Abstract
Identification of tetrodotoxin (TTX) and its derivatives produced from a Vibrio strain in the intestine of the puffer fish Fugu vermicularis radiatus was performed by thin-layer chromatography, electrophoresis, high-performance liquid chromatography, and gas chromatography-mass spectrometry, together with a mouse bioassay for toxicity. It was demonstrated that the isolated bacterium produced TTX, 4-epi-TTX, and anhTTX during cultivation, suggesting that Vibrio strains are responsible for the toxification of the puffer fish.
Tetrodotoxin (TTX) is a strong neurotoxin and is also known as the causative agent of puffer fish poisoning. Moreover, not all species of puffer fish are toxic, and several are only weakly or moderately toxic (4). The toxicity of puffer fish species varies depending on the tissues or organs, geography, season of the year, and sex (3). The female puffer fish is more poisonous than the male, since the ovaries tend to be much more poisonous than the testes (2). TTX is not restricted to puffer fish and is widely distributed among various kinds of animals, such as the California newt Tarichi torosa (14), the goby Gobius criniger (19), Atelopus frogs (9), the gastopod mollusks Charonia sauliae (18) and Babylonia japonica (23, 32), the xanthid crab Atergatis floridus (25), the blue-ringed octopus Octopus maculosus (28), Astropecten starfishes (11, 12, 24), the frog shell Tutufa lissostoma (22), and the small gastropod mollusks Zeuxis siquijorensis (17) and Niotha clathrata (6). These facts indicate that TTX-containing animals may have absorbed and accumulated TTX and its derivatives produced by several marine bacteria (10, 13). The origin of TTX in marine animals has been the subject of a number of recent investigations (16). The probable mechanism of toxification of TTX-bearing animals has recently been discovered: Vibrio fischeri isolated from the xanthid crab Atergatis floridus and Vibrio alginolyticus isolated from the puffer fish Fugu vermicularis vermicularis produced TTX and anhydro-TTX (anh-TTX) (5, 20, 30). Another TTX-producing bacterium has also been found in a calcareous realga, Jania sp. TTX-producing bacteria have been isolated from various marine organisms, including the starfish Astropecten polyacanthus and the blue-ringed octopus O. maculosus (26, 27). The number of bacterial strains reported to produce the toxin has been increasing, and most strains have been identified as members of the genus Vibrio (21). Also, Simidu et al. (29) demonstrated that many species of marine bacteria, including Vibrio spp. (21), Pseudomonas spp. (33), and actinomycetes (1), produce TTX.
Three individual F. vermicularis radiatus puffer fish (male; body weight, 45 g) were collected at Pusan, Korea, in March 1998, transported live to the laboratory, and maintained overnight at 25°C in equipped aquaria. Each puffer fish was dissected for testing of intestine, liver, skin, muscle, testis, and bile under aseptic conditions. When these organs were assayed for toxicity and bacterial population, they were found to be toxic. The intestines were used for the bacteriological examination.
For culture of TTX-producing microflora in the puffer fish intestinal contents, ORI broth containing 0.2% Proteose Peptone no. 3 (Difco Laboratories, Detroit, Mich.), 0.2% Phytone peptone (BBL Microbiology Systems, Cockeysville, Md.), 0.1% yeast extract (Difco), 0.088% ferric citrate, and 3% NaCl was used. The pH of the medium was adjusted to 8.0. In some cases, beef extract broth containing 0.5% glucose, 0.5% polypeptone, 0.5% beef extract, and 3% NaCl (pH 8.0) was also used. For cultivation of Vibrio strains, PCA and TCBS media were also used. PCA medium contained 0.5% Bacto tryptone, 0.25% Bacto yeast extract, 0.1% Bacto dextrose, 1.5% Bacto agar, and 3% NaCl (adjusted to a final pH of 7.0). TCBS medium contained 0.5% yeast extract, 0.5% casein peptone, 0.5% meat peptone, 1.0% sodium citrate, 1.0% sodium thiosulfate, 0.5% Dissect bovine bile, 0.3% sodium cholate, 2% saccharose, 1% sodium chloride, 0.1% ferric citrate, 0.004% thymol blue, 0.004% bromothymol blue, and 1.4% Bacto agar (adjusted to a final pH of 8.6).
To identify TTX-producing bacteria, the intestinal contents were placed in test tubes. Each sample was weighed and serially diluted with 3 volumes of sterilized saline solution. After homogenization, 1 ml of the threefold dilution was spread on PCA medium containing 3% NaCl and on TCBS medium and incubated at 23°C for 1 to 3 days. After incubation, the bacterial colonies on each medium were counted and divided into types according to colony characteristics. Representative colonies of all types were picked up at random and purified by streaking onto the surface of the sample medium for single-colony isolation. Each colony on TCBS medium was selected and incubated with shaking in 200 ml of ORI and beef extract media at 23°C for 48 h. For analysis of the TTX-producing ability of the intestinal bacteria from the puffer fish, the incubated cells were harvested by centrifugation at 5,000 × g for 30 min (7). The bacterial cells were used to examine TTX production. Finally, three strains which clearly exhibited TTX productivity were selected and identified as described by Tansill (31).
For separation of toxin from the bacterial extracts, the extracts were defatted with dichloromethane, and the aqueous layer was concentrated under reduced pressure to remove the dichloromethane. The suspension was subjected to ultrasonic disruption with an ultrasonicator for 10 min. For the extraction of TTXs, the mixture was heated in a boiling water bath, cooled to room temperature, filtered through a Diaflo YM-2 membrane (Amicon), evaporated under vacuum, and freeze-dried. The resulting solid was dissolved in 0.03 M acetic acid and applied to a Bio-Gel P-2 (Bio-Rad Laboratories, Richmond, Calif.) column (2 by 94 cm) equipped with a constant-flow pump (Kyowa Seimitsu Co., Tokyo, Japan). Toxic fractions were combined and lyophilized. The TTX fractions obtained were subjected to mouse assay, gas chromatography-mass spectrometry (GC-MS), and high-performance liquid chromatography (HPLC) for detection and identification of TTXs (8).
One milliliter of test solution prepared through ultrasonication and ultrafiltration of culture broth was used for assay of toxicity. Toxicity was assayed by the official TTX method using mice (5). One hundred microliters of the test solution was injected intraperitoneally into each mouse (strain 3 ddy; male; body weight, 18 to 20 g), and times to death were recorded. The dose injected was calculated from the median death time and the standard dose-death time curve and was expressed in mouse units (MU), which are defined as the amount of TTX which killed a mouse in 30 min after injection.
A fluorometric HPLC method, developed by Nagashima et al. (15), was used for detection of TTX and its derivatives. Authentic standards for TTX, 4-epi-TTX, and anh-TTX were kindly supplied by K. Hashimoto and T. Noguchi, Faculty of Agriculture, The University of Tokyo, Tokyo, Japan. Reverse-phase HPLC was performed on a YMC-pack AM-314 octyldecyl silane column (0.6 by 30 cm) (15). Briefly, the column was prepared by mixing 0.05 M heptanesulfonic acid and methanol in 0.05 M potassium phosphate buffer (pH 7.0) at a flow rate of 1 ml/min. The eluate was mixed with a equal volume of 4 N NaOH and heated in a reaction coil at 100°C. For detection of the fluorescent products, the excitation and emission wavelengths were set at 381 and 505 nm, respectively.
For GC-MS, authentic TTX and related substances or partially purified toxins from bacterial cells and culture broths were converted into the trimethylsilyl-2-amino-6-hydroxylmethyl-8-hydroxyquinazoline (C9-base). Briefly, TTX or bacterial toxins were hydrolyzed in 2 N NaOH for 45 min in a boiling water bath. After cooling, the alkali hydrolyzates were adjusted to pH 4 with 1 N HCl and extracted twice with 5 ml of 1-buthanol. The extracts were combined, freeze-dried, and trimethylsilylated as described by Nouguchi et al. (21). The derivatives obtained were then subjected to GC-MS on a Hitachi M-80 GC-mass spectrometer to examine TMA-C9-base derived from TTX and its related substances. A column of Chromosorb W coated with 1.5% OV 101 was used, and the temperature raised from 165 to 200°C at a rate of 5°C/min. Thin-layer chromatography (TLC) was performed on 5- by 20-cm silica gel (Whatman) LHP-K linear high-performance TLC plates with a solvent system of pyridine-ethyl acetate-acetic acid-water (15:5:3:4). Toxins were visualized as a pink spot after spraying the plate with Weber reagent or as a yellow fluorescent spot under UV light (365 nm) after spraying the plate with 10% KOH and heating.
Electrophoresis was performed on 5- by 18-cm cellulose acetate strips (Chemetron) in 0.08 M Tris-HCl buffer (pH 8.7) at 0.8 mA/cm for 30 min. Authentic TTX prepared from the puffer fish livers was used as a reference standard. Toxins were visualized using the same method as for TLC.
The toxicity of the puffer fish was assayed to show the existence of intestinal bacteria in puffer fish, as a new producer of TTXs, and the mechanism of toxification of the specimen. Three F. vermicularis radiatus puffer fish were moderately toxic, with lethalities of 70 ± 8 MU/g of liver and 45 ± 3 MU/g of skin.
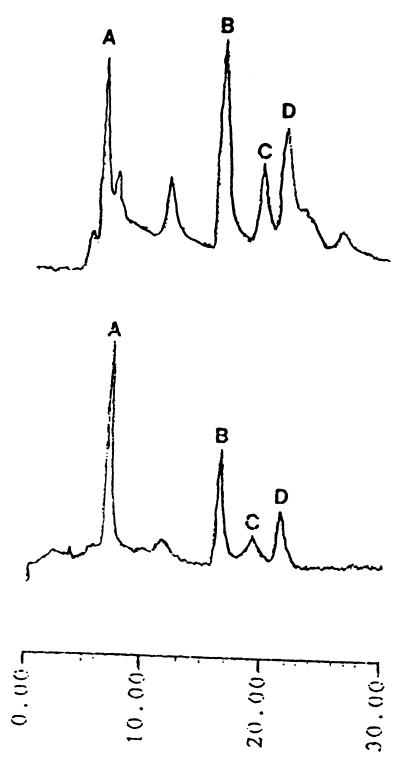
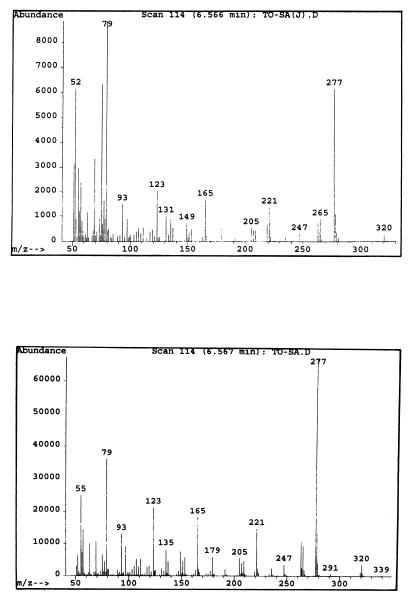
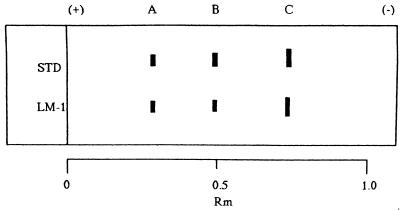
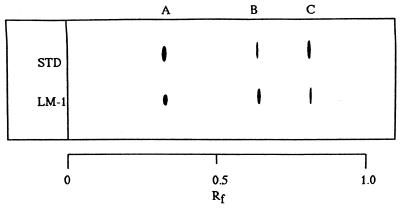
All three dominant Vibrio strains were isolated and examined for the ability to produce TTX and related substances by means of HPLC, GC-MS, electrophoresis, and TLC. Only one strain of the three candidates was further characterized, since it was found to be a TTX producer by HPLC analysis. The HPLC pattern of the TTX fraction from this strain is shown in Fig. 1. The TTX fraction gave rise to several peaks in HPLC, whose retention times (8, 16, 19, and 22 min) were in agreement with, or close to, those of tetrodoic acid (TDA), TTX, 4-epi-TTX, and anh-TTX, respectively. The trimethylsilylated derivative from the alkaline hydrolysate of the TTX fraction exhibited mass fragment ions at m/z 407 (parent peak), 392 (base peak), and 376, which are specific to the corresponding derivatives from authentic TTXs (Fig. 2). With pyridine-ethyl acetate-acetic acid-water (15:5:3:4) as the Solvent system, spraying the plate with 10% KOH revealed three spots with Rf values of 0.4, 0.6, and 0.8 for the bacterial toxins. No further spots emerged after spraying with Weber reagent. The toxins isolated from incubated cells coincided well with those of authentic TDA, TTX, and anh-TTX, respectively (Fig. 3). During electrophoresis, both bacterial toxins and authentic TTXs clearly exhibited two spots when sprayed with 10% KOH, one corresponding to TDA (relative migration distance [Rm]) and the others corresponding to anh-TTX and TTX (Rm, 0.5 and 0.7, respectively) (Fig. 4). It was known that the strains of the family Vibrionaceae showed the ability to produce the anhydrated form of TTX. In contrast, Escherichia coli, which is a typical terrestrial bacterium, does not produce TTXs (14). It was also known that anh-TTX is only slightly toxic but is easily converted into TTX in solution, particularly at lower pH values. TTX also changes into the anhydrated form in solution (33). Although the role of TTX in the bacteria themselves is still unclear, it was postulated that TTXs regulate the transfer of sodium ions through biological membranes (26), and this fact may have some relevance to the function of the toxin in marine bacterial cells.
FIG. 1.
HPLC pattern of the TTX fraction from a Vibrio strain isolated from F. vermicularis radiatus puffer fish intestines (top), along with authentic TTXs (bottom). A, TDA; B, TTX; C, 4-epi-TTX; D, anh-TTX.
FIG. 2.
GC-MS analysis of the trimethylsilyated derivatives from authentic TTXs (top), and of the corresponding derivatives from the TTX fraction of a Vibrio strain isolated from F. vermicularis radiatus puffer fish intestines (bottom).
FIG. 3.
Electrophoresis of the TTX fraction of a Vibrio strain (LM-1) isolated from F. vermicularis radiatus puffer fish intestines, along with authentic TTXs (STD). Electrophoresis was conducted on a cellulose acetate strip (Chemetron) in 0.08 M Tris-HCl buffer (pH 8.7) at 0.8 mA/cm for 30 min. After development, samples were heated for 10 min and visualized under UV light (365 nm). A, TDA; B, anh-TTX; C, TTX.
FIG. 4.
TLC of the TTX fraction of a Vibrio strain (LM-1) isolated from F. vermicularis radiatus puffer fish intestines, along with authentic TTXs (STD). Toxins were developed on a precoated Whatman LHP-K silica gel plate with a solvent system of pyridine-ethyl acetate-acetic acid-water (15:5:3:4). Detection was the same as for Fig. 3. A, TDA; B, TTX; C, anh-TTX.
The results of preliminary tests showed that viable counts of bacteria in the intestinal contents differed significantly depending on the individual, but there was little diversity with respect to the culture medium. In plate count agar with 3% NaCl, 6.4 × 105 cells/g were obtained. A total of three strains of intestinal bacteria were isolated on TCBS agar medium. Moreover, Vibrionaceae were found to be most dominant, using the procedure described above. All of the Vibrionaceae strains were identified as Vibrio sp., and they were divided into three strains on the basis of color, surface appearance, Gram stain reaction, width, length, spore formation, and motility. In the next step, the strains were examined for TTX production. Among these groups, TTXs were detected only in strain 1 when the cellular extracts were assayed by the official method for TTX. The other bacteria of strains 2 and 3 were not positive for production of TTX, 4-epi-TTX, and anh-TTX as analyzed by HPLC (Table 1). Previously, Noguchi et al. (21) reported that the Vibrio strains isolated from the xanthid crab Atergatis floridus and the starfish Astropecten polyacanthus produced TTXs when cultured under conditions similar to those used in this study. Also, several TTX-producing bacteria were isolated from tissues, mainly intestines, of TTX-containing puffer fish (16).
TABLE 1.
Analyses for TTX and its derivatives in extracts of three Vibrio strains isolated from the intestines of F. vermicularis radiatus puffer fish
| Strain | Toxicity | HPLC resulta
|
||
|---|---|---|---|---|
| TTX | 4-Epi-TTX | Anh-TTX | ||
| 1 | − | + | + | + |
| 2 | − | ± | + | + |
| 3 | − | + | ± | + |
+, clearly detected; ±, difficult to detect.
The bacterium was identified at the biochemical and morphological levels (Table 2). The strain was a facultatively aerobic, chemoorganotrophic, and nonsporeforming gram-negative bacterium. The cells were rod shaped (ca. 0.6 by 1.7 μm) in the logarithmic phase of growth. They occurred singly or in chains and were motile by means of flagella, having a single flagellum at one pole.
TABLE 2.
Culture characteristics of surface colonies of isolated strain 1 grown on TCBS agar at 23°C for 24 h
| Characteristic | Result for strain 1 |
|---|---|
| Colony diam (mm) | 3–4 |
| A colony shape | Round |
| Elevation (mm) | 1–2 |
| Color | Yellow |
| Surface appearance | Smooth |
| Density | Transparent |
| Consistency | Mucoid |
| Gram stain reaction | Negative |
| Cell width (μm) | 0.6 |
| Cell length (μm) | 1.7 |
| Spore formation | None |
| Motility | Motile |
In summary, this study revealed for the first time that a Vibrio strain isolated from the intestine of the highly toxic wild puffer fish F. vermicularis radiatus produced TTX, 4-epi-TTX, and anh-TTX and secreted the toxins into the culture broth. This result suggested that the toxification of the puffer fish is likely caused by bacteria. These bacteria are presumably involved in toxin accumulation in the puffer fish, with the following mechanism in TTX-bearing animals. TTX-producing marine Vibrio strains enter and inhibit the intestines of puffer fish. These bacteria produce TTX and/or related substances, which are accumulated in the hosts and then transferred to other organisms. In order to clarify this speculation, we are in the process of obtaining more precise evidence of the biosynthetic derivatives of TTX in the organism.
Acknowledgments
This work was supported in part by a grant (1998-023-H00026) from the Korea Research Foundation, Ministry of Education, Korean Government.
REFERENCES
- 1.Do H K, Kogure K, Imada C, Noguchi T, Ohwada K, Shimidu U. Tetrodotoxin production of actinomycetes isolated from marine sediment. J Appl Bacteriol. 1991;70:464–468. [Google Scholar]
- 2.Goto T, Kishi Y, Takahashi S, Hirate Y. Tetrodotoxin. Tetrahedron. 1965;21:2059–2088. doi: 10.1016/s0040-4020(01)98344-9. [DOI] [PubMed] [Google Scholar]
- 3.Hashimoto Y, Kamiya H. Food chain hypothesis on the origin of marine toxins. Bull Jpn Soc Sci Fish. 1970;36:425–434. [Google Scholar]
- 4.Hwang D, Lin L C, Jeong S S. Occurrence of tetrodotoxin-related toxins in the gastropod mollusk Niotha clathrate from Taiwan. Nippon Suisan Gakkaishi. 1992;58:63–67. [Google Scholar]
- 5.Jensen P R, Fenical W. Strategies for the discovery of secondary metabolites from marine bacteria. Annu Rev Microbiol. 1994;48:559–584. doi: 10.1146/annurev.mi.48.100194.003015. [DOI] [PubMed] [Google Scholar]
- 6.Jeon J K, Miyazawa K, Noguchi T, Narita H, Ito K, Hashimoto K. Occurrence of tetrodotoxin in a gastropod mollusk, “araregai” Niotha clathrata. Bull Jpn Soc Sci Fish. 1984;50:2099–2105. [Google Scholar]
- 7.Kadota H, Taga N. Methods in marine microbiology. Tokyo, Japan: Gakkai Shuppan Center; 1985. [Google Scholar]
- 8.Kawabata T. Food hygiene examination manual. 2. Assay method for tetrodotoxin 1978. . Japan Food Hygiene Association, Tokyo, Japan. [Google Scholar]
- 9.Kim Y H, Brown G B, Fuhrman F A. Tetrodotoxin: occurrence in atelopid frogs of Costa Rica. Science. 1975;189:151–153. doi: 10.1126/science.1138374. [DOI] [PubMed] [Google Scholar]
- 10.Kosuge T, Tsuji K, Hirai K, Fukuyama T. First evidence of toxin production by bacteria in a marine organism. Chem Pharm Bull. 1985;33:3059–3061. doi: 10.1248/cpb.33.3059. [DOI] [PubMed] [Google Scholar]
- 11.Maruyama J, Noguchi T, Jeon J K, Harada T, Hashimoto K. Occurrence of tetrodotoxin in the starfish Astropenten latespinisus. Experientia. 1984;40:1395–1402. [Google Scholar]
- 12.Maruyama J, Noguchi T, Narita H, Nara M, Jeon J K, Otsuka M, Hashimoto K. Occurrence of tetrodotoxin in the starfish Astropenten scoparius. Agric Biol Chem. 1985;49:3069–3075. [Google Scholar]
- 13.Matsui T, Taketsugu S, Kodama K, Ishil A, Yamamori K, Shimizu C. Production of tetrodotoxin by the intestinal bacteria of a puffer fish, Takifugu niphobies. Nippon Suisan Gakkaishi. 1989;55:2199–2203. [Google Scholar]
- 14.Mosher H S, Fuhrman F A, Buchwaid H D, Fischer H G. Tarichatoxin-tetrodotoxin: a potent neurotoxin. Science. 1964;144:1100–1110. doi: 10.1126/science.144.3622.1100. [DOI] [PubMed] [Google Scholar]
- 15.Nagashima Y, Maruyama J, Noguchi T, Hashimoto K. Analysis of paralytic shellfish poison and tetrodotoxin by ion pairing high performance liquid chromatography. Nippon Suisan Gakkaishi. 1984;53:819–823. [Google Scholar]
- 16.Narita H, Matsubara S, Miwa N, Akahane S, Murakami M, Goto T, Nara M, Noguchi T, Saito T, Shida Y, Hashimoto K. Vibrio alginalyticus, a TTX-producing bacterium isolated from the starfish Astropecten polyacanthus. Nippon Suisan Gakkaishi. 1987;53:617–612. [Google Scholar]
- 17.Narita H, Noguchi T, Maruyama J, Nara M, Hashimoto K. Occurence of tetrodotoxin-associated substance in a gastropod, “hanamushirogai” Zeuxis siquijorensis. Bull Jpn Soc Sci Fish. 1984;50:85–89. [Google Scholar]
- 18.Narita H, Noguchi T, Maruyama J, Ueda Y, Hashimoto K, Watanabe Y, Hida K. Occurence of tetrodotoxin in a trumpet shell, “boshubora” Charonia sauliae. Bull Jpn Soc Sci Fish. 1981;47:935–942. [Google Scholar]
- 19.Noguchi T, Hashimoto Y. Isolation of tetrodotoxin from a goby Gobius criniger. Toxin. 1973;11:305–310. doi: 10.1016/0041-0101(73)90060-3. [DOI] [PubMed] [Google Scholar]
- 20.Noguchi T, Hwang D F, Arakawa O, Sugita H, Deguchi Y, Shida Y, Hashimoto K. Vibrio alginolyticus, a tetrodotoxin-producing bacterium, in the intestines of the fish Fugu vermicularis vermicularis. Mar Biol. 1987;94:625–630. [Google Scholar]
- 21.Noguchi T, Jeon J K, Arakawa O, Sugita H, Deguchi Y, Shida Y, Hashimoto K. Occurrence of tetrodotoxin and anhydrotetrodotoxin in Vibrio sp. isolated from the intestines of a xanthid crab, Atergatis floridus. J Biochem. 1986;99:311–314. doi: 10.1093/oxfordjournals.jbchem.a135476. [DOI] [PubMed] [Google Scholar]
- 22.Noguchi T, Maruyama J, Narita H, Hashimoto K. Occurence of tetrodotoxin in the gastropod mollusk Tutifa lissostoma (frog shell) Toxicon. 1984;22:219–224. doi: 10.1016/0041-0101(84)90022-9. [DOI] [PubMed] [Google Scholar]
- 23.Noguchi T, Maruyama J, Ueda Y, Hashimoto K, Harada T. Occurence of tetrodotoxin in the Japanese ivory shell Babylonia japonica. Bull Jpn Soc Sci Fish. 1981;478:909–1013. [Google Scholar]
- 24.Noguchi T, Narita H, Maruyama J, Hashimoto K. Tetrodotoxin in the starfish Astropenten polyacanthus, in association with toxification of a trumpet shell, “boshubora” Charonia sauliae. Bull Jpn Soc Sci Fish. 1982;48:1173–1179. [Google Scholar]
- 25.Noguchi T, Uzu A, Koyama K, Maruyama J, Nagashima Y, Hashimoto K. Occurence of tetrodotoxin as the major toxin in a xanthid crab, Atergatis floridus. Bull Jpn Soc Sci Fish. 1983;49:1887–1892. [Google Scholar]
- 26.Savage I V E, Howden M E H, Spence I. Hapalotoxin, a second lethal toxin from the octopus Hapalochlaena maculosa. Toxicon. 1977;15:463–466. doi: 10.1016/0041-0101(77)90127-1. [DOI] [PubMed] [Google Scholar]
- 27.Sheumack D D, Howden M E H, Spence I. Occurrence of a tetrodotoxin-like compound in the eggs of the venomous blue-ringed octopus (Haplaochlaena maculosa) Toxicon. 1984;22:811–812. doi: 10.1016/0041-0101(84)90164-8. [DOI] [PubMed] [Google Scholar]
- 28.Sheumack D D, Howden M E, Spence I, Quinn R J. Maculotoxin: a neurotoxin from the venom glands of the octopus Hapalochlaena maculosa identified as tetrodotoxin. Science. 1978;199:188–191. doi: 10.1126/science.619451. [DOI] [PubMed] [Google Scholar]
- 29.Simidu U, Noguchi T, Hwang D F, Shida Y, Hashimoto K. Marine bacteria which produce tetrodotoxin. Appl Environ Microbiol. 1987;53:1714–1715. doi: 10.1128/aem.53.7.1714-1715.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sugita H, Ueda R, Noguchi T, Arakawa O, Hashimoto K, Deguchi Y. Identification of a tetrodotoxin-producing bacterium isolated from the xanthid crab Atergatis floridus. Nippon Suisan Gakkaishi. 1987;53:1693. [Google Scholar]
- 31.Tansill B. Family II. Vibrionaceae Veron 1965. In: Krieg N R, Holt J G, editors. Bergey's manual of systematic bacteriology. Vol. 1. Baltimore, Md: Williams & Wilkins; 1984. pp. 516–550. [Google Scholar]
- 32.Yasumoto T, Oshima Y, Hosaka M, Miyakoshi H. Occurence of tetrodotoxin in the ivory shell Babylonia japonica from Wakasa Bay. Bull Jpn Soc Sci Fish. 1981;47:909–914. [Google Scholar]
- 33.Yasumoto T, Yasumura D, Yotsu M, Michishita T, Endo A. Bacterial production of tetrodotoxin and anhydrotetrodotoxin. Agric Biol Chem. 1986;50:793–795. [Google Scholar]