Abstract
Background
Although a variety of data showing that diabetes mellitus (DM) (Type 1 or Type 2) is associated with postoperative complication, there is still a lack of detailed studies that go through the specific diabetic subgroups. The goal of this meta-analysis is to assess the relationship between DM and various complications after non-cardiac surgery.
Methods
We searched articles published in three mainstream electronic databases (PubMed, EMBASE, Web of science) before November, 2020. A random effects model was conducted since heterogeneity always exist when comparing results between different types of surgery.
Results
This paper included 125 studies with a total sample size of 3,208,776 participants. DM was a risk factor for any postoperative complication (Odds ratio (OR)=1.653 [1.487, 1.839]). The risk of insulin-dependent DM (OR=1.895 [1.331, 2.698]) was higher than that of non-insulin-dependent DM (OR=1.554 [1.061, 2.277]) for any postoperative complication. DM had a higher risk of infections (OR=1.537 [1.322, 1.787]), wound healing disorders (OR=2.010 [1.326, 3.046]), hematoma (OR=1.369 [1.120, 1.673]), renal insufficiency (OR=1.987 [1.311, 3.013]), myocardial infarction (OR=1.372 [0.574, 3.278]). Meanwhile, DM was a risk factor for postoperative reoperation (OR=1.568 [1.124, 2.188]), readmission (OR=1.404 [1.274, 1.548]) and death (OR=1.606 [1.178, 2.191]).
Conclusions
DM is a risk factor for any postoperative complications, hospitalization and death after non-cardiac surgery. These findings underscore the importance of preoperative risk factor assessment of DM for the safe outcome of surgical patients.
Keywords: diabetes mellitus, non-cardiac surgery, risk factor, postoperative complication, meta-analysis
1 Introduction
Each year more than 300 million surgeries are performed in the world (1). The baseline 30-day mortality of hospitalized patients undergoing non-cardiac surgery is 1.5% worldwide, primarily depending on surgical method, surgical decision-making or technique, and comorbidities (2). It is important to identify factors that increase the risk of surgery before making clinical decisions (3). The preoperative identification of risk factors has important clinical implications. First, it helps surgeons correct those risk factors that can be optimized prior to surgery to reduce surgical risk. Second, it directs patients to undergo low-risk surgery or transfer to appropriate medical institutions with stronger technical ability. Third, it is beneficial to make correct decisions based on risk-benefit evaluation. To date, it is still a very tough task to preoperatively identify high-risk patients or the subgroup population who would benefit most from surgery.
Diabetes mellitus (DM) (Type 1 or Type 2) is a multifaceted metabolic disease that affects more than 340 million people worldwide (4). They are at high risk for microvascular (neuropathy, nephropathy or retinopathy) or macrovascular (peripheral vascular, cardiovascular disease) complications, both of which increase perioperative morbidity and mortality (5). Surgical patients with DM are more likely to have prolonged hospital stays, admission to intensive care units, myocardial infarction, respiratory infections, poor wound healing, and increased risk of general morbidity and mortality (6–9). It is important for surgeons to be aware of possible complications and associated contributing factors so that they can be appropriately counseled preoperatively. Clinicians should develop direct strategies in the perioperative period to minimize surgical risks based on existing DM screening programs (10).
To date, there seems to lack detailed studies that go through this specific diabetic subgroup, although there are very convincing data showing that DM is associated with a variety of postoperative complications (5). After all, exactly which complications are associated with DM remains controversial. In order to provide clinicians with a reference to assess the surgical risk, we performed meta-analysis and systematic review of various complications after noncardiac surgery in patients with DM.
2 Methods
2.1 Protocol and Guidance
This meta-analysis followed the Preferred Reporting Items for Systematic Reviews and Meta analyses (PRISMA) guidelines (11). No registration details are available.
2.2 Eligibility Criteria
In this manuscript were included studies, which described DM as a preoperative risk factor. These studies that presented postoperative complications, mortality, morbidity, length of ICU stay and prolonged hospital stay, providing adjusted or unadjusted relative risk (RR) or odds ratio (OR) with 95% confidence interval (CI); or providing relevant information that can be used to figure out RR or OR. The studies’ design relied on retrospective data.
2.3 Information Sources and Search Strategy
We searched for articles published before November 30, 2020 regardless of language in a total of three electronic databases (PubMed, EMBASE, Web of science). We restricted our search to human studies. In the search we used the following terms: “diabetes”, “postoperative complications”, “surgical procedures”, “operative,” “hospitalization”, “risk factors”, “treatment outcome”, “perioperative care”, “perioperative period”, “reoperation” and “wound healing”. References of identified studies, recent guidelines and reviews on this topic were also selected by manual screening. Studies on cardiac surgery were excluded.
2.4 Study Selection and Data Collection
Two authors independently selected studies by screening titles and abstracts, and any disagreements were resolved by the senior author. Data extraction was performed independently by two authors. Study characteristics including author, publication year, country, sample size, mean or median age, number of patients with DM, type of DM, and type of surgery were extracted. Data were extracted for pooling, including total number of subjects, number of events of various complications, RR or OR. If the data were only available as graphs, the free software Plot Digitizer was used to estimate from the graphs. Quality assessment was using the Newcastle-Ottawa scale (NOS) for assessing quality of observational studies.
2.5 Definition of Outcomes
Our outcome measure is the OR of the incidence of complications in diabetic versus nondiabetic patients after surgery. It also shows the OR of mortality, readmission, reoperation, and prolonged length of stay (LOS). We have pooled OR for 7 postoperative complications, including any complication, infections, wound healing disorders (WHD), venous thromboembolism (VTE), hematoma, renal insufficiency, and myocardial infarction (MI).
2.6 Statistical Analysis
Homogeneity of effect estimates was tested using the Cochran Q and I² statistics (12, 13). A random effects model was conducted because of heterogeneity always exist when comparing results between different types of surgery, and subgroup analyses were performed. All outcomes were presented as OR with 95% CI. All analyses were performed using Stata/SE version 15.0 (StataCorp, College Station, TX, USA). Publication bias was assessed by evaluating small‐study effects with comparison adjusted funnel plot symmetry if 10 or more studies were available.
3 Results
3.1 Study Selection and Study Characteristics
Our literature search yielded a total of 2,737 retrievals, 125 studies were used for meta-analysis. Figure 1 . A total of 72 studies were from the United States, accounting for more than half of the 125 studies included. The number of patients with DM was 356,300, accounting for 11.1% of the huge sample size of 3,208,776. The vast majority of studies focused on all types of DM, and only eight studies distinguished between IDDM and NIDDM. The types of surgery mainly covered orthopedic surgery, oncological surgery, transplantation surgery, plastic surgery, weight loss surgery, oral surgery, neurological surgery, ophthalmological surgery, etc., with the exception of cardiac surgery. The quality of the included studies was assessed using the NOS criteria. The NOS quality scores of the included studies ranged from 6 to 9 points ( Table 1 ).
Figure 1.
Flowchart of study selection.
Table 1.
Characteristics of included studies.
| Study | Year | Age | Sample size | Number of DM | Country | Surgery | NOS Score | ||
|---|---|---|---|---|---|---|---|---|---|
| Selection | Comparability | Outcome | |||||||
| Afshari et al. (14) | 2016 | – | 1493 | 166 | USA | thighplasty | ☆☆☆☆ | ☆☆ | ☆☆☆ |
| Aigner et al. (15) | 2017 | 72.5(6.1) | 237 | 26 | Germany | open reduction and internal fixation of geriatric ankle fractures | ☆☆☆☆ | ☆☆ | ☆☆☆ |
| Akhter et al. (16) | 2016 | – | 1196 | 133 | India | surgery | ☆☆☆☆ | ☆☆ | ☆☆☆ |
| Ammori et al. (17) | 2018 | 69 | 6985 | 1389 | USA | gastrectomy for malignancy | ☆☆☆ | ☆☆ | ☆☆☆ |
| Arnold et al. (18) | 2014 | 60.1 (10.7) | 278 | 42 | USA | surgical decompression, in cervical spondylotic myelopathy | ☆☆☆☆ | ☆☆ | ☆☆☆ |
| Bailey et al. (19) | 2003 | 63.4(9.9) | 1777 | 221 | USA | esophagectomy | ☆☆☆ | ☆☆ | ☆☆☆ |
| Bailón-Cuadrado et al. (20) | 2019 | 68.6(11.1) | 180 | 26 | Spain | curative surgery for colorectal cancer | ☆☆☆ | ☆☆ | ☆☆☆ |
| Bamba et al. (21) | 2016 | 40.9(13.9) | 129007 | 2368 | USA | aesthetic surgery | ☆☆☆☆ | ☆ | ☆☆ |
| Bascom et al. (22) | 2016 | 43.9 | 829 | 43 | Canada | bulbar urethroplasty | ☆☆☆ | ☆☆ | ☆☆☆ |
| Belmont et al. (23) | 2015 | 67.3(10.2) | 15321 | 2795 | USA | total knee arthroplasty | ☆☆☆☆ | ☆☆ | ☆☆☆ |
| Belmont et al. (24) | 2014 | 50.3 (18.2) | 3328 | 426 | USA | ankle fracture fixation | ☆☆☆☆ | ☆☆ | ☆☆☆ |
| Benrashid et al. (25) | 2020 | 64.7 | 504 | 152 | USA | vascular procedures requiring infrainguinal incisions | ☆☆☆ | ☆☆ | ☆☆☆ |
| Bohl et al. (2) | 2019 | – | 7582 | 842 | USA | open reduction and internal fixation of closed ankle fractures | ☆☆☆☆ | ☆☆ | ☆☆☆ |
| Bolognesi et al. (26) | 2008 | 61.0-76.0 | 751340 | 64262 | USA | total hip and total knee arthroplasty | ☆☆☆☆ | ☆ | ☆☆ |
| Bower et al. (27) | 2010 | 61.6(14.1) | 1343 | 329 | Hong Kong | surgical outcomes of noncardiovascular patients | ☆☆☆☆ | ☆ | ☆☆☆ |
| Browne et al. (28) | 2007 | 48.9(18.16) | 197461 | 11135 | USA | lumbar fusion | ☆☆☆☆ | ☆ | ☆☆ |
| Bruggeman et al. (29) | 2004 | 43 | 167 | 19 | USA | open achilles tendon repair | ☆☆☆☆ | ☆☆ | ☆☆☆ |
| Buchanan et al. (30) | 2018 | >18 | 93920 | 10425 | USA | non-emergent craniotomy | ☆☆☆☆ | ☆☆ | ☆☆☆ |
| Bur et al. (31) | 2016 | 64.2 | 7605 | 844 | USA | head and neck cancer surgery | ☆☆☆ | ☆☆ | ☆☆☆ |
| Cammarata et al. (32) | 2019 | – | 7030 | 770 | USA | abdominal panniculectomy | ☆☆☆ | ☆☆ | ☆☆☆ |
| Chen et al. (33) | 2009 | – | 195 | 30 | USA | spinal arthrodesis | ☆☆☆☆ | ☆☆ | ☆☆☆ |
| Chen et al. (34) | 2019 | 53.9(12.4) | 207 | 23 | China | open hepatectomy | ☆☆☆ | ☆☆ | ☆☆☆ |
| Chiu et al. (35) | 2020 | 54.6(11.5) | 40 | 4 | Taiwan | sequential free flap reconstruction | ☆☆ | ☆☆ | ☆☆ |
| Ciufo et al. (36) | 2019 | 64.5 (13.3) | 4631 | 3233 | USA | below knee amputation | ☆☆☆☆ | ☆☆ | ☆☆☆ |
| Cook et al. (37) | 2008 | 53(13.20) | 37732 | 3432 | USA | cervical fusion | ☆☆☆☆ | ☆☆ | ☆☆ |
| Cote et al. (38) | 2019 | – | 1005 | 112 | USA | microvascular decompression | ☆☆☆ | ☆☆ | ☆☆☆ |
| Courtney et al. (39) | 2017 | 65.9 | 169406 | 25913 | USA | total joint arthroplasty | ☆☆☆☆ | ☆ | ☆☆ |
| Cutler et al. (40) | 2020 | 54-81 | 414 | 29 | USA | total elbow arthroplasty | ☆☆☆☆ | ☆☆ | ☆☆☆ |
| Dodd et al. (41) | 2016 | 53.4(18.4) | 6800 | 836 | USA | ankle fractures | ☆☆☆☆ | ☆☆ | ☆☆☆ |
| Duque et al. (42) | 1997 | – | 605 | 46 | Spain | thoracotomy for bronchogenic carcinoma | ☆☆☆ | ☆☆ | ☆☆☆ |
| Farivar et al. (43) | 2017 | 73 (9) | 5881 | 945 | USA | endovascular aneurysm repair of infrarenal abdominal aortic aneurysms | ☆☆☆ | ☆☆ | ☆☆☆ |
| Fischer et al. (44) | 2014 | 58 | 47443 | 7288 | USA | mastectomy alone compared to immediate breast reconstruction | ☆☆☆ | ☆☆ | ☆☆☆ |
| Franck et al. (45) | 2018 | 55.2 | 60 | 7 | USA | local muscle flap closure following spinal tumor extirpation | ☆☆☆ | ☆☆ | ☆☆☆ |
| Freire et al. (46) | 2015 | 47 (12–79) | 819 | 150 | Brazil | kidney transplantation | ☆☆☆ | ☆☆ | ☆☆☆ |
| Fu et al. (47) | 2016 | – | 3671 | 455 | USA | anterior cervical discectomy and fusion | ☆☆☆☆ | ☆☆ | ☆☆☆ |
| Ganesh et al. (48) | 2005 | 62.9(13.8) | 160598 | 9174 | USA | ankle fracture | ☆☆☆☆ | ☆ | ☆☆ |
| Golinvaux et al. (49) | 2014 | – | 15480 | 2437 | USA | elective lumbar fusion | ☆☆☆☆ | ☆☆ | ☆☆☆ |
| Gupta et al. (50) | 2017 | 40.2(13.9) | 183914 | 20414 | USA | aesthetic surgical | ☆☆☆☆ | ☆ | ☆☆ |
| Gupta et al. (51) | 2016 | 40.9(13.9) | 127961 | 2346 | USA | aesthetic surgery | ☆☆☆☆ | ☆ | ☆☆ |
| Gupta et al. (52) | 2016 | 59.24 (9.4) | 11300 | 303 | USA | facelift | ☆☆☆☆ | ☆ | ☆☆☆ |
| Gupta et al. (53) | 2017 | 40.9 (13.9) | 129007 | 2368 | USA | aesthetic breast surgery | ☆☆☆☆ | ☆ | ☆☆ |
| Hadaya et al. (54) | 2020 | 60.8 (12.6) | 22739 | 2524 | USA | elective pneumonectomy | ☆☆☆ | ☆☆ | ☆☆☆ |
| Hardt et al. (55) | 2017 | 64.4(11.9) | 370 | 37 | Germany | elective rectal cancer resection | ☆☆☆ | ☆☆ | ☆☆☆ |
| Hunecke et al. (56) | 2019 | 43.7(12.7) | 121 | 18 | Germany | abdominoplasty after massive weight loss | ☆☆☆☆ | ☆ | ☆☆☆ |
| Inabnet et al. (57) | 2010 | 44.22 | 3802 | 1323 | USA | non-lap band primary and revisional bariatric surgical procedures | ☆☆☆☆ | ☆ | ☆☆☆ |
| Janczak et al. (58) | 2019 | 67.9(6.7) | 205 | 46 | Poland | elective open surgery for infrarenal aortic aneurysms | ☆☆☆ | ☆☆ | ☆☆☆ |
| John and Thuluvath (59) | 2001 | 53.6(6.7) | 171 | 57 | USA | liver transplantation | ☆☆☆ | ☆☆ | ☆☆☆ |
| Kantar et al. (60) | 2018 | 54.4(11.0) | 7035 | 770 | USA | abdominal panniculectomy | ☆☆☆☆ | ☆ | ☆☆☆ |
| Karthikesalingam et al. (61) | 2011 | 40 (21–70) | 123 | 14 | UK | abdominoplasty | ☆☆☆ | ☆☆ | ☆☆☆ |
| Kauvar et al. (62) | 2017 | 77 (9) | 3344 | 648 | USA | elective endovascular aortic aneurysm repair | ☆☆☆ | ☆☆ | ☆☆☆ |
| Koch et al. (63) | 2015 | 53 (15) | 405 | 79 | Germany | kidney transplantation | ☆☆☆ | ☆☆ | ☆☆☆ |
| Lange et al. (64) | 2009 | 72 (50- 84) | 121 | 27 | Netherlands | peripheral vascular surgery | ☆☆☆ | ☆☆ | ☆☆☆ |
| Lee et al. (65) | 2018 | – | 2301 | 421 | Korea | elective posterior lumbar fusion | ☆☆☆☆ | ☆☆ | ☆☆☆ |
| Lewin et al. (66) | 2014 | 39.6 (13.8) | 512 | 14 | Sweden | breast reduction surgery | ☆☆☆☆ | ☆ | ☆☆☆ |
| Li et al. (67) | 2017 | – | 3024 | 223 | China | gastric cancer | ☆☆☆ | ☆☆ | ☆☆☆ |
| Lindqvist et al. (68) | 2019 | 57.4 (18-91) | 886 | 22 | Sweden | sentinel lymph node biopsy for cutaneous melanoma | ☆☆☆ | ☆☆ | ☆☆☆ |
| Lopez Ramos et al. (69) | 2018 | 61 | 40802 | 4880 | USA | cranial neurosurgery | ☆☆☆ | ☆☆ | ☆☆☆ |
| Louie et al. (70) | 2017 | 51.3 | 3251 | 387 | USA | open reduction internal fixation of ankle fractures | ☆☆☆☆ | ☆☆ | ☆☆☆ |
| Lv et al. (71) | 2015 | 49.7(8.8) | 438 | 140 | China | liver transplantation | ☆☆☆ | ☆☆ | ☆☆☆ |
| Ma et al. (72) | 2019 | 62.6(10.5) | 545 | 61 | China | radical gastrectomy | ☆☆☆ | ☆☆ | ☆☆☆ |
| Maradit Kremers et al. (73) | 2015 | 66.2(12.6) | 20171 | 3507 | USA | total hip and knee arthroplasty | ☆☆☆☆ | ☆☆ | ☆☆ |
| Matsuda et al. (74) | 2009 | 66.2(8.8) | 80 | 9 | Japan | abdominoperineal resection | ☆☆☆ | ☆☆ | ☆☆☆ |
| McElvany et al. (75) | 2019 | 69.5(9.7) | 8819 | 1874 | USA | shoulder arthroplasty | ☆☆☆☆ | ☆☆ | ☆☆☆ |
| Meding et al. (76) | 2003 | – | 5220 | 329 | USA | total knee replacement | ☆☆☆☆ | ☆☆ | ☆☆☆ |
| Menenakos et al. (77) | 2010 | 37 | 261 | 36 | Greece | laparoscopic sleeve gastrectomy | ☆☆☆ | ☆☆ | ☆☆☆ |
| Michalak et al. (78) | 2016 | 53.3(13.5) | 1141 | 115 | USA | cerebrovascular surgery | ☆☆☆ | ☆☆ | ☆☆☆ |
| Moon et al. (79) | 2008 | 67.6 (50–86) | 342 | 171 | Korea | total knee arthroplasty | ☆☆☆☆ | ☆☆ | ☆☆☆ |
| Moon et al. (80) | 2018 | 49.9(11.5) | 5538 | 615 | USA | sleeve gastrectomy | ☆☆☆ | ☆☆ | ☆☆☆ |
| Morgan et al. (81) | 2015 | 48 | 12062 | 1339 | Australia | bariatric surger | ☆☆☆☆ | ☆ | ☆☆☆ |
| Nair et al. (82) | 2009 | 52 (19) | 221 | 55 | USA | liver transplantation | ☆☆☆ | ☆☆ | ☆☆☆ |
| Newman et al. (83) | 2014 | 60.4(12.9) | 3352 | 406 | USA | total knee and total hip arthroplasty | ☆☆☆☆ | ☆☆ | ☆☆☆ |
| Nguyen et al. (84) | 2019 | 62(11.9) | 563 | 69 | Canada | gynecologic oncology | ☆☆☆ | ☆☆ | ☆☆☆ |
| Nguyen et al. (85) | 2016 | 48.65(12.72) | 2294 | 126 | USA | brachioplasty | ☆☆☆☆ | ☆☆ | ☆☆☆ |
| Okamura et al. (86) | 2017 | 63 (8) | 300 | 35 | Japan | esophagectomy | ☆☆☆ | ☆☆ | ☆☆☆ |
| Palmerola et al. (87) | 2016 | 64 (54-94) | 191 | 21 | USA | urologic surgery | ☆☆☆ | ☆☆ | ☆☆☆ |
| Park et al. (88) | 2016 | 51.44(10.8) | 7948 | 1284 | Korea | anterior cervical discectomy and fusion for cervical spondylotic, radiculopathy and myelopathy | ☆☆☆☆ | ☆☆ | ☆☆☆ |
| Patton et al. (89) | 2015 | 55.4 | 87 | 6 | USA | total ankle arthroplast | ☆☆☆☆ | ☆☆ | ☆☆☆ |
| Pearse et al. (90) | 2012 | 56·7 (18·5) | 46539 | 5576 | UK | non-cardiac surgery | ☆☆☆☆ | ☆ | ☆☆ |
| Plano et al. (91) | 2019 | 57.7 (27-86) | 303 | 34 | Spain | unplanned surgery in cervical spondylotic myelopathy surgically treated | ☆☆☆☆ | ☆☆ | ☆☆☆ |
| Ponce et al. (92) | 2014 | 69 (13) | 66485 | 13730 | USA | shoulder arthroplasty | ☆☆☆☆ | ☆ | ☆☆ |
| Pugely et al. (93) | 2013 | 52.6 (16.1) | 4310 | 455 | USA | lumbar discectomy | ☆☆☆☆ | ☆☆ | ☆☆☆ |
| Qin et al. (94) | 2014 | 55.9 (10.2) | 29736 | 1478 | USA | breast reconstruction | ☆☆☆☆ | ☆☆ | ☆☆ |
| Raikin et al. (95) | 2010 | – | 106 | 11 | USA | total ankle arthroplasty | ☆☆☆☆ | ☆☆ | ☆☆☆ |
| Rao et al. (96) | 2020 | 69.9(8.4) | 1074 | 433 | USA | shoulder arthroplasty | ☆☆☆☆ | ☆☆ | ☆☆☆ |
| Rensing et al. (97) | 2017 | 44(13.3) | 1626 | 79 | USA | primary repair of achilles tendon ruptures | ☆☆☆☆ | ☆☆ | ☆☆☆ |
| Roche et al. (98) | 2018 | 61.24 (12.8) | 9439 | 1402 | USA | parathyroidectomy for primary hyperparathyroidism | ☆☆☆☆ | ☆☆ | ☆☆☆ |
| Rubel et al. (99) | 2019 | 57.5(16.2) | 169788 | 31289 | USA | elective primary lumbar spine surgery | ☆☆☆☆ | ☆ | ☆☆ |
| Sakai et al. (100) | 2011 | – | 107 | 12 | Japan | surgery for laryngeal and hypopharyngeal cancers | ☆☆☆ | ☆☆ | ☆☆☆ |
| Sanni et al. (101) | 2014 | 44.0 (12.1) | 20308 | 5268 | USA | bariatric surgery | ☆☆☆☆ | ☆ | ☆☆ |
| Schemitsch et al. (102) | 2015 | 34.9 | 153 | 6 | Canada | plate fixation of the midshaft clavicle | ☆☆☆☆ | ☆☆ | ☆☆☆ |
| Schimmel et al. (103) | 2010 | 51 (16.8) | 171 | 8 | Netherlands | spinal fusion | ☆☆☆☆ | ☆☆ | ☆☆☆ |
| Schipper et al. (104) | 2015 | 65.7(10.1) | 12122 | 2394 | USA | ankle arthrodesis and total ankle arthroplasty | ☆☆☆☆ | ☆☆ | ☆☆☆ |
| Schlottmann et al. (105) | 2017 | 63 (10.3) | 4053 | 229 | USA | esophagectomy | ☆☆☆ | ☆☆ | ☆☆☆ |
| Schürner et al. (106) | 2018 | 40 (32–49) | 711 | 200 | Switzerland | primary roux-en-y gastric bypass surgery | ☆☆☆ | ☆☆ | ☆☆☆ |
| Shah et al. (107) | 2019 | 65 (11) | 3344 | 346 | USA | thumb cmc joint arthroplasty | ☆☆☆☆ | ☆☆ | ☆☆☆ |
| Shigeishi et al. (108) | 2015 | 41(5-84) | 324 | 12 | Japan | oral surgery | ☆☆☆ | ☆☆ | ☆☆☆ |
| Shimada et al. (109) | 1994 | 57.5 | 209 | 23 | Japan. | hepatic resection | ☆☆☆ | ☆☆ | ☆☆☆ |
| Smith et al. (110) | 2017 | 45.78(17.70) | 272 | 30 | USA | tibia fractures treated with intramedullary fixation | ☆☆☆☆ | ☆☆ | ☆☆☆ |
| Söderbäck et al. (111) | 2019 | 71.1 (11.6) | 30050 | 952 | Sweden | colorectal cancer surgery | ☆☆☆ | ☆☆ | ☆☆☆ |
| Sood et al. (112) | 2015 | 62 (54–71) | 3820 | 755 | USA | nephrectomy | ☆☆☆ | ☆☆ | ☆☆☆ |
| Sood et al. (113) | 2017 | 69 (61-76) | 1118 | 214 | USA | radical cystectomy | ☆☆☆ | ☆☆ | ☆☆☆ |
| Sørensen et al. (114) | 2002 | 64 | 425 | 47 | Denmark | breast cancer surgery | ☆☆☆ | ☆☆ | ☆☆☆ |
| Souza et al. (115) | 2007 | 45.6(10.4) | 55 | 4 | Brazil | liver transplantations | ☆☆☆ | ☆ | ☆☆ |
| Spinazzi et al. (116) | 2015 | 55.9(15.2) | 15317 | 2493 | USA | pituitary surgery | ☆☆☆ | ☆☆ | ☆☆☆ |
| Stein et al. (117) | 2011 | – | 221594 | 64569 | USA | cataract surgery | ☆☆☆☆ | ☆ | ☆☆ |
| Suda et al. (118) | 2016 | 57.2 | 108 | 25 | Germany | arthrodesis | ☆☆☆☆ | ☆☆ | ☆☆☆ |
| Takao et al. (119) | 2008 | > 80 | 255 | 68 | Japan | urological surgery | ☆☆☆ | ☆☆ | ☆☆☆ |
| Tang et al. (120) | 2014 | 66.8(5.5) | 236 | 74 | China | spinal fusion and instrumentation | ☆☆☆☆ | ☆☆ | ☆☆☆ |
| Terho et al. (121) | 2016 | 63 (20–94) | 373 | 68 | Finland | laparoscopic cholecystectomy | ☆☆☆ | ☆☆ | ☆☆☆ |
| Tetreault et al. (122) | 2016 | 56.4 (11.9) | 479 | 59 | Canada | surgery for the treatment of cervical spondylotic myelopathy | ☆☆☆☆ | ☆☆ | ☆☆☆ |
| Timmermans et al. (123) | 2018 | 49.1(9.2) | 97 | 5 | Netherlands | free diep flap breast reconstructions | ☆☆☆☆ | ☆☆ | ☆☆☆ |
| Toboni et al. (124) | 2018 | 60.4 | 4260 | 540 | USA | ovarian cancer | ☆☆☆ | ☆☆ | ☆☆☆ |
| Tokgöz et al. (125) | 2011 | 61.6(12.1) | 47 | 8 | Turkey | radical nephrectomy | ☆☆ | ☆☆ | ☆☆☆ |
| Venara et al. (126) | 2014 | 74 (18-109) | 166 | 25 | France | treatment of incarcerated hernias, especially in case of bowel resection | ☆☆☆ | ☆☆ | ☆☆☆ |
| Wadhwa et al. (127) | 2017 | 54.2(16.7) | 9853 | 1690 | USA | surgery for lumbar degenerative disease | ☆☆☆☆ | ☆☆ | ☆☆☆ |
| Wang et al. (128) | 2020 | 72 (65-86) | 118 | 7 | China | radial forearm-free flap | ☆☆☆☆ | ☆☆ | ☆☆☆ |
| Wang et al. (129) | 2017 | – | 1657 | 184 | China | laparoscopy-assisted total gastrectomy | ☆☆☆ | ☆☆ | ☆☆☆ |
| Webb et al. (130) | 2017 | – | 114102 | 20248 | USA | total knee arthroplasty | ☆☆☆☆ | ☆ | ☆☆ |
| Weir et al. (131) | 2019 | 52.2 (14.7) | 5222 | 580 | UK | lumbar spinal surgery | ☆☆☆☆ | ☆☆ | ☆☆☆ |
| Winocour et al. (132) | 2017 | 45.5 (10.3) | 129007 | 2368 | USA | cosmetic surgery | ☆☆☆☆ | ☆ | ☆☆ |
| Wukich et al. (133) | 2010 | 46.7 | 1000 | 190 | USA | foot and ankle surgery | ☆☆☆☆ | ☆☆ | ☆☆☆ |
| Yamauchi et al. (134) | 2013 | – | 1438 | 148 | Japan | lung cancer operations | ☆☆☆ | ☆☆ | ☆☆☆ |
| Zanaty et al. (135) | 2015 | 46.5(12.7) | 348 | 52 | USA | cranioplasty | ☆☆☆ | ☆☆ | ☆☆☆ |
| Zhang et al. (136) | 2015 | 65.8(11.3) | 119 | 39 | China | pancreatoduodenectomy | ☆☆☆ | ☆☆ | ☆☆☆ |
| Zhou et al. (137) | 2016 | 65.9(12.0) | 2795 | 228 | China | gastrectomy for gastric cancer | ☆☆☆ | ☆☆ | ☆☆☆ |
| Total studies 125 | 3208776 | 356300 | |||||||
DM, Diabetes mellitus; NOS, Newcastle-Ottawa scale.
3.2 Synthesis of Results
3.2.1 Any Complication
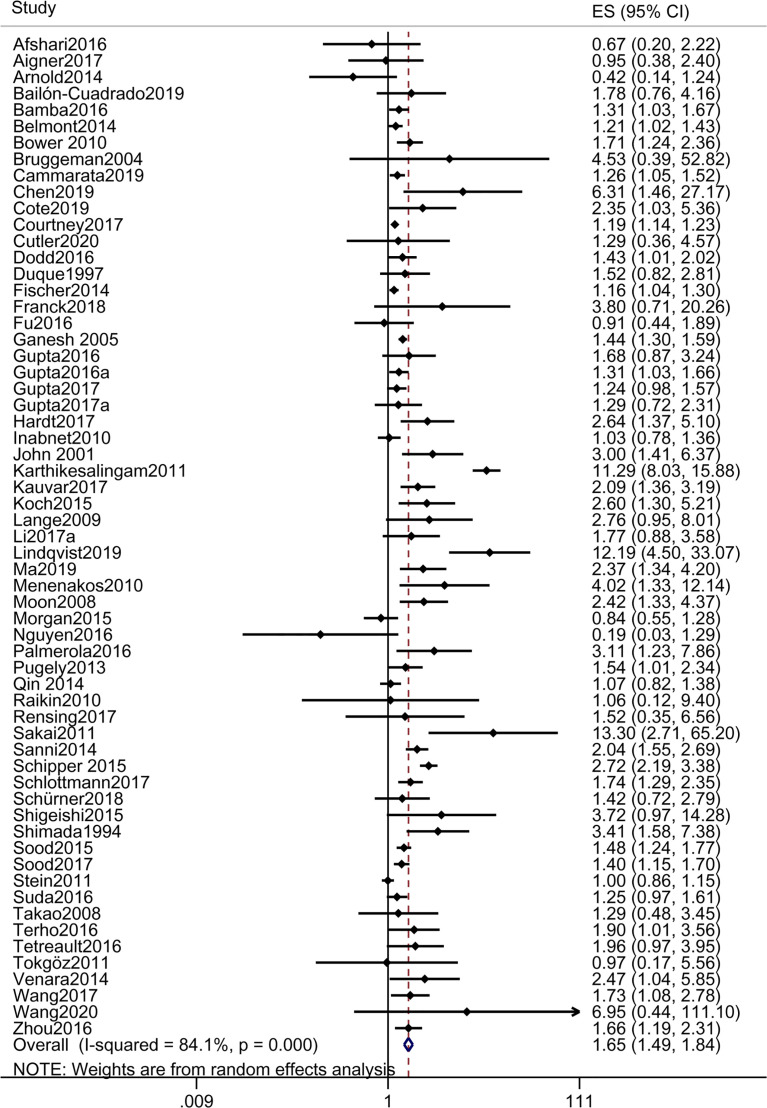
A total of 61 studies reported any complication. The pooled OR of any complication in patients with DM vs those without DM was 1.653 [1.487, 1.839], suggesting that DM was a risk factor for any postoperative complication ( Table 2 and Figure 2 ). The results of subgroup analyses showed that OR of any complication in patients with IDDM and NIDDM vs those without DM were 1.895 [1.331, 2.698] and 1.554 [1.061, 2.277], respectively, suggesting that IDDM and NIDDM were risk factors for any postoperative complication, and the risk of IDDM was higher than that of NIDDM ( Table 2 and Supplementary Figures S1, S2 ).
Table 2.
Outcomes.
| Complications | Odds ratio (95%CI) | Studies included |
|---|---|---|
| Any complications | 1.653 (1.487, 1.839) | 61 |
| Any complications-IDDM | 1.895 (1.331, 2.698) | 8 |
| Any complications-NIDDM | 1.554 (1.061, 2.277) | 7 |
| Infections | 1.537 (1.322, 1.787) | 40 |
| VTE | 1.189 (0.759, 1.864) | 11 |
| Wound healing disorders | 2.010 (1.326, 3.046) | 9 |
| Hematoma | 1.369 (1.120, 1.673) | 8 |
| Renal insufficiency/failure | 1.987 (1.311, 3.013) | 5 |
| MI | 1.372 (0.574, 3.278) | 4 |
| Length of stay | 1.581 (1.271, 1.968) | 7 |
| Readmission | 1.404 (1.274, 1.548) | 15 |
| Reoperation | 1.568 (1.124, 2.188) | 11 |
| Mortality | 1.606 (1.178, 2.191) | 18 |
| Mortality-Cancer surgery | 1.052 (0.419,2.643) | 3 |
| Mortality-Orthopedic surgery | 1.817 (1.136,2.906) | 8 |
| Mortality-Hemangioma resection | 1.509 (0.889,2.561) | 3 |
| Mortality-transplant | 1.214 (0.410,3.592) | 2 |
IDDM, Insulin-Dependent Diabetes Mellitus; MI, Myocardial infarction; NIDDM, Non-Insulin-Dependent Diabetes Mellitus; VTE, Venous Thromboembolism.
Figure 2.
Forest plot of odds ratio of any postoperative complication in patients with DM vs those without DM.
3.2.2 Organ or System Complications
3.2.2.1 Infections
A total of 40 studies reported infections. The pooled OR of infections in patients with DM vs those without DM was 1.537 [1.322, 1.787], suggesting that DM was a risk factor for postoperative infections ( Table 2 and Supplementary Figure S3 ).
3.2.2.2 Venous Thromboembolism
A total of 11 studies reported VTE. The pooled OR of VTE in patients with DM vs those without DM was 1.189 [0.759, 1.864], which was statistically insignificant. This result suggested that DM was not a risk factor for postoperative VTE ( Table 2 and Supplementary Figure S4 ).
3.2.2.3 Wound Healing Disorders
A total of nine studies reported WHD. The pooled OR of WHD in patients with DM vs those without DM was 2.010 [1.326, 3.046], suggesting that DM was a risk factor for postoperative WHD ( Table 2 and Supplementary Figure S5 ).
3.2.2.4 Hematoma
A total of eight studies reported hematoma. The pooled OR of hematoma in patients with DM vs those without DM was 1.369 [1.120, 1.673], suggesting that DM was a risk factor for postoperative hematoma ( Table 2 and Supplementary Figure S6 ).
3.2.2.5 Renal Insufficiency
A total of five studies reported renal insufficiency. The pooled OR of renal insufficiency in patients with DM vs those without DM was 1.987 [1.311, 3.013], suggesting that DM was a risk factor for postoperative renal insufficiency ( Table 2 and Supplementary Figure S7 ).
3.2.2.6 Myocardial Infarction
A total of four studies reported MI. The pooled OR of MI in patients with DM vs those without DM was 1.372 [0.574, 3.278], which was statistically insignificant. This result suggested that DM was not a risk factor for postoperative MI ( Table 2 and Supplementary Figure S8 ).
3.2.3 Hospitalization
A total of seven studies reported LOS. The pooled OR of LOS in patients with DM vs those without DM was 1.987 [1.311, 3.013], suggesting that DM was a risk factor for extended LOS after surgery. A total of eleven and fifteen studies reported reoperation and readmission with the pooled OR 1.568 [1.124, 2.188] and 1.404 [1.274, 1.548], respectively. The results suggested that DM was a risk factor for postoperative reoperation and readmission ( Table 2 and Supplementary Figures S9–S11 ).
3.2.4 Survival
A total of 18 studies reported mortality. The pooled OR of mortality in patients with DM vs those without DM was 1.606 [1.178, 2.191], suggesting that DM was a risk factor for postoperative death ( Figure 3 ). The results of subgroup analyses revealed that the pooled OR of mortality in patients with DM vs those without DM was 1.817 [1.136, 2.906] after orthopedic surgery, while the pooled OR of mortality were 1.052 [0.419, 2.643], 1.509 [0.889, 2.561] and 1.214 [0.410, 3.592] after cancer surgery, hemangioma resection and transplant, respectively. The results suggested that DM was a risk factor for death after orthopedic surgery, not for death after cancer surgery, hemangioma resection and transplant ( Table 2 and Supplementary Figures S12–S15 ).
Figure 3.
Forest plot of odds ratio of postoperative mortality in patients with DM vs those without DM.
3.3 Subgroup Analysis
According to the type of surgery, we had three subgroups: general surgery, orthopedics and aesthetic surgery. Only the results of general surgery are slightly different from the overall results, the pooled OR of VTE in patients with DM vs those without DM was 3.627[2.405, 5.469], suggesting that DM was a risk factor for postoperative VTE. The analysis results of the other two subgroups were consistent with the overall results. ( Table 3 and Supplementary Figures S16–32 ).
Table 3.
Outcomes of subgroup analysis.
| Aesthetic Surgery | OR (95%CI) | Studies included |
|---|---|---|
| Complications | ||
| Any complications | 1.582 (1.044, 2.396) | 10 |
| Infections | 1.670 (1.344, 2.074) | 7 |
| VTE | 0.270 (0.069, 1.059) | 2 |
| Hematoma | 1.362 (1.074, 1.727) | 5 |
| General Surgery | ||
| Complications | OR (95%CI) | Studies included |
| Any complications | 1.847 (1.595, 2.139) | 32 |
| Infections | 1.732 (1.268, 2.364) | 14 |
| VTE | 3.627 (2.405, 5.469) | 2 |
| Wound healing disorders | 2.053 (1.126, 3.740) | 5 |
| Renal insufficiency/failure | 2.259 (1.234, 4.135) | 4 |
| Mortality | 1.400 (0.976, 2.010) | 10 |
| Orthopedic Surgery | ||
| Complications | OR (95%CI) | Studies included |
| Any complications | 1.409 (1.194, 1.664) | 19 |
| Infections | 1.425 (1.136, 1.786) | 19 |
| VTE | 0.975 (0.789, 1.206) | 7 |
| Wound healing disorders | 2.355 (1.380, 4.017) | 3 |
| Hematoma | 1.607 (0.821, 3.145) | 3 |
| MI | 1.372 (0.574, 3.278) | 4 |
| Mortality | 1.817 (1.136, 2.906) | 8 |
MI, Myocardial infarction; VTE, Venous Thromboembolism.
3.4 Publication Bias
We performed Egger’s test based on six comparisons (any complication, infection, VTE, readmission, reoperation, and mortality) with more than ten included studies. P-value of Egger’s test for VTE was 0.688, suggesting no publication bias, while P-values for the other five comparisons were less than 0.1, standing for different degrees of the publication bias. The trim-and-fill procedure was adopted for these 5 comparisons. After four additional studies were filled to “reoperation”, the result of the meta-analysis changed, and the OR changed from statistically to non-statistically significant, suggesting that DM as a risk factor for reoperation are not necessarily reliable and should be interpreted carefully. The other four comparisons (any complication, infection, readmission, and mortality) showed varying degree of changes in the pooled effect values after the adoption of trim-fill method, but without any change in the statistical significance ( Supplementary Figures S33–S38 ).
4 Discussion
As the number of people with DM increases, a large number of diabetic patients are facing various health problems that require surgical treatment. DM is generally considered a major risk factor for postoperative complications (138). Although this is intuitive enough for clinicians, it is unclear which postoperative complications are exactly related to DM because there may be other comorbidities in patients with DM. This meta-analysis included 125 studies with a total sample size of 3,208,776.
We started out with a meta-analysis of any complication. The results showed that DM was a risk factor for any postoperative complication, which was consistent with previous studies (99). Our subgroup analyses showed that both IDDM and NIDDM were risk factors for any postoperative complication, and the risk of IDDM was higher than that of NIDDM. These findings suggested that IDDM, not just DM in general sense, should be an important risk factor in clinical evaluation of patients. This might explain why some diabetic patients, while their blood glucose was under control, still experienced various postoperative complications. The results of our meta-analysis are in accord with Nathan et al. study which found a 2.5-fold increase in the readmission rate of IDDM patients after posterior lumbar fusion. Subgroup analysis showed that readmission rate was nearly the same for patients with NIDDM as for those without DM, while it was twice as high in patients with IDDM as those without DM (65). Similar findings were reported in lumbar fusion surgery. Nicholas et al. suggested that compared with patients without DM, IDDM was more significantly associated with an increased risk of postoperative complications, extended length of hospital stay, postoperative adverse events, and readmission than NIDDM. Furthermore, the complications associated with IDDM were more severe than those associated with NIDDM (49). These findings indicated that whether a patient has IDDM is more important than whether a patient has DM (type 1 or type 2) when considering a patient as a surgical candidate.
It is well known that DM is a risk factor for perioperative complications (4, 46). Our analyses revealed that DM is an independent risk factor for wound infections, WHD, hematoma, and renal insufficiency. DM has been identified as a risk factor for postoperative infection and poor healing because of its vascular lesions and immune effects (139). DM present with neutrophilic dysfunction which increases the risk of infection by the pathogen and decreases healing capacity (52). DM is associated with tissue hypoxia and increased blood viscosity. This slows the inflammatory response, which in turn alters wound healing and increases the risk of infection, especially in the lower extremities (140–143). In addition, several factors prevent wound healing in patients with DM, including reduced angiogenesis, multiple growth factors, and impaired macrophage function (144). These may be responsible for postoperative complications of DM.
Moreover, we also found that DM can increase the incidence of postoperative renal insufficiency, which deserves our attention. After hip and knee arthroplasty, diabetic patients are 1.5 times more likely to develop acute renal failure than nondiabetic patients (92). After orthotopic liver transplantation, renal insufficiency is significantly higher in patients with preexisting DM than in patients without DM (59.7% vs. 20.2%, P < 0.001) (59). Considering the elevated incidence of postoperative renal insufficiency in diabetic patients, surgeons should pay more attention to postoperative fluid management, intraoperative hypotension anesthesia, and perioperative nephrotoxic medications.
A surprising finding in our study is that DM does not significantly increase the risk of VTE and MI. Diabetic patients are prone to hypercoagulable state due to abnormal regulation of coagulation-related plasma proteins caused by prolonged hyperglycemia. Type 2 DM is associated with an increased risk of thrombosis and cardiovascular disease. Therefore, it is also generally accepted that diabetic patients may be at an increased risk of postoperative thrombosis. For example, Rena et al. retrospectively reviewed 5,538 patients who underwent sleeve gastrectomy between January 1, 2008 and September 30, 2016, at 5 weight loss centers in the United States (80). They found that a personal history of malignancy and type 2 DM increased the risk of mesenteric vein thrombosis. However, many studies have shown different results. Ravinder et al. found that DM was not an independent risk factor for the venous thrombosis in various cosmetic procedures, although it was an independent risk factor for major complications, especially infections. The prevalence of DM did not differ significantly between the VTE and non-VTE groups (21) (0.9% vs 1.8%, P = 0.37). VTE and MI are deadly serious postoperative complications with not only high morbidity and mortality, but also prolonged hospital stay and high charges. Accurate identification of which patients are high risk for thromboembolism helps to take more targeted and appropriate preventive measures. A variety of surgeries were included in our study (cardiac surgery was not within the scope of our study). Eleven studies involved VTE. MI was reported in four studies. Our study suggests that DM is not a risk factor for postoperative VTE and MI, and therefore DM should not be considered a priority factor in determining thrombotic risk. Clinicians should pay more attention to age, smoking, and immobility and other factors, which may be associated with thrombosis according to the literature (116).
Our study also found that DM is a risk factor for postoperative reoperation and readmission, and that patients with DM have a higher risk of postoperative death. This is consistent with findings that DM is an independent risk factor for multiple postoperative complications. DM significantly prolongs the hospital stay after ankle fusion and total ankle replacement (104). Ganesh et al. used the NIS database to analyze the effect of DM on the prognosis of patients with ankle fractures and found that DM was associated with a significant increase in hospital stay (4.7 days vs 3.6 days, P < 0.001) (48). These are all consistent with our findings. Postoperative infection, hematoma and WHD are causes of reoperation and readmission. It is not surprising, therefore, that diabetic patients are more prone to reoperation and readmission. Although DM is found to be a risk factor for postoperative death, our subgroup analysis suggests that DM is a risk factor for death after orthopedic surgery but not cancer surgery, hemangioma resection and transplant. This is an important finding in our study. This may be related to the specifics of different types of surgery, such as patient population characteristics, length of surgery, and surgical procedures. Based on this finding, orthopedic surgery should be more strictly controlled by surgical standards and should be performed cautiously in patients with DM.
The strengths of this study lie in the large number of studies included the large sample size, and the exploration of the association of DM with multiple postoperative complications. The limitation of this study is that our study of common complications may have heterogeneity due to differences in the type of surgery. In addition, our combined effect size may be overestimated. The reason is that although a small number of included studies have analyzed a large number of complications, they only show significant differences (P < 0.05). Unfortunately, it is that we can only calculate the combined effect size based on studies that provide OR. Finally, what’s noteworthy is that the OR in some studies is not adjusted for confounders because the incidence of some complications may be affected by potential confounders, such as preoperative diseases other than DM, body weight, or age, etc. Therefore, we stratified the pooled values for any complication by crude OR and adjusted OR, respectively, resulting in consistent results. However, we did not stratify the other subdivided complications in the same way.
In summary, our meta-analysis suggested that DM may significantly affect multiple perioperative complications, hospitalization, and survival (cardiac surgery is not within the scope of our study). DM is a risk factor for postoperative infections, WHD, hematoma, renal insufficiency, reoperation, readmission and death after orthopedic surgery. But DM is not the risk of postoperative VTE, MI and not the risk for death after cancer surgery, hemangioma resection and transplant. These findings underscore the importance of preoperative risk factor assessment for the safe outcome of surgical patients.
Data Availability Statement
The original contributions presented in the study are included in the article/ Supplementary Material . Further inquiries can be directed to the corresponding authors.
Author Contributions
XZ, AH, WM, and JL contributed to the conception or design the study; JC, YL, JSL, and HL contributed to acquisition, analysis of data for the study; XZ, AH, YM, and YS contributed to interpretation of data for the study; XZ and AH wrote the first draft of the manuscript. All authors revised it critically for important intellectual content and approved the final manuscript. All authors agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Funding
This work was supported by the National Key Research and Development Program of China (Grant No. 2018YFC2001900).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2022.841256/full#supplementary-material
Abbreviations
CI, Confidence Intervals; DM, Diabetes mellitus; IDDM, Insulin-Dependent Diabetes Mellitus; LOS, length of stay; MI, Myocardial infarction; NIDDM, Non-Insulin-Dependent Diabetes Mellitus; NOS, Newcastle-Ottawa scale; OR, Odds Ratio; RR, Relative Risk; WHD, Wound Healing Disorders; VTE, Venous Thromboembolism.
References
- 1. Haynes AB, Weiser TG, Berry WR, Lipsitz SR, Breizat AH, Dellinger EP, et al. A Surgical Safety Checklist to Reduce Morbidity and Mortality in a Global Population. N Engl J Med (2009) 360:491–9. doi: 10.1056/NEJMsa0810119 [DOI] [PubMed] [Google Scholar]
- 2. Bohl DD, Idarraga AJ, Holmes GB, Jr., Hamid KS, Lin J, Lee S. Validated Risk-Stratification System for Prediction of Early Adverse Events Following Open Reduction and Internal Fixation of Closed Ankle Fractures. J Bone Joint Surg Am (2019) 101:1768–74. doi: 10.2106/jbjs.19.00203 [DOI] [PubMed] [Google Scholar]
- 3. Quilliot D, Sirveaux MA, Nomine-Criqui C, Fouquet T, Reibel N, Brunaud L. Evaluation of Risk Factors for Complications After Bariatric Surgery. J Visc Surg (2018) 155:201–10. doi: 10.1016/j.jviscsurg.2018.01.004 [DOI] [PubMed] [Google Scholar]
- 4. Patel S, Srivastava S, Singh MR, Singh D. Mechanistic Insight Into Diabetic Wounds: Pathogenesis, Molecular Targets and Treatment Strategies to Pace Wound Healing. BioMed Pharmacother (2019) 112:108615. doi: 10.1016/j.biopha.2019.108615 [DOI] [PubMed] [Google Scholar]
- 5. Rollins KE, Varadhan KK, Dhatariya K, Lobo DN. Systematic Review of the Impact of HbA1c on Outcomes Following Surgery in Patients With Diabetes Mellitus. Clin Nutr (2016) 35:308–16. doi: 10.1016/j.clnu.2015.03.007 [DOI] [PubMed] [Google Scholar]
- 6. Dhatariya K, Levy N, Kilvert A, Watson B, Cousins D, Flanagan D, et al. NHS Diabetes Guideline for the Perioperative Management of the Adult Patient With Diabetes. Diabetes Med (2012) 29:420–33. doi: 10.1111/j.1464-5491.2012.03582.x [DOI] [PubMed] [Google Scholar]
- 7. Chuah LL, Papamargaritis D, Pillai D, Krishnamoorthy A, le Roux CW. Morbidity and Mortality of Diabetes With Surgery. Nutr Hosp (2013) 28 Suppl 2:47–52. doi: 10.3305/nh.2013.28.sup2.6713 [DOI] [PubMed] [Google Scholar]
- 8. King JT, Jr., Goulet JL, Perkal MF, Rosenthal RA. Glycemic Control and Infections in Patients With Diabetes Undergoing Noncardiac Surgery. Ann Surg (2011) 253:158–65. doi: 10.1097/SLA.0b013e3181f9bb3a [DOI] [PubMed] [Google Scholar]
- 9. Carson JL, Scholz PM, Chen AY, Peterson ED, Gold J, Schneider SH. Diabetes Mellitus Increases Short-Term Mortality and Morbidity in Patients Undergoing Coronary Artery Bypass Graft Surgery. J Am Coll Cardiol (2002) 40:418–23. doi: 10.1016/s0735-1097(02)01969-1 [DOI] [PubMed] [Google Scholar]
- 10. Bock M, Johansson T, Fritsch G, Flamm M, Hansbauer B, Mann E, et al. The Impact of Preoperative Testing for Blood Glucose Concentration and Haemoglobin A1c on Mortality, Changes in Management and Complications in Noncardiac Elective Surgery: A Systematic Review. Eur J Anaesthesiol (2015) 32:152–9. doi: 10.1097/eja.0000000000000117 [DOI] [PubMed] [Google Scholar]
- 11. Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, et al. The PRISMA Statement for Reporting Systematic Reviews and Meta-Analyses of Studies That Evaluate Healthcare Interventions: Explanation and Elaboration. Bmj (2009) 339:b2700. doi: 10.1136/bmj.b2700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sedgwick P. Meta-Analyses: What is Heterogeneity? Bmj (2015) 350:h1435. doi: 10.1136/bmj.h1435 [DOI] [PubMed] [Google Scholar]
- 13. Hunter JP, Saratzis A, Sutton AJ, Boucher RH, Sayers RD, Bown MJ. In Meta-Analyses of Proportion Studies, Funnel Plots Were Found to be an Inaccurate Method of Assessing Publication Bias. J Clin Epidemiol (2014) 67:897–903. doi: 10.1016/j.jclinepi.2014.03.003 [DOI] [PubMed] [Google Scholar]
- 14. Afshari A, Gupta V, Nguyen L, Shack RB, Grotting JC, Higdon KK. Preoperative Risk Factors and Complication Rates of Thighplasty: Analysis of 1,493 Patients. Aesthet Surg J (2016) 36:897–907. doi: 10.1093/asj/sjv275 [DOI] [PubMed] [Google Scholar]
- 15. Aigner R, Salomia C, Lechler P, Pahl R, Frink M. Relationship of Prolonged Operative Time and Comorbidities With Complications After Geriatric Ankle Fractures. Foot Ankle Int (2017) 38:41–8. doi: 10.1177/1071100716667315 [DOI] [PubMed] [Google Scholar]
- 16. Akhter MS, Verma R, Madhukar KP, Vaishampayan AR, Unadkat PC. Incidence of Surgical Site Infection in Postoperative Patients at a Tertiary Care Centre in India. J Wound Care (2016) 25:210–2, 4-7. doi: 10.12968/jowc.2016.25.4.210 [DOI] [PubMed] [Google Scholar]
- 17. Ammori JB, Navale S, Schiltz N, Koroukian SM. Predictors of 30-Day Readmissions After Gastrectomy for Malignancy. J Surg Res (2018) 224:176–84. doi: 10.1016/j.jss.2017.12.004 [DOI] [PubMed] [Google Scholar]
- 18. Arnold PM, Fehlings MG, Kopjar B, Yoon ST, Massicotte EM, Vaccaro AR, et al. Mild Diabetes is Not a Contraindication for Surgical Decompression in Cervical Spondylotic Myelopathy: Results of the AOSpine North America Multicenter Prospective Study (CSM). Spine J (2014) 14:65–72. doi: 10.1016/j.spinee.2013.06.016 [DOI] [PubMed] [Google Scholar]
- 19. Bailey SH, Bull DA, Harpole DH, Rentz JJ, Neumayer LA, Pappas TN, et al. Outcomes After Esophagectomy: A Ten-Year Prospective Cohort. Ann Thorac Surg (2003) 75:217–22. doi: 10.1016/s0003-4975(02)04368-0 [DOI] [PubMed] [Google Scholar]
- 20. Bailón-Cuadrado M, Pérez-Saborido B, Sánchez-González J, Rodríguez-López M, Velasco-López R, Sarmentero-Prieto JC, et al. Prognostic Nutritional Index Predicts Morbidity After Curative Surgery for Colorectal Cancer. Cirugía Española (English Ed) (2019) 97:71–80. doi: 10.1016/j.cireng.2019.01.003 [DOI] [PubMed] [Google Scholar]
- 21. Bamba R, Gupta V, Shack RB, Grotting JC, Higdon KK. Evaluation of Diabetes Mellitus as a Risk Factor for Major Complications in Patients Undergoing Aesthetic Surgery. Aesthet Surg J (2016) 36:598–608. doi: 10.1093/asj/sjv241 [DOI] [PubMed] [Google Scholar]
- 22. Bascom A, Ghosh S, Fairey AS, Rourke KF. Assessment of Wound Complications After Bulbar Urethroplasty: The Impact of a Lambda Perineal Incision. Urology (2016) 90:184–8. doi: 10.1016/j.urology.2015.12.047 [DOI] [PubMed] [Google Scholar]
- 23. Belmont PJ, Jr., Davey S, Rensing N, Bader JO, Waterman BR, Orr JD. Patient-Based and Surgical Risk Factors for 30-Day Postoperative Complications and Mortality After Ankle Fracture Fixation. J Orthop Trauma (2015) 29:e476–82. doi: 10.1097/bot.0000000000000328 [DOI] [PubMed] [Google Scholar]
- 24. Belmont PJ, Jr., Goodman GP, Waterman BR, Bader JO, Schoenfeld AJ. Thirty-Day Postoperative Complications and Mortality Following Total Knee Arthroplasty: Incidence and Risk Factors Among a National Sample of 15,321 Patients. J Bone Joint Surg Am (2014) 96:20–6. doi: 10.2106/jbjs.m.00018 [DOI] [PubMed] [Google Scholar]
- 25. Benrashid E, Youngwirth LM, Guest K, Cox MW, Shortell CK, Dillavou ED. Negative Pressure Wound Therapy Reduces Surgical Site Infections. J Vasc Surg (2020) 71:896–904. doi: 10.1016/j.jvs.2019.05.066 [DOI] [PubMed] [Google Scholar]
- 26. Bolognesi MP, Marchant MH, Jr., Viens NA, Cook C, Pietrobon R, Vail TP. The Impact of Diabetes on Perioperative Patient Outcomes After Total Hip and Total Knee Arthroplasty in the United States. J Arthroplasty (2008) 23:92–8. doi: 10.1016/j.arth.2008.05.012 [DOI] [PubMed] [Google Scholar]
- 27. Bower WF, Jin L, Underwood MJ, Lee JF, Lee KF, Lam YH, et al. Overt Diabetes Mellitus Adversely Affects Surgical Outcomes of Noncardiovascular Patients. Surgery (2010) 147:670–5. doi: 10.1016/j.surg.2009.10.070 [DOI] [PubMed] [Google Scholar]
- 28. Browne JA, Cook C, Pietrobon R, Bethel MA, Richardson WJ. Diabetes and Early Postoperative Outcomes Following Lumbar Fusion. Spine (Phila Pa 1976) (2007) 32:2214–9. doi: 10.1097/BRS.0b013e31814b1bc0 [DOI] [PubMed] [Google Scholar]
- 29. Bruggeman NB, Turner NS, Dahm DL, Voll AE, Hoskin TL, Jacofsky DJ, et al. Wound Complications After Open Achilles Tendon Repair: An Analysis of Risk Factors. Clin Orthop Relat Res (2004) 427:63–6. doi: 10.1097/01.blo.0000144475.05543.e7 [DOI] [PubMed] [Google Scholar]
- 30. Buchanan IA, Donoho DA, Patel A, Lin M, Wen T, Ding L, et al. Predictors of Surgical Site Infection After Nonemergent Craniotomy: A Nationwide Readmission Database Analysis. World Neurosurg (2018) 120:e440–e52. doi: 10.1016/j.wneu.2018.08.102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Bur AM, Brant JA, Mulvey CL, Nicolli EA, Brody RM, Fischer JP, et al. Association of Clinical Risk Factors and Postoperative Complications With Unplanned Hospital Readmission After Head and Neck Cancer Surgery. JAMA Otolaryngol Head Neck Surg (2016) 142:1184–90. doi: 10.1001/jamaoto.2016.2807 [DOI] [PubMed] [Google Scholar]
- 32. Cammarata MJ, Kantar RS, Rifkin WJ, Greenfield JA, Levine JP, Ceradini DJ. Advanced Age Is a Risk Factor for Complications Following Abdominal Panniculectomy. Obes Surg (2019) 29:426–33. doi: 10.1007/s11695-018-3492-5 [DOI] [PubMed] [Google Scholar]
- 33. Chen S, Anderson MV, Cheng WK, Wongworawat MD. Diabetes Associated With Increased Surgical Site Infections in Spinal Arthrodesis. Clin Orthop Relat Res (2009) 467:1670–3. doi: 10.1007/s11999-009-0740-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Chen L, Wang YB, Zhang YH, Gong JF, Li Y. Effective Prediction of Postoperative Complications for Patients After Open Hepatectomy: A Simplified Scoring System Based on Perioperative Parameters. BMC Surg (2019) 19:128. doi: 10.1186/s12893-019-0597-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Chiu WK, Chou CY, Chen SG, Chen C, Wang HJ, Yang TF. Is Sequential Free Flap Safe in Oral Cancer Reconstruction in the Same Patient? An Outcome and Complication Analysis. Jpn J Clin Oncol (2020) 50:152–8. doi: 10.1093/jjco/hyz142 [DOI] [PubMed] [Google Scholar]
- 36. Ciufo DJ, Thirukumaran CP, Marchese R, Oh I. Risk Factors for Reoperation, Readmission, and Early Complications After Below Knee Amputation. Injury (2019) 50:462–6. doi: 10.1016/j.injury.2018.10.031 [DOI] [PubMed] [Google Scholar]
- 37. Cook C, Tackett S, Shah A, Pietrobon R, Browne J, Viens N, et al. Diabetes and Perioperative Outcomes Following Cervical Fusion in Patients With Myelopathy. Spine (Phila Pa 1976) (2008) 33:E254–60. doi: 10.1097/BRS.0b013e31816b88ca [DOI] [PubMed] [Google Scholar]
- 38. Cote DJ, Dasenbrock HH, Gormley WB, Smith TR, Dunn IF. Adverse Events After Microvascular Decompression: A National Surgical Quality Improvement Program Analysis. World Neurosurg (2019) 128:e884–e94. doi: 10.1016/j.wneu.2019.05.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Courtney PM, Boniello AJ, Berger RA. Complications Following Outpatient Total Joint Arthroplasty: An Analysis of a National Database. J Arthroplasty (2017) 32:1426–30. doi: 10.1016/j.arth.2016.11.055 [DOI] [PubMed] [Google Scholar]
- 40. Cutler HS, Collett G, Farahani F, Ahn J, Nakonezny P, Koehler D, et al. 30-Day Readmissions and Reoperations After Total Elbow Arthroplasty: A National Database Study. J Shoulder Elbow Surg (2020) 30(2):e41–9. doi: 10.1016/j.jse.2020.06.033 [DOI] [PubMed] [Google Scholar]
- 41. Dodd AC, Lakomkin N, Attum B, Bulka C, Karhade AV, Douleh DG, et al. Predictors of Adverse Events for Ankle Fractures: An Analysis of 6800 Patients. J Foot Ankle Surg (2016) 55:762–6. doi: 10.1053/j.jfas.2016.03.010 [DOI] [PubMed] [Google Scholar]
- 42. Duque JL, Ramos G, Castrodeza J, Cerezal J, Castanedo M, Yuste MG, et al. Early Complications in Surgical Treatment of Lung Cancer: A Prospective, Multicenter Study. Grupo Cooperativo De Carcinoma Broncogénico De La Sociedad Española De Neumología Y Cirugía Torácica. Ann Thorac Surg (1997) 63:944–50. doi: 10.1016/s0003-4975(97)00051-9 [DOI] [PubMed] [Google Scholar]
- 43. Farivar BS, Kalsi R, Drucker CB, Goldstein CB, Sarkar R, Toursavadkohi S. Implications of Concomitant Hypogastric Artery Embolization With Endovascular Repair of Infrarenal Abdominal Aortic Aneurysms. J Vasc Surg (2017) 66:95–101. doi: 10.1016/j.jvs.2016.10.124 [DOI] [PubMed] [Google Scholar]
- 44. Fischer JP, Tuggle CT, Au A, Kovach SJ. A 30-Day Risk Assessment of Mastectomy Alone Compared to Immediate Breast Reconstruction (IBR). J Plast Surg Handb Surg (2014) 48:209–15. doi: 10.3109/2000656x.2013.865633 [DOI] [PubMed] [Google Scholar]
- 45. Franck P, Bernstein JL, Cohen LE, Härtl R, Baaj AA, Spector JA. Local Muscle Flaps Minimize Post-Operative Wound Morbidity in Patients With Neoplastic Disease of the Spine. Clin Neurol Neurosurg (2018) 171:100–5. doi: 10.1016/j.clineuro.2018.05.022 [DOI] [PubMed] [Google Scholar]
- 46. Freire MP, Antonopoulos IM, Piovesan AC, Moura ML, de Paula FJ, Spadão F, et al. Amikacin Prophylaxis and Risk Factors for Surgical Site Infection After Kidney Transplantation. Transplantation (2015) 99:521–7. doi: 10.1097/tp.0000000000000381 [DOI] [PubMed] [Google Scholar]
- 47. Fu MC, Buerba RA, Grauer JN. Preoperative Nutritional Status as an Adjunct Predictor of Major Postoperative Complications Following Anterior Cervical Discectomy and Fusion. Clin Spine Surg (2016) 29:167–72. doi: 10.1097/bsd.0000000000000181 [DOI] [PubMed] [Google Scholar]
- 48. Ganesh SP, Pietrobon R, Cecílio WA, Pan D, Lightdale N, Nunley JA. The Impact of Diabetes on Patient Outcomes After Ankle Fracture. J Bone Joint Surg Am (2005) 87:1712–8. doi: 10.2106/jbjs.d.02625 [DOI] [PubMed] [Google Scholar]
- 49. Golinvaux NS, Varthi AG, Bohl DD, Basques BA, Grauer JN. Complication Rates Following Elective Lumbar Fusion in Patients With Diabetes: Insulin Dependence Makes the Difference. Spine (Phila Pa 1976) (2014) 39:1809–16. doi: 10.1097/brs.0000000000000506 [DOI] [PubMed] [Google Scholar]
- 50. Gupta V, Parikh R, Nguyen L, Afshari A, Shack RB, Grotting JC, et al. Is Office-Based Surgery Safe? Comparing Outcomes of 183,914 Aesthetic Surgical Procedures Across Different Types of Accredited Facilities. Aesthet Surg J (2017) 37:226–35. doi: 10.1093/asj/sjw138 [DOI] [PubMed] [Google Scholar]
- 51. Gupta V, Winocour J, Rodriguez-Feo C, Bamba R, Shack RB, Grotting JC, et al. Safety of Aesthetic Surgery in the Overweight Patient: Analysis of 127,961 Patients. Aesthet Surg J (2016) 36:718–29. doi: 10.1093/asj/sjv268 [DOI] [PubMed] [Google Scholar]
- 52. Gupta V, Winocour J, Shi H, Shack RB, Grotting JC, Higdon KK. Preoperative Risk Factors and Complication Rates in Facelift: Analysis of 11,300 Patients. Aesthet Surg J (2016) 36:1–13. doi: 10.1093/asj/sjv162 [DOI] [PubMed] [Google Scholar]
- 53. Gupta V, Yeslev M, Winocour J, Bamba R, Rodriguez-Feo C, Grotting JC, et al. Aesthetic Breast Surgery and Concomitant Procedures: Incidence and Risk Factors for Major Complications in 73,608 Cases. Aesthet Surg J (2017) 37:515–27. doi: 10.1093/asj/sjw238 [DOI] [PubMed] [Google Scholar]
- 54. Hadaya J, Dobaria V, Aguayo E, Mandelbaum A, Sanaiha Y, Revels SSL, et al. Impact of Hospital Volume on Outcomes of Elective Pneumonectomy in the United States. Ann Thorac Surg (2020) 110(6):1874–81. doi: 10.1016/j.athoracsur.2020.04.115 [DOI] [PubMed] [Google Scholar]
- 55. Hardt J, Pilz L, Magdeburg J, Kienle P, Post S, Magdeburg R. Preoperative Hypoalbuminemia is an Independent Risk Factor for Increased High-Grade Morbidity After Elective Rectal Cancer Resection. Int J Colorect Dis (2017) 32:1439–46. doi: 10.1007/s00384-017-2884-7 [DOI] [PubMed] [Google Scholar]
- 56. Hunecke P, Toll M, Mann O, Izbicki JR, Blessmann M, Grupp K. Clinical Outcome of Patients Undergoing Abdominoplasty After Massive Weight Loss. Surg Obes Relat Dis (2019) 15:1362–6. doi: 10.1016/j.soard.2019.06.001 [DOI] [PubMed] [Google Scholar]
- 57. Inabnet WB, 3rd, Belle SH, Bessler M, Courcoulas A, Dellinger P, Garcia L, et al. Comparison of 30-Day Outcomes After Non-LapBand Primary and Revisional Bariatric Surgical Procedures From the Longitudinal Assessment of Bariatric Surgery Study. Surg Obes Relat Dis (2010) 6:22–30. doi: 10.1016/j.soard.2009.10.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Janczak D, Bakowski W, Bakowska K, Marschollek K, Marschollek P, Malinowski M, et al. Early Complications in Patients Undergoing Elective Open Surgery for Infrarenal Aortic Aneurysms. J Coll Phys Surg Pak (2019) 29:1078–82. doi: 10.29271/jcpsp.2019.11.1078 [DOI] [PubMed] [Google Scholar]
- 59. John PR, Thuluvath PJ. Outcome of Liver Transplantation in Patients With Diabetes Mellitus: A Case-Control Study. Hepatology (2001) 34:889–95. doi: 10.1053/jhep.2001.29134 [DOI] [PubMed] [Google Scholar]
- 60. Kantar RS, Rifkin WJ, Wilson SC, David JA, Diaz-Siso JR, Levine JP, et al. Abdominal Panniculectomy: Determining the Impact of Diabetes on Complications and Risk Factors for Adverse Events. Plast Reconstr Surg (2018) 142:462e–71e. doi: 10.1097/prs.0000000000004732 [DOI] [PubMed] [Google Scholar]
- 61. Karthikesalingam A, Kitcat M, Malata CM. Abdominoplasty in Patients With and Without Pre-Existing Scars: A Retrospective Comparison. J Plast Reconstr Aesthet Surg (2011) 64:369–74. doi: 10.1016/j.bjps.2010.05.025 [DOI] [PubMed] [Google Scholar]
- 62. Kauvar DS, Martin ED, Simon TE, Givens MD. Complication Profile, Failure to Rescue, and Mortality Following Elective Endovascular Aortic Aneurysm Repair. Am J Surg (2017) 214:307–11. doi: 10.1016/j.amjsurg.2017.02.007 [DOI] [PubMed] [Google Scholar]
- 63. Koch M, Kantas A, Ramcke K, Drabik AI, Nashan B. Surgical Complications After Kidney Transplantation: Different Impacts of Immunosuppression, Graft Function, Patient Variables, and Surgical Performance. Clin Transpl (2015) 29:252–60. doi: 10.1111/ctr.12513 [DOI] [PubMed] [Google Scholar]
- 64. Lange CP, Ploeg AJ, Lardenoye JW, Breslau PJ. Patient- and Procedure-Specific Risk Factors for Postoperative Complications in Peripheral Vascular Surgery. Qual Saf Health Care (2009) 18:131–6. doi: 10.1136/qshc.2007.022335 [DOI] [PubMed] [Google Scholar]
- 65. Lee NJ, Kothari P, Phan K, Shin JI, Cutler HS, Lakomkin N, et al. Incidence and Risk Factors for 30-Day Unplanned Readmissions After Elective Posterior Lumbar Fusion. Spine (Phila Pa 1976) (2018) 43:41–8. doi: 10.1097/brs.0000000000001586 [DOI] [PubMed] [Google Scholar]
- 66. Lewin R, Göransson M, Elander A, Thorarinsson A, Lundberg J, Lidén M. Risk Factors for Complications After Breast Reduction Surgery. J Plast Surg Handb Surg (2014) 48:10–4. doi: 10.3109/2000656x.2013.791625 [DOI] [PubMed] [Google Scholar]
- 67. Li Y, Tan B, Fan L, Zhao Q, Tan M, Wang D, et al. Clinicopathologic Characteristics of Elderly With Gastric Cancer, and the Risk Factors of Postoperative Complications. J Invest Surg (2017) 30:394–400. doi: 10.1080/08941939.2016.1265617 [DOI] [PubMed] [Google Scholar]
- 68. Lindqvist EK, Laine E, Kamali A, Sars C, Gillgren P, Schultz I. Risk Factors for Post-Operative Complications After Sentinel Lymph Node Biopsy for Cutaneous Melanoma: Results From a Large Cohort Study. J Plast Reconstr Aesthet Surg (2019) 72:1956–62. doi: 10.1016/j.bjps.2019.08.011 [DOI] [PubMed] [Google Scholar]
- 69. Lopez Ramos C, Brandel MG, Rennert RC, Wali AR, Steinberg JA, Santiago-Dieppa DR, et al. Clinical Risk Factors and Postoperative Complications Associated With Unplanned Hospital Readmissions After Cranial Neurosurgery. World Neurosurg (2018) 119:e294–300. doi: 10.1016/j.wneu.2018.07.136 [DOI] [PubMed] [Google Scholar]
- 70. Louie PK, Schairer WW, Haughom BD, Bell JA, Campbell KJ, Levine BR. Involvement of Residents Does Not Increase Postoperative Complications After Open Reduction Internal Fixation of Ankle Fractures: An Analysis of 3251 Cases. J Foot Ankle Surg (2017) 56:492–6. doi: 10.1053/j.jfas.2017.01.020 [DOI] [PubMed] [Google Scholar]
- 71. Lv C, Zhang Y, Chen X, Huang X, Xue M, Sun Q, et al. New-Onset Diabetes After Liver Transplantation and its Impact on Complications and Patient Survival. J Diabetes (2015) 7:881–90. doi: 10.1111/1753-0407.12275 [DOI] [PubMed] [Google Scholar]
- 72. Ma BW, Chen XY, Fan SD, Zhang FM, Huang DD, Li B, et al. Impact of Sarcopenia on Clinical Outcomes After Radical Gastrectomy for Patients Without Nutritional Risk. Nutrition (2019) 61:61–6. doi: 10.1016/j.nut.2018.10.025 [DOI] [PubMed] [Google Scholar]
- 73. Maradit Kremers H, Lewallen LW, Mabry TM, Berry DJ, Berbari EF, Osmon DR. Diabetes Mellitus, Hyperglycemia, Hemoglobin A1C and the Risk of Prosthetic Joint Infections in Total Hip and Knee Arthroplasty. J Arthroplasty (2015) 30:439–43. doi: 10.1016/j.arth.2014.10.009 [DOI] [PubMed] [Google Scholar]
- 74. Matsuda K, Hotta T, Takifuji K, Yokoyama S, Higashiguchi T, Tominaga T, et al. Long-Term Comorbidity of Diabetes Mellitus is a Risk Factor for Perineal Wound Complications After an Abdominoperineal Resection. Langenbecks Arch Surg (2009) 394:65–70. doi: 10.1007/s00423-008-0381-8 [DOI] [PubMed] [Google Scholar]
- 75. McElvany MD, Chan PH, Prentice HA, Paxton EW, Dillon MT, Navarro RA. Diabetes Disease Severity Was Not Associated With Risk of Deep Infection or Revision After Shoulder Arthroplasty. Clin Orthop Relat Res (2019) 477:1358–69. doi: 10.1097/corr.0000000000000642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Meding JB, Reddleman K, Keating ME, Klay A, Ritter MA, Faris PM, et al. Total Knee Replacement in Patients With Diabetes Mellitus. Clin Orthop Relat Res (2003) 416:208–16. doi: 10.1097/01.blo.0000093002.90435.56 [DOI] [PubMed] [Google Scholar]
- 77. Menenakos E, Stamou KM, Albanopoulos K, Papailiou J, Theodorou D, Leandros E. Laparoscopic Sleeve Gastrectomy Performed With Intent to Treat Morbid Obesity: A Prospective Single-Center Study of 261 Patients With a Median Follow-Up of 1 Year. Obes Surg (2010) 20:276–82. doi: 10.1007/s11695-009-9918-3 [DOI] [PubMed] [Google Scholar]
- 78. Michalak SM, Rolston JD, Lawton MT. Incidence and Predictors of Complications and Mortality in Cerebrovascular Surgery: National Trends From 2007 to 2012. Neurosurgery (2016) 79:182–93. doi: 10.1227/neu.0000000000001251 [DOI] [PubMed] [Google Scholar]
- 79. Moon HK, Han CD, Yang IH, Cha BS. Factors Affecting Outcome After Total Knee Arthroplasty in Patients With Diabetes Mellitus. Yonsei Med J (2008) 49:129–37. doi: 10.3349/ymj.2008.49.1.129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Moon RC, Ghanem M, Teixeira AF, de la Cruz-Munoz N, Young MK, Domkowski P, et al. Assessing Risk Factors, Presentation, and Management of Portomesenteric Vein Thrombosis After Sleeve Gastrectomy: A Multicenter Case-Control Study. Surg Obes Relat Dis (2018) 14:478–83. doi: 10.1016/j.soard.2017.10.013 [DOI] [PubMed] [Google Scholar]
- 81. Morgan DJ, Ho KM, Armstrong J, Baker S. Incidence and Risk Factors for Intensive Care Unit Admission After Bariatric Surgery: A Multicentre Population-Based Cohort Study. Br J Anaesth (2015) 115:873–82. doi: 10.1093/bja/aev364 [DOI] [PubMed] [Google Scholar]
- 82. Nair S, Vanatta JM, Arteh J, Eason JD. Effects of Obesity, Diabetes, and Prior Abdominal Surgery on Resource Utilization in Liver Transplantation: A Single-Center Study. Liver Transpl (2009) 15:1519–24. doi: 10.1002/lt.21889 [DOI] [PubMed] [Google Scholar]
- 83. Newman ET, Watters TS, Lewis JS, Jennings JM, Wellman SS, Attarian DE, et al. Impact of Perioperative Allogeneic and Autologous Blood Transfusion on Acute Wound Infection Following Total Knee and Total Hip Arthroplasty. J Bone Joint Surg Am (2014) 96:279–84. doi: 10.2106/jbjs.l.01041 [DOI] [PubMed] [Google Scholar]
- 84. Nguyen JMV, Sadeghi M, Gien LT, Covens A, Kupets R, Nathens AB, et al. Impact of a Preventive Bundle to Reduce Surgical Site Infections in Gynecologic Oncology. Gynecol Oncol (2019) 152:480–5. doi: 10.1016/j.ygyno.2018.09.008 [DOI] [PubMed] [Google Scholar]
- 85. Nguyen L, Gupta V, Afshari A, Shack RB, Grotting JC, Higdon KK. Incidence and Risk Factors of Major Complications in Brachioplasty: Analysis of 2,294 Patients. Aesthet Surg J (2016) 36:792–803. doi: 10.1093/asj/sjv267 [DOI] [PubMed] [Google Scholar]
- 86. Okamura A, Watanabe M, Imamura Y, Kamiya S, Yamashita K, Kurogochi T, et al. Preoperative Glycosylated Hemoglobin Levels Predict Anastomotic Leak After Esophagectomy With Cervical Esophagogastric Anastomosis. World J Surg (2017) 41:200–7. doi: 10.1007/s00268-016-3763-z [DOI] [PubMed] [Google Scholar]
- 87. Palmerola R, Hartman C, Theckumparampil N, Mukkamala A, Fishbein J, Schwartz M, et al. Surgical Complications and Their Repercussions. J Endourol (2016) 30 Suppl 1:S2–7. doi: 10.1089/end.2015.0705 [DOI] [PubMed] [Google Scholar]
- 88. Park MS, Ju YS, Moon SH, Kim TH, Oh JK, Makhni MC, et al. Reoperation Rates After Anterior Cervical Discectomy and Fusion for Cervical Spondylotic Radiculopathy and Myelopathy: A National Population-Based Study. Spine (Phila Pa 1976) (2016) 41:1593–9. doi: 10.1097/brs.0000000000001590 [DOI] [PubMed] [Google Scholar]
- 89. Patton D, Kiewiet N, Brage M. Infected Total Ankle Arthroplasty: Risk Factors and Treatment Options. Foot Ankle Int (2015) 36:626–34. doi: 10.1177/1071100714568869 [DOI] [PubMed] [Google Scholar]
- 90. Pearse RM, Moreno RP, Bauer P, Pelosi P, Metnitz P, Spies C, et al. Mortality After Surgery in Europe: A 7 Day Cohort Study. Lancet (2012) 380:1059–65. doi: 10.1016/S0140-6736(12)61148-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Plano X, Ramírez M, Matamalas A, Haddad S, García de Frutos A, Casamitjana JM, et al. 30-Day Unplanned Surgery in Cervical Spondylotic Myelopathy Surgically Treated: A Single-Center Experience. Eur Spine J (2019) 28:1209–16. doi: 10.1007/s00586-019-05892-8 [DOI] [PubMed] [Google Scholar]
- 92. Ponce BA, Menendez ME, Oladeji LO, Soldado F. Diabetes as a Risk Factor for Poorer Early Postoperative Outcomes After Shoulder Arthroplasty. J Shoulder Elbow Surg (2014) 23:671–8. doi: 10.1016/j.jse.2014.01.046 [DOI] [PubMed] [Google Scholar]
- 93. Pugely AJ, Martin CT, Gao Y, Mendoza-Lattes SA. Outpatient Surgery Reduces Short-Term Complications in Lumbar Discectomy: An Analysis of 4310 Patients From the ACS-NSQIP Database. Spine (Phila Pa 1976) (2013) 38:264–71. doi: 10.1097/BRS.0b013e3182697b57 [DOI] [PubMed] [Google Scholar]
- 94. Qin C, Vaca E, Lovecchio F, Ver Halen JP, Hansen NM, Kim JY. Differential Impact of Non-Insulin-Dependent Diabetes Mellitus and Insulin-Dependent Diabetes Mellitus on Breast Reconstruction Outcomes. Breast Cancer Res Treat (2014) 146:429–38. doi: 10.1007/s10549-014-3024-5 [DOI] [PubMed] [Google Scholar]
- 95. Raikin SM, Kane J, Ciminiello ME. Risk Factors for Incision-Healing Complications Following Total Ankle Arthroplasty. J Bone Joint Surg Am (2010) 92:2150–5. doi: 10.2106/jbjs.i.00870 [DOI] [PubMed] [Google Scholar]
- 96. Rao AJ, Yeatts NC, Reid RT, Trofa DP, Scarola G, Schiffern SC, et al. Is Postoperative Glucose Variability Associated With Adverse Outcomes Following Shoulder Arthroplasty? J Shoulder Elbow Surg (2020) 30(3):616–24. doi: 10.1016/j.jse.2020.07.005 [DOI] [PubMed] [Google Scholar]
- 97. Rensing N, Waterman BR, Frank RM, Heida KA, Orr JD. Low Risk for Local and Systemic Complications After Primary Repair of 1626 Achilles Tendon Ruptures. Foot Ankle Spec (2017) 10:216–26. doi: 10.1177/1938640016676340 [DOI] [PubMed] [Google Scholar]
- 98. Roche AM, Brant JA, Chai RL. Predictors of Readmission and Reoperation in Patients Undergoing Parathyroidectomy for Primary Hyperparathyroidism. Otolaryngol Head Neck Surg (2018) 158:828–34. doi: 10.1177/0194599818758019 [DOI] [PubMed] [Google Scholar]
- 99. Rubel NC, Chung AS, Wong M, Lara NJ, Makovicka JL, Arvind V, et al. 90-Day Readmission in Elective Primary Lumbar Spine Surgery in the Inpatient Setting: A Nationwide Readmissions Database Sample Analysis. Spine (Phila Pa 1976) (2019) 44:E857–64. doi: 10.1097/brs.0000000000002995 [DOI] [PubMed] [Google Scholar]
- 100. Sakai A, Okami K, Sugimoto R, Ebisumoto K, Yamamoto H, Furuya H, et al. Multivariate Analysis of Wound Complications After Surgery for Laryngeal and Hypopharyngeal Cancers. ORL J Otorhinolaryngol Relat Spec (2011) 73:100–4. doi: 10.1159/000323832 [DOI] [PubMed] [Google Scholar]
- 101. Sanni A, Perez S, Medbery R, Urrego HD, McCready C, Toro JP, et al. Postoperative Complications in Bariatric Surgery Using Age and BMI Stratification: A Study Using ACS-NSQIP Data. Surg Endosc (2014) 28:3302–9. doi: 10.1007/s00464-014-3606-7 [DOI] [PubMed] [Google Scholar]
- 102. Schemitsch LA, Schemitsch EH, Kuzyk P, McKee MD. Prognostic Factors for Reoperation After Plate Fixation of the Midshaft Clavicle. J Orthop Trauma (2015) 29:533–7. doi: 10.1097/bot.0000000000000331 [DOI] [PubMed] [Google Scholar]
- 103. Schimmel JJ, Horsting PP, de Kleuver M, Wonders G, van Limbeek J. Risk Factors for Deep Surgical Site Infections After Spinal Fusion. Eur Spine J (2010) 19:1711–9. doi: 10.1007/s00586-010-1421-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Schipper ON, Jiang JJ, Chen L, Koh J, Toolan BC. Effect of Diabetes Mellitus on Perioperative Complications and Hospital Outcomes After Ankle Arthrodesis and Total Ankle Arthroplasty. Foot Ankle Int (2015) 36:258–67. doi: 10.1177/1071100714555569 [DOI] [PubMed] [Google Scholar]
- 105. Schlottmann F, Strassle PD, Patti MG. Transhiatal vs. Transthoracic Esophagectomy: A NSQIP Analysis of Postoperative Outcomes and Risk Factors for Morbidity. J Gastrointest Surg (2017) 21:1757–63. doi: 10.1007/s11605-017-3572-1 [DOI] [PubMed] [Google Scholar]
- 106. Schürner AM, Manzini G, Bueter M, Schadde E, Beck-Schimmer B, Schläpfer M. Perioperative Surgery- and Anaesthesia-Related Risks of Laparoscopic Roux-En-Y Gastric Bypass - a Single Centre, Retrospective Data Analysis. BMC Anesthesiol (2018) 18:190. doi: 10.1186/s12871-018-0654-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Shah KN, Defroda SF, Wang B, Weiss AC. Risk Factors for 30-Day Complications After Thumb CMC Joint Arthroplasty: An American College of Surgeons National Surgery Quality Improvement Program Study. Handb (N Y) (2019) 14:357–63. doi: 10.1177/1558944717744341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Shigeishi H, Ohta K, Takechi M. Risk Factors for Postoperative Complications Following Oral Surgery. J Appl Oral Sci (2015) 23:419–23. doi: 10.1590/1678-775720150130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Shimada M, Matsumata T, Akazawa K, Kamakura T, Itasaka H, Sugimachi K, et al. Estimation of Risk of Major Complications After Hepatic Resection. Am J Surg (1994) 167:399–403. doi: 10.1016/0002-9610(94)90124-4 [DOI] [PubMed] [Google Scholar]
- 110. Smith EJ, Kuang X, Pandarinath R. Comparing Hospital Outcomes Between Open and Closed Tibia Fractures Treated With Intramedullary Fixation. Injury (2017) 48:1609–12. doi: 10.1016/j.injury.2017.03.038 [DOI] [PubMed] [Google Scholar]
- 111. Söderbäck H, Gunnarsson U, Martling A, Hellman P, Sandblom G. Incidence of Wound Dehiscence After Colorectal Cancer Surgery: Results From a National Population-Based Register for Colorectal Cancer. Int J Colorectal Dis (2019) 34:1757–62. doi: 10.1007/s00384-019-03390-3 [DOI] [PubMed] [Google Scholar]
- 112. Sood A, Abdollah F, Sammon JD, Kapoor V, Rogers CG, Jeong W, et al. An Evaluation of the Timing of Surgical Complications Following Nephrectomy: Data From the American College of Surgeons National Surgical Quality Improvement Program (ACS-NSQIP). World J Urol (2015) 33:2031–8. doi: 10.1007/s00345-015-1564-x [DOI] [PubMed] [Google Scholar]
- 113. Sood A, Kachroo N, Abdollah F, Sammon JD, Löppenberg B, Jindal T, et al. An Evaluation of the Timing of Surgical Complications Following Radical Cystectomy: Data From the American College of Surgeons National Surgical Quality Improvement Program. Urology (2017) 103:91–8. doi: 10.1016/j.urology.2017.01.036 [DOI] [PubMed] [Google Scholar]
- 114. Sørensen LT, Hørby J, Friis E, Pilsgaard B, Jørgensen T. Smoking as a Risk Factor for Wound Healing and Infection in Breast Cancer Surgery. Eur J Surg Oncol (2002) 28:815–20. doi: 10.1053/ejso.2002.1308 [DOI] [PubMed] [Google Scholar]
- 115. Souza MV, Barth AL, Alvares-da-Silva MR, Machado AR. Infections After Liver Transplantation in Adults: Data From a University Hospital in Southern Brazil (1996-2000). Arq Gastroenterol (2007) 44:128–32. doi: 10.1590/s0004-28032007000200008 [DOI] [PubMed] [Google Scholar]
- 116. Spinazzi EF, Pines MJ, Fang CH, Raikundalia MD, Baredes S, Liu JK, et al. Impact and Cost of Care of Venous Thromboembolism Following Pituitary Surgery. Laryngoscope (2015) 125:1563–7. doi: 10.1002/lary.25161 [DOI] [PubMed] [Google Scholar]
- 117. Stein JD, Grossman DS, Mundy KM, Sugar A, Sloan FA. Severe Adverse Events After Cataract Surgery Among Medicare Beneficiaries. Ophthalmology (2011) 118:1716–23. doi: 10.1016/j.ophtha.2011.02.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Suda AJ, Richter A, Abou-Nouar G, Jazzazi M, Tinelli M, Bischel OE. Arthrodesis for Septic Arthritis of the Ankle: Risk Factors and Complications. Arch Orthop Trauma Surg (2016) 136:1343–8. doi: 10.1007/s00402-016-2520-y [DOI] [PubMed] [Google Scholar]
- 119. Takao T, Tsujimura A, Kiuchi H, Komori K, Fujita K, Miyagawa Y, et al. Urological Surgery in Patients Aged 80 Years and Older: A 30-Year Retrospective Clinical Study. Int J Urol (2008) 15:789–93. doi: 10.1111/j.1442-2042.2008.02110.x [DOI] [PubMed] [Google Scholar]
- 120. Tang H, Zhu J, Ji F, Wang S, Xie Y, Fei H. Risk Factors for Postoperative Complication After Spinal Fusion and Instrumentation in Degenerative Lumbar Scoliosis Patients. J Orthop Surg Res (2014) 9:15. doi: 10.1186/1749-799x-9-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Terho PM, Leppäniemi AK, Mentula PJ. Laparoscopic Cholecystectomy for Acute Calculous Cholecystitis: A Retrospective Study Assessing Risk Factors for Conversion and Complications. World J Emerg Surg (2016) 11:54. doi: 10.1186/s13017-016-0111-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Tetreault L, Tan G, Kopjar B, Côté P, Arnold P, Nugaeva N, et al. Clinical and Surgical Predictors of Complications Following Surgery for the Treatment of Cervical Spondylotic Myelopathy: Results From the Multicenter, Prospective AOSpine International Study of 479 Patients. Neurosurgery (2016) 79:33–44. doi: 10.1227/neu.0000000000001151 [DOI] [PubMed] [Google Scholar]
- 123. Timmermans FW, Westland PB, Hummelink S, Schreurs J, Hameeteman M, Ulrich DJO, et al. A Retrospective Investigation of Abdominal Visceral Fat, Body Mass Index (BMI), and Active Smoking as Risk Factors for Donor Site Wound Healing Complications After Free DIEP Flap Breast Reconstructions. J Plast Reconstr Aesthet Surg (2018) 71:827–32. doi: 10.1016/j.bjps.2018.03.003 [DOI] [PubMed] [Google Scholar]
- 124. Toboni MD, Smith HJ, Bae S, Straughn JM, Jr., Leath CA, 3rd. Predictors of Unplanned Reoperation for Ovarian Cancer Patients From the National Surgical Quality Improvement Program Database. Int J Gynecol Cancer (2018) 28:1427–31. doi: 10.1097/igc.0000000000001315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Tokgöz H, Akduman B, Ünal İ, Erol B, Akyürek E, Mungan NA. Chronic Pulmonary Diseases are Independent Risk Factors for Complications After Radical Nephrectomy. Int Urol Nephrol (2011) 43:1025–31. doi: 10.1007/s11255-011-9957-2 [DOI] [PubMed] [Google Scholar]
- 126. Venara A, Hubner M, Le Naoures P, Hamel JF, Hamy A, Demartines N. Surgery for Incarcerated Hernia: Short-Term Outcome With or Without Mesh. Langenbecks Arch Surg (2014) 399:571–7. doi: 10.1007/s00423-014-1202-x [DOI] [PubMed] [Google Scholar]
- 127. Wadhwa RK, Ohya J, Vogel TD, Carreon LY, Asher AL, Knightly JJ, et al. Risk Factors for 30-Day Reoperation and 3-Month Readmission: Analysis From the Quality and Outcomes Database Lumbar Spine Registry. J Neurosurg Spine (2017) 27:131–6. doi: 10.3171/2016.12.spine16714 [DOI] [PubMed] [Google Scholar]
- 128. Wang C, Fu G, Ji F, Cheng S, Liu Z, Cao M. Perioperative Risk Factors for Radial Forearm-Free Flap Complications. J Craniofac Surg (2020) 31:381–4. doi: 10.1097/scs.0000000000006035 [DOI] [PubMed] [Google Scholar]
- 129. Wang JB, Zheng CH, Li P, Xie JW, Lin JX, Lu J, et al. Effect of Comorbidities on Postoperative Complications in Patients With Gastric Cancer After Laparoscopy-Assisted Total Gastrectomy: Results From an 8-Year Experience at a Large-Scale Single Center. Surg Endosc (2017) 31:2651–60. doi: 10.1007/s00464-016-5279-x [DOI] [PubMed] [Google Scholar]
- 130. Webb ML, Golinvaux NS, Ibe IK, Bovonratwet P, Ellman MS, Grauer JN. Comparison of Perioperative Adverse Event Rates After Total Knee Arthroplasty in Patients With Diabetes: Insulin Dependence Makes a Difference. J Arthroplasty (2017) 32:2947–51. doi: 10.1016/j.arth.2017.04.032 [DOI] [PubMed] [Google Scholar]
- 131. Weir S, Kuo TC, Samnaliev M, Tierney TS, Manca A, Taylor RS, et al. Reoperation Following Lumbar Spinal Surgery: Costs and Outcomes in a UK Population Cohort Study Using the Clinical Practice Research Datalink (CPRD) and Hospital Episode Statistics (HES). Eur Spine J (2019) 28:863–71. doi: 10.1007/s00586-018-05871-5 [DOI] [PubMed] [Google Scholar]
- 132. Winocour J, Gupta V, Kaoutzanis C, Shi H, Shack RB, Grotting JC, et al. Venous Thromboembolism in the Cosmetic Patient: Analysis of 129,007 Patients. Aesthet Surg J (2017) 37:337–49. doi: 10.1093/asj/sjw173 [DOI] [PubMed] [Google Scholar]
- 133. Wukich DK, Lowery NJ, McMillen RL, Frykberg RG. Postoperative Infection Rates in Foot and Ankle Surgery: A Comparison of Patients With and Without Diabetes Mellitus. J Bone Joint Surg Am (2010) 92:287–95. doi: 10.2106/jbjs.i.00080 [DOI] [PubMed] [Google Scholar]
- 134. Yamauchi Y, Isaka M, Maniwa T, Takahashi S, Kurai H, Ohde Y. Chest Tube Tip Culture as a Predictor of Postoperative Infection in Lung Cancer Operations. Ann Thorac Surg (2013) 96:1796–802. doi: 10.1016/j.athoracsur.2013.06.043 [DOI] [PubMed] [Google Scholar]
- 135. Zanaty M, Chalouhi N, Starke RM, Clark SW, Bovenzi CD, Saigh M, et al. Complications Following Cranioplasty: Incidence and Predictors in 348 Cases. J Neurosurg (2015) 123:182–8. doi: 10.3171/2014.9.jns14405 [DOI] [PubMed] [Google Scholar]
- 136. Zhang JF, Zhu HY, Sun YW, Liu W, Huo YM, Liu DJ, et al. Pseudomonas Aeruginosa Infection After Pancreatoduodenectomy: Risk Factors and Clinic Impacts. Surg Infect (Larchmt) (2015) 16:769–74. doi: 10.1089/sur.2015.041 [DOI] [PubMed] [Google Scholar]
- 137. Zhou J, Zhou Y, Cao S, Li S, Wang H, Niu Z, et al. Multivariate Logistic Regression Analysis of Postoperative Complications and Risk Model Establishment of Gastrectomy for Gastric Cancer: A Single-Center Cohort Report. Scand J Gastroenterol (2016) 51:8–15. doi: 10.3109/00365521.2015.1063153 [DOI] [PubMed] [Google Scholar]
- 138. Duwayri Y, Jordan WD, Jr. Diabetes, Dysglycemia, and Vascular Surgery. J Vasc Surg (2020) 71:701–11. doi: 10.1016/j.jvs.2019.05.027 [DOI] [PubMed] [Google Scholar]
- 139. Mills JL, Conte MS, Armstrong DG, Pomposelli FB, Schanzer A, Sidawy AN, et al. The Society for Vascular Surgery Lower Extremity Threatened Limb Classification System: Risk Stratification Based on Wound, Ischemia, and Foot Infection (WIfI). J Vasc Surg (2014) 59:220–34.e1-2. doi: 10.1016/j.jvs.2013.08.003 [DOI] [PubMed] [Google Scholar]
- 140. Gortler H, Rusyn J, Godbout C, Chahal J, Schemitsch EH, Nauth A. Diabetes and Healing Outcomes in Lower Extremity Fractures: A Systematic Review. Injury (2018) 49:177–83. doi: 10.1016/j.injury.2017.11.006 [DOI] [PubMed] [Google Scholar]
- 141. Vestergaard P, Rejnmark L, Mosekilde L. Relative Fracture Risk in Patients With Diabetes Mellitus, and the Impact of Insulin and Oral Antidiabetic Medication on Relative Fracture Risk. Diabetologia (2005) 48:1292–9. doi: 10.1007/s00125-005-1786-3 [DOI] [PubMed] [Google Scholar]
- 142. Bibbo C, Lin SS, Beam HA, Behrens FF. Complications of Ankle Fractures in Diabetic Patients. Orthop Clin North Am (2001) 32:113–33. doi: 10.1016/s0030-5898(05)70198-x [DOI] [PubMed] [Google Scholar]
- 143. Falanga V. Wound Healing and its Impairment in the Diabetic Foot. Lancet (2005) 366:1736–43. doi: 10.1016/s0140-6736(05)67700-8 [DOI] [PubMed] [Google Scholar]
- 144. Brem H, Tomic-Canic M. Cellular and Molecular Basis of Wound Healing in Diabetes. J Clin Invest (2007) 117:1219–22. doi: 10.1172/jci32169 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The original contributions presented in the study are included in the article/ Supplementary Material . Further inquiries can be directed to the corresponding authors.