Abstract
Purpose
Use of dual mobility (DM) articulations can reduce the risk of instability in both primary and revision total hip arthroplasty (THA). Knowledge regarding the impact of this design on patient-reported outcome measures (PROMs) is limited. This study aims to compare clinical outcomes between DM and fixed bearing (FB) prostheses following primary THA.
Materials and Methods
All patients who underwent primary THA between 2011-2021 were reviewed retrospectively. Patients were separated into three cohorts: FB vs monoblock-D vs modular-DM. An evaluation of PROMs including HOOS, JR, and FJS-12, as well as discharge-disposition, 90-day readmissions, and revisions rates was performed. Propensity-score matching was performed to limit significant demographic differences, while ANOVA and chi-squared test were used for comparison of outcomes.
Results
Of the 15,184 patients identified, 14,652 patients (96.5%) had a FB, 185 patients (1.2%) had a monoblock-DM, and 347 patients (2.3%) had a modular-DM prosthesis. After propensity-score matching, a total of 447 patients were matched comparison. There was no statistical difference in the 90-day readmission (P=0.584), revision rate (P=0.265), and 90-day readmission (P=0.365) and revision rate due to dislocation (P=0.365) between the cohorts. Discharge disposition was also non-significant (P=0.124). There was no statistical difference in FJS-12 scores at 3-months (P=0.820), 1-year (P=0.982), and 2-years (P=0.608) between the groups.
Conclusion
DM bearings yield PROMs similar to those of FB implants in patients undergoing primary THA. Although DM implants are utilized more often in patients at higher-risk for instability, we suggest that similar patient satisfaction may be attained while achieving similar dislocation rates.
Keywords: Dual mobility, Fixed bearing, Total hip arthroplasty, Outcomes, Patient reported outcome scores
INTRODUCTION
Instability remains a significant mode of failure following total hip arthroplasty (THA)1). It is a multifactorial problem with both patient-related and surgical considerations. Surgical factors that can be modified may include surgical approach, component positioning, and component design2). Abnormal spinopelvic mobility has been implicated in the failure of component placement within traditional “safe zones” for reliable prevention of dislocation; however, there are no definitively established optimal criteria for use in determining patient-specific functional targets. In this context, the dual mobility (DM) articulation has recently gained popularity in the United States (U.S.) as an effective option for increasing stability following THA.
The DM design, which was initially proposed by Gilles Bousquet and Andre Rambert in the 1970s, incorporates a small inner head within a mobile polyethylene bearing, which in turn articulates with a large acetabular shell or liner3). Modular-DM designs combine a separate acetabular shell with or without holes allowing for potential screw fixation and an inner modular liner for articulation with the outer polyethylene head. Monoblock (non-modular) DM designs merge the articulating surface with the acetabular shell, allowing for even larger bearing diameters at the expense of liner modularity and screw fixation capabilities.
Use of DM implants in the settings of revision THA and primary THA for patients at high risk for instability is supported by published data4,5,6). While the use of non-modular DM in primary THA has been prevalent in Europe since its inception and good results have been obtained7,8), introduction of modular options that enable liner modularity and screw fixation and facilitate use in both primary and revision THA resulted in the widespread popularity of DM bearings in the U.S. Reported complications include component loosening, polyethylene wear, intraprosthetic dislocations, and severe corrosion of acetabular components unique to DM implants9,10,11,12); however, modifications to the design of DM implants have resulted in mitigation of several major mechanisms of failure seen in older systems. As a result of these advances, use of DM in the primary THA setting has increased in the U.S13); as reported in 2016, approximately 9% of primary THA and 28% of revisions in the American Joint Replacement Registry utilized a DM device14).
Regarding literature on the use of DM in primary THA, the primary focus has been on implant survivorship and complication rates5,15,16,17,18). Survivorship for first-generation DM implants has been reported as high as 85% at 15-year follow-up and exceeds 99% at 5-year follow-up for second-generation DM implants7,8), while substantially minimizing the risk of instability after both primary and revision THA19). Despite this robust evidence, there is limited data on patient-reported outcome measures (PROMs) in these cohorts. Indeed, there is a theoretical concern that use of prostheses with a large head could result in decreased patient satisfaction due to impingement and irritation of soft tissue 20,21,22,23); this topic was explored by Stavrakis et al.24) however there was no reference to PROMs. In order to gain insight into patient perspective rather than relying on the surgeon’s determination of successful outcomes, it is imperative that we gain an understanding of whether newer implant designs including DM bearings, which are being utilized at higher rates, have any effect on postoperative patient satisfaction. Therefore, the primary purpose of this study is to compare postoperative outcomes, particularly PROMs between patients undergoing primary THA using DM versus FB prostheses.
MATERIALS AND METHODS
1. Study Design
All patients over the age of 18 who underwent primary THA between June 2011 and February 2021 at a single urban institution, which comprises a large academic center and a tertiary orthopedic specialty hospital, were reviewed retrospectively. Patients were separated into three cohorts according to the prostheses type: FB versus monoblock-DM versus modular-DM. Patients undergoing revision THA, as well as THA performed for non-elective or oncologic reasons were excluded from this analysis. Both acetabular cups and femoral stems were selected at the discretion of the surgeon. Cases from 66 surgeons were included; a DM implant was used in 31 cases. Of the 15,184 primary THA cases identified, 14,652 cases (96.5%) had a FB, 185 cases (1.2%) had a monoblock-DM, and 347 cases (2.3%) had a modular-DM prosthesis implanted. All patients included in this study were participants in our institution-wide comprehensive total joint pathway program, which encompasses uniform standardized protocols for all aspects of perioperative care. In addition, all patients followed a standard institutional postoperative rehabilitation protocol, as well as a standard postoperative pain protocol. As part of our institutional quality improvement program, patient records and data were de-identified; therefore, the current study was exempt from human-subjects review by our Institutional Review Board (IRB). Informed consent was obtained from all individual participants included in the study.
2. Monoblock-DM
Of the 185 monoblock-DM cases included in the study, 145 cases (78.4%) were skirted stainless steel (POLARCUP; Smith and Nephew, Memphis, TN, USA) and 40 cases (21.6%) were anatomic cobalt-chromium (Anatomic Dual Mobility [ADM]; Stryker Corporation, Mahwah, NJ, USA). The skirted stainless steel DM cup offers the option of screw fixation via a superior tab (this was not utilized in our patient cohort); however, because the anatomic cobalt-chromium implants lack this capability, both implants were used only in cases where adequate press-fit fixation could be achieved. Both monoblock implants subtend an arc of coverage greater than 180 degrees and thus may be considered variants of a cylindropheric DM design.
3. Modular-DM
Of the 347 modular DM implants included in the study, 210 implants (60.5%) were cylindrospheric (Modular Dual Mobility [MDM]; Stryker Corporation) and 137 implants (39.5%) were subhemispheric. Of the subhemispheric implants, 69 implants (19.9% of the modular DM cohort) were manufactured from cobalt-chromium (CoCr) alloy (G7; Zimmer Biomet, Warsaw, IN, USA), and the remaining 68 implants (19.6% of the modular DM cohort) were manufactured from zirconium (OR3O; Smith and Nephew). All cylindrospheric DM liners were manufactured from CoCr alloy.
4. Data Collection
Patient demographics including age, male/female, race, body mass index (BMI; kg/m2), American Society of Anesthesiology (ASA) classification, Charlson Comorbidity Index (CCI), and smoking status were collected. In addition, admission data including the length of stay (LOS; days), surgical time (minutes), discharge disposition, and 90-day all-cause adverse events (readmissions and revisions) were collected from our electronic patient medical record system, Epic (Epic Caboodle, version 15; Verona, WI, USA) using Microsoft SQL Server Management Studio 2017 (Redmond, WA, USA). Evaluation of LOS was based on days spent in the hospital following surgery and surgical time was determined from calculating the time difference between initial skin incision and skin closure. All patients were either discharged home with self-care or home services or to an acute or subacute rehabilitation facility. Discharge disposition was determined by means of shared decision-making between the operating surgeon and patient prior to surgery.
As part of our institutional standard of care, at the time of surgical scheduling, clinical care coordinators registered patients for an electronic patient engagement application (EPEA; Force Therapeutics, New York, NY, USA). The EPEA, a mobile and web-based technology, performs wireless delivery of digital PROM surveys to patients at predefined time intervals. This application was used for collection of Hip dysfunction and Osteoarthritis Outcome Score for Joint Replacement (HOOS, JR) scores preoperatively, three months, and one year postoperatively as well as the Forgotten Joint Score (FJS-12) at three months, one year, and two years postoperatively.
5. Outcome Measures
Evaluation of the primary outcome included postoperative adverse events such as 90-day all-cause readmission rate, and reoperation rate as well as PROMs as assessed by the HOOS, JR, and FJS-12. An analysis of readmission and reoperation within 90 days for dislocation was also performed. The secondary outcomes included perioperative data such as surgical time, LOS, and discharge disposition.
6. Statistical Analysis
All descriptive data are represented as mean±standard deviation for continuous variables and percentages for categorical variables. ANOVA was used for determination of statistical differences in numeric, continuous demographic variables while chi-squared (χ2) test was performed for categorical variables. A P-value of less than 0.05 was considered significant. All statistical analyses were performed using IBM SPSS Statistics (ver. 25; IBM, Armonk, NY, USA).
Patient demographics were compared between the three study cohorts prior to and after 1:1:1 propensity score matching. Propensity score matching was performed in order to limit the effect of potential differences in demographic variable between the three cohorts25). For this study, the propensity score was defined based on baseline demographic characteristics of the study population including age, sex, race, BMI, ASA classification, CCI, and smoking status. A 1:1:1 match was performed using a balanced, nearest-neighbor propensity score26). This method of cohort matching has previously been established as an optimal method for estimating differences between treatment groups27).
Following propensity score matching, ANOVA and Pearson’s chi-squared test were used for comparison of the demographic variables described previously to ensure these factors were statistically non-significant between the three cohorts. Finally, the same statistical tests described previously were used for comparison of all outcomes of interest between the matched cohorts.
RESULTS
1. Study Population
Prior to propensity score matching, statistical differences in age (63.20±11.69 vs 65.44±12.37 vs 61.45±13.13 years; P=0.001), sex (female, 55.9% vs 55.7% vs 77.8%; P<0.001), race (P<0.001), and CCI (3.60±2.06 vs 4.06±2.35 vs 3.51±2.21; P=0.007) were observed between the three cohorts (FB vs monoblock-DM vs modular-DM). Patients who received a modular-DM prosthesis were statistically more likely to have an ASA classification of III or higher compared with the FB and monoblock-DM cohorts (P=0.036). No statistical differences with regard to BMI (P=0.893) and smoking status (P=0.351) was observed between the three groups.
Following application of the 1:1:1 propensity score matching, each cohort included 149 patients for a total of 447 patients for the matched comparison. Patients who were not matched based on their calculated propensity score were excluded from the statistical analysis. Upon propensity score matching, there were no longer any significant demographic differences between the three cohorts, indicating a successful match for all desired covariates (Table 1).
Table 1. Patient Demographics.
| Variable | Unadjusted cohort comparison (n=15,184) | Matched cohort comparison (n=447) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Fixed bearing (n=14,652) | Monoblock DM (n=185) | Modular DM (n=347) | P-value | Fixed bearing (n=149) | Monoblock DM (n=149) | Modular DM (n=149) | P-value | ||
| Age (yr) | 63.20±11.69 | 65.44±12.37 | 61.45±13.13 | 0.001* | 65.04±11.49 | 64.43±11.61 | 64.51±12.83 | 0.892 | |
| Sex | <0.001* | 0.560 | |||||||
| Female | 8,186 (55.9) | 103 (55.7) | 270 (77.8) | 92 (61.7) | 88 (59.1) | 97 (65.1) | |||
| Male | 6,466 (44.1) | 82 (44.3) | 77 (22.2) | 57 (38.3) | 61 (40.9) | 52 (34.9) | |||
| Race | <0.001* | 0.165 | |||||||
| Caucasian | 10,981 (74.9) | 147 (79.5) | 220 (63.4) | 121 (81.2) | 122 (81.9) | 107 (71.8) | |||
| African-American | 1,792 (12.2) | 19 (10.3) | 48 (13.8) | 16 (10.7) | 15 (10.1) | 21 (14.1) | |||
| Asian | 248 (1.7) | 0 (0.0) | 7 (2.0) | 1 (0.7) | 0 (0.0) | 4 (2.7) | |||
| Other | 1,631 (11.1) | 19 (10.3) | 72 (20.7) | 11 (7.4) | 12 (8.1) | 17 (11.4) | |||
| BMI (kg/m2) | 29.43±41.21 | 29.74±7.13 | 28.37±6.29 | 0.893 | 28.47±5.85 | 29.61±7.45 | 29.33±6.19 | 0.293 | |
| ASA | 0.036* | 0.788 | |||||||
| I | 935 (6.4) | 11 (5.9) | 35 (10.1) | 8 (5.4) | 9 (6.0) | 13 (8.7) | |||
| II | 9,128 (62.3) | 118 (63.8) | 188 (54.2) | 99 (66.4) | 96 (64.4) | 89 (59.7) | |||
| III | 4,353 (29.7) | 54 (29.2) | 117 (33.7) | 40 (26.8) | 43 (28.9) | 44 (29.5) | |||
| IV | 236 (1.6) | 2 (1.1) | 7 (2.0) | 2 (1.3) | 1 (0.7) | 3 (2.0) | |||
| CCI | 3.60±2.06 | 4.06±2.35 | 3.51±2.21 | 0.007* | 3.70±1.91 | 3.72±1.97 | 3.86±2.27 | 0.755 | |
| Smoking status | 0.351 | 0.479 | |||||||
| Never smoker | 7,847 (53.6) | 97 (52.4) | 202 (58.2) | 83 (55.7) | 81 (54.4) | 85 (57.0) | |||
| Former smoker | 5,487 (37.4) | 75 (40.5) | 115 (33.1) | 47 (31.5) | 57 (38.3) | 51 (34.2) | |||
| Current smoker | 1,318 (9.0) | 13 (7.0) | 30 (8.6) | 19 (12.8) | 11 (7.4) | 13 (8.7) | |||
Values are presented as mean±standard deviation or number (%).
P-values are derived using one-way ANOVA for numerical values or χ2 tests for categorical values.
DM: dual mobility, BMI: body mass index, ASA: American Society of Anesthesiology.
*P<0.05.
2. Outcomes
The longest surgical time was observed for the modular-DM cohort compared to the FB and monoblock-DM cohorts, respectively (114.30±39.06 vs 97.51±35.95 vs 95.29±23.56 minutes; P<0.001). Statistically the greatest LOS was observed for those in the FB cohort compared to those in the modular-DM and monoblock-DM cohorts, respectively (2.62±2.62 vs 2.54±1.76 vs 1.94±1.48 days; P=0.007). There was no significant difference in discharge disposition between the three cohorts (P=0.124).
Based on the numbers available for analysis, the 90-day all-cause readmission rate (8.1% vs 5.4% vs 8.1%; P=0.584) and 90-day readmissions for dislocation (1.3% vs 0.0% vs 0.7%; P=0.365) between the FB, monoblock-DM, and modular DM cohorts. In addition, there was no statistical difference in the 90-day all-cause revision rate (3.4% vs 2.7% vs 0.7%; P=0.265) and the 90-day revision rate due to dislocation (1.3% vs 0.7% vs 0.0%; P=0.365) between the modular-DM, FB, and monoblock-DM cohorts, respectively. All findings demonstrated small effect sizes, implying that the differences between the three cohorts may be negligible despite statistical significance. Results of the full outcome comparison between the three propensity matched groups are shown in Table 2.
Table 2. Clinical Outcomes.
| Outcome variable | Fixed bearing (n=149) | Monoblock DM (n=149) | Modular DM (n=149) | Effect size | P-value | |
|---|---|---|---|---|---|---|
| Surgical time (min) | 97.51±35.95 | 95.29±23.56 | 114.30±39.06 | 1.48 | <0.001* | |
| Length of stay (day) | 2.62±2.62 | 1.94±1.48 | 2.54±1.76 | 0.22 | 0.007* | |
| Discharge disposition | 0.10 | 0.124 | ||||
| Home | 125 (83.9) | 129 (86.6) | 116 (77.9) | |||
| Other facility | 24 (16.1) | 20 (13.4) | 33 (22.1) | |||
| 90-day all-cause readmission | 12 (8.1) | 8 (5.4) | 12 (8.1) | 0.05 | 0.584 | |
| Dislocation | 2 (1.3) | 0 (0.0) | 1 (0.7) | 0.07 | 0.365 | |
| 90-day all-cause revision | 4 (2.7) | 1 (0.7) | 5 (3.4) | 0.08 | 0.265 | |
| Dislocation | 1 (0.7) | 0 (0.0) | 2 (1.3) | 0.07 | 0.365 | |
Values are presented as mean±standard deviation or number (%).
P-values are derived using one-way ANOVA for numerical values or χ2 tests for categorical values.
DM: dual mobility.
*P<0.05.
3. PROMs
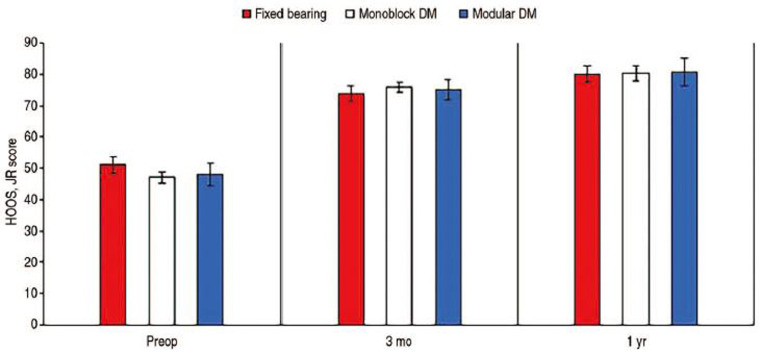
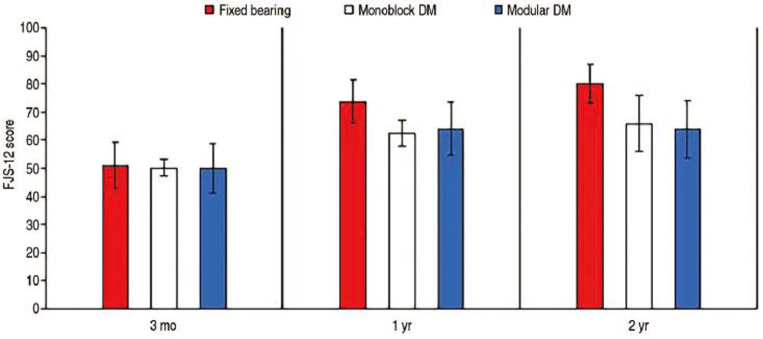
Using the available numbers, no statistical differences in mean HOOS, JR scores preoperatively (P=0.536), three months postoperatively (P=0.795), and one year postoperatively (P=0.993) were observed between the three cohorts. A similar trend was observed for mean FJS-12 as no significant difference in three-month (P=0.820), one-year (P=0.982), and two-year (P=0.608) postoperative scores were observed between the three cohorts. In addition, statistical difference in improvement from baseline to one-year follow-up in HOOS, JR scores was observed between the three groups (P=0.215). In addition, no statistical difference in the delta improvement in FJS-12 scores from three months to one year (P=0.762) and three-month to two-year follow-up (P=0.054) was observed between the three groups. Full details regarding PROMs comparisons are highlighted in Table 3 as well as Fig. 1 and 2.
Table 3. Patient-Reported Outcome Measures (PROMs).
| Fixed bearing | Monoblock DM | Modular DM | P-value | ||
|---|---|---|---|---|---|
| HOOS, JR | |||||
| Preop | 51.09±14.74 (n=29) | 47.12±17.02 (n=97) | 47.99±17.98 (n=23) | 0.536 | |
| 3 mo | 73.75±13.31 (n=28) | 75.84±14.38 (n=82) | 75.10±14.52 (n=21) | 0.795 | |
| 1 yr | 80.07±12.67 (n=24) | 80.24±17.21 (n=52) | 80.69±16.27 (n=14) | 0.993 | |
| FJS-12 | |||||
| 3 mo | 45.29±30.91 (n=15) | 50.20±26.98 (n=77) | 50.01±28.59 (n=11) | 0.820 | |
| 1 yr | 61.58±32.04 (n=18) | 62.47±30.95 (n=47) | 64.07±26.34 (n=8) | 0.982 | |
| 2 yr | 75.90±26.17 (n=14) | 65.94±37.58 (n=14) | 63.90±30.36 (n=9) | 0.608 | |
| Improvement in PROMs | |||||
| ∆HOOS, JR: Preop to 1 yr | 28.98±8.89 | 33.12±10.83 | 32.70±10.95 | 0.215 | |
| ∆FJS-12: 3 mo to 1 yr | 16.29±19.94 | 12.27±18.70 | 14.06±17.50 | 0.762 | |
| ∆FJS-12: 3 mo to 2 yr | 30.61±18.60 | 15.74±22.76 | 13.89±18.72 | 0.054 | |
Values are presented as mean±standard deviation.
P-values are derived using one-way ANOVA.
DM: dual mobility, Preop: preoperative, HOOS, JR: Hip dysfunction and Osteoarthritis Outcome Score for Joint Replacement, FJS-12: Forgotten Joint Score.
Fig. 1. Hip dysfunction and Osteoarthritis Outcome Score for Joint Replacement (HOOS, JR) scores.
Values are presented as mean±standard deviation.
DM: dual mobility, Preop: preoperative.
Fig. 2. Forgotten Joint Score (FJS-12) scores.
Values are presented as mean±standard deviation.
DM: dual mobility.
DISCUSSION
Although THA is among one of the most successful operations to date, instability remains one of its most vexing complications. Many prior studies on DM implants have reported on revision THA, where the problem of instability may be more paramount; however, fewer studies have reported on their utility in primary THA. Our aim was to compare monoblock and modular DM implants with FB prostheses in terms of PROMs as well as clinical outcomes. In our study, we found that HOOS, JR and FJS-12 scores were statistically similar between FB, monoblock-DM, and modular-DM cohorts. Despite significant differences with regard to surgical time and LOS, the effect size for each of these variables was small, suggesting that overall patient-reported and clinical outcomes were similar between all three groups. However, conduct of larger future studies is likely needed in order to uncover small but clinically meaningful differences, which are much more likely to exist than large differences between the three cohorts.
In a recently published multi-center analysis comparing DM and FB prostheses, Dubin and Westrich17) concluded that patients receiving DM bearings have improved PROMs and a lower rate of dislocation, readmission, and revision compared with patients who undergo FB THA. However, their findings with regard to dislocation and readmission were not statistically significant between the two groups. In addition, their study included a single monoblock-DM implant (ADM) and a single modular-DM (MDM) acetabular component by the same manufacturer, which were combined into a single cohort. In the current study monoblock and modular DM articulations were analyzed separately, including several DM constructs not studied in the previous report, potentially providing a clearer comparison of available DM and FB implants in the U.S. market.
Rowan et al.16) reported no statistical difference in postoperative modified Harris hip score (mHHS) between DM and FB cohorts in a matched cohort analysis of patients <55 years old. One recent study reported no significant differences in HHS, HHS function, Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC), pain visual analogue scale, Veterans RAND 12-Item Health Survey, UCLA (University of California, Los Angeles), and HHS total scores between ADM and MDM cups at a follow-up of 2.86 years28). Similarly, results of our study showed no significant differences in HOOS, JR and FJS-12 scores between our three cohorts at any time points, suggesting that DM implant constructs are equally well tolerated by patients as their traditional FB counterparts.
DM implants have large outer-bearing articulations, which can potentially increase the risk of postoperative groin pain. A possible reason for the statistically similar PROMs between our study cohorts may be the lack of a significant increase in groin pain in patients with various DM constructs as demonstrated by Stavrakis et al.24). Findings of their study showed that the odds of groin pain increased with a greater cup-to-head ratio, although, interestingly, DM implants showed no significant association with groin pain one year after surgery compared with conventional THA24). The authors hypothesized that this may be attributable to the fact that the majority of the movement occurs inside the diameter of the cup implant. This may obviate a number of theoretical causes of external sources of hip and groin pain that are associated with large-head bearing surfaces, such as iliopsoas impingement and greater force on the femoral trunnion, which can result in corrosion, metal release, and adverse local tissue reactions29,30,31). Although we did not examine specific individual anatomic factors that may influence PROMs, overall similar PROM improvement rates suggest that the use of DM implants is not associated with decreased patient satisfaction. DM THA may provide a way to increase the functional head size without the increased risk of groin pain seen with HR and large-head THA.
The benefit of employing DM implants over FB constructs in preventing dislocation in primary THA has been demonstrated in some studies16,32). In an analysis of a series of patients at risk of dislocation using DM cups, Prudhon et al.33) found that the dislocation rate at a median follow-up of 91 months was low (0.9%) compared to the dislocation rate in primary THA using a conventional FB cup. Although we found no statistical differences with regard to both 90-day readmission and 90-day revision for dislocation between our three cohorts, DM articulations were used at the discretion of the surgeon, most commonly in patients who were considered to be at a higher risk for instability. At our institution patients considered to be at higher risk for instability include those with a prior history of dislocation of contralateral THA, neurologic disorders such as Parkinson’s disease, hypermobility, and spinopelvic risk factors. Thus achieving a dislocation rate comparable to that of FB implants may indicate that the intrinsic stability of DM cups was sufficient to counter this selection bias, increasing stability by altering the head-neck ratio, increasing jump distance, and increasing the impingement free range of motion in patients who receive DM prosthesis18). A study by Jones et al.34) reported dislocation results (0.66%) with monoblock DM implants that were similar to those of the current study, even in high-risk patients, age >75 years, female sex, and ASA class >3. In addition, Dubin and Westrich28) reported revision rates of 0% in the monoblock-DM (ADM) group and 0% in the modular-DM (MDM) group due to isolated acetabular revisions. In their systematic review that included 12,844 THAs with a mean follow-up of 6.8 years, De Martino et al.19) reported a 0.9% mean rate of dislocation with DM components in primary THA, which is comparable to our findings.
This study is not without limitations. We acknowledge the short follow-up period pertaining to the readmission and revision data, and the retrospective nature of the study may have introduced inherent biases. However, because dislocations occur most frequently within the first three months after surgery35), our data on dislocation at shorter follow-up is very pertinent. Although we did not examine femoral head sizes, there were no statistical differences in dislocation rates irrespective of implant, therefore little can be inferred with regard to the effect of differences in head size and cup size on dislocations. The smaller outer diameter of the polyethylene liner ≤38-mm, 22-mm (vs 28-mm) inner head size, and isolated liner exchange to modular-DM (MDM) with retention of the acetabular component have been reported as risk factors for recurrent dislocation36). While polyethylene wear was not assessed in this study, there were no cases requiring revision surgery for polyethylene wear, osteolysis, or intraprosthetic dislocation. There was heterogeneity of implants used in the FB group as well as the primary diagnosis for requiring surgery in the entire study population. Although iliopsoas impingement due to the large femoral head size in DM constructs was not evaluated in the current study, PROMs were utilized as a proxy to detail the potential for decreased patient satisfaction between DM and FB implant designs. Last, the use of DM articulation was at the discretion of the surgeon, indicating the potential for selection bias; however, the orthopedic literature does not include currently accepted universal criteria for the use of DM implants. Therefore, the most complex and high-risk patients are likely to receive modular-DM. Despite these limitations, because DM bearings are generally reserved for patients who are at increased risk for postoperative instability, these results are valuable and encouraging. With the use of DM implants, these patients may be able to achieve primary THA outcomes comparable to those who receive FB components.
CONCLUSION
DM THAs are a viable alternative to traditional bearing surfaces in patients undergoing primary THA due to the low incidence of postoperative hip instability. Our findings suggest that similar dislocation rates may be achieved with the use of DM implants in primary THA while still maintaining excellent and non-inferior patient satisfaction as measured through PROMs.
Footnotes
CONFLICT OF INTEREST: The authors declare that there is no potential conflict of interest relevant to this article.
References
- 1.Gwam CU, Mistry JB, Mohamed NS, et al. Current epidemiology of revision total hip arthroplasty in the United States: National Inpatient Sample 2009 to 2013. J Arthroplasty. 2017;32:2088–2092. doi: 10.1016/j.arth.2017.02.046. [DOI] [PubMed] [Google Scholar]
- 2.Werner BC, Brown TE. Instability after total hip arthroplasty. World J Orthop. 2012;3:122–130. doi: 10.5312/wjo.v3.i8.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lautridou C, Lebel B, Burdin G, Vielpeau C. [Survival of the cementless Bousquet dual mobility cup: minimum 15-year follow-up of 437 total hip arthroplasties] Rev Chir Orthop Reparatrice Appar Mot. 2008;94:731–739. doi: 10.1016/j.rco.2008.06.001. French. [DOI] [PubMed] [Google Scholar]
- 4.Hartzler MA, Abdel MP, Sculco PK, Taunton MJ, Pagnano MW, Hanssen AD. Otto Aufranc Award: dual-mobility constructs in revision THA reduced dislocation, rerevision, and reoperation compared with large femoral heads. Clin Orthop Relat Res. 2018;476:293–301. doi: 10.1007/s11999.0000000000000035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Levin JM, Sultan AA, O’Donnell JA, et al. Modern dual-mobility cups in revision total hip arthroplasty: a systematic review and meta-analysis. J Arthroplasty. 2018;33:3793–3800. doi: 10.1016/j.arth.2018.08.013. [DOI] [PubMed] [Google Scholar]
- 6.Harwin SF, Sultan AA, Khlopas A, et al. Mid-term outcomes of dual mobility acetabular cups for revision total hip arthroplasty. J Arthroplasty. 2018;33:1494–1500. doi: 10.1016/j.arth.2017.12.008. [DOI] [PubMed] [Google Scholar]
- 7.Vielpeau C, Lebel B, Ardouin L, Burdin G, Lautridou C. The dual mobility socket concept: experience with 668 cases. Int Orthop. 2011;35:225–230. doi: 10.1007/s00264-010-1156-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boyer B, Philippot R, Geringer J, Farizon F. Primary total hip arthroplasty with dual mobility socket to prevent dislocation: a 22-year follow-up of 240 hips. Int Orthop. 2012;36:511–518. doi: 10.1007/s00264-011-1289-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Philippot R, Boyer B, Farizon F. Intraprosthetic dislocation: a specific complication of the dual-mobility system. Clin Orthop Relat Res. 2013;471:965–970. doi: 10.1007/s11999-012-2639-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.De Martino I, D’Apolito R, Waddell BS, McLawhorn AS, Sculco PK, Sculco TP. Early intraprosthetic dislocation in dual-mobility implants: a systematic review. Arthroplast Today. 2017;3:197–202. doi: 10.1016/j.artd.2016.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Abdelaal MS, Zachwieja E, Sharkey PF. Severe corrosion of modular dual mobility acetabular components identified during revision total hip arthroplasty. Arthroplast Today. 2021;8:78–83. doi: 10.1016/j.artd.2021.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kolz JM, Wyles CC, Van Citters DW, Chapman RM, Trousdale RT, Berry DJ. In vivo corrosion of modular dual-mobility implants: a retrieval study. J Arthroplasty. 2020;35:3326–3329. doi: 10.1016/j.arth.2020.05.075. [DOI] [PubMed] [Google Scholar]
- 13.Heckmann N, Weitzman DS, Jaffri H, Berry DJ, Springer BD, Lieberman JR. Trends in the use of dual mobility bearings in hip arthroplasty. Bone Joint J. 2020;102-B(7_Supple_B):27–32. doi: 10.1302/0301-620X.102B7.BJJ-2019-1669.R1. [DOI] [PubMed] [Google Scholar]
- 14.Heckmann N, Ihn H, Stefl M, et al. Early results from the American Joint Replacement Registry: a comparison with other national registries. J Arthroplasty. 2019;34(7S):S125–S134.e1. doi: 10.1016/j.arth.2018.12.027. [DOI] [PubMed] [Google Scholar]
- 15.Harwin SF, Sodhi N, Ehiorobo J, Khlopas A, Sultan AA, Mont MA. Outcomes of dual mobility acetabular cups in total hip arthroplasty patients. Surg Technol Int. 2019;34:367–370. [PubMed] [Google Scholar]
- 16.Rowan FE, Salvatore AJ, Lange JK, Westrich GH. Dual-mobility vs fixed-bearing total hip arthroplasty in patients under 55 years of age: a single-institution, matched-cohort analysis. J Arthroplasty. 2017;32:3076–3081. doi: 10.1016/j.arth.2017.05.004. [DOI] [PubMed] [Google Scholar]
- 17.Dubin JA, Westrich GH. Lack of early dislocation for dual mobility vs. fixed bearing total hip arthroplasty: a multi-center analysis of comparable cohorts. J Orthop. 2020;21:1–5. doi: 10.1016/j.jor.2020.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Darrith B, Courtney PM, Della Valle CJ. Outcomes of dual mobility components in total hip arthroplasty: a systematic review of the literature. Bone Joint J. 2018;100-B:11–19. doi: 10.1302/0301-620X.100B1.BJJ-2017-0462.R1. [DOI] [PubMed] [Google Scholar]
- 19.De Martino I, D’Apolito R, Soranoglou VG, Poultsides LA, Sculco PK, Sculco TP. Dislocation following total hip arthroplasty using dual mobility acetabular components: a systematic review. Bone Joint J. 2017;99-B(ASuppl1):18–24. doi: 10.1302/0301-620X.99B1.BJJ-2016-0398.R1. Erratum in: Bone Joint J. 2017;99-B:702-4. [DOI] [PubMed] [Google Scholar]
- 20.Leiber-Wackenheim F, Brunschweiler B, Ehlinger M, Gabrion A, Mertl P. Treatment of recurrent THR dislocation using of a cementless dual-mobility cup: a 59 cases series with a mean 8 years’ follow-up. Orthop Traumatol Surg Res. 2011;97:8–13. doi: 10.1016/j.otsr.2010.08.003. [DOI] [PubMed] [Google Scholar]
- 21.Vahedi H, Makhdom AM, Parvizi J. Dual mobility acetabular cup for total hip arthroplasty: use with caution. Expert Rev Med Devices. 2017;14:237–243. doi: 10.1080/17434440.2017.1292123. [DOI] [PubMed] [Google Scholar]
- 22.Fessy MH, Riglet L, Gras LL, Neyra H, Pialat JB, Viste A. Ilio-psoas impingement with a dual-mobility liner: an original case report and review of literature. SICOT J. 2020;6:27. doi: 10.1051/sicotj/2020025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee GC, Kamath A, Courtney PM. Clinical concerns with dual mobility-should I avoid it when possible? J Arthroplasty. 2021;36(7S):S88–S91. doi: 10.1016/j.arth.2021.01.006. [DOI] [PubMed] [Google Scholar]
- 24.Stavrakis AI, Khoshbin A, Joseph A, et al. Dual mobility total hip arthroplasty is not associated with a greater incidence of groin pain in comparison with conventional total hip arthroplasty and hip resurfacing: a retrospective comparative study. HSS J. 2020;16(Suppl 2):394–399. doi: 10.1007/s11420-020-09764-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kane LT, Fang T, Galetta MS, et al. Propensity score matching: a statistical method. Clin Spine Surg. 2020;33:120–122. doi: 10.1097/BSD.0000000000000932. [DOI] [PubMed] [Google Scholar]
- 26.Caliendo M, Kopeinig S. Some practical guidance for the implementation of propensity score matching. J Econ Surv. 2008;22:31–72. [Google Scholar]
- 27.Austin PC. Some methods of propensity-score matching had superior performance to others: results of an empirical investigation and Monte Carlo simulations. Biom J. 2009;51:171–184. doi: 10.1002/bimj.200810488. [DOI] [PubMed] [Google Scholar]
- 28.Dubin JA, Westrich GH. Anatomic dual mobility compared to modular dual mobility in primary total hip arthroplasty: a matched cohort study. Arthroplast Today. 2019;5:509–514. doi: 10.1016/j.artd.2019.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bartelt RB, Yuan BJ, Trousdale RT, Sierra RJ. The prevalence of groin pain after metal-on-metal total hip arthroplasty and total hip resurfacing. Clin Orthop Relat Res. 2010;468:2346–2356. doi: 10.1007/s11999-010-1356-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Browne JA, Polga DJ, Sierra RJ, Trousdale RT, Cabanela ME. Failure of larger-diameter metal-on-metal total hip arthroplasty resulting from anterior iliopsoas impingement. J Arthroplasty. 2011;26:978.e5–978.e8. doi: 10.1016/j.arth.2010.10.002. [DOI] [PubMed] [Google Scholar]
- 31.Cooper HJ, Della Valle CJ. Large diameter femoral heads: is bigger always better? Bone Joint J. 2014;96-B(11 Supple A):23–26. doi: 10.1302/0301-620X.96B11.34342. [DOI] [PubMed] [Google Scholar]
- 32.Romagnoli M, Grassi A, Costa GG, Lazaro LE, Lo Presti M, Zaffagnini S. The efficacy of dual-mobility cup in preventing dislocation after total hip arthroplasty: a systematic review and meta-analysis of comparative studies. Int Orthop. 2019;43:1071–1082. doi: 10.1007/s00264-018-4062-0. [DOI] [PubMed] [Google Scholar]
- 33.Prudhon JL, Ferreira A, Verdier R. Dual mobility cup: dislocation rate and survivorship at ten years of follow-up. Int Orthop. 2013;37:2345–2350. doi: 10.1007/s00264-013-2067-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jones CW, De Martino I, D’Apolito R, Nocon AA, Sculco PK, Sculco TP. The use of dual-mobility bearings in patients at high risk of dislocation. Bone Joint J. 2019;101-B(1_Supple_A):41–45. doi: 10.1302/0301-620X.101B1.BJJ-2018-0506.R1. [DOI] [PubMed] [Google Scholar]
- 35.Lu Y, Xiao H, Xue F. Causes of and treatment options for dislocation following total hip arthroplasty. Exp Ther Med. 2019;18:1715–1722. doi: 10.3892/etm.2019.7733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Huang RC, Malkani AL, Harwin SF, et al. Multicenter evaluation of a modular dual mobility construct for revision total hip arthroplasty. J Arthroplasty. 2019;34(7S):S287–S291. doi: 10.1016/j.arth.2019.03.027. [DOI] [PubMed] [Google Scholar]