Abstract
Objective
To determine whether preoperative staging of high-risk prostate cancer with 18F-sodium-fluoride (18F-NaF) positron emission tomography (PET) reduces the risk of skeletal metastases.
Design
Nationwide, population-based cohort study using real-world data.
Setting
The study used national health registries, including all sites in Denmark from 2011 to 2018.
Participants
Newly diagnosed high-risk prostate cancer patients who underwent radical prostatectomy from 2011 to 2018. Patients were stratified into two groups according to the preoperative imaging modality of either 18F-NaF PET or bone scintigraphy.
Main outcome measures
The risk of skeletal-related events (SREs) as a proxy for skeletal metastases following radical prostatectomy. The secondary endpoint was overall survival.
Results
Between 1 January 2011 and 31 December 2018, 4183 high-risk patients underwent radical prostatectomy. Of these patients, 807 (19.3%) underwent 18F-NaF PET and 2161 (51.7%) underwent bone scintigraphy. The remaining 30% were examined by a different imaging method or did not undergo imaging. Using the inverse probability of treatment weighting to control potential confounding, the HR of experiencing an SRE for patients in the 18F-NaF PET group versus the bone scintigraphy group was 1.15 (95% CI 0.86 to 1.54). The 3-year survival rates were 97.4% (95% CI 96.1 to 98.7) and 97.1% (95% CI 96.4 to 97.9) for patients receiving 18F-NaF PET and bone scintigraphy, respectively.
Conclusion
Patients with high-risk prostate cancer undergoing preoperative staging with 18F-NaF PET did not display a lower risk of developing SREs after prostatectomy compared with patients undergoing bone scintigraphy. The survival rates were similar between the two groups.
Keywords: nuclear medicine, oncology, epidemiology, urological tumours
Strengths and limitations of this study.
Registry data provides real-world data on the clinical impact of clinical practices.
This study identified a large cohort from all institutions in Denmark using high-quality registry data.
The routinely collected health data are not specifically registered for the purposes of this research, resulting in a minor degree of missing data.
Regression analysis weighted by the inverse probability of treatment ensured consideration of all measured confounders and addressed confounding by indication.
Introduction
Prostate cancer is one of the most common malignancies in the Western world, with over 1.4 million new cases reported in 2020.1 Prostate cancer frequently metastasizes to the bone, which is associated with significant morbidity and mortality.2 3 Accurate detection of bone metastases at primary staging is essential for decision-making regarding subsequent management. At the time of diagnosis, the risk of recurrence is determined based on the prostate specific antigen (PSA) level, Gleason score and clinical tumour stage (T-stage).4 Patients classified as unfavourable—intermediate risk or high risk will often receive preoperative staging by imaging. International urology and oncology guidelines recommend bone scintigraphy with 99mTechnetium-labelled phosphonate (99mTc) for the assessment of bone metastases at primary staging.4 5
However, several studies have shown that the bone-specific positron emission tomography (PET) tracer 18F-sodium-fluoride (18F-NaF) is superior to bone scintigraphy in terms of its diagnostic accuracy for detecting bone metastases including fewer equivocal findings.6–8 In previous studies, the sensitivity of bone scintigraphy for the detection of bone metastases varied from 57% to 97%, and the specificity varied from 57% to 80%.6–9 In contrast, the sensitivity of 18F-NaF PET for the diagnosis of bone metastases has ranged from 81% to 100% in the majority of studies, with a specificity ranging from 71% to 100%.6–8 10 11 With the purported lower accuracy of bone scintigraphy, the risk of misdiagnosing patients is high, possibly resulting in suboptimal treatment strategies. Among patients referred for suspected metastases, the use of 18F-NaF PET instead of bone scintigraphy in patients with prostate cancer has been shown to affect the patient management strategy in 6%–12% of cases.12 13 However, no studies have documented that the subsequent change in patient management strategies induced by 18F-NaF PET and its improved diagnostic accuracy confer any patient benefit in terms of mortality, morbidity and quality of life. Thus, we performed a cohort study with real-world data of men diagnosed with prostate cancer in Denmark who underwent either bone scintigraphy or 18F-NaF PET as part of primary staging before curative intent prostatectomy to examine whether the type of preoperative imaging modality was associated with overall survival and skeletal-related events (SREs) after radical prostatectomy.
Methods
Study population and data sources
This nationwide register-based cohort study was conducted in Denmark, which has approximately 5.8 million residents. In Denmark, all residents are provided with free, tax-supported healthcare by the National Health Service. A unique 10-digit civil registration number is assigned to all residents at birth by the Central Office of Civil Registration. This number allows unambiguous linkage across all Danish population-based registries.14 Reporting to the registries by clinicians is mandatory, which ensures high completeness of medical information. The applied data included nationwide information from the Danish Cancer Registry,15 the Civil Registration System,16 the Danish National Patient Registry,17 the Register of Laboratory Results for Research,18 the Danish Prostate Cancer Database,19 the Danish National Pathology Register20 and the Register of Causes of Death.21 Online supplemental appendix 1, p1 provides a detailed description of the codes found in the registries for prostate cancer characteristics, treatment, outcomes and covariates. Furthermore, the study is reported in accordance with Strengthening the Reporting of Observational Studies in Epidemiology guidelines, and a checklist is provided in online supplemental files.
bmjopen-2021-058898supp001.pdf (267.9KB, pdf)
Identifying men with prostate cancer
No formal screening programme for prostate cancer existed during the study period. Therefore, men were referred to the urology department on suspicion of prostate cancer. We used the Danish National Patient Registry to identify a cohort consisting of men with a first-time prostate cancer diagnosis from 2011 through 2018 who had undergone radical prostatectomy. This registry was established in 1977 for hospitalised patients; outpatient visits at hospitals have been included since 1995.17 The registry includes dates of admission and discharge, diagnosis (ICD-10 codes), surgical procedures and treatment information. The validity of a prostate cancer diagnosis in this register has previously been evaluated and found to be high, with a positive predictive value of nearly 90%.22
Risk classification
We restricted the cohort to patients we could classify as having a preoperative high risk of cancer recurrence according to the European Association of Urology (EAU) risk classification of prostate cancer. The EAU defines high-risk patients as those with a PSA of more than 20 ng/mL OR a Gleason score >7 OR a T-stage of T2c as the minimum.4 PSA values were retrieved from the Danish Register of Laboratory Results, which includes laboratory data from four of the five regions of Denmark.18 Data from the last region were obtained directly from the relevant regional database. The Gleason score was obtained from the Pathology Register, which contains information on all pathological examinations conducted in Denmark since 1997. T-stage was obtained from the Danish Cancer Registry, which has prospectively recorded all cancers diagnosed in Denmark since 1943, classified according to ICD-10, and ICD Oncology codes (ICD-0-3) for topography and morphology.15 For all three variables, we included the latest recorded value within 6 months prior to surgery. If PSA, Gleason score or T-stage were missing, we used the Danish Prostate Cancer Database to fill in the missing variables. This register is a nationwide clinical cancer database established in 2010 that records data on all incident, historically verified prostate cancer cases.
Imaging modality
We retrieved information on imaging modalities from the Danish National Patient Registry. We identified the preoperative use of bone scintigraphy and 18F-NaF PET, recorded up to 6 months before surgery, combined with CT or MRI. Single-photon emission/CT was conducted according to institutional practices. Patients were categorised according to their preoperative imaging into two groups: those who underwent bone scintigraphy only (bone scintigraphy group) and those who underwent 18F-NaF PET scan with or without bone scintigraphy (18F-NaF PET group). In general, each site performed only one of the two scans; thus, physicians did not stratify patients according to a specific imaging modality. Patients with an 18F-NaF PET scan performed as a part of a clinical research project were excluded from the cohort because the results of these scans were not made available to the referring physician.
SREs and bone metastases
We obtained information on SREs through the Danish National Patient Registry. SREs comprised the following events occurring after the date of radical prostatectomy: radiation to the bone defined as 1–4 treatments with external radiation therapy (standard practice in Denmark for the treatment of bone pain), pathological and osteoporotic fractures, spinal cord compression, surgery to the bone or a first-time bone metastasis diagnosis code.
Mortality
Mortality and migration updates were obtained from the Civil Registration System, which is updated daily.14 The register contains information on the vital status (dead or alive), date of death and migration status of all Danish citizens.
Comorbidity
We used the Charlson comorbidity index to describe preexisting comorbidities in the prostate cancer cohort23 (online supplemental appendix 1, p2). We calculated the index based on diagnoses recorded in the Danish National Patient Registry up to 10 years before the date of surgery. For analysis, we categorised the index into three comorbidity levels, including (1) those without comorbidity, (2) those with a comorbidity index equal to 1 and (3) those with a comorbidity index above 1.
Statistical analysis
Baseline characteristics are reported as frequencies with percentages and medians with IQRs. We estimated the cumulative risk of SREs according to the type of imaging modality and plotted the cumulative risk as a function of time since radical prostatectomy; death was treated as a competing risk event. Patients contributed time at risk from the date of radical prostatectomy until the date of first-time registered SRE, migration, death or 31 December 2018, whichever came first. Finally, we similarly estimated the cumulative incidence of death.
For the main analysis, we used Cox proportional hazards regression analysis to estimate the age-adjusted and multivariate-adjusted HRs of SREs with 95% CIs, comparing those who underwent 18F-NaF PET scans with those who underwent bone scintigraphy. Additionally, to better control potential confounding by indication, analysis of the inverse probability of treatment weighting (IPTW) was performed based on the propensity score for 18F-NaF PET. Propensity scores were calculated using logistic regression with the inclusion of the same variables as in the adjusted Cox analysis. We adjusted for age, Charlson comorbidity index, PSA (categorical variable:<10, 10–20, >20 ng/mL), Gleason score (categorical variable: <7, 7, >7) and T-stage (categorical variable: T1, T2, T3+T4). Adjusting with categorical variables was deemed necessary due to outliers and the limited number of records available on the outer areas of the scales. Furthermore, we stratified the analysis by PSA, Gleason score, T-stage and year of radical prostatectomy. In the stratified analysis, we only adjusted for age and Charlson comorbidity index. An adjusted HR of death was also calculated. No further analyses were performed for patients with other types of imaging or no imaging before surgery.
Several sensitivity analyses were performed to test the robustness of our findings. First, due to potential site-related differences in risk factors among the included patients, we conducted an analysis restricted to the capital region of Denmark, which performed most of the 18F-NaF PET scans. Second, we executed the analysis with a reclassification of the exposure group to include patients with both scans. To account for missing data and enable adjustment for PSA, Gleason score and T-stage we used multiple imputation using splines24 with all the main analysis variables and the outcome variable in the model. We produced and combined 200 sets of imputations.
Statistical software
Data management and analyses were conducted in R V.4.0.3 using RStudio 2020 (RStudio, PBC, Boston, Massachusetts, USA) with the following packages: heaven, data.table, Publish, survival, stringr, mitools, smcfcs and ipw.
Patient and public involvement
This study was observational and based on data from routine healthcare records. No patients were directly involved in the study.
Results
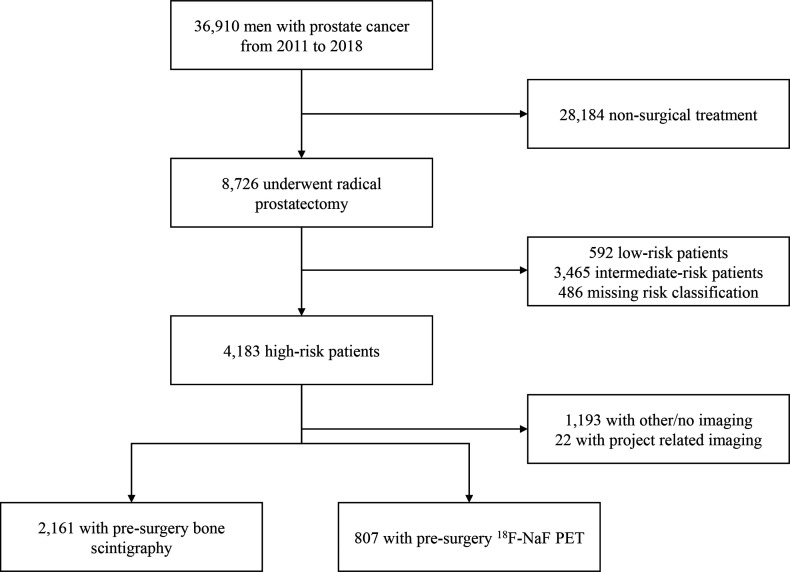
Between 1 January 2011 and 31 December 2018, 36 910 men were diagnosed with prostate cancer in Denmark, of whom 8726 (23.6%) underwent radical prostatectomy (figure 1). Among those who underwent radical prostatectomy, 4183 patients (47.9%) were classified as high risk according to the EAU preoperative staging criteria. A total of 2161 (51.7%) high-risk patients undergoing surgery were evaluated for skeletal metastasis with bone scintigraphy only, and 807 (19.3%) men were evaluated with 18F-NaF PET. Information on the PSA values, Gleason score and T-stage from the registries ensured nearly 90% completeness of the high-risk classification, resulting in a large study population for our analysis. A notable proportion of high-risk patients (28.5%) underwent different imaging modalities or no imaging to evaluate bone metastasis, and a small portion of patients (0.5%) were excluded because they underwent project-related imaging. The median age at the date of radical prostatectomy was 67 years (IQR, 62–70.1), and the median follow-up from surgery was 4.1 years (IQR, 2.4–6.0 years). In general, patients receiving 18F-NaF PET had a higher PSA level, Gleason score, and T-stage at primary staging (table 1).
Figure 1.
Study profile study cohort of 2161 men undergoing presurgical imaging with bone scintigraphy and 807 men undergoing 18F-sodium-fluoride (18F-NaF) positron emission tomography (PET). Patients with no or other imaging were combined since there were no differences between sites performing 18F-NaF PET or bone scintigraphy. Moreover, we experienced inconsistencies in the way CT and MRI scans where coded in the registries, making it difficult to distinguish between imaging of the prostate and other sites.
Table 1.
Baseline patient characteristics by imaging modality
| Bone scintigraphy (n=2161) | 18F-NaF PET (n=807) | All (n=2968) | |
| Age (years, median (IQR)) | 66.3 (61.7, 69.7) | 67.9 (62.9, 71.2) | 66.7 (62.0, 70.1) |
| Year of surgery | |||
| 2011–2013 | 852 (39.4) | 212 (26.3) | 1064 (35.8) |
| 2014–2015 | 602 (27.9) | 235 (29.1) | 837 (28.2) |
| 2016–2018 | 707 (32.7) | 360 (44.6) | 1067 (36.0) |
| Imaging date before prostatectomy (days, median (IQR)) | 46 (32, 65) | 42 (28, 56) | 45 (30, 63) |
| PSA (ng/mL) | |||
| <10 | 955 (45.0) | 263 (33.1) | 1218 (41.8) |
| 10–20 | 642 (30.2) | 292 (36.8) | 934 (32.0) |
| >20 | 526 (24.8) | 239 (30.1) | 765 (26.2) |
| Gleason biopsy score | |||
| <7 | 345 (16.2) | 70 (8.8) | 415 (14.2) |
| 7 | 1225 (57.5) | 469 (58.6) | 1694 (57.8) |
| >7 | 560 (26.3) | 261 (32.6) | 821 (28.0) |
| Clinical T-stage | |||
| T1 | 259 (12.6) | 50 (7.5) | 309 (11.4) |
| T2 | 1260 (61.5) | 241 (36.0) | 1501 (55.2) |
| T3-T4 | 529 (25.8) | 378 (56.5) | 907 (33.4) |
| Comorbidity* | |||
| Cardiovascular diseases | 118 (5.5) | 52 (6.4) | 170 (5.8) |
| Other malignancies | 102 (4.7) | 64 (7.9) | 166 (5.6) |
| Diabetes | 62 (2.9) | 48 (6.0) | 110 (3.7) |
| Charlson comorbidity index | |||
| 1 | 267 (12.4) | 115 (14.3) | 382 (12.9) |
| >1 | 203 (9.4) | 107 (13.3) | 310 (10.4) |
Characteristics on the day of surgery for men with high-risk prostate cancer from 2011 to 2018. Percentages may not add up to 100 due to rounding or missing data.
*Top three comorbidities.
18F-NaF, 18F-sodium- fluoride; PET, positron emission tomography; PSA, prostate specific antigen; T-stage, tumour stage.
SREs and bone metastases
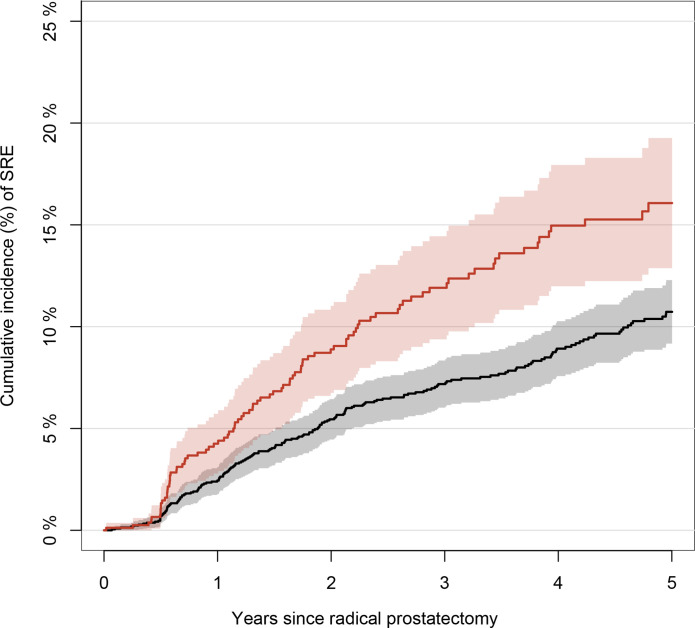
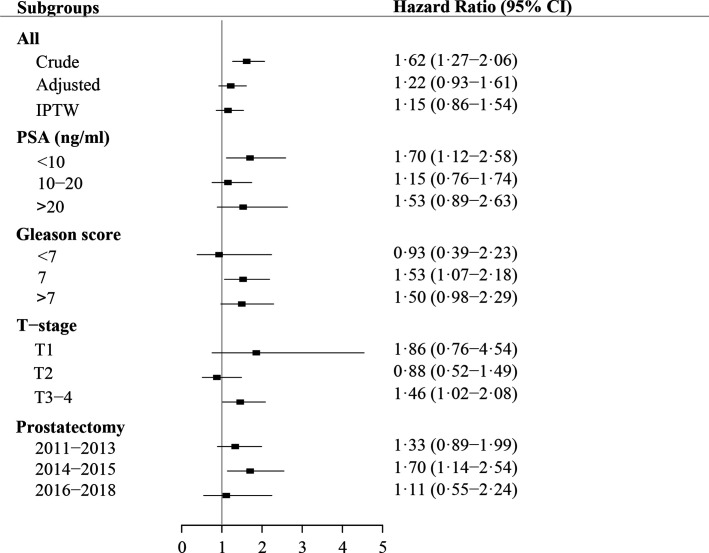
The unadjusted 1-year cumulative risk of SREs was 2.4% (95% CI 1.8 to 3.1) for men who underwent bone scintigraphy and 4.3% (95% CI 2.8 to 5.7) for those who underwent 18F-NaF PET (figure 2). The unadjusted 3-year cumulative risk of SREs was 7.2% (95% CI 6.0 to 8.3) for men undergoing bone scintigraphy and 11.9% (95% CI 9.4 to 14.4) for those undergoing 18F-NaF PET. Of the 300 men with at least one SRE recorded during follow-up, 53.7% had radiation to bone recorded as their first event, 30.7% had a pathological or osteoporotic fracture, 6.3% had spinal cord compression, 6.3% had a code for bone metastases and 3.0% had bone surgery. In the main analysis, we did not find that 18F-NaF PET decreased the HR of experiencing SREs after surgery; in contrast, we observed a slightly increased HR, which was reduced when adjusting the model (adjusted HR, 1.22; 95% CI 0.93 to 1.61; figure 3). When we used IPTW to control for potential confounding factors, the risk of experiencing an SRE was attenuated (IPTW adjusted HR, 1.15: 95% CI 0.86 to 1.54; figure 3). Stratified analyses similarly demonstrated increased HRs for SREs in patients undergoing 18F-NaF PET compared with those undergoing bone scintigraphy, except for patients with stage 2 disease and those with a Gleason score <7 (figure 3).
Figure 2.
Unadjusted cumulative incidence of skeletal-related events (SREs). The unadjusted cumulative incidence with 95% CIs of SREs in men after undergoing radical prostatectomy. Death was treated as a competing event. The red curve represents 18F-sodium-fluoride positron emission tomography and the black curve represents bone scintigraphy.
Figure 3.
Main analysis results HRs for skeletal-related events following radical prostatectomy among patients undergoing 18F-sodium-fluoride positron emission tomography before surgery versus patients undergoing bone scintigraphy. IPTW, inverse probability of treatment weighting; PSA, prostate specific antigen; T-stage, tumour stage.
Survival
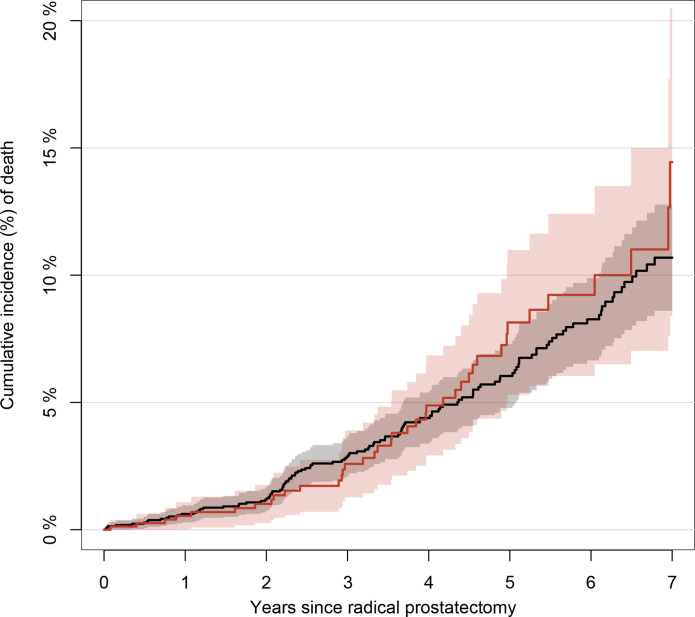
Figure 4 shows the cumulative survival curves of the cohorts for up to 7 years of follow-up. The 1-year survival was 99.4% (95% CI 99.0 to 99.7) in men who underwent bone scintigraphy and 99.5% (95% CI 98.9 to 100) in men who underwent 18F-NaF PET, and the corresponding 3-year survival rates in the cohorts were 97.1% (95% CI 96.4 to 97.9) and 97.4% (95% CI 96.1 to 98.7), respectively. Adjusted analyses showed a modest reduction in mortality for patients who underwent 18F-NaF PET (adjusted HR, 0.89; 95% CI 0.61 to 1.30).
Figure 4.
Unadjusted cumulative incidence of death. Unadjusted cumulative incidence of death with 95% CIs for men with prostate cancer after undergoing radical prostatectomy, stratified by type of imaging modality. The red curve represents 18F-sodium-fluoride positron emission tomography and the black curve represents bone scintigraphy.
Sensitivity analysis
Restricting to patients from the capital region yielded cumulative SRE risk estimates consistent with those of the main analysis (online supplemental appendix 1, p3). Similar to the main analysis, the cumulative risk of SREs was higher for men evaluated with 18F-NaF PET than for those evaluated with bone scintigraphy. Adjusted analysis for the capital region was also comparable to the main analysis (online supplemental appendix 1, p4) and did not suggest any added value of using 18F-NaF PET.
Including patients with both bone scintigraphy and 18F-NaF PET in the bone scintigraphy group or excluding them entirely yielded HRs similar to those of the main analysis. A final analysis with imputed values for PSA, Gleason score and T-stage yielded HRs similar to those of the analysis without imputation.
Discussion
Principal findings
In this nationwide cohort study of Danish patients with high-risk prostate cancer undergoing prostatectomy, we found that primary staging with 18F-NaF PET did not reduce the risk of SREs compared with primary staging with bone scintigraphy, whereas a slight tendency towards a reduction in all-cause mortality was observed in the group undergoing 18F-NaF PET. To the best of our knowledge, this is the first study to evaluate patient-relevant outcomes of using a PET-based method for primary staging.
Comparison with other studies
Prior studies on 18F-NaF PET in prostate cancer have focused on its improvements in diagnostic accuracy compared with bone scintigraphy6–8 or its impact on patient management.12 13 The superior diagnostic performance of 18F-NaF PET when detecting bone metastases, should presumably result in improved patient selection for curative and life-prolonging treatment, resulting in fewer SREs the first few years after surgery. However, in this study, we did not observe any superiority over bone scintigraphy in terms of patient benefit among newly diagnosed, high-risk prostate cancer patients.
Evidence of patient-relevant outcomes is often reported from randomised controlled trials. Randomised trials are, however, not commonly conducted within the field of imaging, and it has previously been debated whether randomised trials are necessary to evaluate diagnostic procedures.25 26 In prostate cancer, only two randomised controlled trials have been published, employing PET in one arm and standard imaging in the other arm. One such trial confirmed the diagnostic superiority of prostate specific membrane antigen (PSMA) PET/CT during primary staging,27 whereas the other trial focused on the changes in patient management based on fluciclovine PET/CT at the time of biochemical recurrence;28 none of these trials were linked to patient-relevant outcomes.
Randomised trials have demonstrated the clinical benefit of PET within other types of cancers, such as haematological and lung cancers.29 Fischer et al compared preoperative staging with FDG PET/CT to conventional staging by CT in patients with lung cancer and found that patients in the PET/CT group showed a reduction in both the total number of thoracotomies and the number of futile thoracotomies; however, they did not observe a decrease in overall mortality.30 Similar results were reported for colorectal liver metastases, with one study finding that FDG PET led to a reduction in futile laparotomies in one of six patients.31 It could be expected that the use of 18F-NaF PET would reduce the number of ‘futile’ prostatectomies in patients harbouring bone metastases at the time of diagnosis, thereby reducing the incidence of SREs postoperatively. With recent trials demonstrating superior diagnostic properties of PSMA PET for primary staging in high-risk prostate cancer, its impact on treatment choice—and perhaps outcome—is likely to be greater than that of 18F-NaF PET.
Strengths and limitations
The major strengths of our study are its national scale, large cohort, high-quality registry data and complete follow-up. The registration of information related to prostate cancer diagnosis and radical prostatectomy, as well as variables defining the high-risk population, is thought to be practically complete because of a uniformly organised healthcare system where healthcare is free (tax-supported) and available to all residents.32 Furthermore, a median follow-up time of 4.1 years is adequate for the purpose of evaluating bone metastases not captured by the imaging modality at primary staging; hence, only patients with a negative scan will undergo radical prostatectomy with curative intend in Denmark.
Nevertheless, our study has several limitations worth considering. The potential of confounding by indication was particularly concerning because of the observed higher values for PSA, Gleason score and T-stage in the 18F-NaF PET group; however, the indication of usage was the same for both scans. Moreover, the demographics of the groups might have been more alike if the International Society of Urological Pathology (ISUP) grading system was used for the Gleason score, which distinguishes between normal high-risk prostate cancer and very high-risk (ISUP grade 5) cancer cases. It was not possible to use the ISUP grading due to unavailability in some of the registers. Furthermore, confounding by indication is only an issue in hospitals that offer both bone scintigraphy and 18F-NaF PET, which is highly uncommon in Denmark. Since sites only used one of the imaging modalities, physicians did not have to choose between the two, resulting in minimal selection bias. We attempted to control for confounding by using a propensity score-based IPTW, but we cannot rule out residual confounding due to misclassified or unmeasured prognostic factors. Multi-parametric MRI (mpMRI) is also a factor worth considering in relation to targeted biopsies in the diagnostic work-up of prostate cancer. This method has been gradually implemented nationally in Denmark and prior to 2018 only very few sites had access to mpMRI for all patients; hence, we do not have data available yet. The introduction of mpMRI targeted biopsy is likely to affect the selection of patients for radical prostatectomy in the future.
In the present study, we defined SREs as either external radiation therapy, pathological or osteoporotic fractures, spinal cord compression, surgery to the bone or a bone metastases code. It can be speculated that patients treated at a site using 18F-NaF PET would undergo 18F-NaF PET rather than bone scintigraphy in case of biochemical recurrence, thereby increasing the detection of bone metastases during follow-up. However, the risk of SREs was primarily driven by a high percentage of radiotherapy of bone or fracture cases, which are not related to 18F-NaF PET. Moreover, with the widespread introduction of prostate specific membrane antigen (PSMA) PET/CT in Denmark from 2015 and onwards, patients with biochemical recurrence would undergo PSMA PET/CT rather than 18F-NaF PET/CT. Information regarding bone metastases was noted in only 6.3% of SREs across the groups.
Conclusions
In conclusion, we found that the use of 18F-NaF PET at primary staging did not improve patient-relevant outcomes in terms of a reduction in SREs compared with that with bone scintigraphy.
Supplementary Material
Footnotes
Contributors: AWM, LJP and HDZ conceived the study and contributed to the literature search. AWM and CT-P had access to the data and carried out the data management and analysis. AWM, CT-P and MN designed the graphs. AWM, LJP, HDZ, CT-P, MN and MTP aided in the interpretation of results. AWM prepared the first draft of the paper. AWM, CT-P and HDZ acts as guarantors for the overall content. All authors contributed to critical revision and approval of the final draft of the paper.
Funding: Funding was received from the North Denmark Region’s Fund for Health Sciences Research and from Knud and Edith Eriksens Mindefond. Award numbers are not applicable.
Competing interests: One author received grants from Bayer and Novo Nordisk for the submitted work.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
All data relevant to the study are included in the article or uploaded as supplementary information.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
The Danish Data Protection Agency approved the use of data for this study (reference number 2008-58-0028). Furthermore, the study was granted approval by the Danish Patient Safety Authority to collect laboratory data (reference numbers 3-3013-3183/1 and 31-1522-37). Ethics approval is not required for historical register-based studies in Denmark.
References
- 1.Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: A Cancer Journal for Clinicians 2021;71:209–49. 10.3322/caac.21660 [DOI] [PubMed] [Google Scholar]
- 2.Logothetis CJ, Lin S-H. Osteoblasts in prostate cancer metastasis to bone. Nat Rev Cancer 2005;5:21–8. 10.1038/nrc1528 [DOI] [PubMed] [Google Scholar]
- 3.Nørgaard M, Jensen Annette Østergaard, Jacobsen JB, et al. Skeletal related events, bone metastasis and survival of prostate cancer: a population based cohort study in Denmark (1999 to 2007). J Urol 2010;184:162–7. 10.1016/j.juro.2010.03.034 [DOI] [PubMed] [Google Scholar]
- 4.Mottet N, van den Bergh RCN, Briers E, et al. EAU-EANM-ESTRO-ESUR-SIOG guidelines on prostate Cancer—2020 update. Part 1: screening, diagnosis, and local treatment with curative intent. Eur Urol 2021;79:243–62. 10.1016/j.eururo.2020.09.042 [DOI] [PubMed] [Google Scholar]
- 5.NCCN . Clinical practice guidelines in oncology, prostate cancer version 2.2020, 2020. Available: http://www.nccn.com
- 6.Jambor I, Kuisma A, Ramadan S, et al. Prospective evaluation of planar bone scintigraphy, SPECT, SPECT/CT, 18F-NaF PET/CT and whole body 1.5T MRI, including DWI, for the detection of bone metastases in high risk breast and prostate cancer patients: SKELETA clinical trial. Acta Oncol 2016;55:59–67. 10.3109/0284186X.2015.1027411 [DOI] [PubMed] [Google Scholar]
- 7.Even-Sapir E, Metser U, Mishani E, et al. The detection of bone metastases in patients with high-risk prostate cancer: 99mTc-MDP planar bone scintigraphy, single- and multi-field-of-view SPECT, 18F-fluoride PET, and 18F-fluoride PET/CT. J Nucl Med 2006;47:287–97. [PubMed] [Google Scholar]
- 8.Iagaru A, Mittra E, Dick DW, et al. Prospective evaluation of (99m)Tc MDP scintigraphy, (18)F NaF PET/CT, and (18)F FDG PET/CT for detection of skeletal metastases. Mol Imaging Biol 2012;14:252–9. 10.1007/s11307-011-0486-2 [DOI] [PubMed] [Google Scholar]
- 9.Palmedo H, Marx C, Ebert A, et al. Whole-Body SPECT/CT for bone scintigraphy: diagnostic value and effect on patient management in oncological patients. Eur J Nucl Med Mol Imaging 2014;41:59–67. 10.1007/s00259-013-2532-6 [DOI] [PubMed] [Google Scholar]
- 10.Langsteger W, Balogova S, Huchet V, et al. Fluorocholine (18F) and sodium fluoride (18F) PET/CT in the detection of prostate cancer: prospective comparison of diagnostic performance determined by masked reading. Q J Nucl Med Mol Imaging 2011;55:448–57. [PubMed] [Google Scholar]
- 11.Mosavi F, Johansson S, Sandberg DT, et al. Whole-Body Diffusion-Weighted MRI Compared With 18 F-NaF PET/CT for Detection of Bone Metastases in Patients With High-Risk Prostate Carcinoma. American Journal of Roentgenology 2012;199:1114–20. 10.2214/AJR.11.8351 [DOI] [PubMed] [Google Scholar]
- 12.Gauthé M, Aveline C, Lecouvet F, et al. Impact of sodium 18F-fluoride PET/CT, 18F-fluorocholine PET/CT and whole-body diffusion-weighted MRI on the management of patients with prostate cancer suspicious for metastasis: a prospective multicentre study. World J Urol 2019;37:1587–95. 10.1007/s00345-018-2547-5 [DOI] [PubMed] [Google Scholar]
- 13.Hillner BE, Hanna L, Makineni R, et al. Intended Versus Inferred Treatment After 18F-Fluoride PET Performed for Evaluation of Osseous Metastatic Disease in the National Oncologic PET Registry. J Nucl Med 2018;59:421–6. 10.2967/jnumed.117.205047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schmidt M, Pedersen L, Sørensen HT. The Danish civil registration system as a tool in epidemiology. Eur J Epidemiol 2014;29:541–9. 10.1007/s10654-014-9930-3 [DOI] [PubMed] [Google Scholar]
- 15.Gjerstorff ML. The Danish cancer registry. Scand J Public Health 2011;39:42–5. 10.1177/1403494810393562 [DOI] [PubMed] [Google Scholar]
- 16.Pedersen CB. The Danish civil registration system. Scand J Public Health 2011;39:22–5. 10.1177/1403494810387965 [DOI] [PubMed] [Google Scholar]
- 17.Schmidt M, Schmidt SAJ, Sandegaard JL, et al. The Danish national patient registry: a review of content, data quality, and research potential. Clin Epidemiol 2015;7:449–90. 10.2147/CLEP.S91125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Arendt JFH, Hansen AT, Ladefoged SA, et al. Existing data sources in clinical epidemiology: laboratory information system databases in Denmark. Clin Epidemiol 2020;12:469–75. 10.2147/CLEP.S245060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nguyen-Nielsen M, Høyer S, Friis S, et al. The Danish prostate cancer database. Clin Epidemiol 2016;8:649–53. 10.2147/CLEP.S100256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Erichsen R, Lash TL, Hamilton-Dutoit SJ, et al. Existing data sources for clinical epidemiology: the Danish national pathology registry and data bank. Clin Epidemiol 2010;2:51–6. 10.2147/clep.s9908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Helweg-Larsen K. The Danish register of causes of death. Scand J Public Health 2011;39:26–9. 10.1177/1403494811399958 [DOI] [PubMed] [Google Scholar]
- 22.Gammelager H, Christiansen CF, Johansen MB, et al. Quality of urological cancer diagnoses in the Danish national Registry of patients. Eur J Cancer Prev 2012;21:545–51. 10.1097/CEJ.0b013e328351c680 [DOI] [PubMed] [Google Scholar]
- 23.Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987;40:373–83. 10.1016/0021-9681(87)90171-8 [DOI] [PubMed] [Google Scholar]
- 24.JBaR K. smcfcs: multiple imputation of covariates by substantive model compatible fully conditional specification, 2020: 17. http://www.missingdata.org.uk,http://thestatsgeek.com [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hicks RJ, Ware RE, Hofman MS. Not-so-random errors: randomized controlled trials are not the only evidence of the value of PET. Journal of Nuclear Medicine 2012;53:1820–2. author reply 22-24. 10.2967/jnumed.112.111351 [DOI] [PubMed] [Google Scholar]
- 26.Lalumera E, Fanti S. Randomized controlled trials for diagnostic imaging: conceptual and Pratical problems. Topoi 2019;38:395–400. 10.1007/s11245-017-9535-z [DOI] [Google Scholar]
- 27.Hofman MS, Lawrentschuk N, Francis RJ, et al. Prostate-Specific membrane antigen PET-CT in patients with high-risk prostate cancer before curative-intent surgery or radiotherapy (proPSMA): a prospective, randomised, multicentre study. The Lancet 2020;395:1208–16. 10.1016/S0140-6736(20)30314-7 [DOI] [PubMed] [Google Scholar]
- 28.Akin-Akintayo OO, Jani AB, Odewole O, et al. Change in salvage radiotherapy management based on guidance with FACBC (Fluciclovine) PET/CT in postprostatectomy recurrent prostate cancer. Clin Nucl Med 2017;42:e22–8. 10.1097/RLU.0000000000001379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Scheibler F, Zumbé P, Janssen I, et al. Randomized controlled trials on PET: a systematic review of topics, design, and quality. J Nucl Med 2012;53:1016–25. 10.2967/jnumed.111.101089 [DOI] [PubMed] [Google Scholar]
- 30.Fischer B, Lassen U, Mortensen J, et al. Preoperative staging of lung cancer with combined PET–CT. N Engl J Med Overseas Ed 2009;361:32–9. 10.1056/NEJMoa0900043 [DOI] [PubMed] [Google Scholar]
- 31.Ruers TJM, Wiering B, van der Sijp JRM, et al. Improved selection of patients for hepatic surgery of colorectal liver metastases with (18)F-FDG PET: a randomized study. J Nucl Med 2009;50:1036–41. 10.2967/jnumed.109.063040 [DOI] [PubMed] [Google Scholar]
- 32.Schmidt M, Schmidt SAJ, Adelborg K, et al. The Danish health care system and epidemiological research: from health care contacts to database records. Clin Epidemiol 2019;11:563–91. 10.2147/CLEP.S179083 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2021-058898supp001.pdf (267.9KB, pdf)
Data Availability Statement
All data relevant to the study are included in the article or uploaded as supplementary information.