undertaking the above aspects of the bedside evaluation.
Subsequently, food and fluid trials may be undertaken to determine which, if any, food or fluid consistency can be swallowed safely. Trials are likely to include a range of fluids from very thick or pudding-like fluids to thin watery fluids, with the trial sequence determined by the preceding clinical examination. The SLP will check for oral residue, voice quality, and breathing rate after each trial. Cervical auscultation may be used to provide additional information about changes in breathing sounds or breathing patterns during or after each trial.[16] If no fluid consistency proves safe, the SLP should advise the multidisciplinary team and consider other sensory stimulation pathways that can be used to promote swallowing activity (eg, tactile, temperature, or taste). If a given consistency appears to enable oral intake without apparent risk to the patient, a diet can be commenced incorporating food and fluids of that consistency. Generally, vitaminised foods are introduced first, as the bedside clinical examination is seldom long enough to detect fatigue either in the swallowing act itself or in the muscles involved in bolus preparation. The final part of a bedside clinical evaluation should be the observation of the patient consuming a full meal of the recommended consistency of food and fluid. At this point, a swallowing management plan has to be developed. This plan should include:
Positioning for oral intake or sensory stimulation (determined after consultation with the physiotherapist and occupational therapist)
A sensory stimulation program to encourage swallowing activity if no level of oral intake appears 'safe'
Texture specification for all oral intake
Recommendations for oral medications
Requirements for meal-time monitoring, including indicators of poor swallow function
Review schedule
A formal review should include evaluation trials of food and fluid consistencies that have previously been considered 'unsafe.' It is important for the patient to move toward normal consistency food and fluids as soon as it is clinically safe. The Consumption of normal food and fluids encourage adequate oral intake,[17] increases saliva flow and therefore taste acuity, increases activity in a range of speech and swallowing muscles, and enhances social interactions and quality of life.[18]
Instrumental Assessment
A thorough bedside clinical examination is a sensitive, but not always specific, tool.[19] The bedside clinical examination may fail to identify some patients at risk of aspiration and may predict aspiration in patients where an instrumental study demonstrates safe swallowing. Nevertheless, routine use of an instrumental examination such as VMBS is unlikely to add significantly to functional outcome for people with post-stroke dysphagia.[9] For many stroke patients, dysphagia will resolve quickly. Instrumental examination should however be considered when:
The clinical examination is inconclusive.
The patient fails to improve in the first week, and additional information is required to inform swallowing management or rehabilitation.
The patient develops clinical signs consistent with aspiration, even though there had been no clinical evidence of dysphagia during screening or despite adherence to the swallowing management plan.
Two instrumental techniques are available for the evaluation of swallowing: FEES and VMBS. Both examinations have similar sensitivity and specificity.[20] The SLP must be present for either of these assessments.
FEES
During the FEES examination, a flexible endoscope is passed transnasally to enable direct viewing of the velopharynx, pharynx, base of tongue, epiglottis, pyriforms, laryngeal vestibule, and vocal cords; the subglottic space may also be viewed. These structures can be examined both at rest and during speech and swallowing, providing information about structural and functional asymmetries and integrity of the swallowing mechanism. Although the movement of the tongue base and epiglottis block the endoscopic view once the involuntary swallow has been initiated, the information provided just prior to this and immediately after swallowing is invaluable.
VMBS
The VMBS is considered the 'gold standard' for the evaluation of swallowing. During the test, the patient swallows a range of fluids and/or foods impregnated with barium and the actual swallow is filmed. The test allows clear identification of laryngeal penetration and aspiration; the timing of that aspiration (before, during, or after the swallow); the function of the velopharyngeal, pharyngeal, and base-of-tongue muscles; excursion of the hyo-laryngeal complex; protection of the airway; pooling in the valleculae or pyriform sinuses; opening of cricopharyngeus; and any pharyngeal residue post swallow.
As well as providing a direct view of swallowing function for food/fluid of different textures, temperatures, or taste, both procedures can be used to test the effectiveness of different postures/positions (eg, head turn, head tilt, chin tuck) and swallowing strategies (eg, supraglottic swallow, Mendelsohn maneuver) and so can inform the swallowing management plan. In conjunction with a thorough clinical examination, either of these tests can be used to develop a swallowing rehabilitation plan.
Swallowing Rehabilitation
Information supporting swallowing rehabilitation is circumstantial rather than evidence based. Carnaby[21] found that stroke patients who received daily swallowing intervention (management and/or rehabilitation) demonstrated better swallowing outcomes at 6 months than those who received less intensive treatment or no treatment. Exercise physiology principles indicate that swallowing rehabilitation is likely to be more effective when coupled with actual swallowing activity. Published exercises which meet this criterion are the Masako maneuver (also known as the tongue-holding swallow) and effortful swallow, both of which promote activity in the base of tongue and pharyngeal wall muscles; effortful swallow supported by sEMG biofeedback; voluntary laryngeal elevation plus suck;[22] sEMG-supported electrical stimulation to support involuntary saliva swallows;[23] faucal arch stimulation and, potentially, taste stimulation. In the majority of patients, these exercises would be used to support saliva swallows. Swallowing maneuvers such as the Mendelsohn maneuver and supraglottic swallow would also meet this criterion if they were employed as an exercise for supporting saliva swallows. Other exercises may also be of benefit; two examples are the Shaker (head-lift) exercise[24] and the Lee Silverman Voice Treatment (LSVT).[25] Exercises that are not accompanied by swallowing activity need to be employed for a minimum of 3 weeks before any decision is made about their effectiveness.
Summary
Dysphagia is a common consequence of stroke, and stroke survivors with dysphagia are likely to have a much poorer outcome than those who do not have dysphagia. Stroke patients should be screened for dysphagia prior to commencing any oral intake. Once dysphagia has been identified, a swallowing management plan should be developed based on the results of the bedside clinical assessment with or without evidence from FEES or VMBS. Often dysphagia will resolve quickly; however, for some patients, a swallowing rehabilitation plan will be required. The SLP plays a major role in all aspects of dysphagia management. The evidence suggests that intensive swallowing exercises will result in optimal swallowing outcomes for patients.
Communication
The communication deficits associated with stroke are diverse; they can be described as affecting one of three main areas: language, motor speech, and/or cognitive communication. The evidence base for the management of post-stroke communication disorders is at best weak.[26,27,28] This is partly because of the diversity of the communication deficits associated with stroke and also reflects the long history of communication intervention. Nevertheless, there is overall agreement in the international evidence-based guidelines regarding intervention for communication impairments associated with acute stroke. These take the form of: communication screening[2,3,4,5,6]; full assessment of those identified with communication impairment by a SLP[2,3,4,5,6]; intensive intervention, which should be initiated as soon as the patient is able to cooperate [2,3,4,5,6]; and education of patient, family and significant others, and involved staff regarding the nature of the communication disorder, strategies to maximize communication, and activities that may support communication recovery. [2,3,4,5] In addition, the SLP should work with the multidisciplinary team to ensure that written information given to stroke patients is provided in a way which maximizes their understanding.[2,14] This holistic approach is supported by the International Classification of Functioning, Disability, and Health (ICF) as it seeks to address issues about the communication environment and the stroke survivor's ability to communicate effectively within that environment, as well as providing direct intervention at the level of impairment.[29]
Communication Screening
Among stroke survivors, 30-60% are likely to experience a communication deficit.[30,31] Communication deficits have been demonstrated to be associated with depression[32,33] and therefore, by inference, with quality of life (QOL); however, any direct relationship between communication involvement and QOL remains unestablished. Many researchers have examined QOL in stroke survivors but have not been able to demonstrate a direct relationship; however, standard QOL measures do not routinely score items that are communication dependent. Results are beginning to emerge from the SAQOL-39 (Stroke and Aphasia Quality of Life Scale-39)[34] which may clarify this. Overall, stroke survivors with acquired communication disorders have poorer outcomes than those who do not have such disorders, and this highlights the need for early diagnosis. The first step in this process is for all stroke survivors to undergo a communication screening test within 48 h of admission. This screening test should, if possible, be done using a validated tool and should be performed by the SLP or suitably trained nursing/other staff.
An effective communication screen needs to check whether the person is able to understand spoken and written material as well as gestures, facial expressions, and prosody; whether they can communicate their ideas effectively through speech, writing, facial expressions, and gestures; and whether or not their speech is easily understood or demands extra effort from the communication partner to decode. Currently, the most commonly used screening test is the Frenchay Aphasia Screening Test (FAST), which is designed to be used by any member of the multidisciplinary team to promote early referral to the SLP.[35] This test however, only examines receptive and expressive language in the domains of speaking, listening, reading, and writing and therefore may fail to identify patients who demonstrate cognitive communication disorders or mild motor speech disorders.
Communication screening could also be seen as part of a more comprehensive cognitive screen. Cognitive screening of all stroke survivors is recommended in some of the clinical guidelines.[2,4,5,6] The Functional Impairment Battery, which includes screening of memory, neglect, aphasia, anomia, hearing, visual acuity, and depression,[31] identifies cognitive deficits in many more stroke survivors than informal screening or a standardized broad instrument such as the National Institutes of Health Stroke Scale (NIHSS).[36] The administration of the Boston Naming Test in addition to FAST in this battery proved effective in identifying a greater number of patients with probable communication involvement than FAST alone. Given the strong relationship between language and memory,[37] and the association between visual neglect and communication disorders associated with right hemisphere involvement,[38] a comprehensive cognitive screen such as this would be more likely to identify stroke survivors with communication deficits.
For any screening tool to be effective, however, it needs to be both linguistically and culturally appropriate. The SLP must therefore be involved in the selection of an appropriate screening tool and in training other staff as required in the administration of that tool. Stroke survivors identified as having communication disorders (including those with memory problems, poor repetition skills, and/or neglect on cognitive screen) should be referred to an SLP for more comprehensive communication assessment. In addition, all stroke survivors who premorbidly used an alternate means of communication, including gesture, sign language, or augmentative device, should be referred for formal communication assessment.
Communication Assessment
Using the ICF as a framework, three areas emerge for comprehensive communication assessment. These are the assessment of the communication impairment experienced by the stroke survivor, the impact that this has on their activity and participation within specific contexts, and the way in which their communication environment influences that activity and participation. Assessment in each of these areas should be both quantitative as well as qualitative so that outcomes of the rehabilitation program can be measured objectively.
Assessment of Communication Impairment
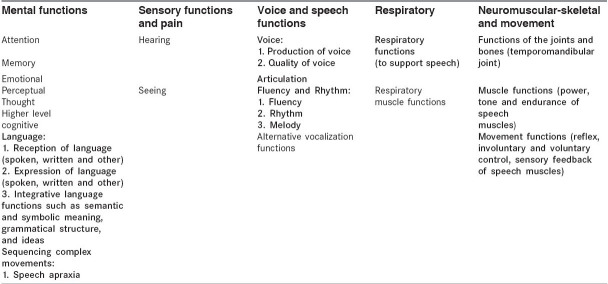
The assessment of the communication impairment should be informed by the cognitive or communication screening test and formal cognitive assessments undertaken by the occupational therapist, psychologist, and neuropsychologist. In addition communication assessments must be linguistically and culturally appropriate. Where possible, the assessment should provide clear direction for intervention strategies.Table 1 summarizes the areas identified by the ICF[29] under the heading of 'Body Functions' that may be affected by stroke and which are likely to have a direct or indirect impact on communication. Areas indicated in bold type demand formal assessment by the SLP, while the other areas demand integrated assessment with other members of the team regarding their impact on communication.
Table 1.
Body functions (ICF framework) likely to be affected by stroke. The SLP provides primary assessment for areas indicated in bold type and supportive assessment for other areas listed.
Activity and participation
All formal communication assessment should be undertaken to inform the intensive rehabilitation program which needs to address real goals identified by the stroke survivor. In some circumstances, however, the communication involvement is so severe that early assessment and intensive therapy must be directed toward the receptive and expressive language disorder to help provide the patient with basic communication skills so that they can be involved in goal setting. In other cases, the stroke survivor may have a range of communication impairments but each of these may not have the same impact on their ability to participate within their communication environment. The rehabilitation program is best directed toward those areas which will have maximum impact on the patient's ability to participate: initially within the hospital environment and subsequently within the home and community. Again the ICF[29] provides a framework for helping the clinicians and the patient evaluate those areas which are most significant [Table 2]. For example, a patient with mild language impairment may have lost the ability to interpret body language and facial expression and this will have a negative impact on activities involving interpersonal interactions and relationships; major life areas; and community, social, and civic life. Rehabilitation directed toward this impairment should be practiced in the context that is most limited by the impairment. For some patients that may be at the level of family relationships, while for others the impact may be most significant in their work life or career.
Table 2.
Activity and participation (ICF framework).

Communication environment
The final part of the communication assessment undertaken by the SLP is to examine the communication environment which impacts on the stroke survivor. During the acute post-stroke stage, this is most likely to be a hospital. The SLP needs to determine:
What communication devices, if any, can support the stroke survivor in their efforts to communicate
What communication strategies can be employed by communication partners to enhance communication with the stroke survivor
What education is required by communication partners (including family, significant others, and staff) to employ the identified strategies
What support the stroke survivor requires to access written information relevant to their care
What support the stroke survivor requires to access leisure time activities in the hospital environment.
Intervention strategies for the patient during the initial acute post-stroke period and the early rehabilitative phase need to address the areas identified by these assessments.
Communication interventions and early rehabilitation
Communication assessment will be an ongoing process throughout rehabilitation; however, once initial assessment is complete, an intervention and rehabilitation plan should be initiated. In the immediate post-stroke period, communication interventions are likely to take precedence over communication rehabilitation. Some strategies for both intervention and rehabilitation are described below; however, the SLP will have access to many other rehabilitation strategies which are accepted practice supported by expert opinion but have not been evaluated in appropriate controlled studies. It is therefore important that the assessment procedures allow adequate measure of outcomes to ensure that the rehabilitation program is effective.
Communication interventions
Communication interventions include strategies that can be employed by the stroke survivor to enhance their communication; strategies used by family, significant others, and staff to promote effective communication with the stroke survivor; and environmental adaptations to enhance communication effectiveness. The aim of these interventions is to maximize communication opportunities and participation for the stroke survivor and also to promote optimal involvement in the rehabilitation and recovery process.
Strategies for the stroke survivor
Where the major communication impairment is in the area of speech rather than language, the patient can be encouraged to use a range of communication supports such as:
Pen and paper (using the non-dominant hand if necessary)
Letter pointing board (either for first letter or whole word)*[39]
Electronic communication devices[40]
Amplification[41]
Reducing speed of speech*
Introducing the topic first*[39]
Using iconic gestures*[42]
Talking one-to-one where possible
*There is emerging evidence from the literature on dysarthria (including that related to etiologies other than stroke) that using letter pointing for initial phoneme in combination with topic nomination or the trained use of gesture is likely to decrease the speed of speech, increase pause times, and improve the intelligibility of the dysarthric speaker.[39,42]
For the patient with aphasia, useful strategies may include:
Strategies for family, significant others, and staff
For all stroke survivors who have communication impairment it is important to:
Reduce distractions
Talk one-to-one where possible
Check that they have understood the message correctly by paraphrasing or reiteration
Seek clarification if you have not understood
Allow sufficient time
Ensure that the area is well lit
Encourage the patient to use any communication device/strategy developed for them
Provide props such as photographs, magazines, or sports results that are important to the patient
Fill in the communication diary at the completion of each visit (see below)
If the patient has aphasia it may also be important to reduce the complexity of sentences, write down key words, use pictures of gesture cues, and ensure that your face is well lit.
Environmental adaptations
A range of environmental adaptations may be considered; for example:
Providing a nurse call button that is more easily recognized
Changing ward rounds such that one team member remains with the patient to reiterate information after the team has moved on.
Developing a communication diary that stays with the patient. Staff, family, and significant others should be encouraged to complete the diary with information about the time/date of the communication, the people involved, and the main elements that were discussed.
The patient with aphasia may require additional adaptations such as:
Development of aphasia-friendly menus
Provision of all essential information in an aphasia-friendly format (information about stroke and its consequences; the rehabilitation team and process; essential tests and medical management routinely used post stroke).[47]
Development of specific information sheets in an aphasia-friendly format where the patient's management varies from the standard protocols.[47]
The SLP is responsible for choosing the optimal strategies and environmental adaptations for each patient; training family, significant others, and staff in the use of those strategies and adaptations; and for providing input for the development of all aphasia-friendly materials.
Rehabilitation
Where rehabilitation principles are included in the clinical stroke guidelines, the consensus is that rehabilitation for communication impairments should be initiated as soon as possible post stroke and as intensively as possible (approximately 3-8 h/week).[48] In the early rehabilitative phase, rehabilitation is likely to be one-to-one. The actual nature of that intervention is determined by the SLP in response to the assessments described above. However, a number of methodologies have evidence base either within the stroke literature or in the broader neurological literature for those patients who demonstrate particular communication impairments.
Motor speech
Dysarthria is underpinned by possible impairment in the respiratory, phonatory, resonance, and/or articulatory systems. This impairment may include weakness, tonicity changes, or incoordination and therefore intervention strategies are inferred by careful assessment. Although evidence from randomized trials is lacking,[27] evidence-based clinical practice guidelines are emerging for the management of dysarthria.[49,50] These guidelines address issues in the areas of respiratory, phonatory and resonance strength, control and coordination.
Apraxia of speech frequently coexists with aphasia and therefore the most appropriate intervention will depend on the functional consequences observed in the individual patient. While there is no clear evidence, intervention strategies such as modeling, visual cueing, integral stimulation,[51] and cueing for articulatory placement Prompts for Restructuring Oral Muscular Phonetic Targets (PROMPT) [52,53,54] have proved effective for some patients.
Respiratory/phonatory
Exercises to improve the respiratory/phonatory parameters underpinning speech may include non-speech, postural, and speech activities. Resistance training techniques can target respiratory and/or phonatory muscles directly when reduced strength is hypothesized, while coordination of respiration and phonation should work toward the fast inspiration and controlled exhalation pattern suitable for supporting speech. Programs need to incorporate motor learning principles with strength and coordination exercises modified quickly to incorporate speech tasks.[50] Patients may initially benefit from blocked practice to develop appropriate motor patterns but may require distributed and randomized practice sessions to promote motor recall.[53,54] Biofeedback will also be an important element to promote effective motor learning across the respiratory/phonatory systems.
Little evidence is available to identify effective programs for patients who demonstrate hyperadduction syndromes. Relaxation with biofeedback may be of benefit, but the SLP should use careful assessment to ensure that the hyperactivity is not a functional adaptation to an underlying weakness.[50]
Velopharyngeal
Increased nasal resonance is common across all types of dysarthria[51] and indicates either velopharyngeal insufficiency or incoordination or a combination of these. A secondary consequence of this is reduced air flow via the oral cavity and therefore reduced resistance during oral articulation. Yorkston[49] provides clinical practice guidelines for the management of velopharyngeal involvement based on a review of the literature and concludes that velopharyngeal prostheses are effective in some instances. Other therapies which directly affect air flow are LSVT[25] and continuous positive airway pressure (CPAP) therapy.[55,56]
Language
Early aphasia rehabilitation should target the areas identified during assessment. It should be initiated as soon as the patient is able to participate in rehabilitative activities and be offered intensively (3-8 h/week).[48] Case study evidence has demonstrated the effectiveness of phonological and semantic interventions,[57] which provide the patient with language models that are systematically made more complex, constraining the patient to produce utterances at the level they have reached.[58]
Cognitive language
Stroke survivors who demonstrate impairment in the areas of attention and memory will frequently have concomitant communication involvement. The speech pathologist should work with the occupational therapist and the neuropsychologist to minimize the impact of these impairments on communication. In particular, areas such as verbal attention, divided attention, sustained attention, auditory memory, verbal memory, and visual neglect are likely to have a direct impact on communication. As yet, there is little evidence on the efficacy of clinical interventions in this area.
Summary: Communication
Communication impairment occurs in 30-60% of stroke survivors. Despite the potential for improvement and recovery being high, communication impairment in stroke survivors is often related to poorer outcomes. Screening for cognitive and communication impairments is therefore critical to ensure that all patients with a communication impairment are referred to the SLP for comprehensive assessment and management.
Conclusion
The SLP plays a significant role in the multidisciplinary team managing patients with acute stroke. Screening for dysphagia (within 24 h) and communication impairment (within 48 h) is critical in working toward optimal outcomes for stroke survivors. The SLP is involved in choosing appropriate screening tools and providing training in the use of those tools. In addition the SLP must provide comprehensive dysphagia and/or communication assessment that takes into account the impairment, the impact that impairment has on the patient's life, and any environmental modifications which may minimize the impact of that impairment. Assessments chosen must be culturally appropriate and provide quantitative as well as qualitative information. The SLP is responsible for developing appropriate management and rehabilitation programs based on the assessments undertaken as well as the goals identified by the patient and the multidisciplinary team and for monitoring the functional gains associated with those programs through careful reassessment.
Acknowledgment
I would like to acknowledge the support of Angie Dobbrick, Senior Speech Pathologist, Royal Brisbane and Women's Hospital, for her suggestions and comments with regard to this manuscript
Footnotes
Source of Support: Angie Dobbrick, Senior Speech Pathologist, Royal Brisbane and Women's Hospital,
Conflict of Interest: Nil.
References
- 1.Stroke Unit Trialists? Collaboration. Organised inpatient (stroke unit) care for stroke. Cochrane Database of Systematic Reviews. 1995;(2) doi: 10.1002/14651858.CD000197. Art. No.:CD000197. DOI:10.1002/14651858.CD000197.pub2. [DOI] [PubMed] [Google Scholar]
- 2.National Stroke Foundation [homepage on the Internet] Melbourne: Clinical Guidelines for Acute Stroke Management; 2007. cited on 2007 Nov 1. Available from: http://www.strokefoundation.com.au/clinical-guidelines . [Google Scholar]
- 3.New Zealand Guidelines Group [homepage o the Internet] New Zealand: Life after stroke: the New Zealand Guidelines for the Management of Stroke;c; 2003. cited 2007 on Nov 1. Available from: http://www.nzgg.org.nz/ [Google Scholar]
- 4.Royal College of Physicians, London: Intercollegiate Stroke Working Party [homepage on the Internet] 2nd Edition. London: National Clinical Guidelines for Stroke; c 2004 June. cited on 2007 Nov 2. Available from: http://www.rcplondon.ac.uk/pubs/books/stroke/ [Google Scholar]
- 5.Duncan PW, Zorowitz R, Bates B, Choy JY, Glasberg JJ, Graham GD, et al. Management of adult stroke rehabilitation care: A clinical practice guideline. Stroke. 2005;36:e100–43. doi: 10.1161/01.STR.0000180861.54180.FF. [DOI] [PubMed] [Google Scholar]
- 6.The European Stroke Initiative Executive Committee and the EUSI Writing Committee. European Stroke Initiative Recommendations for Stroke Management - Update 2003. Cerebrovasc Dis [serial on the Internet]. 2003. cited on 2007 Nov 3. Available from: http://content.karger.com/ProdukteDB/produkte.asp?Aktion=JournalHomeandProduktNr=224153 . [DOI] [PubMed]
- 7.Smithard DG, Smeeton NC, Wolfe CD. Long-term outcome after stroke: Does dysphagia matter? Age Ageing. 2007;36:90–4. doi: 10.1093/ageing/afl149. [DOI] [PubMed] [Google Scholar]
- 8.Mann G, Hankey GJ, Cameron D. Swallowing disorders following acute stroke: Prevalence and diagnostic accuracy. Cerebrovasc Dis. 2000;10:380–6. doi: 10.1159/000016094. [DOI] [PubMed] [Google Scholar]
- 9.Smithard DG, O'Neill PA, Park C, Morris J, Wyatt R, England R, et al. Complications and outcome after acute stroke does dysphagia matter? Stroke. 1996;27:1200–4. doi: 10.1161/01.str.27.7.1200. [DOI] [PubMed] [Google Scholar]
- 10.Perry L, Love CP. Screening for dysphagia and aspiration in acute stroke: A systematic review. Dysphagia. 2001;16:7–18. doi: 10.1007/pl00021290. [DOI] [PubMed] [Google Scholar]
- 11.Daniels SK, McAdam CP, Brailey K, Foundas AL. Clinical assessment of swallowing and prediction of dysphagia severity. Am J Speech Language Pathol. 1997;6:17–24. [Google Scholar]
- 12.Lim SH, Lieu PK, Phua SY, Seshadri R, Venketasubramanian N, Lee SH, et al. Accuracy of bedside clinical methods compared with fiberoptic endoscopic examination of swallowing (FEES) in determining the risk of aspiration in acute stroke patients. Dysphagia. 2001;16:1–6. doi: 10.1007/s004550000038. [DOI] [PubMed] [Google Scholar]
- 13.Langmore SE, Terpenning MS, Schork A, Chen Y. Predictors of Pneumonia: How important is dysphagia? Dysphagia. 1998;13:69–81. doi: 10.1007/PL00009559. [DOI] [PubMed] [Google Scholar]
- 14.Kveton JF, Bartoshuk LM. The effect of unilateral chorda tympani damage on taste. Laryngoscope. 1994;104:25–9. doi: 10.1288/00005537-199401000-00006. [DOI] [PubMed] [Google Scholar]
- 15.Calhoun KH, Gibson B, Hartley L, Minton J, Hokanson JA. Age related changes in oral sensation. Laryngoscope. 1992;102:109–15. doi: 10.1288/00005537-199202000-00001. [DOI] [PubMed] [Google Scholar]
- 16.Borr C, Hielscher-Fastabend M, Lücking A. Reliability and validity of cervical auscultation. Dysphagia. 2007;22:225–34. doi: 10.1007/s00455-007-9078-3. [DOI] [PubMed] [Google Scholar]
- 17.Wright D, Cotter M, Hickson G. Frost. Comparison of energy and protein intakes of older people consuming a texture modified diet with a normal hospital diet. J Hum Nutr Dietet. 2005;18:213–9. doi: 10.1111/j.1365-277X.2005.00605.x. [DOI] [PubMed] [Google Scholar]
- 18.Hickson M, Frost G. An investigation into the relationships between quality of life, nutritional status and physical function. Clin Nutr. 2004;23:213–21. doi: 10.1016/S0261-5614(03)00127-4. [DOI] [PubMed] [Google Scholar]
- 19.Leder SB, Espinosa JF. Aspiration risk after acute stroke: Comparison of clinical examination and fiberoptic endoscopic evaluation of swallowing. Dysphagia. 2002;17:214–8. doi: 10.1007/s00455-002-0054-7. [DOI] [PubMed] [Google Scholar]
- 20.Langmore SE, Schatz K, Olson N. Endoscopic and videofluoroscopic evaluations of swallowing and aspiration. Ann Oto Rhino Laryngol. 1991;100:678–81. doi: 10.1177/000348949110000815. [DOI] [PubMed] [Google Scholar]
- 21.Carnaby G, Hankey GJ, Pizzi J. Behavioural intervention for dysphagia in acute stroke: A randomised controlled trial. Lancet Neurol. 2006;5:31–7. doi: 10.1016/S1474-4422(05)70252-0. [DOI] [PubMed] [Google Scholar]
- 22.Heimlich HJ. Rehabilitation of swallowing after stroke. Ann Otol Rhinol Laryngol. 1983;92:357–9. doi: 10.1177/000348948309200410. [DOI] [PubMed] [Google Scholar]
- 23.Leelamanit V, Limsakul C, Geater A. Synchronized electrical stimulation in treating pharyngeal dysphagia. Laryngoscope. 2002;112:2204–10. doi: 10.1097/00005537-200212000-00015. [DOI] [PubMed] [Google Scholar]
- 24.Shaker R, Easterling C, Kern M, Nitschke T, Massey B, Daniels S, et al. Rehabilitation of swallowing by exercise in tube-fed patients with pharyngeal dysphagia secondary to abnormal UES opening. Gastroenterology. 2002;122:1314–21. doi: 10.1053/gast.2002.32999. [DOI] [PubMed] [Google Scholar]
- 25.El Sharkawi A, Ramig L, Logemann JA, Pauloski BR, Rademaker AW, Smith CH, et al. Swallowing and voice effects of Lee Silverman Voice Treatment (LSVT): A pilot study. J Neurol Neurosurg Psychiatry. 2002;72:31–6. doi: 10.1136/jnnp.72.1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Greener J, Enderby P, Whurr R. Speech and language therapy for aphasia following stroke. Cochrane Database Syst Rev. 2000;2:CD000425. doi: 10.1002/14651858.CD000425. [DOI] [PubMed] [Google Scholar]
- 27.Sellars C, Hughes T, Langhorne P. Speech and language therapy for dysarthria due to non-progressive brain damage. Cochrane Database Syst Rev. 2001;2:CD002088. doi: 10.1002/14651858.CD002088. [DOI] [PubMed] [Google Scholar]
- 28.West C, Hesketh A, Bowen A, Vail A. Interventions for apraxia of speech following stroke. Cochrane Database Syst Rev. 2005;4:CD004298. doi: 10.1002/14651858.CD004298.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.World Health Organization [homepage on the Internet]. International Classiþ cation for Functioning, Disability and Health. C 2001. [last cited on 2007 Nov 4]. Available from: http://www.who.int/classiþ cations/icf/en/
- 30.Engelter ST, Gostynski M, Papa S, Frei M, Born C, Ajdacic-Gross V, et al. Epidemiology of aphasia attributable to first ischemic stroke: Incidence, severity, fluency, etiology, and thrombolysis. Stroke. 2006;37:1379–84. doi: 10.1161/01.STR.0000221815.64093.8c. [DOI] [PubMed] [Google Scholar]
- 31.Edwards DF, Hahn MG, Baum CM, Perlmutter MS, Sheedy C, Dromerick AW. Screening patients with stroke for rehabilitation needs: Validation of the post-stroke rehabilitation guidelines. Neurorehabil Neural Repair. 2006;20:42–8. doi: 10.1177/1545968305283038. [DOI] [PubMed] [Google Scholar]
- 32.Dickson S, Brady M, Barbour RS, Clark AM, Paton G. New York: British Sociological Association Annual Conference; 2000. The psychosocial impact of dysarthria on the individual and their carer. [Google Scholar]
- 33.Kauhanen ML, Korpelainen JT, Hiltunen P, Mδδttδ R, Mononen H, Brusin E, et al. Aphasia, depression, and non-verbal cognitive impairment in ischaemic stroke. Cerebrovasc Dis. 2000;10:455–61. doi: 10.1159/000016107. [DOI] [PubMed] [Google Scholar]
- 34.Hilari K, Byng S, Lamping DL, Smith SC. Stroke and aphasia quality of life scale-39 (SAQOL-39): Evaluation of acceptability, reliability, and validity. Stroke. 2003;34:1944–50. doi: 10.1161/01.STR.0000081987.46660.ED. [DOI] [PubMed] [Google Scholar]
- 35.Salter K, Jutai J, Foley N, Hellings C, Teasell R. Identification of aphasia post stroke: A review of screening assessment tools. Brain Injury. 2006;20:559–68. doi: 10.1080/02699050600744087. [DOI] [PubMed] [Google Scholar]
- 36.Internet Stroke Center [homepage on the Internet] Washington: Stroke Scales and Clinical assessment Tools; C 2007. [last cited on 2007 Nov 10]. Available from: http://www.strokecenter.org/trials/scales/nihss.html . [Google Scholar]
- 37.Jefferies E, Hoffman P, Jones R, Ralph MA. The impact of semantic impairment on verbal short-term memory in stroke aphasia and semantic dementia: A comparative study. J Mem Lang. 2008;58:66–87. doi: 10.1016/j.jml.2007.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Myers, Penelope S. Right hemisphere damage: Disorders of communication and cognition. San Diego: Singular Pub; 1999. [Google Scholar]
- 39.Hustad KC, Jones T, Dailey S. Implementing speech supplementation strategies: Effects on intelligibility and speech rate of individuals with chronic severe dysarthria. J Speech Lang Hear Res. 2003;46:462–74. [PubMed] [Google Scholar]
- 40.Laffont I, Dumas C, Pozzi D, Ruquet M, Tissier AC, Lofaso F, et al. Home trials of a speech synthesizer in severe dysarthria: Patterns of use, satisfaction and utility of word prediction. J Rehabil Med. 2007;39:399–404. doi: 10.2340/16501977-0056. [DOI] [PubMed] [Google Scholar]
- 41.Simpson MB, Till JA, Goff MA. Long term treatment of severe dysarthria: A single case study. J Speech Hear Dis. 1988;53:433–40. doi: 10.1044/jshd.5304.433. [DOI] [PubMed] [Google Scholar]
- 42.Hustad KC, Garcia JM. Aided and unaided speech supplementation strategies: Effect of alphabet cues and iconic hand gestures on dysarthric speech. J Speech Lang Hear Res. 2005;48:996–1012. doi: 10.1044/1092-4388(2005/068). [DOI] [PubMed] [Google Scholar]
- 43.Sacchett C, Byng S, Marshall J, Pound C. Drawing together: Evaluation of a therapy programme for severe aphasia. Int J Lang Commun Dis. 1999;34:265–89. doi: 10.1080/136828299247414. [DOI] [PubMed] [Google Scholar]
- 44.Rao PR. Drawing and gesture as communication options in a person with severe aphasia. Topics Stroke Rehabil. 1995;2:49–56. doi: 10.1080/10749357.1995.11754054. [DOI] [PubMed] [Google Scholar]
- 45.Ho KM. The effect of remnant and pictographic books on the communicative interaction of individuals with global aphasia. Augmentative Alternat Comm. 2005;21:218–32. [Google Scholar]
- 46.Jacobs B, Drew R, Ogletree B, Pierce K. Augmentative and Alternative communication (AAC) for adults with severe aphasia: Where we stand and how we can go further. Disabil Rehabil. 2004;26:1231–40. doi: 10.1080/09638280412331280244. [DOI] [PubMed] [Google Scholar]
- 47.Rose TA, Worrall LE, McKenna KT. The effectiveness of aphasia-friendly principles for printed health education materials for people with aphasia following stroke. Aphasiology. 2003;17:947–63. [Google Scholar]
- 48.Bhogal SK, Teasell R, Speechly M. Intensity of aphasia therapy, impact on recovery. Stroke. 2004;34:987–93. doi: 10.1161/01.STR.0000062343.64383.D0. [DOI] [PubMed] [Google Scholar]
- 49.Yorkston KM, Spencer KA, Duffy JR, Beukelman DR, Golper LA, Miller RM, et al. Evidence-based practice guidelines for dysarthria: Management of velopharyngeal function. J Med Speech Lang Pathol. 2001;9:257–73. [Google Scholar]
- 50.Spencer KA, Yorkston KM, Duffy JR. Behavioral management of respiratory-phonatory dysfunction from dysarthria: A flowchart for guidance in clinical decision-making. J Med Speech Lang Pathol. 2003;11:39–61. [Google Scholar]
- 51.McCaffrey P. [homepae on the Internet]. The Neuroscience on the Web Series: CMSD 642, Neuropathologies of Swallowing and Speech. C 1998-2001. last cited on 2007 Dec 1. Available from: http://www.csuchico.edu/~pmccaffrey/syllabi/SPPA342/342unit14.html .
- 52.Wambaugh JL, Doyle PJ. Treatment for acquired apraxia of speech: Review of efficacy reports. Clin Aphasiol. 1994;22:231–43. [Google Scholar]
- 53.Bose A, Square P, Schlosser R, van Lieshout P. Effects of PROMPT therapy on speech motor function in a person with aphasia and apraxia of speech. Aphasiology. 2001;15:767–85. [Google Scholar]
- 54.Cepeda NJ, Pashler H, Vul E, Wixted JT, Rohrer D. Distributed practice in verbal recall tasks: A review and quantitative synthesis. Psychological Bulletin. 2006;132(3):354–80. doi: 10.1037/0033-2909.132.3.354. [DOI] [PubMed] [Google Scholar]
- 55.Cahill LM, Turner AB, Stabler PA, Addis PE, Theodoros DG, Murdoch BE. An Evaluation of Continuous Positive Airway Pressure (CPAP) therapy in the treatment of hypernasality following traumatic brain injury: A report of 3 cases. J Head Trauma Rehabil. 2004;19:241–53. doi: 10.1097/00001199-200405000-00005. [DOI] [PubMed] [Google Scholar]
- 56.Kuehn DP, Imrey PB, Tomes L, Jones DL, O'Gara MM, Seaver EJ, et al. Efficacy of continuous positive airway pressure for treatment of hypernasality. Cleft Palate Craniofac J. 2002;39:267–76. doi: 10.1597/1545-1569_2002_039_0267_eocpap_2.0.co_2. [DOI] [PubMed] [Google Scholar]
- 57.Doesborgh SJ, van de Sandt-Koenderman MW, Dippel DW, van Harskamp F, Koudstaal PJ, Visch-Brink EG. Effects of semantic treatment on verbal communication and linguistic processing in aphasia after stroke: A randomized controlled trial. Stroke. 2004;35:141–6. doi: 10.1161/01.STR.0000105460.52928.A6. [DOI] [PubMed] [Google Scholar]
- 58.Pulvermuller F, Neininger B, Elbert T, Mohr B, Rockstroh B, Koebbel P, et al. Constraint-induced therapy of chronic aphasia after stroke. Stroke. 2001;32:1621–6. doi: 10.1161/01.str.32.7.1621. [DOI] [PubMed] [Google Scholar]
- 59.Bakheit AM. Drug treatment of post stroke aphasia. Exp Rev Neurother. 2004;4:211–7. doi: 10.1586/14737175.4.2.211. [DOI] [PubMed] [Google Scholar]