Abstract
Following ischemic stroke, interventions to bring about reperfusion must be implemented within the recognized timeframe; this means that timely clinical recognition of this condition is vital. The process of diagnosis begins with the initial bedside assessment of the patient to be followed by appropriate imaging studies. However, because reperfusion therapy may be attended by significant adverse consequences, and since imaging may be negative for many hours after stroke onset, the clinician must be aware of conditions that mimic cerebral ischemia. Depending on the timing and nature of ancillary testing, stroke mimics can be identified in 3-30% of patients presenting with the acute onset of a neurological deficit. These mimics include metabolic, traumatic, migrainous, neoplastic, endocrine, convulsive, and psychiatric disorders. Interestingly, the nature of these mimics, their frequency of occurrence, and presentation may vary between different geographical regions; however, detailed information regarding such variations is not available at present. This review provides an overview of the conditions that can masquerade as stroke, and includes information that may aid in their early detection or, at the very least, serve to warn the clinician that the patient is presenting with something other than cerebral ischemia.
Keywords: Bedside assessment, stroke, stroke mimics
Stroke is the sudden onset of a focal neurologic deficit in a recognizable vascular distribution with a common presentation. However, differential diagnosis is often a problem because there are several subtypes of stroke and also because some nonvascular disorders may have clinical pictures that resemble that of strokes. These nonvascular conditions that simulate stroke are referred to as stroke 'mimics.' In the current era, stroke is often treatable in the acute setting with modalities such as thrombolysis with recombinant tissue plasminogen activator (rtPA), but treatment is also associated with a substantial risk of hemorrhage; it is, therefore, of prime importance to distinguish stroke from its mimics. Thrombolytic therapy given to a stroke mimic exposes the patient to the risk of hemorrhage, without providing any benefit. Blood tests and brain imaging are useful adjuncts to bedside assessment and the latter, especially, may be critical for identifying stroke, for arriving at the decision to treat, and for prioritization of tests, because thrombolytic therapy in stroke is often limited by a narrow time window of opportunity. This paper reviews the differential diagnoses of stroke, starting with the stroke subtypes. The final part of the discussion addresses unusual clinical pictures that may result from stroke, i.e., strokes that take on the appearances of other diseases.
Stroke subtypes
The abrupt onset of acute ischemic stroke results from the sudden disruption of blood flow to a part of the brain. The TOAST classification for ischemic stroke subtypes is a standard and widely-used method.[1] It characterizes each stroke according to its causal mechanism, taking into account the history, neurological signs, and the findings from head CT scan, carotid and transcranial duplex sonography, magnetic resonance angiography (MRA), electrocardiography, and echocardiography. Ischemic strokes are classified into the following categories: 1) large-artery disease, either intracranial or extracranial; 2) small-artery disease (lacune); 3) cardioembolism; 4) stroke of other determined etiology; 5) stroke of undetermined etiology; and 6) stroke of undetermined etiology-incomplete evaluation. Intracranial hemorrhage from a variety of causes- spontaneous, hypertension, and vascular malformations, eg, cerebral aneurysm-is frequently encountered clinically and figure prominently in the initial differential diagnoses of stroke. These are different processes but may be considered stroke subtypes for classification purposes and are listed in the first section of Table 1.
Table 1.
Differential diagnosis of acute stroke syndromes, mimics, and atypical stroke presentations

Little is known about stroke etiology in India. Available data suggest that hemorrhagic stroke is more common in India compared to the West.[2] There is not much data available on the prevalence of the different stroke subtypes in India. According to the stroke registry of the Nizam's Institute of Medical Sciences, which has data for the past few years, intracranial large-artery atherosclerotic disease seems to be the most common stroke mechanism in India, followed by lacunar, cardioembolic, and extracranial carotid disease respectively.[3,4,5] Two earlier studies from India, one based on conventional angiography and the other on MRA, also reported the high frequency of intracranial lesions.[6,7] The risk factors for the development of large- and small-artery disease are the same: hypertension, diabetes, and smoking.[8] No significant differences have been found between the risk factors for extra- and intracranial large-artery disease.[9] For cardioembolic stroke, rheumatic heart disease and ischemic heart disease seem to be the dominant risk factors in India.[10]
Stroke mimics
Stroke mimic is the term for a nonvascular disease process that produces a stroke-like clinical picture. The presentation resembles or may even be indistinguishable from a stroke syndrome. The mimics include processes occurring within the central nervous system (CNS) as well as systemic disease processes. Distinguishing these stroke mimics from genuine strokes is important as stroke therapies that are used today have potentially serious adverse effects.
Stroke mimics may be discovered at different stages during the clinical investigation of a patient. The advent of neuroimaging allowed the first estimates to be made of the frequency of stroke mimics. One of the earliest studies, which included patients with non-acute illness, showed the frequency of stroke mimics to be as high as 30%.[11] A prospective study published 25 years ago reviewed consecutive patients admitted to a stroke unit from the emergency department of a hospital.[12] They found that the initial diagnosis of stroke was incorrect in 13% of patients. A recent article by Libman et al. investigated the variables that might help discriminate stroke from stroke mimics.[13] They looked at consecutive patients presenting to an emergency department with an initial diagnosis of stroke over a 2-year period and found that 19% of these cases were actually stroke mimics. Four conditions comprised the majority of the mimics in this study: unrecognized seizures with postictal deficits, systemic infections, brain tumor, and toxic-metabolic disturbances. Kothari et al. reviewed the admission diagnoses of stroke (ischemic and hemorrhagic) for patients evaluated in an emergency department and admitted to a hospital.[14] They found that there was disagreement between the admission diagnosis of ischemic stroke or TIA and the final discharge diagnosis in only 4% of cases. A recent small study used extensive imaging, including magnetic resonance imaging (MRI), MRA, diffusion-weighted imaging (DWI), and perfusion-weighted imaging (PWI), to investigate patients thought to have anterior circulation stroke. They found that 9% of patients initially diagnosed with stroke were 'misdiagnosed,' which they defined as completely normal detailed MR studies with a probable alternative clinical diagnosis.[15] Diffusion-weighted MRI was used to investigate stroke-like events in a study by Ay et al.[16] They found that 3.5% of the patients with enduring deficits who were thought to have ischemic stroke had normal diffusion-weighted MRI. It is clear that the incidence of stroke mimics in any study depends upon the time at which the acute stroke syndrome is assessed. In the study by Libman et al., in which the initial assessment was made after history and physical examination alone, stroke mimics made up 19% of the cases.[13] In the study by Kothari et al., when the assignment of stroke syndrome was made after routine laboratory work and CT scanning, the incidence of stroke mimics was about 4%.[14] In the study by Ay et al., which employed MRI techniques in addition to laboratory workup and CT scanning, the incidence of stroke mimics decreased to 1-2%.[16] There are technical limitations in detecting cerebral ischemia using these techniques, but each investigation-be it a laboratory test, CT, or MRI-increases the specificity of the diagnosis of ischemic stroke. Information on stroke mimics in India is lacking, and readers are asked to be cautious in interpretation of the stroke mimics based on data from the Western population.
Specific stroke mimics
Broadly, stroke mimics may be grouped into those that are likely to have and those less likely to have focal neurological findings. Those that are likely to present with focal neurological findings are seizure, complicated migraine, structural brain lesions, hypoglycemia, rare manifestation of multiple sclerosis, and functional hemiparesis.
Seizure
Every study on stroke mimics identifies seizures and post-seizure events as common causes of stroke-like conditions. Traditional thought is that these postictal symptoms are manifestations of seizure-induced alterations in neuronal function that are reversible; structural neuronal alterations are not present. Postictal weakness or Todd's paralysis usually follows partial motor seizures but may follow generalized seizures as well. The duration of such weakness or paralysis is usually brief, but it may last up to 48 h.[17] Rare inhibitory seizures, with extremity weakness as a manifestation of the seizure event, have been reported as well.[17] Seizures may also present as a complication of acute stroke or develop in a patient with a history of stroke.[18] Most studies have identified postictal states indirectly, after further seizures were observed or after additional history was obtained that suggested a history of seizure disorder.
Migraine mimicking stroke
Migraine may actually precipitate a stroke, but there is also a variant of migraine-hemiplegic migraine-where unilateral hemiparesis outlasts the headache.[19] This is difficult, if not impossible, to diagnose correctly at first presentation; it must be regarded as a diagnosis of exclusion. Only with recurrent, stereotypic attacks can this diagnosis be suspected. Cases with alternating hemiplegia have been reported. In some cases, this disorder has been shown to be familial.[20]
Mass lesions
Subdural hematoma, cerebral abscess, primary CNS tumors, and metastatic tumors are among the clinical conditions simulating stroke in the studies cited above. A slowly growing mass typically produces a progressive syndrome; an abrupt onset of symptoms of these cases seems counterintuitive. A review of patients with brain tumors presenting to an Emergency Department (ED) showed that 6% of patients had symptoms that were of less than 1 day's duration; it was thought that these patients with brief symptom duration might represent a sub-population who suffer acute deterioration due to hemorrhage into the tumor or who develop obstructive hydrocephalus.[21] Secondary effects of mass or edema on cerebral vasculature have been identified as possible causes for the abrupt onset of seizures as well. Chronic subdural hematoma has been frequently reported as a cause of stroke and TIA-like symptoms.[22]
Hypoglycemia
That transient hypoglycemia may produce a stroke-like picture with hemiplegia and aphasia has been known for years.[23] These patients are usually drowsy but can also often be alert, without the more common features of hypoglycemia of confusion, diminished level of consciousness, or coma.[24] Aphasia may make the history of diabetes more difficult to discover. The syndrome has also been reported in alcoholics with hypoglycemia.[25] The pathogenesis of this focal CNS dysfunction is unclear. Hypoglycemia is generally defined as a blood glucose level of less than 45 mg/dl in these studies. The wide use of rapid bedside testing for glucose now makes this condition easily detectable and treatable. The hemiplegia may resolve immediately with the administration of intravenous glucose but resolution over a few hours is also reported.[26]
Paroxysmal manifestation of multiple sclerosis (MS)
A sudden-onset neurological deficit resembling a TIA may be the first manifestation of MS more often than is generally realized. This presentation is more common in the younger age-groups, the neurological deficits tending to be of briefer duration and recurring more frequently. Attention has been focused on paroxysmal symptoms of brain-stem and spinal-cord origin of the following types: paroxysmal dysarthria and ataxia, diplopia, tonic seizures, paroxysmal akinesia, and paroxysmal sensory disturbances and pains. All of these have been reported as the first symptoms of MS, with remissions lasting from less than 1 year to 21 years before other manifestations of MS developed.[27]
Functional hemiparesis
Some patients initially thought to have cerebrovascular disease are later determined to have a functional cause for the hemiparesis or other stroke-like syndrome. Conversion disorder is the most commonly diagnosed psychiatric illness in these patients. One study of emergency department presentations of conversion disorder noted that symptoms of paresis, paralysis, or movement disorders were common, being the presentation in almost 30% of patients.[28] Significant comorbidities were seen in this population, with other psychiatric disorders often present, and the authors emphasized that conversion disorder is a diagnosis of exclusion. Patients often undergo multiple diagnostic tests before the diagnosis is assigned. The history of the onset of the symptoms can be particularly helpful. Patients with functional weakness will often describe symptoms suggestive of dissociation at the onset-either occurring in combination with panic or a physical trauma (often minor) or spontaneously. Dissociation refers to the reduction or loss of the normal sense of ownership of one's actions. The history and the physical signs enable the diagnosis of functional hemiparesis to be made. In terms of physical findings, positive evidence of functional weakness (eg, Hoover's sign) and lack of objective signs of organic disease, are both supportive of the diagnosis of functional hemiparesis.[29]
Malingering or factitious hemiparesis
Discriminating between consciously produced and unconsciously produced functional hemiparesis is often more difficult. Among those patients who are consciously generating symptoms and signs, it is important to distinguish between those whose aim is to obtain 'medical care' and those in pursuit of material gain. Behavior of the first kind comes under the diagnosis of factitious disorder. Those who simulate illness for financial or other material gains are called malingerers. Determination of the nature of the gain that is being sought by the patient requires assessment by a psychiatrist.[29]
Encephalopathies and other toxic-metabolic conditions
Hyperglycemia with a hyperosmolar state may be associated with focal neurologic deficits simulating stroke; focal seizures have been reported in this condition as well. Focal neurologic signs seen in hyperglycemia may include aphasia, homonymous hemianopsia, hemisensory deficits, hemiparesis, unilateral hyperreflexia, and the presence of the Babinski sign.[17] Other metabolic encephalopathies reported to cause stroke-like conditions include hyponatremia and hepatic encephalopathy.[30,31]
Stroke in the young Indian population
Most of the studies carried out in India have shown that about 10-15% of strokes occur in those below 40 years, which is high compared to other countries.[32,33] This could be due to many local etiological factors. Previously, causes contributing to stroke in the young were reported as meningovascular syphilis in men, puerperal cerebral venous thrombosis in women, and rheumatic heart disease in both sexes.[34] A disturbance in the balance of coagulation and fibrinolysis has been suggested in the etiopathology of nonembolic cerebral infarction in the young.[35] Other studies have incriminated subacute tubercular meningitis, leading to arteritis or autoimmune angiitis, as an important risk factor in India.[36] More recently reported risk factors among the young include viper envenomation, elevated lipoprotein (a), and elevated anticardiolipin antibodies.[37,38,39] A recent Indian study suggests that the squatting posture adopted in the toilet could be an important triggering factor for stroke in Indians, by the mechanism of raising the blood pressure.[40]
Other unusual causes for stroke mimics in children
Stroke is always a consideration when a previously healthy child or infant suddenly develops focal neurological disturbance. Half of these cases are of ischemic etiology and half are nontraumatic intracerebral and subarachnoid hemorrhages arising from vascular malformation.[41] In children, ischemic stroke may be precipitated by a hemoglobinopathy (eg, sickle cell anemia), hypercoagulable state, congenital and rheumatic heart disease, trauma, vasculitis, vasculopathies such as MELAS (mitochondrial myopathy, encephalopathy, lactic acidosis, and stroke-like episodes). Nonvascular causes of focal neurological disturbances include, alternating hemiplegia, migraine, seizures, Kawasaki disease, trauma, and space occupying lesions.[42] Detailed descriptions of these conditions is outside the scope of this paper.
Atypical stroke presentations
Strokes with atypical presentations that take on the appearance of other disease process may change and evolve with time. The clinician is left with the daunting problem of discovering the unusual manifestation of an uncommon clinical process. A seemingly infinite number of unusual clinical syndromes have been attributed to ischemic stroke after thorough investigation. The presence of historical risk factors for cerebrovascular disease and the abrupt onset of symptoms may be the best clues available to the emergency physician to detect these unusual stroke syndromes. A few that are of clinical importance will be briefly summarized.
Most strokes present as a deficit or loss of function. Uncommonly, movement disorders will present due to a focal lesion such as an ischemic stroke or hemorrhage. Acute hemiballismus or unilateral dyskinesias often result from acute vascular lesions in the subthalamic nucleus or connections.[43] The movements may vary from wild flinging movements to mild uncontrollable unilateral movements. The key to diagnosis is the abrupt onset of symptoms and the presence of risk factors for cerebrovascular disease. A review notes that any kind of dyskinesia, hypokinetic as well as hyperkinetic, may be found from lesions at many different levels in the frontal motor cortical and subcortical regions.[44]
Confusional states, agitation, and delirium have all been reported as a consequence of focal neurologic injury; structures involving the limbic cortex of the temporal lobes and the orbitofrontal regions are commonly involved.[45] These states must be distinguished from the neglect syndromes and fluent aphasias in which patients are often reported as confused but careful examination demonstrates a clear focal deficit. In syndromes of visual neglect especially, testing for visual fields will reveal a dramatic field cut that the patient cannot report since he or she was unaware of the deficit.
Sensory complaints of either unusual sensations or loss of sensation are common in parietal and thalamic strokes. At times, the sensory manifestation of a stroke may take on the characteristics of another clinical condition. Chest pain and limb pain that mimicked that of myocardial infarction were reported in a small series of patients; most had thalamic strokes but one had a lateral medullary infarct.[46] Sensory symptoms may occur with lesions in many places in the central nervous system. Cortical involvement is usually accompanied by other neurologic deficits such as hemiparesis, aphasia, hemineglect, or visual field abnormalities.[47]
Cortical blindness is unusual but may occur; it can be distinguished from bilateral ocular disease by the normal pupillary light responses and normal optic disks. As many as 10% of patients with cortical blindness deny visual symptoms (Anton's syndrome); at times there is an element of 'blindsight,' with patients retaining some residual visual ability in their blind areas. For example, patients with blindsight may make correct 'guesses' about movements or colors of objects in the visually deficient areas, demonstrating some remnant perception of which they are not consciously aware.[48]
Bedside assessment of stroke
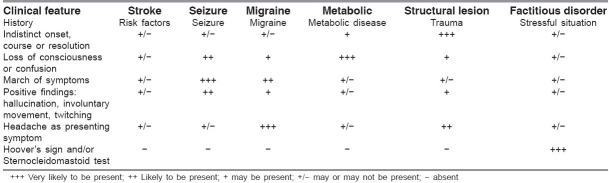
Simultaneous to the process of confirming the diagnosis of stroke, there needs to be an ongoing assessment at the bedside. The initial evaluation of a potential stroke patient is similar to that of other critically ill patients: stabilization of airway, breathing, and circulation (ABC). This is quickly followed by a secondary assessment of the neurological deficits and possible comorbidities. The overall goal is not only to identify patients with possible stroke but also to exclude stroke mimics, identify other conditions requiring immediate intervention, and determine potential causes of the stroke for early secondary prevention [Table 2]. A good history and a thorough physical examination to elicit the associated signs may help in differentiating stroke from the common mimics.
Table 2.
Clinical features of common stroke mimics that may distinguish it from stroke
1. History
Although it may not be absolutely accurate, some early historical data and clinical findings may direct the physician toward a diagnosis of another cause for the patient's symptoms [Table 1]. Some of the salient historical features that aid in distinction of stroke from the common stroke mimics are outlined in Table 2. An indistinct onset, course, or resolution point towards a stroke mimic being present.[49] Alteration of mental status or loss of consciousness, in the absence of lateralizing symptoms or signs, points towards metabolic or other causes of encephalopathy.[49] March of symptoms or positive symptoms is indicative of increased brain activity as seen in seizures. Finally, headache as the presenting symptom in the appropriate (young) age-group is more likely to represent migraine though it is also a feature seen commonly in stroke.
In addition to the historical aspects of the symptoms, it is important to ask about risk factors for atherosclerosis and cardiac disease in all patients, as well as any history of cigarette smoking, migraine, seizure, infection, trauma, or pregnancy. Historical data necessary for deciding the eligibility of the patient for therapeutic interventions in acute ischemic stroke are equally important.[50] Bystanders or family witnesses should be asked for information about onset time and historical issues, especially when patients are unable to speak or provide history. Validated tools for identification of stroke patients within an ED are available.[51] Telephone numbers, including cellular telephone numbers, of witnesses or relatives may help the ED physician to clarify the history or seek consent for treatment. A list of the patient's medications, or the medication containers themselves, should be sought, with particular attention paid to identifying any anticoagulant (both oral and injectable), antiplatelet, and antihypertensive drug use.
2. Physical examination
The general physical examination continues from the original assessment of the Airway, Breathing and Circulation (ABC) and should include pulse oximetry and body temperature. Examination of the head and neck may reveal signs of trauma or seizure activity (eg, contusions or tongue biting), carotid disease (bruits), or congestive heart failure (jugular venous distention). The cardiac examination focuses on identifying concurrent myocardial ischemia, valvular conditions, irregular rhythm and, in rare cases, aortic dissection, which could precipitate a cardioembolic event. Similarly, the respiratory and abdominal examinations seek to identify other comorbidities. Examination of the skin and extremities may also provide insight into important systemic conditions such as hepatic dysfunction, coagulopathies, or platelet disorders (eg, jaundice, purpura, or petechia).
3. Neurological examination and stroke scale scores
The emergency physician's neurological examination should be brief but thorough. It is enhanced by use of a formal stroke score or scale, such as the NIH Stroke Scale (NIHSS). It enables examiners to rapidly detect focal neurological deficits. In addition it may help quantify the neurological deficit resulting from a stroke and is useful in monitoring progress with stroke treatment such as thrombolysis. The scale can be used by a broad spectrum of non-neurological healthcare providers.[51,52] Use of a standardized examination helps to ensure that the major components of a neurological examination are performed in a timely fashion. These scores not only help to quantify the degree of neurological deficit but also facilitate communication between healthcare professionals, identify the possible location of vessel occlusion, provide early prognosis, and help to identify patient eligibility for various interventions and the potential for complications.[53,54,55] It may also have some predictive value in detecting stroke mimics. One study found a higher proportion of stroke mimics among patients with NIHSS 0-10 compared to those presenting with NIHSS >10.[49] Several studies have demonstrated that emergency physicians committed to stroke care may correctly identify and safely treat stroke patients, especially with the use of such standardized scales.[56,57] Access to neurological expertise when required may benefit care of the stroke patient.[58]
4. Diagnostic tests
Diagnostic tests should be performed routinely in patients with suspected ischemic stroke to identify systemic conditions that may mimic or cause stroke or that may influence therapeutic options [Table 3]. Neuroimaging in the form of CT and MRI are critically important. While non-contrast CT scan is useful in distinguishing hemorrhagic from ischemic stroke, it is of limited diagnostic value in differentiating stroke from stroke mimics. It may remain normal up to 24 h from symptom onset in ischemic stroke patients. Contrast CT, including CT perfusion (CTP) and CT angiogram (CTA), can contribute significantly to this differentiation. An abnormal CTP or CTA will not only aid in confirming the diagnosis of ischemic stroke, but also enable detection of contrast-enhancing lesions such as tumor and abscess. MR diffusion-weighted imaging has been found to have a high sensitivity and specificity in the early diagnosis of ischemic stroke. Perfusion-weighted imaging, which requires MR imaging with contrast, may be a useful adjunct to non-contrast DWI in confirming the diagnosis of ischemic stroke.
Table 3.
Immediate diagnostic studies: Evaluation of a patient with suspected acute stroke
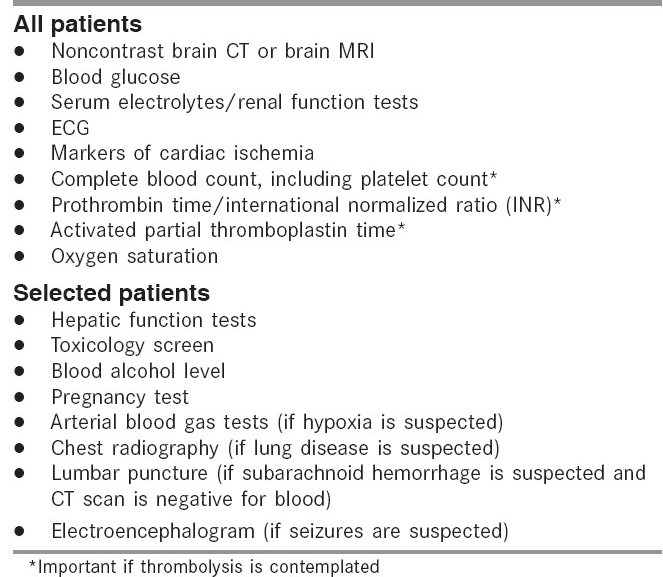
In addition to the neuroimaging modalities, blood tests are useful in the diagnosis of the stroke mimics. These tests include blood glucose measurement, complete blood count with platelet count, prothrombin time, activated partial thromboplastin time, international normalized ratio, and renal function studies. Hypoglycemia may cause focal symptoms and signs that mimic stroke, and hyperglycemia is associated with unfavorable outcomes. Determination of the platelet count and, in patients taking warfarin or with liver dysfunction, the prothrombin time/international normalized ratio is important. Because time is critical, it is advocated that thrombolytic therapy should be started for stroke patients while awaiting the results of the prothrombin time, activated partial thromboplastin time, or platelet count; therapy with thrombolytics is withheld in absence of these test results, if a bleeding abnormality or thrombocytopenia is suspected, if the patient has been taking warfarin and heparin, or if there is any uncertainty regarding anticoagulation use.
5. Other tests
A clinical cardiovascular examination, measurement of serum levels of cardiac enzymes, and a 12-lead ECG may be performed in all stroke patients [Table 3].[59] Cardiac abnormalities are common among patients with stroke, and the patient can have an acute cardiac condition that mandates urgent treatment. For example, acute myocardial infarction can lead to stroke, and acute stroke can lead to myocardial ischemia.[60,61,62] In addition, cardiac arrhythmias can occur among patients with acute ischemic stroke.[60,61,62,63,64] Atrial fibrillation, an important potential cause of stroke, can be detected in the acute setting.[65] Cardiac monitoring should be conducted routinely after an acute cerebrovascular event to screen for serious cardiac arrhythmias.[65] Examination of the cerebrospinal fluid is indicated if the patient has symptoms suggestive of subarachnoid hemorrhage and a CT scan does not demonstrate blood. Although CT scan is more sensitive than MRI in detecting subarachnoid blood in the acute phase, in the subacute phase, MRI sequences, in particular gradient-echo T2 images followed by fluid-attenuated inversion recovery (FLAIR) images, are considered to be the most sensitive. The clinical features of subarachnoid hemorrhage differ considerably from those of ischemic stroke. Cerebrospinal fluid analysis may be of additional value when CNS infection needs to be excluded as the cause for the stroke-like presentation. Electroencephalography may be helpful for evaluating patients in whom seizures are suspected as the cause of the neurological deficits or in whom seizures could have been a complication of the stroke.[66] Seizure in the absence of imaging confirmation of acute ischemia is a relative contraindication for the use of rtPA in acute ischemic stroke.
Additional tests may be performed as indicated by the patient's history, symptoms, physical findings, or comorbidities [Table 3]. A toxicology screen, blood alcohol level, arterial blood gas, and pregnancy test should be obtained if the physician is uncertain about the patient's history or if suggested by findings on examination.
In summary, bedside assessment is important in distinguishing stroke from stroke mimics. Blood tests and brain imaging are often useful adjuncts to bedside assessment. The later may play critical roles in identification of stroke, decision to treat, and prioritization of tests, in view of the fact that thrombolytic therapy carries the risk of bleeding and is often limited by a narrow time window of opportunity
Footnotes
Source of Support: Nil
Conflict of Interest: Nil.
References
- 1.Adams HP, Bendixen BH, Jap Kappelle L, Biller J, Love BB, Gordon DL, et al. Classification of subtypes of acute ischemic stroke: Definitions for use in a multicenter clinical trial. Stroke. 1993;24:35–41. doi: 10.1161/01.str.24.1.35. [DOI] [PubMed] [Google Scholar]
- 2.Das SK, Banerjee TK, Biswas A, Roy T, Raut DK, Mukherjee CS, et al. A prospective community based study of stroke in Kolkata, India. Stroke. 2007;38:906–10. doi: 10.1161/01.STR.0000258111.00319.58. [DOI] [PubMed] [Google Scholar]
- 3.Kaul S, Sunitha P, Suvarna A, Meena AK, Uma M, Reddy JM. Subtypes of Ischemic Stroke in a Metropolitan City of South India (One year data from a hospital based stroke registry) Neurol India. 2002;50:S8–14. [Google Scholar]
- 4.Varalakshmi EA, Kaul S, Ramamurty S, Uma DM, Suvarna A, Murthy JM. The frequency, risk factors and pattern of intracranial vascular disease in patients of ischemic stroke. Neurol India. 1999;47:83. [Google Scholar]
- 5.Researchers identify stroke subtypes in India. Lancet. 2002;359:499. [Google Scholar]
- 6.Padma MV, Gaikwad S, Jain S, Maheshwari MC, Misra NK. Distribution of vascular lesions in ischemic stroke: A magnetic resonance angiographic study. Natl Med J India. 1997;10:217–20. [PubMed] [Google Scholar]
- 7.Dalal PM, Shah PM, Aiyar RR, Kikani BJ. Cerebrovascular diseases in West Central India: A report on angiographic findings from a prospective study. BMJ. 1968;3:769–74. doi: 10.1136/bmj.3.5621.769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kaul S, Venketswamy P, Meena AK, Sahay R, Murthy JM. Frequency, clinical features and risk factors of lacunar infarction: Data from a stroke registry from South India. Neurol India. 2000;2:116–9. [PubMed] [Google Scholar]
- 9.Kaul S, Sunitha P, Meena AK, Suvarna A. Subtypes of ischemic stroke in Hyderabad (South India): Data from a hospital based stroke registry. Neurol India. 2008 in press. [Google Scholar]
- 10.Kaul S, Laxmi GS, Meena AK, Murthy JM. Aetiological spectrum of cardioembolic stroke in India. Lancet. 1998;352:4. [Google Scholar]
- 11.Weisberg LA, Nice CN. Intracranial tumors simulating the presentation of cerebrovascular disease. Am J Med. 1977;63:517–24. doi: 10.1016/0002-9343(77)90196-6. [DOI] [PubMed] [Google Scholar]
- 12.Norris JW, Hachinski VC. Misdiagnosis of stroke. Lancet. 1982;1:328–31. doi: 10.1016/s0140-6736(82)91580-x. [DOI] [PubMed] [Google Scholar]
- 13.Libman RB, Wirkowski E, Alvir J, Rao TH. Conditions that mimic stroke in the emergency department. Implications for acute stroke trials. Arch Neurol. 1995;52:1119–22. doi: 10.1001/archneur.1995.00540350113023. [DOI] [PubMed] [Google Scholar]
- 14.Kothari RU, Brott T, Broderick JP, Hamilton CA. Emergency physicians: Accuracy in diagnosis of stroke. Stroke. 1995;26:2238–41. doi: 10.1161/01.str.26.12.2238. [DOI] [PubMed] [Google Scholar]
- 15.Allder SJ, Moody AR, Martel AL, Morgan PS, Delay GS, Gladman JR, et al. Limitations of clinical diagnosis in acute stroke. Lancet. 1999;354:1523. doi: 10.1016/s0140-6736(99)04360-3. [DOI] [PubMed] [Google Scholar]
- 16.Ay H, Buonanno FS, Rordorf G, Schaefer PW, Schwamm LH, Wu O, et al. Normal diffusion-weighted MRI during stroke-like deficits. Neurology. 1999;52:1784–92. doi: 10.1212/wnl.52.9.1784. [DOI] [PubMed] [Google Scholar]
- 17.Blume WT. Focal motor seizures and epilepsia partialis continua. In: Wyllie E, editor. The treatment of epilepsy: Principles and practice. Philadelphia: Lea and Febiger; 1993. pp. 393–400. [Google Scholar]
- 18.Abbot AL, Bladin PF, Donnan GA. Seizures and stroke. In: Bogousslavsky J, Caplan LR, editors. Stroke syndromes. 2nd ed. Cambridge: Cambridge University Press; 2001. pp. 182–91. [Google Scholar]
- 19.Thomsen LL, Ostergaard E, Olesen J, Russell MB. Evidence for a separate type of migraine with aura: Sporadic hemiplegic migraine. Neurology. 2003;60:595–601. doi: 10.1212/01.wnl.0000046524.25369.7d. [DOI] [PubMed] [Google Scholar]
- 20.Headache Classification Subcommittee of the International Headache Society. The International Classification of Headache Disorders, 2nd ed. Cephalalgia. 2004;24:9–160. doi: 10.1111/j.1468-2982.2003.00824.x. [DOI] [PubMed] [Google Scholar]
- 21.Snyder H, Robinson K, Shah D, Brennan R, Handrigan M. Signs and symptoms of patients with brain tumors presenting to the emergency department. J Emerg Med. 1993;11:253–8. doi: 10.1016/0736-4679(93)90042-6. [DOI] [PubMed] [Google Scholar]
- 22.Moster ML, Johnston DE, Reinmuth OM. Chronic subdural hematoma with transient neurological deficits: A review of 15 cases. Ann Neurol. 1983;14:539–42. doi: 10.1002/ana.410140508. [DOI] [PubMed] [Google Scholar]
- 23.Montgomery BM, Pinner CA, Newberry SC. Transient hypoglycemic hemiplegia. Arch Intern Med. 1964;114:680–4. doi: 10.1001/archinte.1964.03860110150017. [DOI] [PubMed] [Google Scholar]
- 24.Malouf R, Brust JC. Hypoglycemia: Causes, neurological manifestations, and outcome. Ann Neurol. 1985;17:421–30. doi: 10.1002/ana.410170502. [DOI] [PubMed] [Google Scholar]
- 25.Andrade R, Mathew V, Morgenstern MJ, Roberge R, Rubin K, Senekjian D, et al. Hypoglycemic hemiplegic syndrome. Ann Emerg Med. 1984;13:529–31. doi: 10.1016/s0196-0644(84)80521-1. [DOI] [PubMed] [Google Scholar]
- 26.Wallis WE, Donaldson I, Scott RS, Wilson J. Hypoglycemia masquerading as cerebrovascular disease (hypoglycemic hemiplegia) Ann Neurol. 1985;18:510–2. doi: 10.1002/ana.410180415. [DOI] [PubMed] [Google Scholar]
- 27.Espir ML, Watkins SM, Smith HV. Paroxysmal dysarthria and other transient neurological disturbances in disseminated sclerosis. J Neurol Neurosurg Psychiatry. 1966;29:323–30. doi: 10.1136/jnnp.29.4.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dula DJ, DeNaples L. Emergency department presentation of patients with conversion disorder. Acad Emerg Med. 1995;2:120–3. doi: 10.1111/j.1553-2712.1995.tb03174.x. [DOI] [PubMed] [Google Scholar]
- 29.Stone J, Zeman A, Sharpe M. Functional weakness and sensory disturbance. J Neurol Neurosurg Psychiatry. 2002;73:241–5. doi: 10.1136/jnnp.73.3.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Maccario M. Neurological dysfunction associated with nonketotic hyperglycemia. Arch Neurol. 1968;19:525–34. doi: 10.1001/archneur.1968.00480050095009. [DOI] [PubMed] [Google Scholar]
- 31.Berkovic SF, Bladin PF, Darby DG. Metabolic disorders presenting as stroke. Med J Aust. 1984;140:421–4. doi: 10.5694/j.1326-5377.1984.tb108105.x. [DOI] [PubMed] [Google Scholar]
- 32.Razdan S, Kaul RL, Motta A, Kaul S. Cerebrovascular disease in Rural Kashmir, India. Stroke. 1989;20:1691–3. doi: 10.1161/01.str.20.12.1691. [DOI] [PubMed] [Google Scholar]
- 33.Srinivasan K. Ischemic cerebrovascular disease in the young: Two common causes in India. Stroke. 1984;15:733–5. doi: 10.1161/01.str.15.4.733. [DOI] [PubMed] [Google Scholar]
- 34.Panicker JN, Madhusudanan S. Cerebral infarction in a young male following viper envenomation. J Assoc Physicians India. 2000;48:744–5. [PubMed] [Google Scholar]
- 35.Chopra JS, Prabhakar SK. Clinical features and risk factors in stroke in young. Acta Neurol Scand. 1979;60:289–300. doi: 10.1111/j.1600-0404.1979.tb02984.x. [DOI] [PubMed] [Google Scholar]
- 36.Abraham J. Tropical neurology. In: Spilllane JD, editor. London: Oxford University Press; 1973. pp. 86–91. [Google Scholar]
- 37.Christopher R, Kailasanatha KM, Nagaraja D, Tripathi M. Case-control study of serum lipoprotein(a) and apolipoproteins A-1 and B in stroke in the young. Acta Neurol Scand. 1996;94:127–30. doi: 10.1111/j.1600-0404.1996.tb07042.x. [DOI] [PubMed] [Google Scholar]
- 38.Nagaraja D, Christopher R, Manjari T. Anticardiolipin antibodies in ischemic stroke in the young: Indian experience. J Neurol Sci. 1997;150:137–42. doi: 10.1016/s0022-510x(97)00071-3. [DOI] [PubMed] [Google Scholar]
- 39.Chakrabarti SD, Ganguly R, Chatterjee SK, Chakaravarty A. Is squatting a triggering factor for stroke in Indians. Acta Neurol Scand. 2002;105:124–7. doi: 10.1034/j.1600-0404.2002.1o196.x. [DOI] [PubMed] [Google Scholar]
- 40.Feldmann E, Daneault N, Kwan E, Ho KJ, Pessin MS, Langenberg P, et al. Chinese-white differences in the distribution of occlusive cerebrovascular disease. Neurology. 1990;40:1541–2. doi: 10.1212/wnl.40.10.1540. [DOI] [PubMed] [Google Scholar]
- 41.Lanthier S, Carmant L, David M, Larbrisseau A, de Veber G. Stroke in children: The coexistence of multiple risk factors predicts poor outcome. Neurology. 2000;54:371–8. doi: 10.1212/wnl.54.2.371. [DOI] [PubMed] [Google Scholar]
- 42.Fenichel GM. Clinical pediatric neurology, a signs and symptoms approach. 5th ed. Elsevier Saunders; 2005. pp. 239–53. [Google Scholar]
- 43.Atchison JW, Pellegrino M, Herbers P, Tipton B, Matkovic V. Hepatic encephalopathy mimicking stroke. Am J Phys Med Rehabil. 1992;71:114–8. doi: 10.1097/00002060-199204000-00010. [DOI] [PubMed] [Google Scholar]
- 44.Ghika J, Bogousslavsky J. Abnormal movements. In: Bogousslavsky J, Caplan LR, editors. Stroke syndromes. 2nd ed. Cambridge: Cambridge University Press; 2001. pp. 162–81. [Google Scholar]
- 45.Brust JC, Caplan LR. Agitation and delirium. In: Bogousslavsky J, Caplan LR, editors. Stroke syndromes. 2nd ed. Cambridge: Cambridge University Press; 2001. pp. 222–31. [Google Scholar]
- 46.Gorson KC, Pessin MS, DeWitt LD, Caplan LR. Stroke with sensory symptoms mimicking myocardial ischemia. Neurology. 1996;46:548–51. doi: 10.1212/wnl.46.2.548. [DOI] [PubMed] [Google Scholar]
- 47.Kim JS. Sensory abnormality. In: Bogousslavsky J, Caplan LR, editors. Stroke syndromes. 2nd ed. Cambridge: Cambridge University Press; 2001. pp. 34–47. [Google Scholar]
- 48.Barton JS, Caplan LR. Cerebral visual dysfunction. In: Bogousslavsky J, Caplan LR, editors. Stroke syndromes. 2nd ed. Cambridge: Cambridge University Press; 2001. pp. 87–110. [Google Scholar]
- 49.Hand PJ, Kwan J, Lindley RI, Dennis MS, Wardlaw JM. Distinguishing between stroke and mimic at the bedside: The brain attack study. Stroke. 2006;37:769–75. doi: 10.1161/01.STR.0000204041.13466.4c. [DOI] [PubMed] [Google Scholar]
- 50.The National Institute of Neurological Disorders and Stroke tPA Stroke Study Group. Tissue plasminogen activator for acute ischemic stroke. N Engl J Med. 1995;333:1581–7. doi: 10.1056/NEJM199512143332401. [DOI] [PubMed] [Google Scholar]
- 51.Nor AM, Davis J, Sen B, Shipsey D, Louw SJ, Dyker AG, et al. The Recognition of Stroke in the Emergency Room (ROSIER) scale: Development and validation of a stroke recognition instrument. Lancet Neurol. 2005;4:727–34. doi: 10.1016/S1474-4422(05)70201-5. [DOI] [PubMed] [Google Scholar]
- 52.Goldstein LB, Samsa GP. Reliability of the National Institutes of Health Stroke Scale: Extension to non-neurologists in the context of a clinical trial. Stroke. 1997;28:307–10. doi: 10.1161/01.str.28.2.307. [DOI] [PubMed] [Google Scholar]
- 53.Krieger DW. Should a hospital without a neurologist use t-PA to treat stroke? Cleve Clin J Med. 1999;66:585–6. doi: 10.3949/ccjm.66.10.585. [DOI] [PubMed] [Google Scholar]
- 54.Frankel MR, Morgenstern LB, Kwiatkowski T, Lu M, Tilley BC, Broderick JP, et al. Predicting prognosis after stroke: A placebo group analysis from the National Institute of Neurological Disorders and Stroke rt-PA Stroke Trial. Neurology. 2000;55:952–9. doi: 10.1212/wnl.55.7.952. [DOI] [PubMed] [Google Scholar]
- 55.Lewandowski CA, Frankel M, Tomsick TA, Broderick J, Frey J, Clark W, et al. Combined intravenous and intra-arterial r-TPA versus intra-arterial therapy of acute ischemic stroke: Emergency Management of Stroke (EMS) Bridging Trial. Stroke. 1999;30:2598–605. doi: 10.1161/01.str.30.12.2598. [DOI] [PubMed] [Google Scholar]
- 56.Kothari RU, Brott T, Broderick JP, Hamilton CA. Emergency physicians: Accuracy in the diagnosis of stroke. Stroke. 1995;26:2238–41. doi: 10.1161/01.str.26.12.2238. [DOI] [PubMed] [Google Scholar]
- 57.Morgenstern LB, Lisabeth LD, Mecozzi AC, Smith MA, Longwell PJ, McFarling DA, et al. A population-based study of acute stroke and TIA diagnosis. Neurology. 2004;62:895–900. doi: 10.1212/01.wnl.0000115103.49326.5e. [DOI] [PubMed] [Google Scholar]
- 58.Goldstein LB, Simel DL. Is this patient having a stroke? JAMA. 2005;293:2391–402. doi: 10.1001/jama.293.19.2391. [DOI] [PubMed] [Google Scholar]
- 59.Christensen H, Fogh Christensen A, Boysen G. Abnormalities on ECG and telemetry predict stroke outcome at 3 months. J Neurol Sci. 2005;234:99–103. doi: 10.1016/j.jns.2005.03.039. [DOI] [PubMed] [Google Scholar]
- 60.Oppenheimer SM, Hachinski VC. The cardiac consequences of stroke. Neurol Clin. 1992;10:167–76. [PubMed] [Google Scholar]
- 61.Oppenheimer SM. Neurogenic cardiac effects of cerebrovascular disease. Curr Opin Neurol. 1994;7:20–4. doi: 10.1097/00019052-199402000-00005. [DOI] [PubMed] [Google Scholar]
- 62.Dimant J, Grob D. Electrocardiographic changes and myocardial damage in patients with acute cerebrovascular accidents. Stroke. 1977;8:448–55. doi: 10.1161/01.str.8.4.448. [DOI] [PubMed] [Google Scholar]
- 63.Norris JW, Froggatt GM, Hachinski VC. Cardiac arrhythmias in acute stroke. Stroke. 1978;9:392–6. doi: 10.1161/01.str.9.4.392. [DOI] [PubMed] [Google Scholar]
- 64.Mikolich JR, Jacobs WC, Fletcher GF. Cardiac arrhythmias in patients with acute cerebrovascular accidents. JAMA. 1981;246:1314–7. [PubMed] [Google Scholar]
- 65.Vingerhoets F, Bogousslavsky J, Regli F, Van Melle G. Atrial fibrillation after acute stroke. Stroke. 1993;24:26–30. doi: 10.1161/01.str.24.1.26. [DOI] [PubMed] [Google Scholar]
- 66.Bladin CF, Alexandrov AV, Bellavance A, Bornstein N, Chambers B, Cote R, et al. Seizures after stroke: A prospective multicenter study. Arch Neurol. 2000;57:1617–22. doi: 10.1001/archneur.57.11.1617. [DOI] [PubMed] [Google Scholar]