Highlights
-
•
Improving response to vaccination is a key public health issue.
-
•
Morning vaccination shows some benefit to the antibody response.
-
•
Previous analyses have focused on T-dependent vaccines.
-
•
These analyses examined pneumococcal T-independent responses.
-
•
Time of day of vaccination had no impact on T-independent responses.
Keywords: Antibody response, Diurnal, Thymus-independent, Time of day, Vaccination
Abstract
Variable responses to vaccination are of historical and current concern, particularly among vulnerable groups. Biochemical and behavioural methods of improving vaccination response have been examined. There is some evidence that vaccinating in the morning could enhance vaccine responses, however, this has consistently been shown in thymus-dependent vaccinations, such as influenza. The present analysis of data from two observational studies of the association between psychosocial factors and vaccination response. These data included response to a thymus-independent vaccination - pneumococcal polysaccharide vaccine, examined morning versus afternoon vaccine administration in 75 healthy young adults and 61 parents, including 32 caregivers of a child with a development disability and 29 control parents. In both datasets, timing of vaccination was not related to antibody response. This suggests that effects of time of day may be limited to thymus-dependent vaccinations although replication in a large randomised controlled trial using other thymus-dependent vaccinations is required.
Vaccination is a key strategy for preventing or reducing illness and mortality from infectious diseases and has regained attention in recent months due to the Covid19 pandemic. However, it is commonly understood that some vulnerable groups such as older people and stressed individuals, such as caregivers or those reporting experiencing stressful events or high perceived stress, [1] may have altered antibody responses possibly resulting in weaker disease protection. Consequently, substantial effort has gone into researching chemical adjuvants to improve vaccine response but with limited impact and some undesirable side effects [2], [3].
Behavioural adjuvants i.e., circumstances surrounding vaccine administration, such as acute stress or exercise [4] and time of day have also received attention. Early small-scale studies showed mixed evidence as to whether morning or afternoon vaccination related to higher antibody responses [5], [6], [7]. It has been suggested that manipulating diurnal rhythms within the immune system would be a simple way to improve disease protection [8] so time of day has been revisited in existing datasets developed to examine the impact of psychosocial factors on vaccine response. First, this involved pseudo-randomisation to morning or early evening vaccination in university students, where men vaccinated in the morning mounted a greater antibody response at 4-weeks post-vaccination to the hepatitis A vaccine. Second, male older adults non-randomised to morning or afternoon vaccination times similarly showed a better antibody response 4-weeks post-vaccination to the A/Panama influenza strain than men vaccinated in the afternoon/evening [9]. These non-randomised analyses prompted a larger scale randomised controlled trial (RCT) of the impact of time of day of vaccination in older adults receiving the annual influenza vaccination [10]. In this cluster-randomised trial of 276 older adults, standard annual influenza vaccinations were administered either between 9 and 11 am or 3 and 5 pm to fit in with regular UK General Practice availability and pragmatism for older people to attend; medical practices were the clusters randomised to either morning or afternoon vaccination. Blood samples were taken at baseline and one-month post-vaccination and revealed that participants vaccinated in the morning showed marginally significant evidence of a better response to morning vaccination to the H1N1 strains and a trend in the same direction for the B strains of the annual vaccination but no significant effect for the H3N2 strains [10]. However, this study was underpowered due to recruitment issues, and effect sizes suggest that a larger study might yield more conclusive results. The Covid-19 pandemic has provided an opportunity to examine the question of vaccine timing again more recently, and evidence of a stronger antibody response to morning versus afternoon vaccination has been found in one study [11] but in a large, randomised trial with a broad age range (16-74yrs) those vaccinated later in the afternoon showed higher anti-Spike antibody responses [12]. Finally, a further large cross-sectional non-randomised study of young adults observed no effect of vaccine timing on anti-Spike protein antibodies [13]. Taken together, these studies provide mixed evidence for favouring morning or afternoon vaccination which may reflect differences in type of vaccine, immune status of the participants involved [12] or antigen load in the vaccine and after which dose sampling took place [13]; definitive results would inform a simple strategy to adopt in practice.
These time of day effects have only been demonstrated for thymus-dependent vaccinations that involve T-cell help in the recognition of protein antigens and formation of antibodies and have not been examined in a range of vaccines. The present analyses revisit datasets from two previous longitudinal observational studies conducted at the University of Birmingham School of Sport and Exercise Sciences which included a thymus-independent polysaccharide pneumococcal vaccine, to examine morning versus afternoon vaccination on antibody response. This study tested the null hypothesis that there would be no effect of vaccination timing on antibody response.
1. Method
1.1. Participants
For study 1 [14], [15], participants were 75 (41 women) students (mean age: 22.9 (SD = 3.89) years; 89% white). Participants were excluded if they had received the pneumococcal vaccination previously, were suffering from medical conditions that could affect antibody response e.g., current cancer, glandular fever, acute infection, were pregnant or taking prescribed medication excluding contraceptives. For study 2 [16], [17], data were available for 61 parents (43 women, aged 41.4 (SD = 5.31) years; 91% white) of children aged 3–18 years including 32 parents caring for children with developmental disabilities and 29 control parents of a typically developing child. The studies were approved by the local Research Ethics Committee.
1.2. Procedures
Both studies were longitudinal observational studies investigating the association between psychosocial factors (measured using psychometric questionnaires) and antibody response to different types of vaccination in order to examine which aspects of the immune response are influenced by psychological/behavioural factors. At baseline, participants provided a blood sample before vaccination to determine antibody status. In study 1, participants were recruited from the undergraduate population at the University of Birmingham and were offered course credits for participation. They were individually randomly allocated to either a morning (10am to 12 pm; n = 39) or early evening (4 pm to 6 pm; n = 36) vaccination session; although given academic timetabling practicalities, about 30% of participants could only attend a specific session. At baseline, they also completed psychometric questionnaires regarding life events stress (Life Events Scale for Students [18]) and social support (Medical Outcomes Study Social Support Survey [19]) alongside standardised questionnaires on socio-demographics and health behaviours (for full details see [14], [15]), and were vaccinated with the 23-valent polysaccharide pneumococcal vaccine (Pneumovax II; Sanofi Pasteur MSD) and returned to provide a further blood sample for antibody analysis five days later (range 3–7 days; mean = 5 and SD = 0.72 days) then at four- and 18-weeks. In study 2, participants were either parents who were caregivers of child(ren) with a developmental disability or control group parents of a typically developing child(ren); caregiving/control status was assessed at the point of recruitment. Caregivers were recruited via invitation letters distributed by their respective developmental disability Associations, advertising in syndrome newsletters, and by direct contact with family support groups, whereas control parents were recruited via local schools, media campaigns and newspaper advertisements. They attended the laboratory in the morning (n = 32) or afternoon (n = 29) as available, as time of vaccination was not part of the initial investigation. At baseline, parents provided a blood sample, completed psychometric questionnaires on perceived stress, social support, caregiver burden and child problem behaviour alongside socio-demographics and health behaviours (full details in [16]) and then were vaccinated with the same pneumococcal vaccine. They returned one month later (mean lag = 31, SD = 4, days) and six months later (mean lag = 183, SD = 5, days) to provide samples for antibody measurement.
1.3. Sample preparation and immunological assays
Venous blood specimens were collected from an ante-cubital vein into two 7-ml plain tubes (BD Vacutainer, Meylan Cedex) to assess antibody titres. Samples were allowed to clot at room temperature for 1 h and centrifuged at 3500 rpm for 5 min. The separated serum was frozen at − 20 °C until assayed. Luminex technology was used to assess seven pneumococcal (Pn) IgG antibody serotypes (types 1, 3, 6, 9, 14, 19 and 23) contained in the pneumococcal vaccine. We selected to assess these specific Pn serotypes based on clinical observations linking these common serotypes to invasive disease in Europe [20], [21], thus these would seem to be the most important to focus on. Further details of this assay are described elsewhere [22], [23]. Serum samples were diluted 1:400 in diluent buffer that additionally contained 5μg/ml purified pneumococcal serotype 22F in accordance with the WHO protocol for ELISA detection of Pn antibody (http://www.vaccine.uab.edu/#), were run in duplicate, and read on a Luminex 100 machine (Luminex Corp, TX, USA). Acquisition software (BioPlex Software Manager (version 4, BioRads, Labs, CA, USA) was used to generate serotype antibody concentrations from a 5-parameter logistic curve fit. Serum Pn IgM and IgG levels are reported in μg/L.
1.4. Data analysis
Antibody titres were log10 transformed due to their non-normal distribution and skew. Chi-square and ANOVA were used to examine whether any socio-demographics were associated with timing of vaccination, to include these as confounding variables. Repeated-measures ANOVAs were applied to log antibody titres at each time point (baseline, follow-up). Morning/afternoon vaccination was entered as a fixed factor. Given that previous analyses showed interactions of sex with time of day [9], models were repeated as ANCOVAs with sex added. Further, given the influence of social support score in study 1 [14], [15] and caregiver status in study 2 [16], ANCOVAs were rerun with social support total score, or caregiver group entered as a covariate, respectively. Analyses were Greenhouse-Geisser corrected due to the violation of sphericity with the repeated-measures ANOVA. Finally, whether participants mounted a two-fold increase in antibody titre overall to all serotypes from baseline was assessed with chi-squares to provide an estimate of the clinical implications of any diurnal variation. This fits as closely as we were able with the criterion of a 2-fold rise in antibody concentration in at least 70% of serotypes tested [24] although we acknowledge protective thresholds may vary across serotypes [25]. Small variations in n or degrees of freedom reflect occasional missing data, e.g., where it was not possible to get an assay readout for a participant for all serotypes.
2. Results
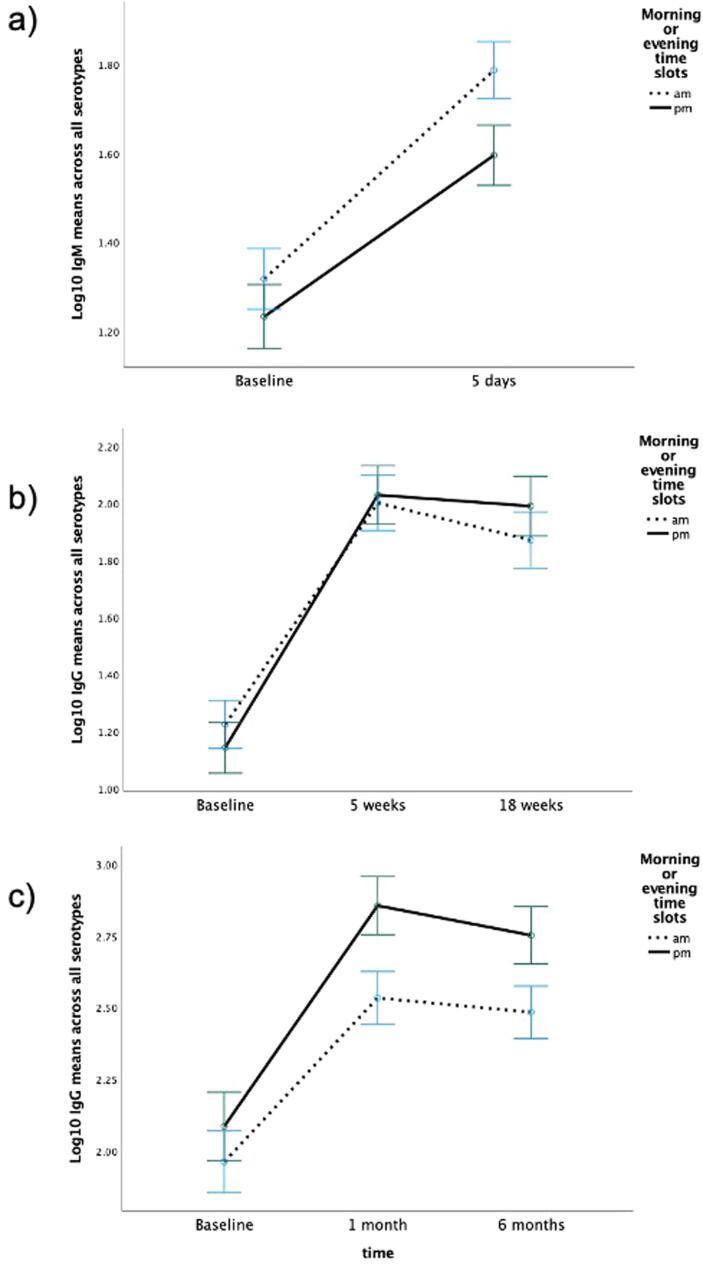
There was a significant increase in antibody titre for all antigens from baseline to each follow-up (see Table 1). In study 1, there were no significant interaction effects of timepoint with time of day of vaccination for average IgM response overall (F(1,72) = 1.54, p =.22, η2 = 0.021) or to each individual serotype (type1 p =.16; type 3p =.71, type 14p =.41, type 19p =.47, and type 23p =.78). For IgG response, there were no significant interaction effects between timepoint and of time of vaccination overall (F(2,132) = 1.54, p =.22) or for each serotype (type 1 p =.78, type 3 p =.06, type 9 p =.17, type 19p =.84, and type 23p =.08) For type 3, on closer examination in 2-time point ANOVAs, the trend for an interaction effect was being driven by a marginally significant response at 4 weeks (p =.05) rather than 18-weeks (p =.14); the morning response was lower than the afternoon response. Similarly for type 23, the trend appeared to be driven by the response at four weeks (p =.03) rather than at 18-weeks (p =.06); again the morning response was lower than the afternoon response. The average response by time of day for IgM and IgG is shown in Fig. 1a-b.
Table 1.
Log10 Mean (SD) Pneumococcal Antibody Titres at Baseline and Follow-up.
| Study 1: Healthy Young adults | |||
|---|---|---|---|
| Vaccine serotype | Time point |
||
| (N = 74) | Baseline | Five-days | |
| Pneumococcal IgM | |||
| Type 1 | 0.89 (0.38) | 1.72 (0.51)*** | – |
| Type 3 | 0.46 (0.44) | 0.82 (0.51)*** | – |
| Type 14 | 1.52 (0.50) | 1.88 (0.46)*** | – |
| Type 19 | 1.30 (0.42) | 1.54 (0.42)*** | – |
| Type 23 | 0.97 (0.62) | 1.25 (0.60)*** | – |
| All serotypes | 1.28 (0.42) | 1.70 (0.41)*** | |
| (N = 68–75) | Baseline | 4-weeks | 18-weeks |
| Pneumococcal IgG | |||
| Type 1 | 0.87 (0.46) | 1.69(0.66)*** | 1.64 (0.69)*** |
| Type 3 | 0.24 (0.52) | 0.95 (0.62)*** | 0.89 (0.62)*** |
| Type 9 | 0.98 (0.57) | 1.57 (0.57)*** | 1.58 (0.66)*** |
| Type 19 | 1.26 (0.48) | 1.87 (0.70)*** | 1.92 (0.71)*** |
| Type 23 | 1.21 (0.69) | 2.04 (0.76)*** | 1.92 (0.72)*** |
| All serotypes | 1.19 (0.50) | 2.01 (0.58)*** | 1.93 (0.59)*** |
| Study 2: Parents | |||
|---|---|---|---|
| (N = 54–59) | Baseline | 1-month | 6-months |
| Pneumococcal IgG | |||
| Type 1 | 1.05 (0.55) | 1.73 (0.72)*** | 1.63 (0.79)*** |
| Type 3 | 0.54 (0.66) | 1.22 (0.76)*** | 1.12 (0.75)*** |
| Type 6 | 2.43 (0.74) | 2.92 (0.68)*** | 2.80 (0.61)*** |
| Type 9 | 1.05 (0.73) | 1.81 (0.72)*** | 1.68 (0.78)*** |
| Type 14 | 1.85 (0.80) | 2.79 (0.83)*** | 2.76 (0.84)*** |
| Type 19 | 1.50 (0.64) | 2.11 (0.78)*** | 2.08 (0.80)*** |
| Type 23 | 1.39 (0.67) | 2.10 (0.61)*** | 1.98 (0.66)*** |
| All serotypes | 2.02 (0.58) | 2.68 (0.52)*** | 2.61 (0.50)*** |
Asterisks indicate that increase in antibodies from baseline is significant ***p <.001.
Fig. 1.
Log10 antibody titre averaged across all serotypes tested at each time point by time of day (am/pm). Error bars = +/- 1 Standard Error.
There were no significant differences between morning/afternoon vaccination for whether participants achieved a two-fold IgM response at 5 days (χ2(1) = 0.05, p =.82) or IgG 5 week (χ2(1) = 0.30, p =.59) or 18 week (χ2(1) = 0.17, p =.68) response across all serotypes from baseline.
In study 2, there were no significant interaction effects with time of vaccination for antibody titres averaged across serotypes (F(2,102) = 2.52, p =.10) or for each serotype (type 1p =.52, type 3p =.57, type 6 p =.34, type 9 p =.37, type 14p =.89, type 19p =.55, and type 23p =.35). Fig. 1c shows the average response across all serotypes by time of day. There was also no significant effect of timepoint on two-fold response at one month (χ2(1) = 1.14, p =.29) or six months (χ2(1) = 0.05, p =.82).
Study 1 analyses were repeated first with adjustment for sex, then for social support (measured using the Medical Outcomes Survey Social Support Scale [19]). There were no significant 3-way interactions between timepoint, time of vaccination and sex for IgM across all serotypes (F(1,70) = <0.001, p =.995) or individual types (1p =.66, 3p =.85, 14p =.94, 19p =.98 and 23p =.50). Further, for IgG responses, there was no significant 3-way interactions between timepoint, time of vaccination and sex overall (F(2,128) = 1.30, p =.27) or for any individual serotype (1p =.83, 3 p =.97, 9 p =.37, 19p =.67 and 23p =.43).
Adding social support into the models as a covariate did not alter the null results described above for IgM overall (F(1, 71) = 1.52, p =.22) or for IgM individual serotypes (1p =.17, 3p =.72, 14p =.40. 19p =.47 and 23p =.78). Similarly, adding social support did not influence the null results for IgG overall (F(2,130) = 1.49, p =.23) or for any specific serotype (1p =.79, 3 p =.07, 9 p =.17, 19p =.84, and 23p =.09). As before, types 3 and 23 showed some evidence of a non-significant trend where morning vaccination yielded a weaker response at each time point, particularly at 5 weeks for type 3 (p =.05) and type 23 (p =.02).
For study 2, there were no significant time point × time of vaccination × caregiver group interactions overall (F(2,98) = 0.67, p =.48) or for any specific serotype (1p =.05, 3p =.12, 6 p =.64, 9 p =.70, 14p =.79, 19p =.31, and 23p =.59). The trend for type 1 showed that morning vaccination yielded a stronger response but only in the control group not the caregiver group at one month follow-up and conversely a weaker response in the caregiver group at six months.
3. Discussion
Previous research on the effects of time of day of vaccine administration on antibody response has yielded inconsistent or limited findings, e.g., only in one gender or for certain vaccine strains/serotypes. However, overall effects have tended towards favouring morning over afternoon vaccination, at least for typical full dose thymus-dependent vaccines. The recent Covid-19 vaccinations yielded mixed findings for time of vaccination [11], [12], [13]. The present analyses in two different samples show no consistent advantage of morning versus afternoon vaccination for the thymus-independent pneumococcal vaccination. There were some non-significant trends for favouring afternoon vaccination for some serotypes but not consistently across time or when taking into account sub-groups based on sex or caregiver status, which make it difficult to suggest strong evidence for this effect.
These findings contrast with earlier studies reporting preliminary effects of time of day, at least for males, to the hepatitis and influenza vaccinations [9], the indication of some morning benefit in the RCT in older adults receiving the influenza vaccination [10], and contradictory evidence supporting morning vaccination for inactivated BBIBP-CorV Covid-19 [11] but later afternoon vaccination for first dose mRNA or Adenovirus based Covid-19 vaccinations, respectively [12]. They also contrast with the mixed findings from the earlier small-scale studies. However, the most recent Covid-19 vaccination study also showed no significant effect of time of vaccination following the full two doses of mRNA-based BNT162b2 vaccine [13]. It remains to be tested whether the null effect in the present study would extend to other thymus-independent vaccines that are an important public health strategy.
The explanation for this null result might be that a thymus-independent vaccine such as pneumococcal pure polysaccharide not conjugated to a protein can only stimulate B cells without T cell help because it contains no peptides for presentation to T cells. The native polysaccharide antigen binds surface IgM on either naïve and memory B cells or binds to immunoglobulin G or A on memory B cells and then activates these cells to directly produce antibodies. There is also potentially a costimulatory signal through B cell membrane CD21 if the polysaccharide vaccine activated Alternate Pathway Complement or pre-existing antibodies from prior natural exposure to pneumococci activated Classical Pathway Complement. Antibody to pneumococcal polysaccharides is common in the population due to natural exposure to pneumococcal commensalisation or infection, which means most individuals have memory B cells for pneumococcal polysaccharides. However, the present evidence suggests B cells are not influenced by time of day and that any T-cell or complement pathway activation by this type of vaccine is not sufficient to be affected by time of day. Time of day effects may be restricted to thymus-dependent vaccines perhaps through influence on T-cells or antigen presentation to T-cells by the innate immune system and resulting interaction with newly activated B-cells. This more complex process of vaccine response has more components than the thymus-independent vaccine response, each of which may be susceptible to diurnal rhythm effects. The exact mechanism remains to be elucidated and may emerge from contemporary RCTs.
An alternative explanation for the present findings is that the analyses were underpowered to find significant effects of time of vaccination or time × sex interactions as we have observed previously. However, this might be discounted for several reasons: 1) the finding of no consistent significant interaction with time of vaccination across two datasets with different populations but the same vaccine; 2) that any non-significant trends detected were attenuated by inclusion of key covariates e.g., sex, or were inconsistent in direction across sub-groups and time points and 3) perhaps most importantly, these analyses were sufficiently powered according to post-hoc power analysis from the previous study which detected a time of vaccination × sex interaction effect for hepatitis A in Study 1. Using the derived f effect size of 0.31, power at 0.74, and correlation between repeated measures as 0.6 from that analysis, and setting p at 0.05, the required sample size to detect similar effects was 54 for analyses with three repeated measures time points and 60 for two timepoints. When applying these effect sizes to Study 2, considering the slightly higher correlation between repeated measures of 0.7, the calculated sample size was 62. The studies included in the present analysis numbered 75 and 61, thus, were deemed adequately powered to detect significant effects or at least indicative trends in Study 2.
3.1. Limitations and future directions
One limitation is that only seven polysaccharide serotypes of the pneumonia vaccine were examined as this was the thymus-independent vaccine in both previous studies of stress and vaccination response where time of vaccination was also measured, thus replication with other thymus-independent vaccines is necessary. Second, the present analyses were restricted to young and middle-aged samples, thus it would be important to examine whether diurnal rhythm effects might be more likely to emerge in groups with poorer immunity and greater risk such as older adults. In groups where the antibody response is lower, effects of extrinsic variables might be more evident; we have observed this phenomenon previously to two serotypes where the average response was lower [26], [27]. However, some parents included in the present Study 2 did show lower responses, and although psychological factors were associated with fewer antibodies time of day was not. This suggests lack of variability in response is not the source of the null effect here. Further, it is possible that sampling time at follow-up blood samples might also have altered antibody levels [28]. In the current absence of sampling times at follow-ups in these datasets, we were unable to account for this in analysis. This would be important to include in future studies of vaccination timing, although we note other large trials have failed to detect an effect of sample timing [12]. Finally, the present analyses were on small samples not fully randomised to time of vaccination, making it difficult to discount alternative explanations, such as those vaccinated in the morning had higher stress thus a lower antibody response making them comparable to the afternoon groups. However, this bias is unlikely given that stress and caregiving status were not associated with time of day, and the present analyses statistically adjusted for these variables.
The present findings should be regarded as tentative. However, data from two different groups (young healthy, and middle-aged parents) suggest that time of day of vaccination does not consistently have an impact on the antibody response to the thymus-independent pneumococcal vaccination, and thus may not be a helpful adjuvant for thymus-independent vaccines. Results from RCTs comparing both types of vaccine, with appropriate adjustment for confounding variables, would help to confirm this hypothesis, as well as providing an opportunity for closer examination of the immune mechanisms potentially influenced by diurnal rhythms.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgements
The authors would like to thank all the participants for their contribution to this research.
This research was funded by a University of Birmingham PhD studentship for S.Gallagher.
References
- 1.Phillips A.C. Psychosocial influences on vaccine responses. Soc Pers Psychol Compass. 2011;5:621–633. doi: 10.1111/j.1751-9004.2011.00378.x. [DOI] [Google Scholar]
- 2.Jefferson T. Influenza vaccination: policy versus evidence. BMJ. 2006;333(7574):912–915. doi: 10.1136/bmj.38995.531701.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pollard A.J., Bijker E.M. A guide to vaccinology: from basic principles to new developments. Nat Rev Immunol. 2021;21(2):83–100. doi: 10.1038/s41577-020-00479-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Edwards K.M., Burns V.E., Reynolds T., Carroll D., Drayson M., Ring C. Acute stress exposure prior to influenza vaccination enhances antibody response in women. Brain Behav Immun. 2006;20(2):159–168. doi: 10.1016/j.bbi.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 5.Feigin R.D., Jaeger R.F., McKinney R.W., Live A.AC. attenuated Venezuelan equine encephalomyelitis virus vaccine. II. Whole-blood amino-acid and fluorescent-antibody studies following immunization. Am J Trop Med Hyg. 1967;16:769–777. [PubMed] [Google Scholar]
- 6.Pollman L., Pollman B. Circadian variations of the efficiency of hepatitis B vaccination. Annu Rev Chronopharmacol. 1988;5:45–48. [Google Scholar]
- 7.Langlois P.H., Smolensky M.H., Glezen W.P., Keitel W.A. Diurnal variation in responses to influenza vaccine. Chronobiol Int. 1995;12(1):28–36. doi: 10.3109/07420529509064497. [DOI] [PubMed] [Google Scholar]
- 8.Petrovsky N., McNair P., Harrison L.C. Diurnal rhythms of pro-inflammatory cytokines: regulation by plasma cortisol and therapeutic implications. Cytokine. 1998;10(4):307–312. doi: 10.1006/cyto.1997.0289. [DOI] [PubMed] [Google Scholar]
- 9.Phillips A.C., Gallagher S., Carroll D., Drayson M. Preliminary evidence that morning vaccination is associated with an enhanced antibody response in men. Psychophysiology. 2008;45(4):663–666. doi: 10.1111/j.1469-8986.2008.00662.x. [DOI] [PubMed] [Google Scholar]
- 10.Long J.E., Drayson M.T., Taylor A.E., Toellner K.M., Lord J.M., Phillips A.C. Morning vaccination enhances antibody response over afternoon vaccination: a cluster-randomised trial. Vaccine. 2016;34(24):2679–2685. doi: 10.1016/j.vaccine.2016.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang H, Liu Y, Liu D, Zeng Q, Li L, Zhou Q, et al. Time of day influences immune response to an inactivated vaccine against SARS-CoV-2. Cell Res 2021;31:1215–7. doi: 10.1038/s41422-021-00541-6. [DOI] [PMC free article] [PubMed]
- 12.Wang W., Balfe P., Eyre D.W., Lumley S.F., O’Donnell D., Warren F., et al. Time of day of vaccination affects SARS-CoV-2 antibody responses in an observational study of health care workers. J Biol Rhythms. 2022;37:124. doi: 10.1177/07487304211059315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Matryba P., Gawalski K., Ciesielska I., Horvath A., Bartoszewicz Z., Sienko J., et al. The influence of time of day of vaccination with BNT162b2 on the adverse drug reactions and efficacy of humoral response against SARS-CoV-2 in an observational study of young adults. Vaccines (Basel) 2022;10:443. doi: 10.3390/VACCINES10030443/S1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gallagher S., Phillips A.C., Ferraro A.J., Drayson M.T., Carroll D. Social support is positively associated with the immunoglobulin M response to vaccination with pneumococcal polysaccharides. Biol Psychol. 2008;78:211–215. doi: 10.1016/j.biopsycho.2008.01.001. [DOI] [PubMed] [Google Scholar]
- 15.Gallagher S., Phillips A.C., Ferraro A.J., Drayson M.T., Carroll D. Psychosocial factors are associated with the antibody response to both thymus-dependent and thymus-independent vaccines. Brain Behav Immun. 2008;22:456–460. doi: 10.1016/j.bbi.2007.10.018. [DOI] [PubMed] [Google Scholar]
- 16.Gallagher S., Phillips A.C., Drayson M.T., Carroll D. Parental caregivers of children with developmental disabilities mount a poor antibody response to pneumococcal vaccination. Brain Behav Immun. 2009;23:338–346. doi: 10.1016/j.bbi.2008.05.006. [DOI] [PubMed] [Google Scholar]
- 17.Gallagher S., Phillips A.C., Drayson M.T., Carroll D. Caregiving for children with developmental disabilities is associated with a poor antibody response to influenza vaccination. Psychosom Med. 2009;71:341–344. doi: 10.1097/PSY.0b013e31819d1910. [DOI] [PubMed] [Google Scholar]
- 18.Linden W. Development and initial validation of a life event scale for students. Canadian Counsellor. 1984;18:106–110. [Google Scholar]
- 19.Sherbourne C.D., Stewart A.L. The MOS social support survey. Social Science Medicine. 1991;32:705–714. doi: 10.1016/0277-9536(91)90150-b. [DOI] [PubMed] [Google Scholar]
- 20.Denham B.C., Clarke S.C. Serotype incidence and antibiotic susceptibility of Streptococcus pneumoniae causing invasive disease in Scotland, 1999–2002. J Med Microbiol. 2005;54:327–331. doi: 10.1099/jmm.0.45718-0. [DOI] [PubMed] [Google Scholar]
- 21.Sleeman K., Knox K., George R., Miller E., Waight P., Griffiths D., et al. Invasive pneumococcal disease in England and wales: vaccination implications. J Infect Dis. 2001;183:239–246. doi: 10.1086/317924. [DOI] [PubMed] [Google Scholar]
- 22.Ferraro A.J., Drayson M.T., Savage C.O.S., MacLennan I.C.M. Levels of autoantibodies, unlike antibodies to all extrinsic antigen groups, fall following B cell depletion with Rituximab. Eur J Immunol. 2008;38:292–298. doi: 10.1002/eji.200737557. [DOI] [PubMed] [Google Scholar]
- 23.Lal G., Balmer P., Stanford E., Martin S., Warrington R., Borrow R. Development and validation of a nonaplex assay for the simultaneous quantitation of antibodies to nine Streptococcus pneumoniae serotypes. J Immunol Methods. 2005;296:135–147. doi: 10.1016/j.jim.2004.11.006. [DOI] [PubMed] [Google Scholar]
- 24.Orange J.S., Ballow M., Stiehm E.R., Ballas Z.K., Chinen J., de La Morena M., et al. Use and interpretation of diagnostic vaccination in primary immunodeficiency: a working group report of the Basic and Clinical Immunology Interest Section of the American Academy of Allergy, Asthma & Immunology. J Allergy Clin Immunol. 2012;130:S1–S24. doi: 10.1016/j.jaci.2012.07.002. [DOI] [PubMed] [Google Scholar]
- 25.LaFon D.C., Nahm M.H. Measuring immune responses to pneumococcal vaccines. J Immunol Methods. 2018;461:37–43. doi: 10.1016/j.jim.2018.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Phillips A.C., Burns V.E., Carroll D., Ring C., Drayson M. The association between life events, social support and antibody status following thymus-dependent and thymus-independent vaccinations in healthy young adults. Brain Behav Immun. 2005;19:325–333. doi: 10.1016/j.bbi.2004.10.004. [DOI] [PubMed] [Google Scholar]
- 27.Phillips A.C., Carroll D., Bums V.E., Ring C., Macleod J., Drayson M. Bereavement and marriage are associated with antibody response to influenza vaccination in the elderly. Brain Behav Immun. 2006;20:279–289. doi: 10.1016/j.bbi.2005.08.003. [DOI] [PubMed] [Google Scholar]
- 28.Kurupati R.K., Kossenkoff A., Kannan S., Haut L.H., Doyle S., Yin X., et al. The effect of timing of influenza vaccination and sample collection on antibody titers and responses in the aged. Vaccine. 2017;35:3700–3708. doi: 10.1016/J.VACCINE.2017.05.074. [DOI] [PubMed] [Google Scholar]