Abstract
Noonan syndrome (NS) is associated with short stature. Growth hormone treatment has been FDA approved for use in these patients. Children with NS are at a higher risk of developing benign and malignant proliferative disorders, primary brain tumors being one of them. Since growth hormone therapy can worsen the tumor burden, screening with a brain MRI prior to growth hormone initiation in NS patients is strongly recommended. Here we present two NS patients who developed different primary brain tumors while being on growth hormone therapy.
Keywords: Noonan syndrome, Brain tumors, Growth hormone
Background
Noonan syndrome (NS) is an autosomal dominant condition affecting 1:1,000–1:2,500 live births. NS was defined by van der Burgt [1] using major and minor criteria including dysmorphic facial features (hypertelorism, down-slanting palpebral fissures, eyelid ptosis, broad forehead, high-arched palate, and low-set posteriorly rotated ears), webbed neck, congenital heart defects, deafness, cryptorchidism, lymphatic dysplasia, bleeding diathesis, mild mental retardation, chest-wall abnormalities, and short stature.
NS is associated with a higher risk of benign and malignant proliferative disorders. More recently, an increased association between NS and primary brain tumors has been recognized; however, data remains limited [2, 3].
The majority of NS patients have short stature, and growth hormone (GH) therapy is an FDA-approved treatment. It has been described that GH and IGF-1 could have a significant impact on the genesis and growth of various tumors. This has led to concerns about a possible increased risk of tumor development and progression in patients with NS. The potential risk for tumorigenesis with GH treatment is linked to overexpression of IGF-1 receptors by tumor cells [4].
Current clinical guidelines (Pediatric Endocrine Society clinical practice guidelines) do not recommend screening for brain tumors (obtaining a brain MRI or other central nervous system [CNS] imaging studies) prior to initiating GH treatment in patients with NS. Here, we present two patients with NS who developed a primary brain tumor while being on GH treatment for more than 1 year.
Clinical Case No. 1
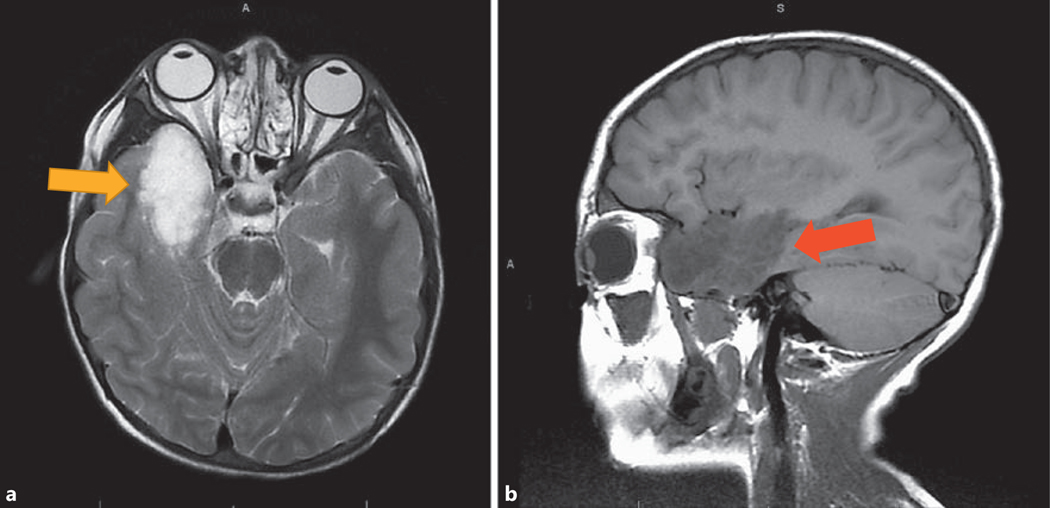
An 8-year-old white, adopted male with facial features suggestive of NS, a history of pulmonary stenosis, Crohn’s disease, bilateral cryptorchidism, and unexplained pancytopenia was evaluated for short stature. His height Z-score (Ht SDS) was –3.5 in the CDC standards (10th percentile on NS height chart) and BMI was at the 50th percentile. He was found to have NS with genetically confirmed mutation in the PTPN11 gene (p.Asp61Gly, Exon3, PTPN11). Bone age was concordant with chronological age and IGF-1 level was low (86 ng/mL, reference range 88–474). He was started on a standard GH dose (0.3 mg/kg/week), without prior GH stimulation testing. Within 6 months of GH therapy, IGF-1 level was 115 ng/mL. His growth velocity (GV) improved to 7 cm/year. Fifteen months after GH therapy was initiated, he presented to the Emergency Department with lethargy and altered mental status with a 3-week history of early-morning vomiting and headaches. An MRI of the brain showed a large right temporal lobe tumor and a small right cerebellar tumor (Fig. 1). He underwent subtotal resection of the tumors, which showed histologic and immunohistochemical features of dysembryoplastic neuroepithelial tumor, WHO grade 1 (p53 negative, Ki67 proliferation index <2%). GH therapy was discontinued following this diagnosis. A year after tumor resection, his GV was noted to be suboptimal at 3.6 cm/year. Following a multidisciplinary team discussion, GH therapy was restarted with a plan for close follow-up and regular brain MRI. Repeat MRIs in the past 1 year showed no change in residual tumor size. He continues to take GH. IGF-1 levels remain between 1 and 2 SDS for age and pubertal stage.
Fig. 1.
Case No. 1. Transverse view (a) and sagittal view (b) of MRI of brain showing two separate lesions in the right anteromedial temporal lobe (yellow arrow) and right medial cerebellum (red arrow) with similar signal characteristics.
Clinical Case No. 2
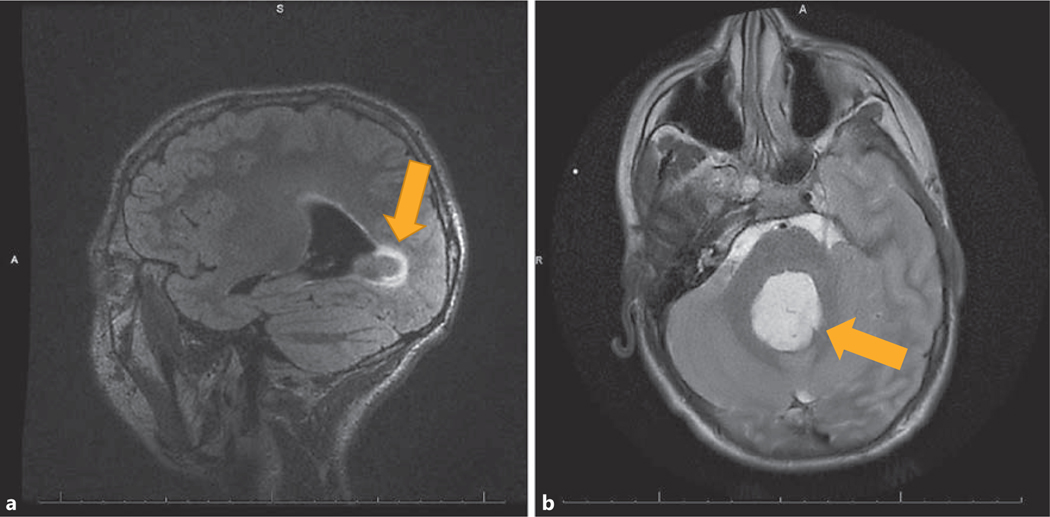
A 16-year-old Caucasian male diagnosed with NS at approximately 7.5 years of age had a genetically confirmed mutation in the PTPN11 gene (922A>G [N308D], Exon3, PTPN11 mutation). He also had a known history of spontaneously closed ventricular septal defect, bilateral cryptorchidism, history of migraines, and short stature, for which he was evaluated at 13 years and 4 months. At the initial evaluation, his height Z-score (Ht SDS) was –2.6 for the CDC standards (between 25th and 50th percentiles on NS height chart) and BMI was at the 8th percentile. Bone age was concordant with chronological age and IGF-1 level was low (145 ng/mL, reference range 202–957). GH therapy was initiated at 0.35 mg/kg/week, without prior GH stimulation testing. After 4 months of therapy, IGF-1 level increased to 250 ng/ mL, and given the borderline IGF-1 levels, in spite of acceptable GV of 7.5 cm/year, GH dose was increased to 0.4 mg/kg/week. Ten months after the initiation of growth hormone therapy, his GV had increased to 9.3 cm/year, and at that point considering the favorable response, repeat IGF-1 level was not obtained. On the follow-up visit, at age 14 years and 7 months, approximately 13 months after the start of GH therapy, he revealed a history of episodically worsening migraine headaches. Migraine headaches were monitored and treated by neurology. He was initially receiving cyproheptadine, which was later changed to topiramate. A nondilated funduscopic exam, at this visit, was not indicative of increased intracranial pressure and a neurology evaluation 2 days later revealed no abnormalities. On that visit, his IGF-1 level was 149 ng/mL and GV slowed down to 6 cm/year. GH dose was adjusted to 0.4 mg/kg/week. A subsequent clinic follow-up showed an IGF-1 level of 500 ng/mL with a GV of 6.9 cm/year. His migraines appeared to be well controlled. At age 15 years and 10 months, 18 months after GH therapy was initiated, he presented to the Emergency Department with altered mental status, lethargy, right upper extremity weakness, and aphasia. Initial head CT findings were concerning for hydrocephalus with a possible cerebellar mass; thus, an extraventricular drain was placed emergently. He then had an MRI, which showed a heterogeneously enhancing tumor within the fourth ventricle and a nonenhancing nodule in the left lateral ventricle suggestive of drop metastasis (Fig. 2). He underwent subtotal resection of the tumors, which showed histologic and immunohistochemical features of pilocytic astrocytoma, WHO grade 1 (BRAF negative, Ki67 proliferation index <1%). GH therapy was permanently discontinued following this diagnosis. A repeat MRI performed at age 16 years and 10 months, approximately 12 months after the resection, showed a stable appearance of the residual primary tumor and metastatic nodule.
Fig. 2.
Case No. 2. Sagittal view (a) and transverse view (b) of MRI of brain showing a heterogeneously enhancing tumor within the fourth ventricle with drop metastasis in the anterior recess of the third ventricle (indicated by arrows).
Discussion
The patient in case No. 1 with NS did not have any clinical evidence of CNS involvement and/or neoplastic process in the brain at the initiation of GH therapy. Interestingly, 15 months after GH treatment was initiated, he was diagnosed with a dysembryoplastic neuroepithelial tumor in the brain. The patient in case No. 2, in contrast, had a prior history of migraines and may have had related symptoms overlapping with the underlying CNS tumor. Interestingly, as a part of the previous workup for migraine headaches, he did have a normal brain MRI 6 years prior to the diagnosis of the pilocytic astrocytoma (and 4 years prior to the initiation of GH therapy). Of note, only 3 other cases of NS with pilocytic astrocytoma have been reported in the medical literature [2, 5]. Only one of the cases had confirmatory genetic testing, which was positive for the PTPN11 mutation (c.1471 C>T and c.1472 C>T). Our case would therefore be the second reported case with a confirmed genetic mutation for NS.
It is challenging to say whether the respective tumors in these patients were present before GH was started and increased in size with GH treatment, or whether the tumor origin and progression was dependent on GH treatment. Data from the KIGS database showed a standardized incidence ratio of 2.24 (95% CI 1.02–4.25) for brain cancers in GH-treated patients who were “not at high risk” [6]. This suggests a higher incidence in a “neoplasmsusceptible” population like patients with NS. The CNS remains the most common site for childhood neoplasms. Given that GH therapy is approved for use in the treatment of short stature in patients with NS, screening with a brain MRI should be an integral part of the screening process. Heterozygous germline mutations in 6 genes encoding components of the RAS/MAPK pathway, namely, PTPN11, KRAS, SOS1, RAF1, NRAS, and SHOC2, have been identified in NS. The RAS/MAPK pathway represents a signal transduction cascade involved in processes of cell proliferation, differentiation, survival, and death. Mutations in PTPN11, which encodes the non-receptor protein tyrosine phosphatase SHP-2, are most frequently observed and account for ~50% of NS cases [7]. Considering the universal role of increased RAS-MAPK signaling in oncogenesis, patients with NS might have an increased risk for developing a broad range of malignancies. Previous studies have shown a 3.5-fold increase in the overall cancer risk up to the age of 55 years in NS patients with a PTPN11 mutation, compared with that in the general population. Activation of proteins in the RAS-RAF-MEK pathway may result in phosphorylation of ERK and subsequent downstream signaling in a wide spectrum of grade I and grade II gliomas. Another focus of interest is BRAF-activating mutations, as BRAF is a downstream effector of RAS signaling and it has been found in previously reported cases of pediatric pilocytic astrocytomas, two of which were NF-1 associated [5].
Cancer survivors treated with GH require close monitoring during therapy and long-term follow-up [8]. However, since the potential malignancies for patients with NS involve multiple sites and develop throughout life, a routine tumor surveillance program is not warranted. Reported cases (11) of CNS neoplasms in NS patients continue emerging, calling for a more conservative approach and judicious surveillance. Thus, considering the possible association of NS and primary brain tumors, we recommend obtaining a brain MRI at baseline prior to initiation of GH treatment in patients with NS, particularly in those with PTPN11 mutation as they appear to have higher risk for cranial neoplasms.
Established Facts
• Growth hormone therapy is an approved indication for treatment of short stature in Noonan syndrome patients, with no current guideline to obtain brain MRI prior to treatment.
Novel Insights
• Given the increased risk for primary brain tumors, we recommend obtaining a brain MRI prior to initiating growth hormone therapy.
Footnotes
Statement of Ethics
Consent was obtained from both families regarding use of their information for the purpose of learning. No patient identifiers are disclosed to the best of our knowledge.
Disclosure Statement
All authors have no disclosures/conflict of interest except as mentioned below: Oscar Escobar served as a member of an Advisory Board meeting for Versartis, Inc.
References
- 1.van der Burgt I: Noonan syndrome. Orphanet J Rare Dis 2007; 2:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fryssira H, Leventopoulos G, Psoni S, KitsiouTzeli S, Stavrianeas N, Kanavakis E: Tumor development in three patients with Noonan syndrome. Eur J Pediatr 2008; 167:1025–1031. [DOI] [PubMed] [Google Scholar]
- 3.Karafin M, Jallo GI, Ayars M, Eberhart CG, Rodriguez FJ: Rosette forming glioneuronal tumor in association with Noonan syndrome: pathobiological implications. Clin Neuropathol 2011; 30:297–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Perry JK, Emerald BS, Mertani HC, Lobie PE: The oncogenic potential of growth hormone. Growth Horm IGF Res 2006; 16:277–289. [DOI] [PubMed] [Google Scholar]
- 5.Schuettpelz LG, McDonald S, Whitesell K, Desruisseau DM, Grange DK, Gurnett CA, Wilson DB: Pilocytic astrocytoma in a child with Noonan syndrome. Pediatr Blood Cancer 2009; 53:1147–1149. [DOI] [PubMed] [Google Scholar]
- 6.Wilton P, Mattsson AF, Darendeliler F: Growth hormone treatment in children is not associated with an increase in the incidence of cancer: experience from KIGS (Pfizer International Growth Database). J Pediatr 2010; 157:265–270. [DOI] [PubMed] [Google Scholar]
- 7.Jongmans MC, van der Burgt I, Hoogerbrugge PM, Noordam K, Yntema HG, Nillesen WM, Kuiper RP, Ligtenberg MJ, van Kessel AG, van Krieken JH, Kiemeney LA, Hoogerbrugge N: Cancer risk in patients with Noonan syndrome carrying a PTPN11 mutation. Eur J Hum Genet 2011; 19:870–874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chemaitilly W, Robison LL: Safety of growth hormone treatment in patients previously treated for cancer. Endocrinol Metab Clin North Am 2012; 41:785–792. [DOI] [PubMed] [Google Scholar]