Abstract
Germline genetic testing is recommended for all patients with pancreatic cancer (PC) but uptake rates are low. We implemented a mainstreaming program in oncology clinics to increase testing for PC patients. Genetic counselors trained oncology providers to offer a standardized multigene panel and obtain informed consent using an educational video. Pre-test genetic counseling was available upon request. Otherwise, patients with identified pathogenic variants, strong family history, or questions regarding their results were referred for post-test genetic counseling. We measured rates of testing and genetic counseling visits. From September 2019 to April 2021, 245 patients with PC underwent genetic testing. This represents a 6.5-fold increase in germline testing volume (95% confidence interval 5.2–8.1) compared to previous years. At least one pathogenic or likely pathogenic variant (PV/LPV) was found in 34 (13.9%) patients, including 17 (6.9%) PV/LPVs in high or moderate risk genes and 18 (7.3%) in low risk or recessive genes. Five (2.0%) PVs had implications on treatment selection. 22 of the positive patients (64.7%) and an additional 8 PC patients (1 negative, 3 VUS, and 4 pre-test) underwent genetic counseling during the study period. Genetic counselors saw 2.0 PC patients/month prior to this project, 1.6 PC patients/month during this project, and would have seen 2.2 PC patients/month if all patients with pathogenic variants attended post-test counseling. Conclusions Mainstreaming genetic testing expands access for PC patients without overwhelming genetic counseling resources.
Supplementary Information
The online version contains supplementary material available at 10.1007/s10689-022-00300-5.
Keywords: BRCA1/2, PALB2, Pancreatic ductal adenocarcinoma, Healthcare delivery
Introduction
Germline genetic testing has been recommended for all patients diagnosed with pancreatic cancer (PC) by the National Comprehensive Cancer Network’s (NCCN) guidelines since 2019 [1, 2]. Genetic testing may identify therapeutic targets, such as the use of immune checkpoint inhibitors for patients with mismatch repair deficiency or poly-ADP ribose polymerase (PARP) inhibitors for patients with variants affecting DNA damage repair [3–5]. There are also implications for family members, who may require intensive cancer surveillance protocols if a pathogenic variant (PV) is discovered. Despite these benefits, only 32% of patients with PC undergo germline testing [6].
A dedicated pre-test genetic counseling appointment to discuss genetic testing presents a barrier to test completion, especially among underserved populations [7]. Additionally, genetic counselor availability may be limited and long wait times are common [8]. Due to the poor prognosis for patients with PC, these delays in accessing pre-test genetic counseling can eliminate the opportunity to test the affected individual. Novel strategies to improve access to genetic testing among patients with PC are needed.
One opportunity to increase germline testing among patients with PC is to shift from genetic counselor-led pre-test genetic counseling to pre-test informed consent in the oncology clinic with post-test genetic counseling for those found to have a pathogenic or likely pathogenic germline variant (PV/LPV) in a cancer susceptibility gene. This approach is called “mainstreaming” genetic testing and has been shown to be acceptable and feasible in a variety of cancer types, including breast cancer [9] and, very recently, pancreatic cancer [10–12]. Mainstreaming is particularly useful when universal testing is recommended, as this eliminates the need for providers to apply complex criteria to identify the patients who are eligible for genetic testing. In this model, oncology clinicians obtain informed consent and refer patients to a genetic counselor when an abnormal result is encountered although the specifics of post-test counseling can vary by institution. Post-test counseling options range from a uniform requirement to meet a genetic counselor regardless of results to a consultant or collaborative model where the clinician might ask the genetic counselor for risk assessment and then relay this information to the patient [13].
Pancreatic cancer is a good candidate for the mainstreaming approach given the barriers discussed above. Additionally, the prevalence of PV/LPVs is between 8 and 20% among PC patients [12, 14–16]. This means that 80–92% of PC patients will test negative or have a variant of uncertain significance (VUS) in a cancer gene and may not require an additional visit for post-test genetic counseling. Of note, PC patients with negative or VUS results can have post-test genetic counseling upon request and the oncology clinicians are trained to refer those with a strong family history to Genetics despite their negative or VUS result.
We implemented a mainstreaming project to offer germline testing in our oncology clinics for patients with PC followed by post-test genetic counseling for those with a PV/LPV in a cancer susceptibility gene. We hypothesized that eliminating the pre-test genetic counseling visit would increase the rate of germline testing at our institution without dramatically affecting genetic counseling patient volumes.
Methods
Beginning in September 2019, all patients treated for PC at our center were offered germline testing in medical oncology clinics, and this was expanded to include surgical oncology clinics in November 2020. This was a retrospective cohort study; no power calculation was performed. No study blinding was performed. Descriptive statistics were used to summarize the number of tests completed before and during the mainstreaming program. Genetic counselors trained non-physician providers (RN, APRN-CNP) to show an informational video, obtain informed consent, complete the test requisition, and collect and ship the sample. Training of non-physician providers consisted of an hour-long educational session run by GCs that described the importance of germline testing in PC, the specifics of informed consent, the process of collecting and shipping samples, and when to refer patients to Genetics. The session was recorded and made available to new nurses in medical and surgical oncology clinics. The lead genetic counselor (HH) was copied on all germline testing orders for quality assurance and provided interpretation of PV/LPV results to clinicians. Testing with an 86-gene panel (Supplemental Table 1) was performed through a free sponsored testing program thus eliminating complex discussions about cost. The test offer and result disclosure were documented in the electronic medical record (EMR) by the oncology team using templates developed by the genetic counselors. The EMR and project spreadsheet were used to quantify patient volumes throughout the duration of the study.
Description of mainstreaming approach
The short informational video (approximately 7 min in duration) described the indications for testing, implications of potential results, and the availability of genetic counseling after the test result is known [17]. This video is readily available online (https://www.invitae.com/en/pretest-video-cancer/), and the clinic nurse would start the video online using the computers in the exam rooms after completing the check-in and rooming process. Once the video was playing, the clinic nurse left the room to attend to other tasks, and the patient and their caregivers could watch the video while waiting for their oncologist. The video could be paused and restarted as needed, thus it did not interrupt clinic flow. The next provider to enter the room (either the oncology provider or clinic nurse) would then obtain informed consent, and the clinic nurse would collect and ship the sample at the end of the patient visit. This process places most of the testing burden on the clinic nurse and may prolong clinic visits slightly, but it does not require additional providers or separate patient encounters to complete germline testing. Those involved in implementing this project have not experienced any negative impact to their clinic workflow or overall workload.
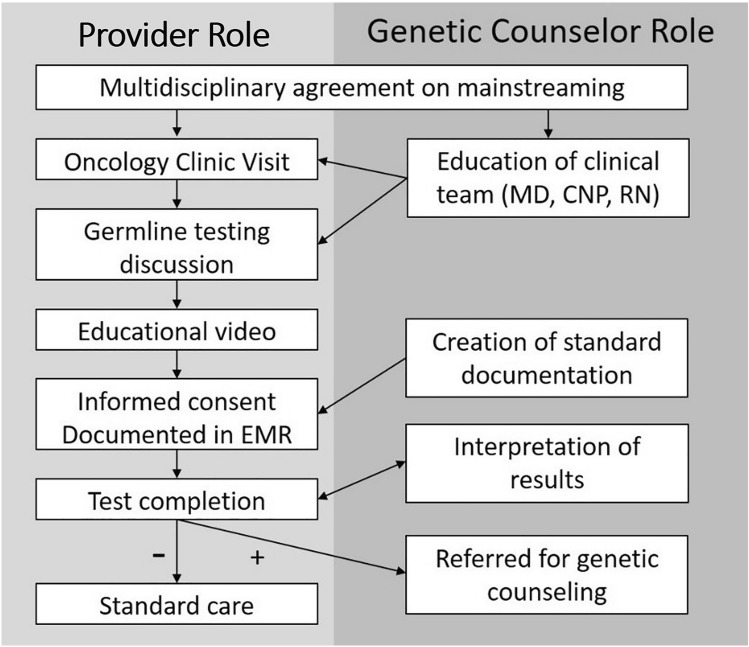
Patients with PV/LPV in cancer genes were referred for post-test genetic counseling (Fig. 1). Patients with variants of uncertain significance (VUS) or negative results were referred for post-test genetic counseling upon request or if they had a significant family history of cancer and required additional follow-up despite the negative result. Genetic counseling visits occurred in person until March 2020 when our institution shifted all visits to video conferencing due to the SARS-CoV-2 pandemic.
Fig. 1.
Clinician and genetic counselor roles during the mainstreaming project
Results
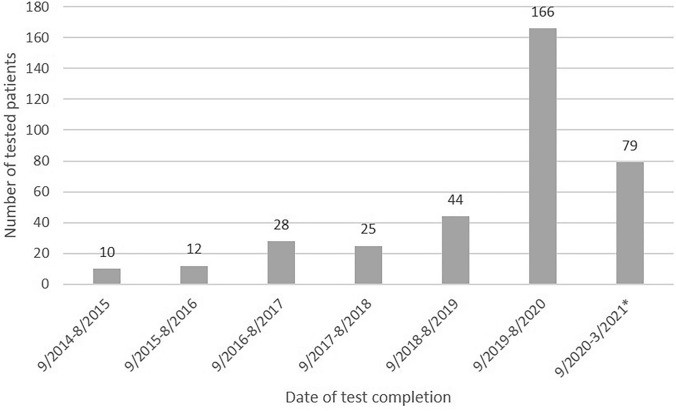
From September 2019 until April 2021, 241 patients underwent genetic testing through this mainstreaming program. An additional 4 patients received traditional pre-test genetic counseling and elected to pursue genetic testing for a total of 245 patients tested during the study period. Median age was 66 (range 38–88) and 52.5% were male. During the same time, 369 patients with PC were seen at our institution so genetic testing was performed on 66.4% of patients. No information is available for the 124 patients who did not undergo testing. Prior to this program, our center had completed genetic testing for 119 PC patients out of 1192 PC patients seen over the previous 5 years (10%). The mainstreaming project, therefore, increased genetic testing among PC patients 6.5-fold (95% confidence interval 5.2–8.1), from 2.0 patients per month prior to the program implementation to 12.9 patients per month during the program (Fig. 2).
Fig. 2.
Number of patients with pancreatic ductal adenocarcinoma who underwent germline testing. *6 month period
Of the 245 subjects tested during the study period, 34 (13.9%) patients had a PV/LPV in at least one gene; 17 (6.9%) of which occurred in moderate/high risk genes. Eight patients were found to have PV/LPV in high-penetrance genes (1 BRCA1, 3 BRCA2, 1 PALB2, 1 FH, 1 PRSS1, 1 RUNX1), 8 in moderate-penetrance genes (4 ATM, 4 CHEK2), and 18 in low-penetrance or recessive genes (3 heterozygous CFTR, 1 MITF, 3 RAD50, 1 BLM, 6 heterozygous MUTYH, 1 RECQL, 1 heterozygous NTHL1, 1 CHEK2 I157T, 1 NBN). Of the tested population, 5 (2.0%) subjects were found to have variants with genotype-directed therapies (4 BRCA1/2 and 1 PALB2) and one was treated with a PARP inhibitor. One subject with BRCA2 and prostatomegaly underwent a prostate biopsy to assess for comorbid prostate cancer, but the other subjects did not have additional cancer surveillance or risk reducing procedures.
Post-test genetic counseling occurred for 22/34 (64.7%) patients (or a relative if deceased) at a median of 33.5 days after result receipt (range: 4–196). The remaining 12 patients did not complete post-test counseling because they declined the referral or did not schedule the visit (n = 7), were not referred (n = 1, heterozygous for an autosomal recessive condition), or died prior to completing counseling (n = 4). In addition to the PV/LPV patients who attended counseling, 4 patients were seen for pre-test counseling, 3 patients with VUS were seen for post-test genetic counseling, and 1 patient with negative results but a strong family history of PC was seen for post-test genetic counseling. Prior to this mainstreaming project, genetic counselors were counseling 2.0 PC patients/month. During the study, 1.6 patients/month were counseled. If all of the PC patients with a PV/LPV in a cancer gene had attended post-test genetic counseling, genetic counselors would be seeing 2.2 PC patients/month.
Discussion
Mainstreaming germline genetic testing for patients with PC facilitated a 6.5-fold increase in the number of patients undergoing testing compared to the traditional pre-test genetic counseling model. The increase in testing was proportional to the rate of positive results, such that the number of genetic counseling visits for PC would remain relatively stable if all of the individuals with PV/LPV variants in cancer genes followed through with their post-test genetic counseling. However, the content of the visit shifted from pre-test education to PV-specific post-test genetic counseling with a focus on cascade testing in at-risk relatives and intensive surveillance recommendations for PV-positive family members. This suggests that mainstreaming of genetic testing for PC increases detection of PV/LPV without significantly impacting genetic counselor visit volumes.
The primary goal of this mainstreaming project was to increase the proportion of PC patients undergoing germline genetic testing, while still providing thorough genetic counseling to those who would have the greatest benefit. We achieved this by educating oncology providers to order germline testing from their clinics. Similar strategies have been implemented in more common malignancies, such as breast and colorectal cancer, and have increased the rates of germline testing in those conditions as well [9, 18]. However, differences in the quality of pre-test counseling provided by oncology staff compared to genetic counselors have been detected [19]. In our study, an educational video was utilized to address this gap, as automation of components of pre-test counseling has been previously shown to improve patient knowledge of germline testing and feelings of empowerment [20]. While the patients did not receive full pre-test genetic counseling, they did receive the information necessary to provide informed consent for testing. This reserves limited genetic counseling resources for those with hereditary cancer syndromes or concerning family histories who need it most.
In a mainstreaming model, genetic counselors are involved on a consultation basis after genetic test results are available. In our study, genetic counselors informed clinicians to refer PC patients with PV/LPVs to Genetics, reviewed all results, and provided post-test genetic counseling to the PC patients with PV/LPVS and to PC patients with negative results or variants of unknown significance that had concerning family histories or questions about their results. This model of post-test counseling is most closely aligned with the “post-test counseling—complex” model described by Trepanier, et al. [13]. This model is appropriate for scenarios where pre-test risk assessment is straightforward, which would include cancers with recommendations for universal genetic testing, including ovarian and pancreatic [2, 21]. In our study, all subjects with PC underwent the same 86-gene pan-cancer panel as determined by the sponsored program, so there was no need for additional individual risk assessment prior to test selection. In contrast, scenarios where pre-test risk assessment is more complex should employ either a traditional or collaborative model, engaging a genetic counselor for assistance with pre-test risk assessment and post-test counseling [13]. In these scenarios, involvement of a genetic counselor at the outset improves adherence to testing recommendations and improves the quality of pre-test education and counseling [22]. As indications for universal genetic testing expand, collaboration between genetic counselors and clinicians to create appropriate referral patterns will need to be established. Universal genetic testing guidelines are ideal for implementing mainstreaming models as they reduce or eliminate the need for providers to apply complex criteria to identify the patients who are eligible for genetic testing. Cost of testing is another component of pre-test counseling, and a systematic way of handling this aspect of germline testing is necessary to implement mainstreaming. In our case, the testing was free through a sponsored testing program at the commercial laboratory. However, if such a program was not available, costs of testing could be included in the pre-test educational video, or by creating a fact sheet with information about the billing policies of the laboratory and maximum out-of-pocket costs (e.g. “The laboratory will contact you if you are expected to owe > $100 out of pocket for the testing. At that time, you can choose to stop the test, apply for the financial assistance program at the laboratory, pay the out-of-pocket maximum of $250, or proceed with testing knowing your estimated out of pocket expense.”). We suggest that genetic counselors and clinicians collaborate to create local clinical practice guidelines according to each indication for testing, followed by automation of eligible components in order to maximize access and throughput.
Our results are similar to those of other groups who have recently applied the mainstreaming approach to PC. Some recent publications describe the mainstreaming approach among subjects with PC, and report germline test completion in 65–71% of patients with PC [12, 23]. In contrast, during this same period another group systematized the traditional genetic counseling and testing approach and only tested 38% of eligible subjects, [16] highlighting the strength of mainstreaming approaches. Importantly, Hamilton, et al. report that patient satisfaction was high with the mainstreaming approach [23] and Bokkers et al. report that pre-test counseling by a non-genetic counselor does not necessarily lead to a large time investment [24]. Mainstreaming was accomplished in one group by implementing a Genetic Testing Station in the oncology clinic, which was staffed by genetic counseling assistants who guided patients through a pre-recorded video and brochure [12]. While effective, this required additional personnel and added an additional component to the patient’s visit (i.e., visit to the kiosk after other components of the oncology visit are complete). Our approach involved additional training of existing personnel who are already interacting with the patient, and the education components were built into existing downtime during the visit (i.e., while waiting in room for provider). Furthermore, our post-test counseling method of reserving counseling only for those with PV/LPV or with strong family history further reduces the resources required to complete the germline testing process.
Interestingly, the volume of patients with PC seen by genetic counselors during our study decreased slightly since not all of the patients with PV/LPV pursued post-test genetic counseling. If all patients with PV/LPV had attended post-test counseling, then the volume would have increased by 10%. The rate of PV/LPV in our study (13.9%) is similar to previous reports [12, 14, 15]. For more prevalent cancers, like colorectal cancer, mainstreaming might lead to a dramatic increase in uptake of germline testing, which could increase genetic counseling visit volumes. One recent study automated the risk assessment and pre-test counseling and completed testing on 129 subjects attending colonoscopy who might have otherwise never been tested, 9% of which had a germline PV [25]. Efficient post-test counseling strategies, such as group or telemedicine visits, may need to be developed to accommodate any increase in post-test visits [13].
We recognize several limitations to our study. Genetic testing in our study was performed at no cost to participants, which may have increased completion of genetic testing regardless of the genetic counseling approach. However, this is unlikely to increase test completion to the extent observed in our study as low- or no-cost testing is now widely available and all of the patients met NCCN guidelines for genetic testing which generally leads to good insurance coverage. Additionally, our study period coincided with a change in NCCN guidelines to offer universal testing, which certainly increased the number of patients eligible for testing and presents a confounding variable for measuring the efficacy of mainstreaming. However, recommendations for universal testing in PC were issued in July 2018—14 months prior to the implementation of our mainstreaming program, which began in September of 2019. During the period from September 2018 to August 2019, 44 out of 269 PC subjects were tested (16.4%), representing an increase of 76% compared to the previous year. However, once the mainstreaming project was implemented, 166 out of 240 (69.1%) PC subjects completed testing, representing a 377% increase in the rate of test completions. This high rate of test completion persisted throughout the study period, which indicates that the initial increase was not entirely attributed to legacy patients obtaining testing under the new NCCN recommendations. Lastly, the SARS-CoV-2 pandemic occurred during the study period and affected healthcare delivery in many ways. Switching from in-person to telehealth visits is one potential confounding variable, although this is unlikely to have altered the rate of germline test uptake. Finally, this reports a single center experience and the findings may not be replicable in other centers or countries.
Despite these limitations, our study has many strengths. This project was conducted for over 18 months and encompasses a great number of patients seen in both medical and surgical oncology clinics. We measure the impact of this change compared to 5 years of historical control data. Additionally, we completed germline testing for a majority of PC patients seen at our institution, which minimizes selection bias and improves internal validity.
Conclusions
Mainstreaming germline genetic testing increases test completion 6.5-fold among patients with PC, without increasing genetic counselor visit volumes. This represents an opportunity to increase identification of hereditary cancer syndromes without overwhelming genetic counseling service lines. Genetic counselors should be involved in establishing appropriate pre-test education and should be available to provide post-test counseling for those with PV/LPV and those with negative or VUS results who have a strong family history or are concerned about their result. Assessment of patient and family satisfaction with the post-testing approach should be performed prior to widespread adoption in pancreatic cancer and other cancer types.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
We acknowledge the contributions of The Ohio State University Medical Oncology nursing pool, who contributed to patient recruitment and education throughout this project.
Funding
Germline genetic testing was provided by Invitae at no cost to patients throughout this project.
Data availability
De-identified data may be made available to researchers on reasonable request.
Declarations
Conflict of interest
PPS receives research support from Emtora Biosciences, Freenome Holdings, Janssen Pharmaceuticals Inc., Pfizer Inc. and the PTEN Research foundation. HH is on the scientific advisory boards for Invitae, Promega, and Genome Medical and has stock/stock options in Genome Medical and GI OnDemand. The remaining authors declare no conflict of interest related to this research.
Ethical approval
This project was reviewed and approved by The Ohio State University Institutional Review Board (protocol 2021E0849).
Consent to participate
Given the retrospective nature of this study and minimal risk of reporting aggregate data, a waiver of informed consent was granted by the Institutional Review Board.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Mitchell L. Ramsey and Jewel Tomlinson have contributed equally to this work.
References
- 1.Tempero MA. NCCN guidelines updates: pancreatic cancer. J Natl Compr Cancer Netw. 2019;17(55):603–605. doi: 10.6004/jnccn.2019.5007. [DOI] [PubMed] [Google Scholar]
- 2.Daly MB, Pal T, Berry MP, Buys SS, Dickson P, Domchek SM, et al. Genetic/familial high-risk assessment: breast, ovarian, and pancreatic, version 2.2021, NCCN clinical practice guidelines in oncology. J Natl Compr Cancer Netw. 2021;19(1):77–102. doi: 10.6004/jnccn.2021.0001. [DOI] [PubMed] [Google Scholar]
- 3.Marabelle A, Le DT, Ascierto PA, Di Giacomo AM, De Jesus-Acosta A, Delord JP, et al. Efficacy of pembrolizumab in patients with noncolorectal high microsatellite instability/mismatch repair-deficient cancer: results from the phase II KEYNOTE-158 study. J Clin Oncol. 2020;38(1):1–10. doi: 10.1200/JCO.19.02105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Golan T, Hammel P, Reni M, Van Cutsem E, Macarulla T, Hall MJ, et al. Maintenance olaparib for germline BRCA-mutated metastatic pancreatic cancer. N Engl J Med. 2019;381(4):317–327. doi: 10.1056/NEJMoa1903387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stadler ZK, Maio A, Chakravarty D, Kemel Y, Sheehan M, Salo-Mullen E, et al. Therapeutic implications of germline testing in patients with advanced cancers. J Clin Oncol. 2021;39(24):2698–2709. doi: 10.1200/JCO.20.03661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Walker EJ, Carnevale J, Pedley C, Blanco A, Chan S, Collisson EA, et al. Referral frequency, attrition rate, and outcomes of germline testing in patients with pancreatic adenocarcinoma. Fam Cancer. 2019;18(2):241–251. doi: 10.1007/s10689-018-0106-2. [DOI] [PubMed] [Google Scholar]
- 7.Stenehjem DD, Au T, Sainski AM, Bauer H, Brown K, Lancaster J, et al. Impact of a genetic counseling requirement prior to genetic testing. BMC Health Serv Res. 2018;18(1):165. doi: 10.1186/s12913-018-2957-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cohen SA, Gustafson SL, Marvin ML, Riley BD, Uhlmann WR, Liebers SB, et al. Report from the national society of genetic counselors service delivery model task force: a proposal to define models, components, and modes of referral. J Genet Couns. 2012;21(5):645–651. doi: 10.1007/s10897-012-9505-y. [DOI] [PubMed] [Google Scholar]
- 9.Beard C, Monohan K, Cicciarelli L, James PA. Mainstream genetic testing for breast cancer patients: early experiences from the Parkville familial cancer centre. Eur J Hum Genetics. 2021 doi: 10.1038/s41431-021-00848-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Scheinberg T, Young A, Woo H, Goodwin A, Mahon KL, Horvath LG. Mainstream consent programs for genetic counseling in cancer patients: a systematic review. Asia-Pac J Clin Oncol. 2020 doi: 10.1111/ajco.13334. [DOI] [PubMed] [Google Scholar]
- 11.Hamilton JG, Symecko H, Spielman K, Breen K, Mueller R, Catchings A et al (2021) Uptake and acceptability of a mainstreaming model of hereditary cancer multigene panel testing among patients with ovarian, pancreatic, and prostate cancer. Genetics Med 23(11):2105–2113. 10.1038/s41436-021-01262-2 [DOI] [PMC free article] [PubMed]
- 12.Walker EJ, Goldberg D, Gordon KM, Pedley C, Carnevale J, Cinar P, et al. Implementation of an embedded in-clinic genetic testing station to optimize germline testing for patients with pancreatic adenocarcinoma. Oncologist. 2021;26(11):e1982–e91. doi: 10.1002/onco.13968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Trepanier AM, Allain DC. Models of service delivery for cancer genetic risk assessment and counseling. J Genet Couns. 2014;23(2):239–253. doi: 10.1007/s10897-013-9655-6. [DOI] [PubMed] [Google Scholar]
- 14.Hu C, Hart SN, Polley EC, Gnanaolivu R, Shimelis H, Lee KY, et al. Association between inherited germline mutations in cancer predisposition genes and risk of pancreatic cancer. JAMA. 2018;319(23):2401–2409. doi: 10.1001/jama.2018.6228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Salo-Mullen EE, O’Reilly EM, Kelsen DP, Ashraf AM, Lowery MA, Yu KH, et al. Identification of germline genetic mutations in patients with pancreatic cancer. Cancer. 2015;121(24):4382–4388. doi: 10.1002/cncr.29664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chittenden A, Haraldsdottir S, Ukaegbu C, Underhill-Blazey M, Gaonkar S, Uno H, et al. Implementing systematic genetic counseling and multigene germline testing for individuals with pancreatic cancer. JCO Oncol Pract. 2021;17(2):e236–e47. doi: 10.1200/OP.20.00678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Strassberg M. Genetic testing for hereditary cancer. Available from: https://www.invitae.com/en/pretest-video-cancer/. Accessed 01 June 2021
- 18.O’Shea R, Rankin NM, Kentwell M, Gleeson M, Tucker KM, Hampel H. Stakeholders’ views of integrating universal tumour screening and genetic testing for colorectal and endometrial cancer into routine oncology. Eur J Hum Genetics. 2021 doi: 10.1038/s41431-021-00871-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McCuaig JM, Thain E, Malcolmson J, Keshavarzi S, Armel SR, Kim RH. A comparison of patient-reported outcomes following consent for genetic testing using an oncologist- or genetic counselor-mediated model of care. Curr Oncol (Toronto Ont) 2021;28(2):1459–1471. doi: 10.3390/curroncol28020138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cragun D, Weidner A, Tezak A, Zuniga B, Wiesner GL, Pal T. A web-based tool to automate portions of pretest genetic counseling for inherited cancer. J Natl Compr Canc Netw. 2020;18(7):841–847. doi: 10.6004/jnccn.2020.7546. [DOI] [PubMed] [Google Scholar]
- 21.Manahan ER, Kuerer HM, Sebastian M, Hughes KS, Boughey JC, Euhus DM, et al. Consensus guidelines on genetic` testing for hereditary breast cancer from the American society of breast surgeons. Ann Surg Oncol. 2019;26(10):3025–3031. doi: 10.1245/s10434-019-07549-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cragun D, Camperlengo L, Robinson E, Caldwell M, Kim J, Phelan C, et al. Differences in BRCA counseling and testing practices based on ordering provider type. Genet Med. 2015;17(1):51–57. doi: 10.1038/gim.2014.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hamilton JG, Symecko H, Spielman K, Breen K, Mueller R, Catchings A, et al. Uptake and acceptability of a mainstreaming model of hereditary cancer multigene panel testing among patients with ovarian, pancreatic, and prostate cancer. Genet Med. 2021;23(11):2105–2113. doi: 10.1038/s41436-021-01262-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bokkers K, Zweemer RP, Koudijs MJ, Stehouwer S, Velthuizen ME, Bleiker EMA, et al. Positive experiences of healthcare professionals with a mainstreaming approach of germline genetic testing for women with ovarian cancer. Fam Cancer. 2021 doi: 10.1007/s10689-021-00277-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Heald B, Keel E, Marquard J, Burke CA, Kalady MF, Church JM et al (2020) Using chatbots to screen for heritable cancer syndromes in patients undergoing routine colonoscopy. J Med Genet 58(12):807–814. 10.1136/jmedgenet-2020-107294 [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
De-identified data may be made available to researchers on reasonable request.