Abstract
Background
Cancer comprises a high burden on health systems. Performance indicators monitoring cancer outcomes are routinely used in OECD countries. However, the development of process and cancer-pathway based information is essential to guide health care delivery, allowing for better monitoring of changes in the quality of care provided. Assessing the changes in the quality of cancer care during the COVID-19 pandemic requires a structured approach considering the high volume of publications. This study aims to summarize performance indicators used in the literature to evaluate the impact of the COVID-19 pandemic on cancer care (January-June 2020) in OECD countries and to assess changes in the quality of care as reported via selected indicators.
Methods
Search conducted in MEDLINE and Embase databases. Performance indicators and their trends were collated according to the cancer care pathway.
Results
This study included 135 articles, from which 1013 indicators were retrieved. Indicators assessing the diagnostic process showed a decreasing trend: from 33 indicators reporting on screening, 30 (91%) signalled a decrease during the pandemic (n = 30 indicators, 91%). A reduction was also observed in the number of diagnostic procedures (n = 64, 58%) and diagnoses (n = 130, 89%). The proportion of diagnoses in the emergency setting and waiting times showed increasing trends (n = 8, 89% and n = 14, 56%, respectively). A decreasing trend in the proportion of earliest stage cancers was reported by 63% of indicators (n = 9), and 70% (n = 43) of indicators showed an increasing trend in the proportion of advanced-stage cancers. Indicators reflecting the treatment process signalled a reduction in the number of procedures: 79%(n = 82) of indicators concerning surgeries, 72%(n = 41) of indicators assessing radiotherapy, and 93%(n = 40) of indicators related to systemic therapies. Modifications in cancer treatment were frequently reported: 64%(n = 195) of indicators revealed changes in treatment.
Conclusions
This study provides a summary of performance indicators used in the literature to assess the cancer care pathway from January 2020 to June 2020 in OECD countries, and the changes in the quality of care signalled by these indicators. The trends reported inform on potential bottlenecks of the cancer care pathway. Monitoring this information closely could contribute to identifying moments for intervention during crises.
Supplementary information
The online version contains supplementary material available at 10.1186/s12913-022-08166-0.
Keywords: Quality of Health Care; Quality Indicators, Health Care [MeSH]; Continuity of Patient Care; Cancer; COVID-19
Background
The COVID-19 outbreak was declared a public health emergency of international concern by the World Health Organization on the 30th of January, 2020 [1]. Since the beginning of the pandemic, health systems have struggled to cope with the high numbers of people infected with SARS-CoV-2, while maintaining adequate care for people with other conditions [2].
Cancer is the second cause of death in Organisation for Economic Co-operation and Development (OECD) countries [3], comprising a high burden on OECD’s health systems. The COVID-19 pandemic has widely affected cancer care delivery in these countries. Substantial declines in the number of cancer diagnoses have been reported in the Netherlands [4–6], Spain [7], Belgium [8], and Denmark [9]. While trying to minimize the risk of COVID-19 disease in cancer patients, changes in practice were pursued by oncologists, according to each setting’s capacity and recommendations released by oncology societies [10–12]. Changes in the treatments prescribed were also reported [13].
Improving cancer care was already on the international health agenda before this pandemic, namely in the 2030 Agenda for Sustainable Development adopted in 2015 at the United Nations Sustainable Development Summit [14], in the Resolution “Cancer prevention and control in the context of an integrated approach” approved in 2017 by the World Health Assembly [15]. At the same time, systematic efforts to capture the outcomes of cancer care globally are in place such as the CONCORD study [16, 17] (via cancer registries), OECD reports on cancer care as part of its Health at a Glance series [3] and several European Union (joint) actions [18]. During the COVID-19 pandemic, efforts continued to be pursued to improve prevention, diagnostics, treatment, and the quality of life of cancer patients and to monitor and report upon inequalities, such as the “Europe's Beating Cancer Plan” [19], the European Cancer Inequalities Registry [20] and the launch of the European Commission’s Knowledge Centre on Cancer [21]. These efforts are necessary to address the backlog this pandemic is creating [22–24].
Healthcare performance measurement is key to evaluate and improve healthcare systems, informing policy decisions and quality of care improvement initiatives [25]. Healthcare quality indicators are “quantitative measures that provide information about the effectiveness, safety and/or people-centeredness of care” [26]. The development of performance indicators following fit for purpose and use considerations [27] underpinned by robust health information systems is key to monitoring changes in care quality during crises, allowing for comparisons within and between countries’ health systems [28]. A common conceptual framework for health system performance measurement developed by the OECD aims to help member countries to prioritize areas to improve quality of care [29]. Indicators monitoring cancer patients’ outcomes, like 5-year survival, are already routinely used in OECD countries [3]. However, although improving outcomes is the ultimate aim, for guiding health care delivery systems towards that goal, process and cancer-pathway based information is essential. Therefore, indicators should ideally inform on the whole pathway of care, which is a reality in a few OECD countries [30]. Additionally, only a small percentage of scientific literature assessed in a previous review focused on hospital performance indicators [31] considered a clinical pathway perspective.
In this study, we focus on the cancer care pathway in OECD countries, which share a common conceptual framework for health system performance measurement [29]. Additionally, considering that the literature published on the consequences of the pandemic on cancer care is vast, assessing its impact requires a structured approach. Thus, this study aims to: 1) provide a structured summary of cancer care performance indicators used in the literature, regarding various cancers, across the care pathway, from early detection to outcomes, within OECD member countries; 2) assess the main trends of the changes in the quality of cancer care during the first wave of the COVID-19 pandemic, from January to June 2020, in these countries.
Methods
We conducted a scoping review, following Arksey and O’Malley methodological framework [32], further developed by Levac et al. [33]. Given the heterogeneous methods across countries for data collection on health care system performance and their translation to indicators [28, 34], a scoping review methodology allows to map large sums and heterogeneity of literature available [33, 35, 36], namely cancer care performance indicators. It also enables the reporting of emerging evidence [35], to summarize, and communicate findings [32], including trends revealed by indicators. The PRISMA extension for scoping reviews [37] was used for reporting (Additional file 1).
Eligibility criteria of studies
We considered the following inclusion criteria: 1) studies using empirical data on the use of health services in OECD countries, 2) studies that described health outcomes and/or performance indicators related to NCDs during the COVID-19 pandemic, 3) original journal articles using quantitative or qualitative methods (cohort studies, case–control, cross-sectional, case reports, systematic reviews, surveys, and meta-analyses). Studies were excluded if they did not provide empirical data on health services and NCDs, namely: 1) editorials and commentaries, 2) prediction models, 3) clinical case reports; 4) diseases management or health services organization guidelines, 5) studies about the impact on healthcare workers, patients diagnosed with COVID-19, children, or pregnant women; 6) studies primarily performed in non-OECD countries. No limitations were set regarding language or year of publication.
Data sources and search strategy
MEDLINE and Embase databases were selected to search for this study, given the large number of articles and their comprehensive coverage of the literature [38]. Pilot searches were conducted to identify a list of relevant search terms. An experienced medical information specialist was consulted to improve the search strategy, which was refined with discussion among co-authors. The comprehensive search included search terms grouped by key concepts (COVID-19, pandemic, non-communicable disease, chronic disease, performance indicator, healthcare quality, healthcare utilization, healthcare delivery and other closely related terms). The search was adapted to both databases and conducted by the information specialist on 17–03-2021. The full search strategy for Embase can be found in Additional file 2. Duplicates were removed using EndNote software. Additional articles of relevance were added by hand-searching the reference lists of the included studies.
Study selection
Title and abstract screening was performed independently by two researchers (ASC, OBF) using Rayyan [39]. Studies considered relevant were exported to a spreadsheet to support full-text screening. For this study, only articles related to cancer care were analysed. Full-text screening was performed independently by two researchers (ASC, MdL). The reason for the exclusion of articles was recorded at this point, and both researchers agreed on the excluded studies. In case of doubt, the other co-authors were consulted.
Data collection
Data extracted were collated in a spreadsheet (Additional file 3) piloted on 15 studies. Before extracting data from all studies, two researchers (ASC, MdL) compared data collected from a sample of 10 selected articles to enhance data extraction consistency among researchers. Then, data was charted independently by ASC and MdL. Extracted data included information on generic and methodological aspects of the article (e.g., authors, title, setting) and information about the indicators collected (e.g., indicator title and data inclusion/exclusion considerations). We identified the trend reported in the articles (increase/decrease/stable) for every indicator, if any.
Synthesis of results
The indicators collected that measured similar clinical procedures, identical patients’ characteristics, or similar outcomes were grouped together. Since there was some heterogeneity in indicators’ titles referring to identical measures, a common indicator title was given by the authors to each category of similar indicators (e.g.: “Number of screening procedures”), using the language from the studies as much as possible. Then, these indicators’ categories were organized and reported according to the different phases of the cancer care pathway, namely: early detection, diagnosis, treatment, and outcomes. Quantitative indicators’ trends were collated, and the percentage of indicators reporting each trend (increase/decrease/stable) computed for each category. This evidence is presented in the text of this study, in a table and in a diagram informing about each phase of the cancer care pathway. Qualitative information extracted from surveys is presented in the text, and it was not considered to compute trends. In the category “changes in treatment” quantitative indicators and qualitative information were grouped and categorized according to clinical reasoning to present an overview of the modifications in cancer treatment reported.
Results
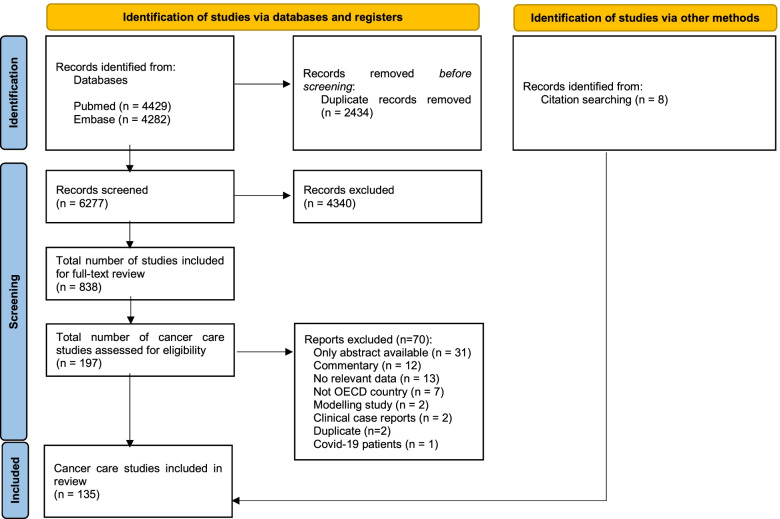
Database searches retrieved 6277 articles. Of these articles, 838 met the inclusion criteria, from which 197 articles on cancer care were identified. Eight records were identified via hand-searching. After full-text screening, 135 articles were included in this study (From: Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021;372:n71. doi: 10.1136/bmj.n71. http://www.prisma-statement.org/Fig. 1.)
Fig. 1.
PRISMA flow diagram of the literature search performed on 17th March, 2021. From: Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021;372:n71. doi: 10.1136/bmj.n71
General characteristics of the included articles
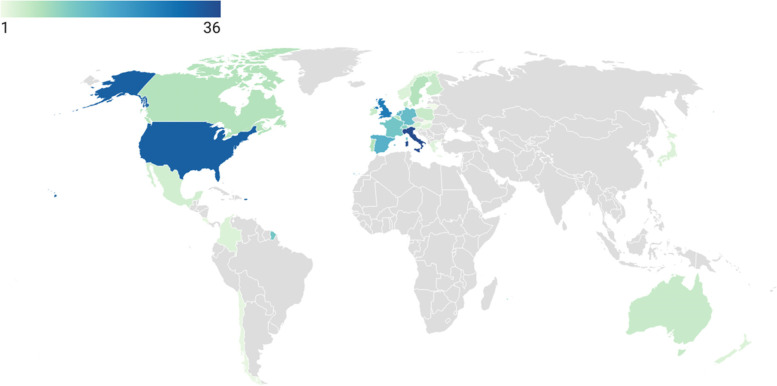
In total, 135 studies were included, reporting on 94 countries (Additional file 4). Of these studies, 26 (19%) provided information on multiple countries, from which 14 (10%) specified all the countries included. Of those, 8 (6%) included non-OECD countries. Most of the studies including more than one country were surveys (n = 23, 89%). The most frequent countries reported on were Italy (n = 36, 29%), US (n = 32, 26%) and UK (n = 27, 22%) (Fig. 2). Most articles used a retrospective cohort design (n = 82, 61%), followed by surveys (n = 44, 33%). Surveys were often directed to health professionals (n = 37/44, 84%) and to patients (n = 6/44, 14%) to a lesser extent. Other study designs that were applied included prospective cohorts (n = 5), observational retrospective cohorts (n = 3), and a combination of prospective and retrospective cohorts (n = 1). Studies reported indicators’ trends during the first phase of the COVID-19 pandemic (from January to June 2020) and, in some cases, after the (in many countries implemented) lockdown period (from May to October 2020). The magnitude of each indicator in 2020 was compared with its magnitude in the same period of 2019 (n = 51, 38%), to a period immediately before (n = 33, 24%) or to the average of the same period in previous years (ranging from 2017 to 2019) (n = 26, 19%).
Fig. 2.
OECD countries reported on, color-graded according to the number of papers (n = 122;90% of included articles)
Cancer care indicators
A total of 1013 quantitative indicators from 91 articles were retrieved and grouped into categories (Table 1).
Table 1.
Number of quantitative indicators retrieved, grouped in categories according to the cancer care pathway (n = 1013)
| Number of indicators with quantitative information a) | Number of studies | ||
|---|---|---|---|
| n | % | ||
| Early detection | 59 | 6% | |
| Number of screening procedures | 33 | 13 | |
| Early diagnosis and predisposition exams | 12 | 7 | |
| Screening detection rates | 14 | 5 | |
| Diagnosis and staging | 418 | 41% | |
| Delay in access to diagnostic procedures | 9 | 2 | |
| Clinical severity at diagnosis | 14 | 7 | |
| Changes in cancer staging | 110 | 21 | |
| Proportion of urgent/emergent referrals and procedures | 25 | 5 | |
| Number of diagnostic, surveillance, and staging exams/procedures | 90 | 17 | |
| Number of cancer diagnoses | 157 | 40 | |
| Cancer detection rate | 13 | 5 | |
| Treatment | 497 | 49% | |
| Delay in treatment | 42 | 18 | |
| Number of treatments | |||
| •Surgeries & loco-regional therapies | 104 | 30 | |
| • Radiotherapy | 57 | 8 | |
| • Systemic therapy | 71 | 12 | |
| Number of referrals / first encounters | 41 | 9 | |
| Outpatient volume | 47 | 14 | |
| Changes in treatment | 119 | 21 | |
| Number of visits and hospital admissions | 12 | 4 | |
| Telemedicine utilization | 4 | 4 | |
| Outcomes | 39 | 4% | |
| Surgical and procedures outcome measures | 15 | 8 | |
| Mortality | 24 | 4 | |
| 1013 | 100% | ||
a)A total of 338 indicators were not included in the analysis since they were too specific to be grouped into the defined categories
The first stage of the care pathway looks at early detection. Regarding the “number of screening and other early diagnosis procedures”, a total of 33 indicators from 13 articles [40–52] have been reported. Most indicators on the number of screening procedures signaled a decreasing trend (n = 30, 91%), namely in US, UK, and Italy. One international survey answered by physicians [53] (including Italy, Iran, Spain, UK, US, China, Denmark, Sweden, and Switzerland) reported the suspension of breast screening programs in all countries, except 2. Only one American paper [45] addressed cervical cancer screening, showing a decrease in number of screenings during stay-at-home order, compared with 2019. Three articles [42, 46, 49] addressed colorectal cancer screening, from US, UK and Spain and have also revealed a reduction when compared with previous year.
Seven papers [40, 41, 43–45, 47, 49] (12 indicators) reported on “early diagnosis and predisposition exams”, namely: screening visit for prostate cancer (US), gBRCA testing (Italy), human papillomavirus tests rate, low-dose computed tomography and prostate-specific antigen measurement (all from the US). All these indicators have shown a decreasing trend in the number of diagnostic procedures.
“Screening detection rates” were reported by 14 indicators from 5 articles [22, 43, 44, 54, 55], from the US and Italy. Most of the indicators (n = 9, 75%) showed an increase in screening detection rates. These included an increase in high-risk adenomas and colorectal cancer detection rates during the lockdown period, along with a decrease in low-risk adenoma detection rates in one Italian study [54].
The next stage of the care pathway focuses on diagnosis and staging. The “number of diagnostic, surveillance and staging procedures” was reported in 17 articles [48, 50–52, 55–67] (from the UK, Italy, US, France, Australia, the Netherlands, Turkey, Ireland, and Slovenia), comprising a total of 90 indicators. Most of those indicators (n = 58, 64%) showed a decreasing trend in the number of procedures, namely cystoscopy, diagnostic mammograms, breast cancer wire-guided biopsy, gastroscopy, colonoscopy, computed tomography (CT), magnetic resonance imaging (MRI), and endoscopic retrograde cholangiopancreatography.
A total of 147 indicators from 35 articles [9, 42, 46, 54, 55, 59, 61, 62, 66–90] reported on the “number of cancer diagnosis”. Most of these indicators (n = 130, 89%) signalled a decrease in the number of cancer diagnoses. Data came from registries in the Netherlands, Denmark, Germany, and Italy, cytology laboratories, tumor boards numbers, and administrative sources. One international survey to laboratories from 23 countries [73] showed an absolute reduction in the number of cytological samples regarding all anatomic sites.
Five studies [44, 62, 74, 82, 89] (from the US, UK, France, and Italy) addressed the number of cancer diagnoses after the lockdown period (10 indicators). Three of these indicators (30%) showed a higher number of diagnoses, when compared with the period of lockdown.
Five surveys [91–95] reported “delays concerning different aspects of cancer diagnostics”. One international survey [92] focusing on colorectal cancer care, with professionals from 84 countries, reported delays in radiologic exams and endoscopic procedures. Other surveys mentioned limited access to hospital facilities (Italy) [96], delays in tissue diagnosis (UK) [95], delays in diagnostics of patients with neuroendocrine tumors (Germany, Austria, Switzerland) [94], and genetic testing or counseling (US) [93]. The other two articles [50, 97] addressed the delays in access to diagnostics, comprising a total of 9 quantitative indicators, from which 8 (89%) signalled an increase in the waiting time to diagnostic procedures.
The “cancer detection rate in referrals and diagnostic exams” was assessed in 5 articles, from UK [66, 98], Italy [55], Ireland [50], and one international survey of 23 laboratories worldwide [73]. The 13 indicators collected signalled an increasing trend in cancer detection rate in 9 indicators (69%). The survey reported an increase of 5.5% in the malignancy rate in nongynecological samples, when compared with the corresponding period in 2019.
Twenty-five indicators from 5 studies [46, 64, 66, 99, 100] (from Spain, UK, Australia, and Croatia) reported on the proportion of “urgent/emergent referrals and procedures”. Most of these (n = 14, 56%) showed an increase in the proportion of urgent procedures (endoscopy and colonoscopies), diagnosis in emergency setting or operations that followed an emergency admission.
The indicators that reported on “changes in cancer staging” were grouped in 3 different categories: general staging indicators (14 indicators from 8 articles [82, 84, 88, 101–105]), proportion of earliest-stage cancers (35 indicators from 9 articles [48, 78, 88, 106–111]), and proportion of advanced-stage cancers (61 indicators from 17 articles [46, 64, 70, 73, 84, 86, 94, 99, 100, 102–109]).
Most of the general staging indicators (n = 9, 64%) showed stability in cancer stages distribution at diagnosis, when comparing the pre- and post-lockdown periods (data from Italy, US, UK, France, US, and Portugal). These indicators included stages of gynecological cancer, breast cancer, lung cancer, and hepatocellular carcinoma. With respect to the proportion of earliest stage cancers, most of the indicators (n = 9, 63%) showed a lower proportion of these cancers when compared with pre-pandemic period, 9 (26%) signalled a stable trend, and 4 (11%) showed an increasing trend. From the indicators reporting on the proportion of advanced-stage cancers, 43 (70%) showed an increasing trend in this proportion after the beginning of the pandemic, 14 (23%) signalled a stable proportion, and 4 (7%) reported a decreasing trend.
Fourteen indicators from 7 articles [48, 54, 64, 76, 104, 107, 112] evaluated the “clinical severity at diagnosis”, which included symptoms, scores, and biomarkers. The majority (n = 8 indicators, 57%) showed patients presenting in a more severe clinical condition than before the pandemic, namely in US (endometrial cancer), Portugal (hepatocellular carcinoma), Italy, and Turkey (colorectal cancer).
The following stage of the care pathway reported on is cancer treatment. A total of 41 indicators (from 9 articles [42, 67, 72, 79, 98, 103, 110, 113, 114]) reported on the “number of cancer patients’ referrals”. Most of the indicators (37, 90%) signalled a decrease in the number of first encounters for oncological examination, namely in Slovenia, UK, US, France, Spain, and the Netherlands. Four surveys (from UK [95], US, Italy [115] and one international study [116] reported a decrease in the number of new referrals.
A total of 47 indicators were identified from 14 articles [42, 49, 51, 59, 62, 67, 72, 97, 98, 100, 103, 113, 117, 118] regarding the outpatient volume of patients diagnosed with cancer. Of these indicators, 46 (93%) showed a decrease in the number of outpatient visits (in Korea, US, France, UK, Spain, Slovenia, and Italy). Thirteen surveys [13, 53, 77, 93, 94, 96, 115, 119–124] disclosed information concerning outpatient care. Ten were answered by oncologists and 3 were answered by patients. The latter studies reported consequences on treatment or follow-up (Netherlands) [121], namely treatment adjustment, postponement, delay, or discontinuation; delay in routine or follow-up clinic appointment (US) [93] and postponements of physician appointments (Germany). A substantial percentage of physicians reported cancellation or deferral of follow-up visits.
Regarding the “volume of cancer treatment”, indicators regarding the 3 main therapeutic components were reported: surgeries and loco-regional therapies, radiotherapy, and systemic therapy.
Concerning surgeries and loco-regional therapies, 104 indicators were identified from 30 articles [49, 55–58, 62, 69, 72, 72, 82, 88, 97, 99–101, 104–106, 108–110, 118, 125–132]. Of those, 82 indicators (79%) showed a reduction in the number of treatment procedures, namely in Italy, France, Germany, Ireland, Netherlands, Spain, Turkey, UK, US, Australia, and in 1 international study. Nineteen articles were surveys directed to physicians [53, 65, 77, 85, 91, 92, 95, 97, 115, 122, 133–141], out of which 8 were international. All have shown significant reductions in surgical activity regarding different cancers.
Regarding radiotherapy treatments, 57 indicators from 8 articles [62, 97, 99, 128, 142–145] were identified. Most of these indicators signalled a reduction of the number of treatments (n = 41, 72%), namely in UK, US, and Canada. Five international surveys to physicians [119, 121, 133, 139, 144] also reported that this type of cancer therapy was affected.
With respect to systemic anticancer therapy, 43 indicators were identified from 9 articles [49, 60, 62, 79, 103, 113, 118, 146, 147], concerning Italy, France, UK, Spain, and the US. The majority (n = 40, 93%) showed a decrease in requests for initial treatment and in the number of chemotherapy administrations. Six surveys [65, 116, 119, 122, 141, 148] reported on this treatment modality. One study including 54 countries [53] showed that 88.2% of centers reported a reduction in their usual level of care, while 9.83% of those reported lack of access to medications. A European study with 29 countries [116] reported that 6% of centers revealed shortages of drugs.
A total of 28 indicators (from 3 articles) [62, 146, 147] reported on systemic anticancer therapy after lockdown ending (from May to October 2020). Of these indicators, 14 (50%) reported an increase in the number of treatments (in UK and France).
Regarding “delay in treatment”, a total of 42 indicators from 18 articles [46, 59, 79, 84, 88, 96, 98, 99, 103, 104, 106, 108, 145, 149–153] were identified. Of those, 18 (43%) reported an increasing trend in waiting time to treatment, namely in France, Portugal, Canada, US, and Italy. Sixteen indicators (38%) signalled stable waiting times and 8 (19%) a reduction in time to treatment. Twenty-one surveys [65, 77, 91–93, 95, 115, 116, 121, 124, 139–141, 148, 154–160], 2 studies using administrative data and surveys [128, 145] and 1 prospective study [124] reported on delays in cancer treatment. From those, eleven articles were international studies. All have reported delays or interruptions on different aspects of cancer treatment, namely in Canada, France, Germany, Italy, Netherlands, US, and UK.
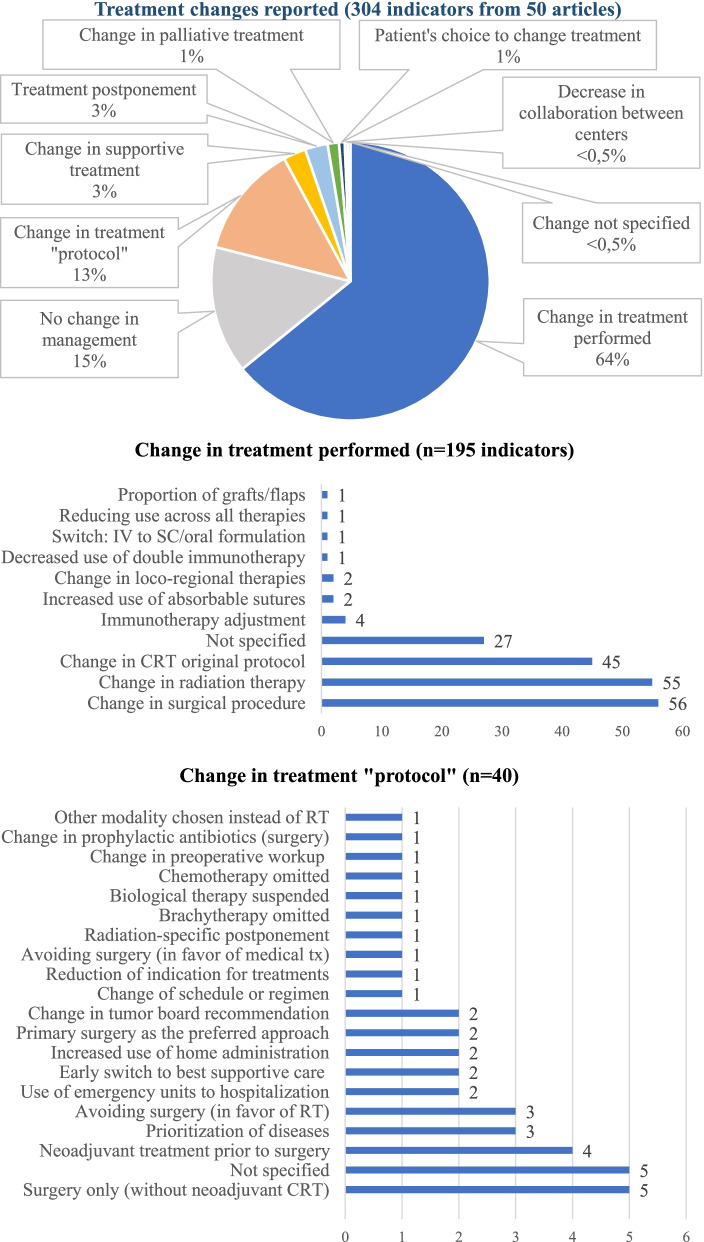
Fifty articles reported on “changes in treatment”, resulting in 304 indicators collated. A total of 106 indicators with quantitative information (from 19 articles [57, 59, 72, 82, 84, 97, 103–105, 108, 109, 113, 125, 128, 142, 144, 145, 149, 161]) were collected from administrative data and 13 indicators from registry data [141, 145]. A total of 185 indicators were survey-base information [13, 53, 65, 77, 85, 92, 95, 120–123, 133, 137, 148, 152, 155–158, 162–166]. Changes in treatment including all the indicators are summarized in Fig. 3.
Fig. 3.
Treatment changes reported in the articles (n = 304 indicators from 50 articles). CRT: chemotherapy; IV: intravenous; RT: radiotherapy; SC: subcutaneous; tx: treatment
Modifications in treatment were reported in 195 (64%) of indicators. The most frequent changes in treatment were in surgical procedures (n = 56 indicators, 29%), radiation-specific changes (n = 55 indicators, 28%) and change of original protocol of chemotherapy (n = 45 indicators, 23%). Modifications documented in surgical procedures were a decrease in use of laparoscopic surgery together with an increase of open or radical surgery, an increase in stoma formation rate, and a decrease of immediate breast reconstruction rate in breast cancer patients. The radiation-specific care variations identified were radiotherapy hypofractionation, treatment disruptions, increase in short-course treatments, and physicians being less likely to prescribe adjuvant radiotherapy.
Treatment “protocol” changes were reported by 40 indicators. The most frequent change was performing surgery without neoadjuvant chemotherapy (Fig. 3).
A total of 12 indicators from 4 articles [49, 62, 100, 106] reported on the “number of visits and admissions” of cancer patients to the hospital. Of these indicators, 10 (83%) signalled a decreasing trend. Two international surveys [62, 92] to health professionals have also disclosed a decrease in the number of oncology unit hospitalizations.
Regarding the “utilization of telemedicine”, 4 indicators were identified from 4 articles [75, 93, 110, 159]. Fourteen surveys [13, 65, 77, 116, 119–122, 141, 148, 152, 156, 159, 164] provided information on telemedicine use, from which ten were international studies. Three were patients’ surveys. All the survey-based information and quantitative indicators reported an increase in the use of telehealth to provide cancer care. One article [167] assessed patient and providers’ satisfaction regarding the use of telemedicine in rehabilitation of cancer patients. The proportion of patients that provided good feedback ranged from 63 to 84%, and the physicians’ perspective was also satisfactory, ranging from 66 to 83% of physicians reporting positive feedback. Four international surveys [122, 148, 152, 157] addressed the implementation of virtual multidisciplinary tumor boards, showing a marked increase in the use of web-based platforms.
Two main outcomes were addressed in the included articles: “procedures and surgical outcome measures” and “mortality”. Fifteen indicators from 8 articles [100, 104–110] conveyed information regarding procedures’ outcomes. From these indicators, 11 (73%) showed similar complication rates. One Italian survey [134] has also documented a stable number of complications after esophageal resections.
With respect to mortality in cancer patients, 24 indicators were identified from 4 articles. Twenty of these indicators resulted from one Portuguese study [112], the other 3 indicators showed a stable postoperative death rate in patients with head and neck cancer (France) [109], and a stable in-hospital mortality rate for orthopedic tumors at the traumatology department (Germany) [130]. One Turkish study [107] documented increased mortality in occlusive colorectal cancers patients.
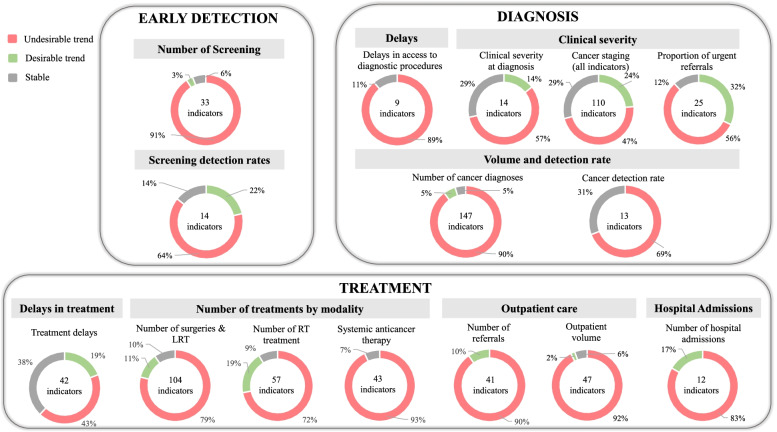
The trends of the indicators comparing the COVID-19 period with a previous time period are summarized in Fig. 4.
Fig. 4.
Cancer care indicators’ trends (%) comparing the COVID-19 period (Jan-Jun 2020) with a previous one
Discussion
In this study, we performed a scoping review to identify the indicators used in the literature to measure the impact of the COVID-19 pandemic on the cancer care pathway from January to June 2020, and the changes in the quality of care signalled by these indicators in OECD countries. We identified 135 articles, with a total of 1013 quantitative indicators collected, reporting on 94 countries. Changes in the quality of care are spread across the care pathway. This performance information suggests capacity constraints, it shows quick adaptations and innovations in cancer management. If collected in near-real-time and processed into actionable information, it would allow monitoring changes in care during the current pandemic and in future events more efficiently, supporting timely and adequate responses.
Our findings signal a major impact on the diagnosis of cancer: a decreasing trend in the number of screenings, diagnostic procedures, and, consequently, in the number of cancer diagnoses, resulting in increasing screening detection rates, and delays in diagnostic care. Indeed, in a recent OECD report, reductions in screening rates were reported by 15 out of 16 OECD countries that had data available and a decline in the number of new diagnoses was reported in the 12 countries with information available [168]. The cancelation of elective procedures [22], and patients’ avoidance of going to healthcare facilities [169, 170]were other contributing factors. Previous studies documented the decreasing number of primary care consultations for a wide range of clinical conditions [171, 172] and a relevant fall in new cancer diagnoses in primary care [83].
Trends regarding cancer staging at diagnosis portray a mixed picture. The reduction in the number of elective diagnostic procedures could explain stage-shifts in cancer presentation. We also found an increasing trend in the proportion of urgent/emergent cancer referrals and in the proportion of emergent procedures, as well as evidence of patients presenting with more clinically advanced conditions to the hospital than before the pandemic. These trends suggest patients with advanced-stage cancers continued to seek care. It could also signal that those patients waited longer before receiving care, with potential deleterious outcomes. These results highlight the need to closely monitor the impact of the COVID-19 pandemic on shifts in cancer staging at diagnosis and the relevance of collecting this data systematically, which is a reality only in a few OECD countries, such as the Netherlands and Slovenia [168].
With respect to treatment, about 40% of the indicators reporting on waiting times to treatment signalled an increasing trend, we observe a decreasing trend in the volume of the three modalities of cancer treatment, and a large number and diversity of information reporting on treatment changes. These results reveal the remarkable influence of the pandemic on patterns of cancer care in the first half of 2020. These changes result from postponements of care decided by physicians to decrease patients’ exposure to hospitals [120], switch to audio- or video-consultations, and deferrals and treatment modifications guided by updated recommendations by many medical societies [11, 12], which were used by some OECD countries at a national level [168]. Almost two-thirds of the indicators reported changes in treatment, which shows that providers have quickly adapted their care practices, which strengthens the argument for the need for monitoring closely these changes. The relevant number of indicators collected from surveys, mostly conducted by international societies and networks of providers, highlight how medical societies and countries were unable to obtain these data using current health information systems and data infrastructures. While some of these care modifications could be learning opportunities for the future, this information should be standardised, transparent, and timely, allowing to appraise the modifications in care provided during crises regarding access, quality, and outcomes.
The increase in telemedicine utilization we report is a generalized trend across a range of medical specialties [3] and it constitutes a hallmark of the innovation triggered by this pandemic. Albeit the positive feedback by physicians and patients we report, telehealth risks to increase inequalities in access to care [173, 174].
Short-term oncological outcomes were addressed by a few indicators and are reported as being stable, which is in line with a recent international cohort study including 61 countries and 15 tumor types [22]. However, deferred care will most likely lead to worse long-term outcomes, which needs to be monitored. Attempts to quantify this impact were developed, for instance, by a British nation-wide modelling study where the authors estimated a total of 59 204–63 229 additional years of life lost attributed to four major cancers [175], compared with pre-pandemic data.
Previous works addressed the impact of the COVID-19 pandemic on regional or national settings [4, 57], on specific cancers [4, 5, 176], specific stages of the care pathway [61], or treatment modalities [22]. This scoping review provides a summary of cancer care performance indicators, concerning various diseases, from early detection to the treatment phase of the care pathway, within OECD countries. Additionally, we report changes in the quality of cancer care based on indicators’ trends, from January to June 2020, which constitutes an innovative approach to assess changes in healthcare performance.
Our study has some limitations. The heterogeneity of study designs, populations, diseases, indicators, indicators’ definitions and distribution of studies per country do not allow the application of a meta-analysis approach to quantify the real impact of the pandemic on cancer care and the generalization of these trends to all included countries. Furthermore, the time needed to collect, organize, and synthesize a relevant number of indicators in a meaningful way to inform decision-making explains why this study reports on the first semester of 2020. Nevertheless, this study presents a comprehensive overview of the cancer care pathway and the modifications and adaptations that occurred during this period in a broad range of countries. The indicators we collated could already comprise useful tools to assess the health systems’ response and changes in the quality of care after this period.
The COVID-19 pandemic keeps evolving until the present time and postponing of care was reported in some countries by the end of 2021 and the beginning of 2022 [177, 178], which means that this impact is adding up. Figures concerning new COVID-19 cases, mortality, and vaccination coverage, are presented daily to the public since the beginning of 2020. Additional and considerable efforts are needed to expose the effects caused by this pandemic in non-COVID-19 care, and some of the indicators we present could be useful to convey that message. Care inequalities could have been exacerbated during this pandemic, which also needs to be further studied regarding cancer care.
As new waves keep evolving, it is crucial to monitor performance indicators, such as shifts in cancer staging or worsening of outcomes. Also, the link between structure, process, and (short- and long-term) outcome indicators should be undertaken to allow an accurate and timely evaluation of the changes in the care provided during crises and in regular times. Tools like the “Time to Act Data Navigator” [179] developed by the European Cancer Organization and the “Global Cancer Observatory” [180] signal the ambition to address this lack of standardised and regular collection of data and indicators. Within the scope of Europe’s Beating Cancer Plan, the European Cancer Inequalities Registry [20] aims to monitor inequalities across Europe, by providing reliable data on cancer prevention and care.
Conclusion
This scoping review provides a structured summary of performance indicators used in the literature to assess the cancer care pathway from January 2020 to June 2020, and the changes in the quality of care signalled by these indicators in OECD countries. This study shows health systems have struggled to ensure the continuity of care to cancer patients. It also highlights adaptations and innovations in cancer management, as well as the importance of monitoring these changes closely, notably during crises. These performance measures could inform on the bottlenecks of the cancer care pathway, as well as moments for intervention during the evolving pandemic and in future crises. Furthermore, it could contribute to identifying disparities between and within countries and to better address the backlog this pandemic has created. To ensure the continuity of regular care pathways and enhance health systems’ resilience and adaptability, further research and investment are necessary. It is critical to develop system-wide oriented intelligence and strengthen data infrastructures worldwide to constantly monitor changes in care provision. This would support timely and adequate health policy responses and improve the preparedness for future crises.
Supplementary Information
Additional file 1. Preferred Reporting Items for Systematic reviews and Meta-Analyses extension for Scoping Reviews(PRISMA-ScR) Checklist
Acknowledgements
This research paper was elaborated as part of the first author's research project for the Research Master in Health Sciences at the Netherlands Institute for Health Sciences, Rotterdam, The Netherlands. The authors wish to thank Wichor Bramer from the Erasmus MC Medical Library for developing and updating the search strategies used in this study.
The authors wish to thank Rie Fujisawa from the Organisation for Economic Co-operation and Development (OECD), Directorate for Employment, Labour and Social Affairs, Health Division, for relevant comments provided to this study.
Abbreviations
- ASC
Ana Sofia Carvalho
- CRT
Chemotherapy
- IV
intravenous
- MdL
Mats de Lange
- NCDs
Non-communicable diseases
- OBF
Óscar Brito Fernandes
- OECD
Organisation for Economic Co-operation and Development
Authors’ contributions
All authors contributed to conceptualize the study. ASC and MdL performed the data collection. ASC performed the data analysis and drafted the article. All authors provided feedback and contributed to revising the manuscript. All authors approved the final version of the manuscript.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Availability of data and materials
The datasets generated and analysed during the current study are available in the Zenodo.org repository, at https://doi.org/10.5281/zenodo.6129839. [181]
Declarations
Ethics approval and consent to participate
Not required.
Consent for publication
Not applicable.
Competing interests
None declared.
Footnotes
Publisher’ s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.World Health Organization. A year without precedent: WHO’s COVID-19 response [Internet]. [cited 2022 Feb 11]. Available from: https://www.who.int/news-room/spotlight/a-year-without-precedent-who-s-covid-19-response
- 2.World Health Organization. Pulse survey on continuity of essential health services during the COVID-19 pandemic - Key informant findings from 135 countries, territories and areas - Quarter 1 2021 Reporting period: 3 months preceding date of survey submission [Internet]. [cited 2022 Jan 18]. Available from: https://www.who.int/docs/default-source/coronaviruse/finalupdate_22-april-2021_summary-ppt_ehs-pulse-survey_second-round.pdf?sfvrsn=a965e121_8
- 3.OECD. Health at a Glance 2021: OECD Indicators, OECD Publishing, Paris. [Internet]. 2021. Available from: 10.1787/ae3016b9-en.
- 4.Eijkelboom AH, de Munck L, Vrancken PeetersBroeders M-JTFDMJM, Strobbe LJA, Bos MEMM, et al. Impact of the COVID-19 pandemic on diagnosis, stage, and initial treatment of breast cancer in the Netherlands: a population-based study. J Hematol Oncol. 2021;14:64. doi: 10.1186/s13045-021-01073-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Toes-Zoutendijk E, Vink G, Nagtegaal ID, Spaander MCW, Dekker E, van Leerdam ME, et al. Impact of COVID-19 and suspension of colorectal cancer screening on incidence and stage distribution of colorectal cancers in the Netherlands. Eur J Cancer. 2022;161:38–43. doi: 10.1016/j.ejca.2021.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.van Velthuysen MLF, van Eeden S, le Cessie S, de Boer M, van Boven H, Koomen BM, et al. Impact of COVID-19 pandemic on diagnostic pathology in the Netherlands. BMC Health Serv Res. 2022;22:166. doi: 10.1186/s12913-022-07546-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Amador M, Matias-Guiu X, Sancho-Pardo G, Contreras Martinez J, de la Torre-Montero JC, Peñuelas Saiz A, et al. Impact of the COVID-19 pandemic on the care of cancer patients in Spain. ESMO Open. 2021;6:100157. doi: 10.1016/j.esmoop.2021.100157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Peacock HM, Tambuyzer T, Verdoodt F, Calay F, Poirel HA, De Schutter H, et al. Decline and incomplete recovery in cancer diagnoses during the COVID-19 pandemic in Belgium: a year-long, population-level analysis. ESMO Open. 2021;6:100197. doi: 10.1016/j.esmoop.2021.100197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Skovlund CW, Friis S, Dehlendorff C, Nilbert MC, Mørch LS. Hidden morbidities: drop in cancer diagnoses during the COVID-19 pandemic in Denmark. Acta Oncol. 2021;60:20–3. doi: 10.1080/0284186X.2020.1858235. [DOI] [PubMed] [Google Scholar]
- 10.Burki TK. Cancer guidelines during the COVID-19 pandemic. Lancet Oncol. 2020;21:629–30. doi: 10.1016/S1470-2045(20)30217-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.BASO~The Association for Cancer Surgery BASO Guidance. Strategy for Cancer Surgery sustainability and recovery in the COVID 19 pandemic. [cited 2021 Dec 9]. Available from: https://baso.org.uk/media/99217/baso_guidance_for_cancer_surgery_9th_april_2020_v7.pdf
- 12.Curigliano G, Banerjee S, Cervantes A, Garassino MC, Garrido P, Girard N, et al. Managing cancer patients during the COVID-19 pandemic: an ESMO multidisciplinary expert consensus. Ann Oncol. 2020;31:1320–35. doi: 10.1016/j.annonc.2020.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chazan G, Franchini F, Alexander M, Banerjee S, Mileshkin L, Blinman P, et al. Impact of COVID-19 on cancer service delivery: results from an international survey of oncology clinicians. ESMO Open. 2020;5:e001090. doi: 10.1136/esmoopen-2020-001090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.United Nations - Department of Economic and Social Affairs. Sustainable Development. The 17 Goals. [cited 2022 Jan 11]. Available from: https://sdgs.un.org/goals
- 15.World Health Organization. World Health Assembly 70. Cancer prevention and control in the context of an integrated approach. 2017 [cited 2022 Jan 11]. Available from: https://apps.who.int/iris/bitstream/handle/10665/275676/A70_R12-en.pdf?sequence=1&isAllowed=y
- 16.Coleman MP, Quaresma M, Berrino F, Lutz J-M, De Angelis R, Capocaccia R, et al. Cancer survival in five continents: a worldwide population-based study (CONCORD) Lancet Oncol. 2008;9:730–56. doi: 10.1016/S1470-2045(08)70179-7. [DOI] [PubMed] [Google Scholar]
- 17.Allemani C, Weir HK, Carreira H, Harewood R, Spika D, Wang X-S, et al. Global surveillance of cancer survival 1995–2009: analysis of individual data for 25 676 887 patients from 279 population-based registries in 67 countries (CONCORD-2) The Lancet. 2015;385:977–1010. doi: 10.1016/S0140-6736(14)62038-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.European Comission. Public health - Non-communicable diseases - Cancer. [cited 2022 Jan 17]. Available from: https://ec.europa.eu/health/non-communicable-diseases/cancer_en
- 19.European Comission. Communication from the Commission to the European Parliament and the Council - Europe’s Beating Cancer Plan. 2021 [cited 2022 Jan 11]. Available from: https://ec.europa.eu/health/sites/default/files/non_communicable_diseases/docs/eu_cancer-plan_en.pdf
- 20.European Comission. European Cancer Inequalities Registry. [cited 2022 Jan 24]. Available from: https://cancer-inequalities.jrc.ec.europa.eu/
- 21.European Comission. EU Science Hub. Launch of the EC Knowledge Centre on Cancer. [cited 2022 Jan 11]. Available from: https://ec.europa.eu/jrc/en/event/conference/launch-ec-knowledge-centre-cancer
- 22.Glasbey J, Ademuyiwa A, Adisa A, AlAmeer E, Arnaud AP, Ayasra F, et al. Effect of COVID-19 pandemic lockdowns on planned cancer surgery for 15 tumour types in 61 countries: an international, prospective, cohort study. Lancet Oncol. 2021;22:1507–17. doi: 10.1016/S1470-2045(21)00493-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Blay JY, Boucher S, Le Vu B, Cropet C, Chabaud S, Perol D, et al. Delayed care for patients with newly diagnosed cancer due to COVID-19 and estimated impact on cancer mortality in France. ESMO Open. 2021;6:100134. doi: 10.1016/j.esmoop.2021.100134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kirchberg J, Rentsch A, Klimova A, Vovk V, Hempel S, Folprecht G, et al. Influence of the First Wave of the COVID-19 Pandemic on Cancer Care in a German Comprehensive Cancer Center. Front Public Health. 2021;9:750479. doi: 10.3389/fpubh.2021.750479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Leatherman S. Performance measurement for health system improvement: Experiences, challenges and prospects [Internet]. Smith PC, Mossialos E, Papanicolas I, editors. Cambridge: Cambridge University Press; 2010 [cited 2022 Apr 21]. Available from: http://ebooks.cambridge.org/ref/id/CBO9780511711800
- 26.Busse R, Klazinga N, Panteli D, Quentin W. Improving Healthcare Quality in Europe: Characteristics, Effectiveness and Implementation of Different Strategies OECD; 2019 [cited 2022 Mar 2]. Available from: https://www.oecd-ilibrary.org/social-issues-migration-health/improving-healthcare-quality-in-europe_b11a6e8f-en [PubMed]
- 27.Barbazza E, Klazinga NS, Kringos DS. Exploring the actionability of healthcare performance indicators for quality of care: a qualitative analysis of the literature, expert opinion and user experience. BMJ Qual Saf. 2021;30:1010–20. doi: 10.1136/bmjqs-2020-011247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kringos D, Carinci F, Barbazza E, Bos V, Gilmore K, Groene O, et al. Managing COVID-19 within and across health systems: why we need performance intelligence to coordinate a global response. Health Res Policy Syst. 2020;18:80. doi: 10.1186/s12961-020-00593-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Towards actionable international comparisons of health system performance: expert revision of the OECD framework and quality indicators. Int J Qual Health Care. 2015 [cited 2022 Apr 24]; Available from: https://academic.oup.com/intqhc/article/27/2/137/1787909/Towards-actionable-international-comparisons-of [DOI] [PubMed]
- 30.OECD. Caring for Quality in Health: Lessons Learnt from 15 Reviews of Health Care Quality. OECD; 2017 [cited 2022 Apr 24]. Available from: https://www.oecd-ilibrary.org/social-issues-migration-health/caring-for-quality-in-health_9789264267787-en
- 31.Carini E, Gabutti I, Frisicale EM, Di Pilla A, Pezzullo AM, de Waure C, et al. Assessing hospital performance indicators. What dimensions? Evidence from an umbrella review. BMC Health Serv Res. 2020;20:1038. doi: 10.1186/s12913-020-05879-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Arksey H, O’Malley L. Scoping studies: towards a methodological framework. Int J Soc Res Methodol. 2005;8:19–32. doi: 10.1080/1364557032000119616. [DOI] [Google Scholar]
- 33.Levac D, Colquhoun H, O’Brien KK. Scoping studies: advancing the methodology. Implement Sci. 2010;5:69. doi: 10.1186/1748-5908-5-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tolonen H, Reinikainen J, Koponen P, Elonheimo H, Palmieri L, Tijhuis MJ, et al. Cross-national comparisons of health indicators require standardized definitions and common data sources. Arch Public Health. 2021;79:208. doi: 10.1186/s13690-021-00734-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lockwood C, dos Santos KB, Pap R. Practical Guidance for Knowledge Synthesis: Scoping Review Methods. Asian Nurs Res. 2019;13:287–94. doi: 10.1016/j.anr.2019.11.002. [DOI] [PubMed] [Google Scholar]
- 36.Munn Z, Peters MDJ, Stern C, Tufanaru C, McArthur A, Aromataris E. Systematic review or scoping review? Guidance for authors when choosing between a systematic or scoping review approach. BMC Med Res Methodol. 2018;18:143. doi: 10.1186/s12874-018-0611-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tricco AC, Lillie E, Zarin W, O’Brien KK, Colquhoun H, Levac D, et al. PRISMA Extension for Scoping Reviews (PRISMA-ScR): Checklist and Explanation. Ann Intern Med. 2018;169:467–73. doi: 10.7326/M18-0850. [DOI] [PubMed] [Google Scholar]
- 38.Bramer WM, Rethlefsen ML, Kleijnen J, Franco OH. Optimal database combinations for literature searches in systematic reviews: a prospective exploratory study. Syst Rev. 2017;6:245. doi: 10.1186/s13643-017-0644-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ouzzani M, Hammady H, Fedorowicz Z, Elmagarmid A. Rayyan—a web and mobile app for systematic reviews. Syst Rev. 2016;5:210. doi: 10.1186/s13643-016-0384-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Minucci A, Scambia G, Santonocito C, Concolino P, Urbani A. BRCA testing in a genomic diagnostics referral center during the COVID-19 pandemic. Mol Biol Rep. 2020;47:4857–60. doi: 10.1007/s11033-020-05479-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Minucci A, Scambia G, De Bonis M, De Paolis E, Santonocito C, Fagotti A, et al. BRCA testing delay during the COVID-19 pandemic: How to act? Mol Biol Rep. 2021;48:983–7. doi: 10.1007/s11033-020-06060-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.London JW, Fazio-Eynullayeva E, Palchuk MB, Sankey P, McNair C. Effects of the COVID-19 Pandemic on Cancer-Related Patient Encounters. JCO Clin Cancer Inform. 2020;4:657–65. [DOI] [PMC free article] [PubMed]
- 43.Henderson LM, Benefield T, Bosemani T, Long JM, Rivera MP. Impact of the COVID-19 Pandemic on Volumes and Disparities in Lung Cancer Screening. Chest. 2021;160:379–82. doi: 10.1016/j.chest.2020.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bakouny Z, Paciotti M, Schmidt AL, Lipsitz SR, Choueiri TK, Trinh Q-D. Cancer Screening Tests and Cancer Diagnoses During the COVID-19 Pandemic. JAMA Oncol. 2021;7:458. doi: 10.1001/jamaoncol.2020.7600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Miller MJ, Xu L, Qin J, Hahn EE, Ngo-Metzger Q, Mittman B, et al. Impact of COVID-19 on Cervical Cancer Screening Rates Among Women Aged 21–65 Years in a Large Integrated Health Care System — Southern California, January 1–September 30, 2019, and January 1–September 30, 2020. MMWR Morb Mortal Wkly Rep. 2021;70:109–13. doi: 10.15585/mmwr.mm7004a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Suárez J, Mata E, Guerra A, Jiménez G, Montes M, Arias F, et al. Impact of the COVID-19 pandemic during Spain’s state of emergency on the diagnosis of colorectal cancer. J Surg Oncol. 2021;123:32–6. doi: 10.1002/jso.26263. [DOI] [PubMed] [Google Scholar]
- 47.Maganty A, Yu M, Anyaeche VI, Zhu T, Hay JM, Davies BJ, et al. Referral pattern for urologic malignancies before and during the COVID-19 pandemic. Urol Oncol Semin Orig Investig. 2021;39:268–76. doi: 10.1016/j.urolonc.2020.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Toss A, Isca C, Venturelli M, Nasso C, Ficarra G, Bellelli V, et al. Two-month stop in mammographic screening significantly impacts on breast cancer stage at diagnosis and upfront treatment in the COVID era. ESMO Open. 2021;6:100055. doi: 10.1016/j.esmoop.2021.100055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Patt D, Gordan L, Diaz M, Okon T, Grady L, Harmison M, et al. Impact of COVID-19 on Cancer Care: How the Pandemic Is Delaying Cancer Diagnosis and Treatment for American Seniors. JCO Clin Cancer Inform. 2020;4:1059–71. [DOI] [PMC free article] [PubMed]
- 50.Song H, Bergman A, Chen AT, Ellis D, David G, Friedman AB, et al. Disruptions in preventive care: Mammograms during the COVID-19 pandemic. Health Serv Res. 2021;56:95–101. doi: 10.1111/1475-6773.13596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yin K, Singh P, Drohan B, Hughes KS. Breast imaging, breast surgery, and cancer genetics in the age of COVID-19. Cancer. 2020;126:4466–72. doi: 10.1002/cncr.33113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nyante SJ, Benefield TS, Kuzmiak CM, Earnhardt K, Pritchard M, Henderson LM. Population-level impact of coronavirus disease 2019 on breast cancer screening and diagnostic procedures. Cancer. 2021;127:2111–21. doi: 10.1002/cncr.33460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rocco N, Montagna G, Di Micco R, Benson J, Criscitiello C, Chen L, et al. The impact of the COVID-19 pandemic on surgical management of breast cancer: global trends and future perspectives. The Oncologist. 2021;26:e66–77. doi: 10.1002/onco.13560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.D’Ovidio V, Lucidi C, Bruno G, Lisi D, Miglioresi L, Bazuro ME. Impact of COVID-19 pandemic on colorectal cancer screening program. Clin Colorectal Cancer. 2021;20:e5–11. doi: 10.1016/j.clcc.2020.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Furnari M, Eusebi LH, Savarino E, Petruzzellis C, Esposito G, Maida M, et al. Effects of SARS-CoV-2 emergency measures on high-risk lesions detection: a multicentre cross-sectional study. Gut. 2021;70:1241–3. doi: 10.1136/gutjnl-2020-323116. [DOI] [PubMed] [Google Scholar]
- 56.John A, Mian M, Sreedharan S, Kahokehr AA. The impact of the coronavirus disease 2019 pandemic on elective urological procedures in Australia. Asian J Urol. 2022;9:35–41. doi: 10.1016/j.ajur.2021.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kiziltan G, Tumer CBK, Ozaslan C, Guler OC. Effects of COVID-19 pandemics in a Breast unit. Eur J Cancer. 2020;138:S116. doi: 10.1016/S0959-8049(20)30849-2. [DOI] [Google Scholar]
- 58.O’Connor E, O’Dowd G, Phelan S. Impact of COVID-19 on small biopsy diagnostic procedures and cancer resection surgeries in the North-West of Ireland. J Clin Pathol. 2022;75(4):270–3. [DOI] [PubMed]
- 59.Amaddeo G, Brustia R, Allaire M, Lequoy M, Hollande C, Regnault H, et al. Impact of COVID-19 on the management of hepatocellular carcinoma in a high-prevalence area. JHEP Rep. 2021;3:100199. doi: 10.1016/j.jhepr.2020.100199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Quaquarini E, Saltalamacchia G, Presti D, Caldana G, Tibollo V, Malovini A, et al. Impact of COVID-19 outbreak on cancer patient care and treatment: data from an outpatient oncology clinic in lombardy (Italy) Cancers. 2020;12:2941. doi: 10.3390/cancers12102941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lantinga MA, Theunissen F, ter Borg PCJ, Bruno MJ, Ouwendijk RJT, Siersema PD, et al. Impact of the COVID-19 pandemic on gastrointestinal endoscopy in the Netherlands: analysis of a prospective endoscopy database. Endoscopy. 2021;53:166–70. doi: 10.1055/a-1272-3788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Penel N, Hammoudi A, Marliot G, De Courreges A, Cucchi M, Mirabel X, et al. Major impact of COVID-19 national containment on activities in the French northern comprehensive cancer center. Med Oncol. 2021;38:28. doi: 10.1007/s12032-021-01467-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Markar SR, Clarke J, Kinross J. Practice patterns of diagnostic upper gastrointestinal endoscopy during the initial COVID-19 outbreak in England. Lancet Gastroenterol Hepatol. 2020;5:804–5. doi: 10.1016/S2468-1253(20)30236-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wassie MM, Agaciak M, Cock C, Bampton P, Young GP, Symonds EL. The impact of coronavirus disease 2019 on surveillance colonoscopies in South Australia. JGH Open. 2021;5:486–92. doi: 10.1002/jgh3.12525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.CRC Covid Research Collaborative The impact of the COVID-19 pandemic on colorectal cancer service provision. Br J Surg. 2020;107:e521–2. doi: 10.1002/bjs.11706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rutter MD, Brookes M, Lee TJ, Rogers P, Sharp L. Impact of the COVID-19 pandemic on UK endoscopic activity and cancer detection: a National Endoscopy Database Analysis. Gut. 2021;70:537–43. doi: 10.1136/gutjnl-2020-322179. [DOI] [PubMed] [Google Scholar]
- 67.Zadnik V, Mihor A, Tomsic S, Zagar T, Bric N, Lokar K, et al. Impact of COVID-19 on cancer diagnosis and management in Slovenia – preliminary results. Radiol Oncol. 2020;54:329–34. doi: 10.2478/raon-2020-0048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Uyl-de Groot CA, Schuurman MS, Huijgens PC, Praagman J. Fewer cancer diagnoses during the COVID-19 epidemic according to diagnosis, age and region. TSG - Tijdschr Voor Gezondheidswetenschappen. 2021;99:1–8. doi: 10.1007/s12508-020-00289-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Barruscotti S, Giorgini C, Brazzelli V, Vassallo C, Michelerio A, Klersy C, et al. A significant reduction in the diagnosis of melanoma during the COVID‐19 lockdown in a third‐level center in the Northern Italy. Dermatol Ther. 2020 [cited 2022 Feb 17];33. Available from: https://onlinelibrary.wiley.com/doi/10.1111/dth.14074 [DOI] [PubMed]
- 70.Ferrara G, De Vincentiis L, Ambrosini-Spaltro A, Barbareschi M, Bertolini V, Contato E, et al. Cancer Diagnostic Delay in Northern and Central Italy During the 2020 Lockdown Due to the Coronavirus Disease 2019 Pandemic. Am J Clin Pathol. 2021;155:64–8. doi: 10.1093/ajcp/aqaa177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kaufman HW, Chen Z, Niles J, Fesko Y. Changes in the Number of US Patients With Newly Identified Cancer Before and During the Coronavirus Disease 2019 (COVID-19) Pandemic. JAMA Netw Open. 2020;3:e2017267. doi: 10.1001/jamanetworkopen.2020.17267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kiong KL, Guo T, Yao CMKL, Gross ND, Hanasono MM, Ferrarotto R, et al. Changing practice patterns in head and neck oncologic surgery in the early COVID -19 era. Head Neck. 2020;42:1179–86. doi: 10.1002/hed.26202. [DOI] [PubMed] [Google Scholar]
- 73.Vigliar E, Cepurnaite R, Alcaraz-Mateos E, Ali SZ, Baloch ZW, Bellevicine C, et al. Global impact of the COVID-19 pandemic on cytopathology practice: Results from an international survey of laboratories in 23 countries. Cancer Cytopathol. 2020;128:885–94. doi: 10.1002/cncy.22373. [DOI] [PubMed] [Google Scholar]
- 74.Ricci F, Fania L, Paradisi A, Di Lella G, Pallotta S, Sobrino L, et al. Delayed melanoma diagnosis in the COVID‐19 era: increased breslow thickness in primary melanomas seen after the COVID‐19 lockdown. J Eur Acad Dermatol Venereol. 2020 [cited 2022 Feb 17];34. Available from: https://onlinelibrary.wiley.com/doi/10.1111/jdv.16874 [DOI] [PMC free article] [PubMed]
- 75.De Vincentiis L, Mariani MP, Ferrara G. Dermatologic Oncology and Histopathology at a Secondary Care Centre During the Coronavirus Disease 2019 Pandemic. Am J Dermatopathol. 2021;43:160–2. doi: 10.1097/DAD.0000000000001814. [DOI] [PubMed] [Google Scholar]
- 76.Suh-Burgmann EJ, Alavi M, Schmittdiel J. Endometrial cancer detection during the coronavirus disease 2019 (COVID-19) pandemic. Obstet Gynecol. 2020;136:842–3. doi: 10.1097/AOG.0000000000004087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Panzuto F, Maccauro M, Campana D, Faggiano A, Massironi S, Pusceddu S, et al. Impact of the SARS-CoV2 pandemic dissemination on the management of neuroendocrine neoplasia in Italy: a report from the Italian Association for Neuroendocrine Tumors (Itanet) J Endocrinol Invest. 2021;44:989–94. doi: 10.1007/s40618-020-01393-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Fernández Canedo MI, de Troya Martín M, Rivas Ruíz F. Impacto de la pandemia SARS-CoV-2 en el diagnóstico precoz del melanoma. Med Clínica. 2021;156:356–7. doi: 10.1016/j.medcli.2020.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Gathani T, Clayton G, MacInnes E, Horgan K. The COVID-19 pandemic and impact on breast cancer diagnoses: what happened in England in the first half of 2020. Br J Cancer. 2021;124:710–2. doi: 10.1038/s41416-020-01182-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Shannon AB, Sharon CE, Straker RJ, Miura JT, Ming ME, Chu EY, et al. The impact of the COVID-19 pandemic on the presentation status of newly diagnosed melanoma: A single institution experience. J Am Acad Dermatol. 2021;84:1096–8. doi: 10.1016/j.jaad.2020.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Dinmohamed AG, Cellamare M, Visser O, de Munck L, Elferink MAG, Westenend PJ, et al. The impact of the temporary suspension of national cancer screening programmes due to the COVID-19 epidemic on the diagnosis of breast and colorectal cancer in the Netherlands. J Hematol OncolJ Hematol Oncol. 2020;13:147. doi: 10.1186/s13045-020-00984-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wang H, Elsheikh M, Gilmour K, Cohen V, Sagoo MS, Damato B, et al. Impact of COVID-19 pandemic on eye cancer care in United Kingdom. Br J Cancer. 2021;124:1357–60. doi: 10.1038/s41416-021-01274-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Jacob L, Loosen SH, Kalder M, Luedde T, Roderburg C, Kostev K. Impact of the COVID-19 pandemic on cancer diagnoses in general and specialized practices in Germany. Cancers. 2021;13:408. doi: 10.3390/cancers13030408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Van Haren RM, Delman AM, Turner KM, Waits B, Hemingway M, Shah SA, et al. Impact of the COVID-19 pandemic on lung cancer screening program and subsequent lung cancer. J Am Coll Surg. 2021;232:600–5. doi: 10.1016/j.jamcollsurg.2020.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Price SJ, Joannides A, Plaha P, Afshari FT, Albanese E, Barua NU, et al. Impact of COVID-19 pandemic on surgical neuro-oncology multi-disciplinary team decision making: a national survey (COVID-CNSMDT Study) BMJ Open. 2020;10:e040898. doi: 10.1136/bmjopen-2020-040898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Dinmohamed AG, Visser O, Verhoeven RHA, Louwman MWJ, van Nederveen FH, Willems SM, et al. Fewer cancer diagnoses during the COVID-19 epidemic in the Netherlands. Lancet Oncol. 2020;21:750–1. doi: 10.1016/S1470-2045(20)30265-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Valenti M, Pavia G, Gargiulo L, Facheris P, Nucca O, Mancini L, et al. Impact of delay in follow-up due to COVID-19 pandemic on skin cancer progression: a real-life experience from an Italian hub hospital. Int J Dermatol. 2021;60:860–3. doi: 10.1111/ijd.15501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kiong KL, Diaz EM, Gross ND, Diaz EM, Hanna EY. The impact of COVID-19 on head and neck cancer diagnosis and disease extent. Head Neck. 2021;43:1890–7. doi: 10.1002/hed.26665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Marson JW, Maner BS, Harding TP, Meisenheimer J, Solomon JA, Leavitt M, et al. The magnitude of COVID-19’s effect on the timely management of melanoma and nonmelanoma skin cancers. J Am Acad Dermatol. 2021;84:1100–3. doi: 10.1016/j.jaad.2020.12.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Vigliar E, Cepurnaite R, Alcaraz-Mateos E, Ali S, Baloch Z, Bongiovanni M, et al. COVID-19 Pandemic effect on Cytopathology Practice: Results from 23 Laboratories in 11 Countries. J Am Soc Cytopathol. 2020;9:S51. doi: 10.1016/j.jasc.2020.07.103. [DOI] [PubMed] [Google Scholar]
- 91.Torzilli G, Viganò L, Galvanin J, Castoro C, Quagliuolo V, Spinelli A, et al. A snapshot of elective oncological surgery in Italy during COVID-19 emergency: pearls, pitfalls, and perspectives. Ann Surg. 2020;272:e112–7. doi: 10.1097/SLA.0000000000004081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Santoro GA, Grossi U, Murad-Regadas S, Nunoo-Mensah JW, Mellgren A, Di Tanna GL, et al. DElayed COloRectal cancer care during COVID-19 Pandemic (DECOR-19): Global perspective from an international survey. Surgery. 2021;169:796–807. doi: 10.1016/j.surg.2020.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Papautsky EL, Hamlish T. Patient-reported treatment delays in breast cancer care during the COVID-19 pandemic. Breast Cancer Res Treat. 2020;184:249–54. doi: 10.1007/s10549-020-05828-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Krug S, Garbe J, König S, Ungewiss H, Michl P, Rinke A, et al. Professional assessment of the impact of COVID-19 on handling NET patients. J Clin Med. 2020;9:3633. doi: 10.3390/jcm9113633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Boyle JM, Kuryba A, Blake HA, Aggarwal A, Meulen J, Walker K, et al. The impact of the first peak of the COVID-19 pandemic on colorectal cancer services in England and Wales: A national survey. Colorectal Dis. 2021;23:1733–44. doi: 10.1111/codi.15622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Andrew TW, Alrawi M, Lovat P. Reduction in skin cancer diagnoses in the UK during the COVID-19 pandemic. Clin Exp Dermatol. 2021;46:145–6. doi: 10.1111/ced.14411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Nolan GS, Dunne JA, Kiely AL, Pritchard Jones RO, Gardiner M, Jain A. The effect of the COVID-19 pandemic on skin cancer surgery in the United Kingdom: a national, multi-centre, prospective cohort study and survey of Plastic Surgeons. J Br Surg. 2020;107:e598–600. doi: 10.1002/bjs.12047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Taylor R, Omakobia E, Sood S, Glore RJ. The impact of coronavirus disease 2019 on head and neck cancer services: a UK tertiary centre study. J Laryngol Otol. 2020;134:684–7. doi: 10.1017/S0022215120001735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Morris EJA, Goldacre R, Spata E, Mafham M, Finan PJ, Shelton J, et al. Impact of the COVID-19 pandemic on the detection and management of colorectal cancer in England: a population-based study. Lancet Gastroenterol Hepatol. 2021;6:199–208. doi: 10.1016/S2468-1253(21)00005-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kvolik Pavić A, Zubčić V, Kvolik S. Workload changes during the COVID-19 pandemic and effects on the flow of cancer patients in the Maxillofacial Surgery Department. Med Glas Ljek Komore Zenicko-Doboj Kantona. 2021 [cited 2022 Feb 17]; Available from: 10.17392/1308-21 [DOI] [PubMed]
- 101.Vissio E, Falco EC, Collemi G, Borella F, Papotti M, Scarmozzino A, et al. Impact of COVID-19 lockdown measures on oncological surgical activity: Analysis of the surgical pathology caseload of a tertiary referral hospital in Northwestern Italy. J Surg Oncol. 2021;123:24–31. doi: 10.1002/jso.26256. [DOI] [PubMed] [Google Scholar]
- 102.Weston GK, Jeong HS, Mu EW, Polsky D, Meehan SA. Impact of COVID-19 on melanoma diagnosis. Melanoma Res. 2021;31:280–1. doi: 10.1097/CMR.0000000000000717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Gosset M, Gal J, Schiappa R, Dejode M, Fouché Y, Alazet F, et al. Impact de la pandémie de COVID-19 sur les prises en charge pour cancer du sein et gynécologique. Bull Cancer (Paris). 2021;108:3–11. doi: 10.1016/j.bulcan.2020.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Veiga J, Amante S, Costa NV, Luz JH, Gomes FV, Coimbra É, et al. The Covid-19 pandemic constraints may lead to disease progression for patients with liver cancer scheduled to receive locoregional therapies: single-centre retrospective analysis in an interventional radiology unit. Cardiovasc Intervent Radiol. 2021;44:669–72. doi: 10.1007/s00270-021-02774-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Leung E, Pervaiz Z, Lowe-Zinola J, Cree S, Kwong A, Marriott N, et al. Maintaining surgical care delivery during the COVID-19 pandemic: A comparative cohort study at a tertiary gynecological cancer centre. Gynecol Oncol. 2021;160:649–54. doi: 10.1016/j.ygyno.2020.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Rodrigues-Pinto E, Ferreira-Silva J, Fugazza A, Capogreco A, Repici A, Everett S, et al. Upper gastrointestinal stenting during the SARS-CoV-2 outbreak: impact of mitigation measures and risk of contamination for patients and staff. Endosc Int Open. 2021;09:E76–86. doi: 10.1055/a-1319-1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Ferahman S, Dönmez T, Sürek A, Aydın H, Gümüşoğlu AY, Karabulut M. Effects of COVID-19 outbreak on emergency surgeries for occlusive colorectal cancers. Turk J Colorectal Dis. 2020;30:237–45. doi: 10.4274/tjcd.galenos.2020.2020-7-2. [DOI] [Google Scholar]
- 108.Vanni G, Tazzioli G, Pellicciaro M, Materazzo M, Paolo O, Cattadori F, et al. Delay in Breast Cancer Treatments During the First COVID-19 Lockdown. A Multicentric Analysis of 432 Patients. Anticancer Res. 2020;40:7119–25. doi: 10.21873/anticanres.14741. [DOI] [PubMed] [Google Scholar]
- 109.Laccourreye O, Mirghani H, Evrard D, Bonnefont P, Brugel L, Tankere F, et al. Impact of the first month of Covid-19 lockdown on oncologic surgical activity in the Ile de France region university hospital otorhinolaryngology departments. Eur Ann Otorhinolaryngol Head Neck Dis. 2020;137:273–6. doi: 10.1016/j.anorl.2020.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Filipe MD, van Deukeren D, Kip M, Doeksen A, Pronk A, Verheijen PM, et al. Effect of the COVID-19 pandemic on surgical breast cancer care in the Netherlands: A multicenter retrospective cohort study. Clin Breast Cancer. 2020;20:454–61. doi: 10.1016/j.clbc.2020.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Purushotham A, Roberts G, Haire K, Dodkins J, Harvey-Jones E, Han L, et al. The impact of national non-pharmaceutical interventions (‘lockdowns’) on the presentation of cancer patients. ecancermedicalscience. 2021 [cited 2022 Feb 17];15. Available from: https://ecancer.org/en/journal/article/1180-the-impact-of-national-non-pharmaceutical-interventions-lockdowns-on-the-presentation-of-cancer-patients [DOI] [PMC free article] [PubMed]
- 112.Morais S, Antunes L, Rodrigues J, Fontes F, Bento MJ, Lunet N. The impact of the COVID-19 pandemic on the short-term survival of patients with cancer in Northern Portugal. Int J Cancer. 2021;149:287–96. doi: 10.1002/ijc.33532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Manso L, De Velasco G, Paz-Ares L. Impact of the COVID-19 outbreak on cancer patient flow and management: experience from a large university hospital in Spain. ESMO Open. 2020;5:e000828. doi: 10.1136/esmoopen-2020-000828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Rogado J, Obispo B, Gullón P, Lara MÁ. Impact of the COVID-19 pandemic in cancer diagnosis in the first and second waves in one of the most affected cancer areas in the city of Madrid (Spain) Int J Cancer. 2021;148:1794–5. doi: 10.1002/ijc.33462. [DOI] [PubMed] [Google Scholar]
- 115.Oderda M, Calleris G, Falcone M, Fasolis G, Muto G, Oderda G, et al. How uro-oncology has been affected by COVID-19 emergency? Data from Piedmont/Valle d’Aosta Oncological Network. Italy. Urol J. 2021;88:3–8. doi: 10.1177/0391560320946186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Slotman BJ, Lievens Y, Poortmans P, Cremades V, Eichler T, Wakefield DV, et al. Effect of COVID-19 pandemic on practice in European radiation oncology centers. Radiother Oncol. 2020;150:40–2. doi: 10.1016/j.radonc.2020.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Park JY, Lee YJ, Kim T, Lee CY, Kim HI, Kim J-H, et al. Collateral effects of the coronavirus disease 2019 pandemic on lung cancer diagnosis in Korea. BMC Cancer. 2020;20:1040. doi: 10.1186/s12885-020-07544-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Zuliani S, Zampiva I, Tregnago D, Casali M, Cavaliere A, Fumagalli A, et al. Organisational challenges, volumes of oncological activity and patients’ perception during the severe acute respiratory syndrome coronavirus 2 epidemic. Eur J Cancer. 2020;135:159–69. doi: 10.1016/j.ejca.2020.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Gordan LN, Weidner S. Impact of the COVID-19 pandemic on specialty community practices: an oncology perspective. Am J Manag Care. 2020;26:SP333–5. doi: 10.37765/ajmc.2020.88569. [DOI] [PubMed] [Google Scholar]
- 120.Gill S, Hao D, Hirte H, Campbell A, Colwell B. Impact of COVID-19 on Canadian Medical Oncologists and Cancer Care: Canadian Association of Medical Oncologists Survey Report. Curr Oncol. 2020;27:71–4. doi: 10.3747/co.27.6643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.de Joode K, Dumoulin DW, Engelen V, Bloemendal HJ, Verheij M, van Laarhoven HWM, et al. Impact of the coronavirus disease 2019 pandemic on cancer treatment: the patients’ perspective. Eur J Cancer. 2020;136:132–9. doi: 10.1016/j.ejca.2020.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Onesti CE, Tagliamento M, Curigliano G, Harbeck N, Bartsch R, Wildiers H, et al. Expected Medium- and Long-Term Impact of the COVID-19 Outbreak in Oncology. JCO Glob Oncol. 2021;5(1):162–72. [DOI] [PMC free article] [PubMed]
- 123.Nicholson P, Ali FR, Mallipeddi R. Impact of COVID-19 on Mohs micrographic surgery: UK-wide survey and recommendations for practice. Clin Exp Dermatol. 2020;45:901–2. doi: 10.1111/ced.14356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Walter J, Sellmer L, Kahnert K, Zauber R, Syunyaeva Z, Kauffmann-Guerrero D, et al. Daily routine and access to care: initial patient reported experiences at a German lung cancer center during the COVID-19 pandemic. Respiration. 2021;100:90–2. doi: 10.1159/000513849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Lamblin G, Golfier F, Peron J, Moret S, Chene G, Nohuz E, et al. Impact de la pandémie de Covid-19 sur les modifications thérapeutiques des patientes atteintes de cancers gynécologiques. Gynécologie Obstétrique Fertil Sénologie. 2020;48:777–83. doi: 10.1016/j.gofs.2020.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Medas F, Ansaldo GL, Avenia N, Basili G, Bononi M, Bove A, et al. Impact of the COVID-19 pandemic on surgery for thyroid cancer in Italy: nationwide retrospective study. Br J Surg. 2021;108:e166–7. doi: 10.1093/bjs/znab012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Tejedor P, Simó V, Arredondo J, López-Rojo I, Baixauli J, Jiménez LM, et al. The impact of SARS-CoV-2 infection on the surgical management of colorectal cancer: Lessons learned from a multicenter study in Spain. Rev Esp Enfermedades Dig. 2020 [cited 2022 Feb 17];113. Available from: https://online.reed.es/fichaArticulo.aspx?iarf=687768749234-416277197164 [DOI] [PubMed]
- 128.Teckie S, Andrews JZ, Chen WCY, Goenka A, Koffler D, Adair N, et al. Impact of the COVID-19 Pandemic Surge on Radiation Treatment: Report From a Multicenter New York Area Institution. JCO Oncol Pract. 2021;17:e1270–7. doi: 10.1200/OP.20.00619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Chang EI, Liu JJ. Flattening the curve in oncologic surgery: Impact of Covid-19 on surgery at tertiary care cancer center. J Surg Oncol. 2020;122:602–7. doi: 10.1002/jso.26056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Bolenz C, Vogel T, Morakis P, Mayr R, Marx M, Burger M. Uroonkologische Therapie in der ersten Welle der COVID-19-Pandemie: Was sagen uns die Krebsregister der deutschen Bundesländer? Urol. 2021;60:291–300. doi: 10.1007/s00120-021-01454-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Maluchnik M, Podwójcic K, Więckowska B. Decreasing access to cancer diagnosis and treatment during the COVID-19 pandemic in Poland. Acta Oncol. 2021;60:28–31. doi: 10.1080/0284186X.2020.1837392. [DOI] [PubMed] [Google Scholar]
- 132.Polan C, Burggraf M, Kauther MD, Meyer H-L, Rademacher F, Braitsch H, et al. Development of case numbers during the COVID-19 pandemic in a center of maximum-care for traumatology and orthopedic oncology. Healthcare. 2020;9:3. doi: 10.3390/healthcare9010003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Oba A, Stoop TF, Löhr M, Hackert T, Zyromski N, Nealon WH, et al. Global survey on pancreatic surgery during the COVID-19 pandemic. Ann Surg. 2020;272:e87–93. doi: 10.1097/SLA.0000000000004006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Rebecchi F, Arolfo S, Ugliono E, Morino M, Asti E, Bonavina L, et al. Impact of COVID-19 outbreak on esophageal cancer surgery in Northern Italy: lessons learned from a multicentric snapshot. Dis Esophagus. 2021;34:doaa124. doi: 10.1093/dote/doaa124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.PelvEx Collaborative, Chok AY, Kontovounisios C, Rasheed S, Kelly ME, Agj A, et al. The impact of the COVID-19 pandemic on the Management of Locally Advanced Primary/Recurrent Rectal Cancer. Br J Surg. 2020;107:e547–8. doi: 10.1002/bjs.11893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Maccagnano C, Rocchini L, Montanari E, Conti GN, Petralia G, Dehò F, et al. Overview of the italian experience in surgical management of bladder cancer during first month of COVID-19 pandemic. Arch Ital Urol E Androl. 2020 [cited 2022 Feb 17];92. Available from: https://www.pagepressjournals.org/index.php/aiua/article/view/aiua.2020.4.275 [DOI] [PubMed]
- 137.de la Portilla de Juan F, Reyes Díaz ML, Ramallo Solía I. Impact of the pandemic on surgical activity in colorectal cancer in Spain. Results of a national survey. Cir Esp Engl Ed. 2021;99:500–5. doi: 10.1016/j.ciresp.2020.07.011. [DOI] [PubMed] [Google Scholar]
- 138.Brunner M, Krautz C, Kersting S, Weber GF, Stinner B, Benz SR, et al. Oncological colorectal surgery during the COVID-19pandemic—a national survey. Int J Colorectal Dis. 2020;35:2219–25. doi: 10.1007/s00384-020-03697-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Gasparri ML, Gentilini OD, Lueftner D, Kuehn T, Kaidar-Person O, Poortmans P. Changes in breast cancer management during the Corona Virus Disease 19 pandemic: An international survey of the European Breast Cancer Research Association of Surgical Trialists (EUBREAST) The Breast. 2020;52:110–5. doi: 10.1016/j.breast.2020.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.The Italian Colorectal Anastomotic Leakage (iCral) study group. Caricato M, Baiocchi GL, Crafa F, Scabini S, Brisinda G, et al. Colorectal surgery in Italy during the Covid19 outbreak: a survey from the iCral study group. Updat Surg. 2020;72:249–57. doi: 10.1007/s13304-020-00760-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Thaler M, Khosravi I, Leithner A, Papagelopoulos PJ, Ruggieri P. Impact of the COVID-19 pandemic on patients suffering from musculoskeletal tumours. Int Orthop. 2020;44:1503–9. doi: 10.1007/s00264-020-04636-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Higgins E, Walters S, Powell E, Staffurth J. The Impact of the Acute Phase of COVID-19 on Radiotherapy Demand in South East Wales. Clin Oncol. 2020;32:e217. doi: 10.1016/j.clon.2020.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Spencer K, Jones CM, Girdler R, Roe C, Sharpe M, Lawton S, et al. The impact of the COVID-19 pandemic on radiotherapy services in England, UK: a population-based study. Lancet Oncol. 2021;22:309–20. doi: 10.1016/S1470-2045(20)30743-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Nieder C, Haukland EC, Mannsaker B, Yobuta R. Palliative Radiotherapy During the Last Month of Life: Have COVID-19 Recommendations Led to Reduced Utilization? In Vivo. 2021;35:649–52. doi: 10.21873/invivo.12304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Roberge D, Delouya G, Bohigas A, Michalowski S. Catching the Wave: Quantifying the Impact of COVID on Radiotherapy Delivery. Curr Oncol. 2020;28:152–8. doi: 10.3390/curroncol28010018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Clark JJ, Dwyer D, Pinwill N, Clark P, Johnson P, Hackshaw A. The effect of clinical decision making for initiation of systemic anticancer treatments in response to the COVID-19 pandemic in England: a retrospective analysis. Lancet Oncol. 2021;22:66–73. doi: 10.1016/S1470-2045(20)30619-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Baxter MA, Murphy J, Cameron D, Jordan J, Crearie C, Lilley C, et al. The impact of COVID-19 on systemic anticancer treatment delivery in Scotland. Br J Cancer. 2021;124:1353–6. doi: 10.1038/s41416-021-01262-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Jazieh AR, Akbulut H, Curigliano G, Rogado A, Alsharm AA, Razis ED, et al. Impact of the COVID-19 Pandemic on Cancer Care: A Global Collaborative Study. JCO Glob Oncol. 2020;6:1428–38. [DOI] [PMC free article] [PubMed]
- 149.Pendyala P, Goglia AG, Mattes MD, Grann A, Huang D, Wagman RT, et al. Impact of the Coronavirus Disease of 2019 Pandemic on Radiation Oncology Clinical Decision Making in a High-Prevalence Environment. Adv Radiat Oncol. 2021;6:100680. doi: 10.1016/j.adro.2021.100680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Kuitunen I, Ponkilainen VT, Uimonen MM, Paloneva J, Launonen AP, Mattila VM. Postponing elective surgery due to COVID-19 did not decrease the oncological surgery rate in Finland. Br J Surg. 2021;108:e191–3. doi: 10.1093/bjs/znab046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Engelina S, Watson L, O’Connell D, Kennedy M, Ilchyshyn A. Effects of COVID-19 on the 2-week-wait dermatology services at a regional centre between 2019 and 2020. Br J Dermatol. 2020;183(SUPPL1):208.
- 152.Depypere LP, Daddi N, Gooseman MR, Batirel HF, Brunelli A. The impact of coronavirus disease 2019 on the practice of thoracic oncology surgery: a survey of members of the European Society of Thoracic Surgeons (ESTS) Eur J Cardiothorac Surg. 2020;58:752–62. doi: 10.1093/ejcts/ezaa284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Earnshaw CH, Hunter HJA, McMullen E, Griffiths CEM, Warren RB. Reduction in skin cancer diagnosis, and overall cancer referrals, during the COVID-19 pandemic. Br J Dermatol. 2020;183:792–4. doi: 10.1111/bjd.19267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Muñoz-Martínez S, Sapena V, Forner A, Nault J-C, Sapisochin G, Rimassa L, et al. Assessing the impact of COVID-19 on liver cancer management (CERO-19) JHEP Rep. 2021;3:100260. doi: 10.1016/j.jhepr.2021.100260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Bogani G, Apolone G, Ditto A, Scambia G, Panici PB, Angioli R, et al. Impact of COVID-19 in gynecologic oncology: a Nationwide Italian Survey of the SIGO and MITO groups. J Gynecol Oncol. 2020;31:e92. doi: 10.3802/jgo.2020.31.e92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Sadler D, DeCara JM, Herrmann J, Arnold A, Ghosh AK, Abdel-Qadir H, et al. Perspectives on the COVID-19 pandemic impact on cardio-oncology: results from the COVID-19 International Collaborative Network survey. Cardio-Oncol. 2020;6:1–13. doi: 10.1186/s40959-019-0056-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Kamarajah SK, Markar SR, Singh P, Griffiths EA, Oesophagogastric Anastomosis Audit Group The influence of the SARS-CoV-2 pandemic on esophagogastric cancer services: an international survey of esophagogastric surgeons. Dis Esophagus. 2020;33:doaa054. doi: 10.1093/dote/doaa054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Mason SE, Scott AJ, Markar SR, Clarke JM, Martin G, Winter Beatty J, et al. Insights from a global snapshot of the change in elective colorectal practice due to the COVID-19 pandemic. Fong ZV, editor. Plos One. 2020;15:e0240397. doi: 10.1371/journal.pone.0240397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Frey MK, Ellis AE, Zeligs K, Chapman-Davis E, Thomas C, Christos PJ, et al. Impact of the coronavirus disease 2019 pandemic on the quality of life for women with ovarian cancer. Am J Obstet Gynecol. 2020;223:725.e1–725.e9. doi: 10.1016/j.ajog.2020.06.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Martinelli F, Garbi A. Change in practice in gynecologic oncology during the COVID-19 pandemic: a social media survey. Int J Gynecol Cancer. 2020;30:1101–7. doi: 10.1136/ijgc-2020-001585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Elkrief A, Kazandjian S, Bouganim N. Changes in Lung Cancer Treatment as a Result of the Coronavirus Disease 2019 Pandemic. JAMA Oncol. 2020;6:1805. doi: 10.1001/jamaoncol.2020.4408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Reuter-Oppermann M, Müller-Polyzou R, Wirtz H, Georgiadis A. A flash survey in Germany, Austria and Switzerland. Chun S, editor. PLoS One. 2020;15:e0233330. doi: 10.1371/journal.pone.0233330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Bartels MMTJ, Gal R, van der Velden JM, Verhoeff JJC, Verlaan JJ, Verkooijen HM. Impact of the COVID-19 pandemic on quality of life and emotional wellbeing in patients with bone metastases treated with radiotherapy: a prospective cohort study. Clin Exp Metastasis. 2021;38:209–17. doi: 10.1007/s10585-021-10079-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Ürün Y, Hussain SA, Bakouny Z, Castellano D, Kılıçkap S, Morgan G, et al. Survey of the Impact of COVID-19 on Oncologists’ Decision Making in Cancer. JCO Glob Oncol. 2020;6:1248–57. [DOI] [PMC free article] [PubMed]
- 165.Baffert K-A, Darbas T, Lebrun-Ly V, Pestre-Munier J, Peyramaure C, Descours C, et al. Quality of Life of Patients With Cancer During the COVID-19 Pandemic. In Vivo. 2021;35:663–70. doi: 10.21873/invivo.12306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Aeppli S, Eboulet E, Eisen T, Escudier B, Fischer S, Larkin J, et al. Impact of COVID-19 pandemic on treatment patterns in metastatic clear cell renal cell carcinoma. ESMO Open. 2020;5:e000852. doi: 10.1136/esmoopen-2020-000852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Chang PJ, Jay GM, Kalpakjian C, Andrews C, Smith S. Patient and provider-reported satisfaction of cancer rehabilitation telemedicine visits during the COVID-19 pandemic. PM&R. 2021;13:1362–8. doi: 10.1002/pmrj.12552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Fujisawa, Rie. Impact of the COVID-19 pandemic on cancer care in OECD countries. OECD Health Working Papers. [Internet]. 2022 May. Report No.: 141. Available from: https://www.oecd-ilibrary.org/social-issues-migration-health/impact-of-the-covid-19-pandemic-on-cancer-care-in-oecd-countries_c74a5899-en
- 169.Mitchell RD, O’Reilly GM, Mitra B, Smit DV, Miller J, Cameron PA. Impact of COVID-19 State of Emergency restrictions on presentations to two Victorian emergency departments. Emerg Med Australas. 2020;32:1027–33. doi: 10.1111/1742-6723.13606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Vanni G, Legramante JM, Pellicciaro M, De Carolis G, Cotesta M, Materazzo M, et al. Effect of Lockdown in Surgical Emergency Accesses: Experience of a COVID-19 Hospital. In Vivo. 2020;34:3033–8. doi: 10.21873/invivo.12137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Mansfield KE, Mathur R, Tazare J, Henderson AD, Mulick AR, Carreira H, et al. Indirect acute effects of the COVID-19 pandemic on physical and mental health in the UK: a population-based study. Lancet Digit Health. 2021;3:e217–30. doi: 10.1016/S2589-7500(21)00017-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Stephenson E, Butt DA, Gronsbell J, Ji C, O’Neill B, Crampton N, et al. Changes in the top 25 reasons for primary care visits during the COVID-19 pandemic in a high-COVID region of Canada. Leong C, editor. PLoS One. 2021;16:e0255992. doi: 10.1371/journal.pone.0255992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Sloan M, Lever E, Harwood R, Gordon C, Wincup C, Blane M, et al. Telemedicine in rheumatology: a mixed methods study exploring acceptability, preferences and experiences among patients and clinicians. Rheumatology. 2022;61(6):2262–74. [DOI] [PMC free article] [PubMed]
- 174.Elam AR, Sidhom D, Ugoh P, Andrews CA, De Lott LB, Woodward MA, et al. Disparities in Eye Care Utilization During the COVID-19 Pandemic. Am J Ophthalmol. 2022;233:163–70. doi: 10.1016/j.ajo.2021.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Maringe C, Spicer J, Morris M, Purushotham A, Nolte E, Sullivan R, et al. The impact of the COVID-19 pandemic on cancer deaths due to delays in diagnosis in England, UK: a national, population-based, modelling study. Lancet Oncol. 2020;21:1023–34. doi: 10.1016/S1470-2045(20)30388-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Seretis K, Boptsi E, Boptsi A, Lykoudis EG. The impact of treatment delay on skin cancer in COVID-19 era: a case-control study. World J Surg Oncol. 2021;19:350. doi: 10.1186/s12957-021-02468-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Reuters. Facing new COVID wave, Dutch delay care for cancer, heart patients. [Internet]. 2021 [cited 2022 Jan 18]. Available from: https://www.reuters.com/world/europe/facing-new-covid-wave-dutch-delay-care-cancer-heart-patients-2021-11-19/
- 178.Aboulenein A. (Reuters). Overwhelmed by Omicron surge, U.S. hospitals delay surgeries [Internet]. [cited 2022 Jan 25]. Available from: https://www.reuters.com/world/us/overwhelmed-by-omicron-surge-us-hospitals-delay-surgeries-2022-01-07/
- 179.European Cancer Organization. “Time to Act Data Navigator”. [cited 2021 Dec 9]. Available from: https://www.europeancancer.org/data-navigator/#
- 180.International Agency for Research on Cancer WHO. Global Cancer Observatory. [cited 2022 Jan 8]. Available from: https://gco.iarc.fr/about-the-gco
- 181.Carvalho, Ana Sofia, Brito Fernandes, Óscar, de Lange, Mats, Lingsma, Hester, Klazinga, Niek, Kringos, Dionne. Dataset_Scoping review_Changes in quality of cancer care assessed through performance indicators. Zenodo. 2022. Available from: 10.5281/zenodo.6129839 [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1. Preferred Reporting Items for Systematic reviews and Meta-Analyses extension for Scoping Reviews(PRISMA-ScR) Checklist
Data Availability Statement
The datasets generated and analysed during the current study are available in the Zenodo.org repository, at https://doi.org/10.5281/zenodo.6129839. [181]