Abstract
Introduction
Inducible laryngeal obstruction (ILO) describes transient laryngeal closure during respiration and can cause significant morbidity. Non-pharmacological behavioural therapy is the commonly cited treatment but efficacy is largely unknown.
Aim
To synthesise the current evidence base on the effectiveness of non-pharmacological interventions used to treat adults with ILO.
Methods
Electronic databases (Medline/Embase/CINAHL/PsycINFO/AMED/CENTRAL) were systematically searched, informed by a population, intervention, comparison, outcome framework. Two reviewers independently screened a representative sample, with lead-author completion due to excellent inter-rater reliability. Data was extracted using a predefined piloted form. Methodological quality was appraised (blindly by two reviewers) using the Joanna Briggs Institute Critical Appraisal Tools. A narrative synthesis was performed due to heterogeneity of studies (PROSPERO registration number: CRD42020213187).
Results
Initial searching identified 3359 records. Full-text screening occurred in 92 records and 14 studies, comprising 527 participants, were deemed eligible. All studies were low-level evidence (observational by design, with four case reports), with a high risk of bias; none contained control arms for comparison. Intervention description was inconsistently and poorly described but direction of effect was positive in 76% of outcomes measured. The majority of studies showed a reduction in symptom scores and improved direct laryngeal imaging post intervention; there was an overall reduction, 59.5%, in healthcare utilisation.
Discussion
The literature is in an embryonic state and lacks robust data to truly inform on the effectiveness of non-pharmacological interventions used to treat adults with ILO. However, positive signals in the synthesis performed support non-pharmacological treatment approaches and further development is warranted.
Keywords: asthma, not applicable
What is already known on this topic?
Non-pharmacological behavioural therapy is the commonly cited treatment for adults suffering with inducible laryngeal obstruction (ILO). Despite this, there is limited awareness on the efficacy of treatment approaches used and no comprehensive synthesis of the current evidence-base exists.
What this study adds
This study provides a robust synthesis on the effectiveness of non-pharmacological interventions used to treat adults with ILO. Evidence is in an embryonic state with marked heterogeneity, low-level evidence and high risk of bias.
How this study might affect research, practice or policy
Despite the poor quality of evidence, positive signals in the synthesis performed support non-pharmacological treatment approaches. Further development, guided by robust scientific principles, is warranted to inform future practice.
Introduction
Inappropriate laryngeal closure during respiration can cause symptoms of respiratory distress. When this occurs transiently, in the absence of any structural or neurological abnormalities, management is often challenging. Inducible laryngeal obstruction (ILO) defines this phenomena and describes reoccurring airflow obstruction at the glottic and/or supraglottic level. Vocal cord dysfunction or paradoxical vocal fold motion disorder are the most commonly recognised terms related to inappropriate glottic closure, although numerous terminology exist which has impeded cohesive research advances within the field. ILO is the recommend nomenclature by an international multiprofessional body task force and is increasingly adopted in research and clinical settings.1 Due to the transient nature of ILO symptoms, the consensus gold standard for diagnosis is direct visualisation of the larynx during an episodic attack.2 3
Individuals suffering with ILO present across varied healthcare settings with symptoms ranging from mild dyspnoea to acute respiratory distress.4 Typically, symptom onset is rapid and in response to an aggravating trigger5; commonly reported triggers include mechanical, scent, environmental and emotional factors.6 ILO induced by exercise is referred to as E-ILO (exercise induced laryngeal obstruction).7 Associated throat tightness, stridor and voice change are common and respiratory distress usually occurs during inspiration.3 8 9 Despite a clearly distinct pathophysiology, the symptoms of ILO are akin to asthma and therefore many individuals are mismanaged as such.10–12 The average time for misdiagnosis is lengthy (5.4 years), meaning escalating pharmacological burden with high healthcare utilisation is prominent, which in turn leads to significant levels of patient morbidity.9 10 12–14
The mechanistic drivers for ILO are poorly understood. In recent years, the concept of ‘laryngeal dysfunction’ has been proposed, which suggests a laryngeal sensitisation and a consequential laryngeal hyper-responsiveness.8 15 16 However, such hypothesises are yet to be tested robustly meaning that there is little consensus on ILO treatment approaches and no standardised protocol exist. Despite this, therapy-based management approaches are regularly cited within the literature and are often employed in the clinical context; speech and language therapy (SLT) is commonly referred to as the beneficial gold standard.17 18 However, although Patel et al’s systematic review highlights preliminary support for SLT as an effective ILO treatment it concludes the evidence supporting efficacy is in its infancy.19 Similarly, Mahoney et al’s recent work to determine treatment effectiveness for a range of vocal cord dysfunction interventions (pharmacological and non-pharmacological) concluded limited objective data exists to support the effectiveness of these interventions.20
More broadly, SLT involves delivery of multimodal interacting components in an attempt to effect change and as such meets criteria as a complex intervention.21 It is unknown if all components of the intervention are essential or if it is acceptable to patients. Further, other non-pharmacological interventions are reported beneficial, for example, airway device techniques.20 22 23
Before establishing and recommending a standardised ILO therapy approach, it is important to develop an intervention worthy of evaluation. Inline with the MRC framework for developing and evaluating complex interventions the first step in this robust process mandates identification of existing evidence, in the form of a systematic review.21 The overall aim of this review therefore is to identify the effectiveness of existing non-pharmacological interventions used to treat adults with ILO. Specifically, we aim to establish: (1) what are the key components, if any (2) what outcome measures have been used, if any (3) what is the effectiveness, if any
Methods
Cochrane methodology and the Preferred Reporting Items for Systematic Reviews and Meta-Analyses 2020 guidelines were used to perform this systematic review.24 25 The review protocol was registered on the international Prospective Register of Systematic Reviews database on 9 December 2020 (registration number: CRD42020213187).
Inclusion and exclusion criteria
A population, intervention, comparison, outcome framework was applied to identify studies of adults with ILO (population) who had received non-pharmacological treatment (intervention) with or without a control intervention (comparison) and where effectiveness of these interventions were considered (outcome).26 All types of studies, except expert opinion and reviews, were included; no publication date limitation was applied. Case reports and case series were excluded if no pre–post outcomes were available. Studies were limited to those in the English language.
For inclusion, ILO had to be objectively diagnosed, either by direct laryngeal imaging (ie, endoscopic and/or scanning confirmation) or via the validated diagnostic Pittsburgh questionnaire.27 Due to the recognised differences in paediatric and adult respiratory clinical care management, together with the variances in paediatric and adult larynges, publications reporting data from participants under 16 years of age were excluded. Further, if a study did not report any age demographic data it was excluded from review.
‘Non pharmacological interventions’ were classed as any interventions not classified as a pharmacological intervention under the EU Directive 2001/83/EEC (ie, the intervention contained no administration of a substance or combination of substances aiming to modify physiological function).
To ensure included studies had meaningful and non-trivial outcomes, only those reporting outcome measures explicit to ILO (eg, symptoms, direct visualisation, healthcare utilisation) were included.24
Search strategy
Electronic databases (Medline, Embase, CINAHL, PsycINFO, AMED and CENTRAL) were systematically searched (10 January 2021) to identity relevant publications for inclusion. Search domain one (ILO and associated terms) was combined using “AND” with search domain two (terms relating to non-pharmacological intervention). Terms within each domain were combined using “OR”. Due to varying taxonomy within the literature for ILO, search domain one terms were developed and agreed by representatives of a national expert SLT Respiratory Forum group. The final search strategy is detailed in online supplemental file 1 search strategy.
bmjresp-2022-001199supp001.pdf (14.9KB, pdf)
To check for any extra studies, grey searching in respiratory guidelines and policy documents was completed and expert authors in the field had their work searched; all identified relevant studies had their reference lists hand searched, together with forward citation searching. Prior to final analysis, searches were rerun on 1 July 2021.
Data management, selection and extraction
All identified studies were stored in Endnote V.X9, which facilitated deduplication of records. References were exported to Rayyan Qatar Computing Research Institute Systematic Review Web application to enable efficient screening of records. A predefined inclusion–exclusion screening checklist (as agreed by the study team) was applied (online supplemental file 2 screening checklist); reviewer one (JH) and reviewer two (JW-D) blindly screened 150 (7%) of the titles and abstracts in duplicate. There was an excellent 98% inter-rater agreement and a substantial kappa agreement (k=0.718, p=0.000). In view of such agreement, JH continued to screen the remainder of records. Full texts of all records meeting eligibility criteria and those with questionable eligibility were retrieved. To finalise studies eligible for inclusion, full text screening was performed by JH. If additional information was required to inform selection decision, the relevant corresponding author was contacted (three authors) for further clarification of unreported data.
bmjresp-2022-001199supp002.pdf (196.3KB, pdf)
Data were extracted from all eligible studies using a predefined data extraction form, which was piloted on three studies (by JH and JW-D) to ensure all relevant information was captured. Extracted data included characteristics of methods, study participates, intervention group(s), outcome(s) used and data analysis. Consistency of data extracted in the piloting phase was extremely high and therefore JH completed the remaining data extraction.
Grouping for synthesis
Methodological diversity across all included studies resulted in highly diverse characteristics and excessive statistical heterogeneity so meta-analysis was not possible. Studies were therefore synthesised in accordance with the Synthesis Without Meta-analysis guideline.28 Studies were grouped for synthesis based on the outcome measures reported. The broad outcome measure categories applied were (1) symptoms (validated (defined as those scales with published evidence of validity) and non-validated), (2) objective evidence on imaging, (3) healthcare utilisation.
Quality assessment and risk of bias
The Joanna Briggs Institute critical appraisal tools and level of evidence were used to assess evidence of effectiveness, trustworthiness, relevance and results.29 All studies were blindly rated by JH and JW-D; high agreement was achieved so third reviewer adjudication was unnecessary.
Patient and public involvement
The development of this work was indirectly informed by patient feedback about the need for effective interventions for ILO. Patients and the public were not involved in the study design as the nature of research mandates a standardised methodological approach. However, the findings of this review and how it will inform future therapeutic interventions will be disseminated across existing national patient support group networks.
Results
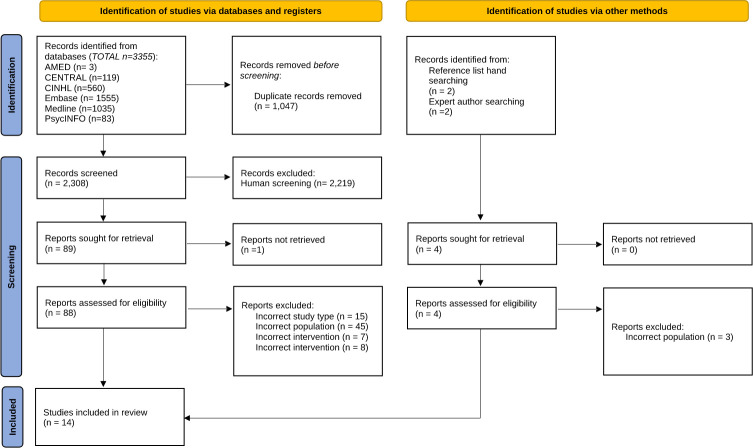
Electronic database searching retrieved 3355 records. A further four records were identified via other methods (figure 1), resulting in a total of 3359 records identified on initial searching. Following deduplication, a total of 2308 were screened for eligibility. Full-text screening occurred for 92 records, with a final inclusion of 14 studies; of those excluded, 60% (n=47) were due to inadequate ILO diagnosis, not satisfying the review criteria. Specific participant information could not be extrapolated from the overall study data in several studies so were excluded.5 30–32
Figure 1.
Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) 2020 flow diagram showing the screening and eligibility process using inclusion/exclusion criteria
Key characteristics of the included studies, comprising 527 participants, are summarised in the online supplemental file 3 table 1].18 22 33–44 All studies were observational by design and no randomised control trials were identified. Only one inclusion presented limited comparison data and there were no cohort or cross sectional studies.33 Four studies were case reports and four were reported in abstract form only.
bmjresp-2022-001199supp003.pdf (261.8KB, pdf)
Methodological quality
JBI levels of evidence for effectiveness were generally very low. Only one study achieved level 2 evidence, but was the lowest quality within the level at 2.d (retrospective control group study).33 The majority (n=9) of studies were level 3.e (observational-analytical design; observation study without a control group). The four included case reports were the lowest level of evidence, level 4.d (observational-descriptive design; case study).
Risk of bias was high across the studies, with all having risk of bias in multiple domains (online supplemental file 4 critical appraisal). No study achieved a low risk of bias. Specifically internal validity was questionable throughout; all but two studies did not report multiple measurements pre and post intervention exposure. Predefined primary outcomes were only identified in one study resulting in high risk of bias for selective outcome reporting across the studies.34 Statistical analysis was unclear for the majority and limited in those reported; none reported CIs and only one study included a calculation of statistical power.36 Collectively, the case reports achieved 60% of the quality and bias checklist parameters positively, evidencing the issue of their inherent bias.
bmjresp-2022-001199supp004.pdf (267.3KB, pdf)
It was not clear in the majority of studies if any additional intervention(s) were occurring concurrently, thus potentially introducing confounding, and meaning possible bias in the true estimate of intervention impact. Further, the most commonly reported outcome measures were those obtained through self-reporting methods, which resulted in performance biases in the measurement of the intervention.
Interventions
Intervention description, duration of treatment, frequency, timing and fidelity were inconsistently and poorly described. Overall reported treatment duration ranged from 2.5 weeks to 12 months,.34 Frequency of sessions and the number received had marked heterogeneity meaning combined calculations was unfeasible. Weekly intervention sessions were reported in two studies, but the duration over which they occurred varied.37–41 Individual session duration was rarely detailed but when reported ranged from 30 min to an hour.40 41 The most time onerous intervention described involved 45-min daily therapy sessions, on five consecutive days, for 3 weeks, with additional home practice requirements,.37 Similarly, those interventions comprising non-invasive treatment tools were time intensive, reporting 30-min patient led training sessions 5–7 days a week for 5–6 weeks,.22
Only a third of studies provided a theoretical basis for the intervention applied,.22 36–38 Of those studies reporting the providers of intervention (n=10), 90% were delivered by a speech and language therapist. Physiotherapy delivered care was reported in one study abstract and a traditional physiotherapy led therapy approach, Buteyko Breathing technique, was delivered by a speech and language therapist,.36 40
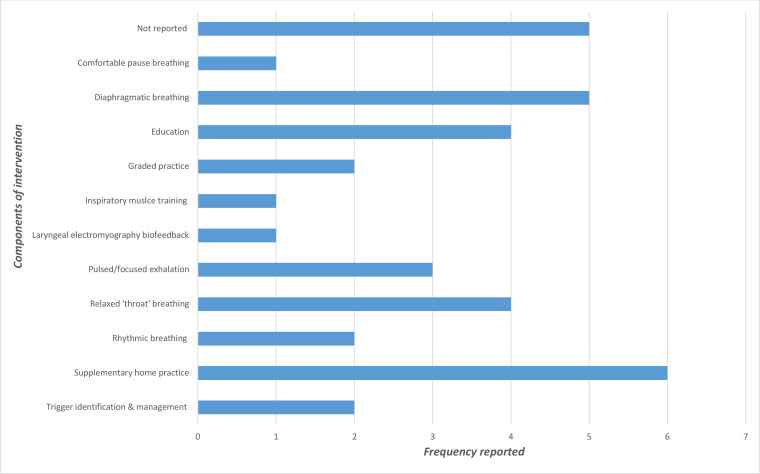
Components of intervention were varied, with some commonalties across the studies (figure 2). However, non-reporting of intervention components was frequent. Often multiple components were reported, but there was no identifiable common combinations of components within interventions. Six studies included supplementary home practice as part of the intervention but detail regarding frequency, monitoring and content of this was not reported except in those using non-invasive treatment tools (n=2) and the Buteyko breathing technique,.18 22 36–38 41
Figure 2.
Components of interventions of included studies
Outcome measures and descriptive analysis
The most commonly used outcome measures were non-validated symptom scales, reported in nine studies, with a similar number utilising objective measures. Validated symptom measures were applied in a third of studies, with the effects of intervention on healthcare utilisation only measured in a fifth. Nine of the studies used more than one outcome measure to monitor intervention effect, and two reported three outcome domains.34 38
Overall direction of effect was positive in 19/25 (76%) measures reported (table 1). Only one study showed a negative direction of effect (for validated symptom domain).34 Three of the five studies reported an improvement in validated symptom scores following intervention, compared with eight out of nine studies reporting non-validated measures. Of those studies using objective outcome measures three found conflicting findings of intervention direction effect in the in-study objective measures used, with the remaining five demonstrating a positive impact. All studies measuring healthcare utilisation impact (n=3) reported a positive effect direction.
Table 1.
Effect direction plot summarising direction of outcome domains of included studies
| Study | Study design | Symptoms (validated) | Symptoms (non-validated) | Objective measures | Healthcare utilisation |
| Hatzelis et al, 201237 | CS | ▲ | ▲ | ||
| Nacci et al39 2011 |
O/U | ▲ | |||
| Pinho et al,42 1997 |
CS | ▲ | |||
| Baxter et al, 201934 |
O/U | ▼ | ◄► | ▲ | |
| Haines et al, 201635 |
O/U | ▲ | |||
| Halevi-Katz, 201936 |
O/U | ▲ | ◄► | ||
| Krammer et al, 201733 | O/C | ▲ | ▲ | ||
| Marcinow et al, 201518 | O/U | ▲ | |||
| Mathers-Schmidt & Brilla, 200522 | CS | ▲ | ◄► | ||
| Murry et al, 201038 |
O/U | ◄► | ▲ | ▲ | |
| Olley et al, 201340 |
O/U | ▲ | ▲ | ||
| Pargeter & Mansur, 201641 | O/U | ▲ | ▲ | ||
| Shin et al, 201843 |
O/U | ◄► | |||
| Warnes et al, 200544 | CS | ▲ | ▲ |
Study design: O/U observational non-randomised uncontrolled before and after; O/C observational non-randomised controlled before and after; CS case study.
Effect direction: upward arrow ▲=positive impact; downward arrow ▼=negative impact; sideways arrow ◄►=no change/mixed effects/conflicting findings.
Sample size: final sample size (individuals) in intervention group. Large arrow ▲>300; medium arrow ▲ 50–300; small arrow ▲<50.
Study quality: denoted by row colour and JBI ratings assigned: green=low risk of bias; amber=some concerns; red=high risk of bias.
Symptom score outcomes
Of the validated symptom scores used, all except two demonstrated an improvement in reported symptoms following intervention (table 2). The heterogeneity of non-validated measures applied, together with inconsistent reporting methods makes meaningful synthesis challenging. Polar questions, ordinal severity scales, symptom quantification, symptom questionnaire and multi-choice question answer were reported and the outcomes following intervention are described in table 3. Only one study reported significance values for intervention effects on ordinal severity scales showing significant improvement 24 months from baseline, with a more significant change for those participants receiving eight cycles of intervention compared with three (−0.1 vs 3.7, p<0.01).39 A dramatic post intervention reduction (62%), in the largest included patient population (n=249), was reported for the frequency of patient reported daily attacks.41 A worsening of symptoms was reported post intervention in 22% of a population with associated chronic obstructive pulmonary disease.43
Table 2.
Symptom score outcomes of included studies pre–post completed intervention
| Validated scales | ||||||
| Study | N* | Validated symptom scale | Time point pre–post data collected | Premean/median† (SD/range) | Post mean/median† (SD/range) | P value |
| Baxter et al, 201934 | 35 | ACQ | 12 months | 2.50 (1.32) | 2.05 (1.14) | 0.19 |
| ACT | 12 months | 13.21 (4.73) | 14.69 (4.94) | 0.28 | ||
| Haines et al,35 2016‡ | 16 | VCDQ | NR | 46† (20–60) | 38† (12–50)§ | 0.017 |
| Halevi-Katz,36 2019‡ | 12 | DI | 3 weeks | 2.00 (5.39) | 16.83 (3.31)§ | 0.0016 |
| Murry et al,38 2010‡ | 12¶ | RSI-7 | NR | NR | NR | 0.05** |
| Olley et al, 201340 | 4 | D12 | NR | 18.6 (7.8) | 6.3 (5.7) | NR |
| Non-Validated scales | ||||||
| 1) Polar question—‘Have your symptoms improved?’ | ||||||
| Study | N* | Time pre–post | % responding YES | P value | ||
| Krammer et al, 201733 | 25 | NR | 92 | NR | ||
| Marcinow et al, 201518 | 34 | NR | 100 (>2 sessions) 29 (1 session) | NR | ||
| Murry et al,38 2010‡ | 16 | NR | NR (asked separately for cough, throat clear and hoarseness) | <0.01 | ||
| 2) Ordinal severity scales | ||||||
| Study | N* | Time pre–post | Scale description; range†† | Pre measure(mean(SD) if applicable) | Post measure(mean(SD) if applicable) | P value |
| Hatzelis et al, 201237 | 1 | 12 months | Severity of symptoms; 1–5 | 5 | 1 | NR |
| Mathers-Schmidt and Brilla, 200522 | 1 | 16 weeks | Dyspnoea rating scale; 1–3 | 2.4 | 1.3 | NR |
| Nacci et al, 2011 ‡39 | 10 | 24 months (received three intervention cycles) | Severity of symptoms; 1–10 | 9.2 | 6.2 | p<0.01 |
| 10 | 24 months (received eight intervention cycles) | 9.3 | 2.5 | p<0.01 | ||
| Warnes et al, 200544 | 1 | NR | Severity adaptive functioning; 1–6 | 5 | 0 | NR |
| 3) Symptom quantification | ||||||
| Study | N* | Time pre–post | Description | Pre measure(mean(SD) if applicable) | Post measure(mean(SD) if applicable) | P value |
| Mathers-Schmidt and Brilla, 200518 | 1 | 16 weeks | Time to symptom onset | 23 s | 30 s | NR |
| Pargeter & Mansur, 201641 | 249 | NR | Frequency of patient reported daily attacks | 72% | 10% | NR |
| 4) Symptom questionnaire | ||||||
| Study | N* | Time pre–post | Description | Pre measure (mean(SD) if applicable) | Post measure (mean(SD) if applicable) | P value |
| Pargeter & Mansur,41 2016‡ | 249 | NR | In-house ILO symptom questionnaire; high score indicating poor control | 16.57 (3.96) | 7.75 (4.82) | <0.001 |
| 5) Multichoice question | ||||||
| Study | N* | Time pre–post | Description | Pre measure (mean (SD) if applicable) | Post measure (mean (SD) if applicable) | P value |
| Shin et al, 201843 | 46 | NR | Have symptoms a) not improved b) improved c) worsened | NA | 50% improve 22% worsen | NR |
*Number of participants.
†Median.
‡Studies reported statistically significant improvements pre–post intervention.
§Improved by the reported clinically meaningful response reported DI Dyspnoea Index Questionnaire; RSI Reflux Symptom Index item 7.
¶Only reported in 12/16 participants.
**Mean difference in score 3.76, no pre–post scores reported; D12 Dysponea12.
††Highest value most impairment.
ACQ, Asthma Control Questionnaire; ACT, Asthma Control Test; NR, Not reported; VCDQ, Vocal Cord Dysfunction Questionnaire.
Table 3.
Healthcare utilisation of included studies pre–post completed intervention
| Study | N* | Healthcare utilisation measure | Time pre–post (months) | Pre mean (SD) | Post mean (SD) | P value | % change | Overall (mean) % change |
| Baxter et al, 201934 | 35 | General practice visits | 12 | 10.17 (9.09) | 5.26 (5.36) | <0.001 | − 48% | |
| Baxter et al, 201934 | 35 | Hospital admissions | 12 | 4.20 (4.66) | 2.40 (5.33) | 0.001 | − 43% | |
| Krammer et al, 201733 | 25 | Asthma medication score | 6 | 4.6 (NR) |
1.92 (NR) |
NR | − 58% | |
| Pargeter and Mansur, 201641 | 249 | Hospital admissions | 12 | 2.44 (4.84) | 0.31 (1.01) | <0.001 | − 87% | |
| Total | 344 | − 59.5% |
*Number of participants.
NR, not reported.
Objective outcome measures
Direct imaging was the most commonly reported objective measure across studies, reported in five papers. Four of those studies performed no statistical analysis of change pre post intervention, with polar reporting of the presence of ILO. Of the 39 participants within those studies 56% improved after intervention, but there was no consistent time point on endpoint data collection. Baxter et al34 demonstrated a reduced but non-significant frequency of ILO during laryngoscopy, 12 months post intervention (72% vs 60%, p=0.98). There was however significant improvement in the number of participants presenting with a lower limit of normal on CT larynx post intervention (38% vs 11%, p=0.02). However, pre and post participant numbers differed (34 vs 27) and no comparison between laryngoscopy and CT larynx outcome was made.
Four studies reported physiological measures (cardiopulmonary parameters and electromyography data) in addition to other outcome measures explicit to ILO. In all but one study, such measures were applied to monitor effects of intervention in E-ILO.44
Healthcare utilisation outcomes
There was an overall 59.5% reduction in healthcare utilisation following intervention (table 3). However, only three studies reported data relating to healthcare utilisation and study design differed as well as the measures used. Krammer et al33 reported a decrease in the asthma medication score following intervention but there was no significant difference in score reduction between those completing therapy and those not (2.3 vs 1.92, p=0.71).33
Discussion
This systematic review investigates the effectiveness of non-pharmacological interventions used to treat adults with ILO. The fourteen studies were evaluated to (1) determine the key components of non-pharmacological interventions, if any (2) the outcome measures used, if any (3) the effectiveness of intervention, if any. The overall quality of the current evidence is low and the risk of bias high. In addition, there was significant heterogeneity across the studies precluding any meaningful comparisons. However, despite these limitations, there are positive signals from the evidence to support the effectiveness of non-pharmacological interventions as a treatment for adults with ILO.
Despite review inclusion criteria aiming to homogenise the population there was still variance in the ILO presentations reported. It is not yet known whether ILO induced by exercise, noxious stimulus or mechanical factors differ with regard to pathophysiology. There is suggestion ILO induced by exercise represents a manifestation of increased respiratory neural drive whereas laryngeal hyper-responsiveness accounts for ILO induced by other factors.8 45 Similarly, differences in visualised anatomical presentations (ie, glottic, supraglottic, inspiratory or expiratory ILO) may indicate more than one pathophysiological mechanism exists. Shin’s study reported a deterioration in symptoms post treatment when intervention was applied to an ILO population with co-existing COPD.43 Participants may have had ‘compensatory’ expiratory ILO in an effort to create external positive end-expiratory pressure, as previously reported in ILO patients with co-morbid obstructive lung disease.32 45–47 This therefore may explain why interventions applied in cohorts with inspiratory ILO may not be appropriate. Indeed, if there are ILO phenotypes, this has implications for interventions applied, primary endpoints used and effectiveness.
Interventions
Intervention reporting was inconsistent and varied in the level of detail and description. As a result, meaningful between-study appraisals was difficult and there is no clear configuration on intervention components and mode of delivery. Education was only reported in a quarter of studies as an intervention component. It is likely this is not representative of actual frequency as clinical diagnostic feedback often includes education on contributory factors to an individual’s presentation. SLT was the most reported intervention and reflects previous cited claims it is the gold standard treatment for ILO. However, this review is in common with Patel’s previous review specifically evaluating speech pathology interventions in ILO, and highlights the state of the evidence precludes concrete gold-standard recommendations of use.19
Outcomes
There was no uniformed approach in the selected outcomes used to monitor treatment effectiveness, which likely reflects of the lack of a robust standardised evaluation tool for ILO. Variable outcomes were used with non-validated and non-quantifiable results, thus impeding accurate comparisons. Two studies selected outcomes intended to monitor other disease processes.34 36 Baxter et al34 applied a primary end point validated to evaluate the effectiveness of intervention in asthma. It is therefore unsurprising the non-significant impact of ILO intervention reported and is more likely a reflection of the tool used, rather than the effectiveness of intervention. Similarly, ILO interventions appear not to have effectiveness on cardiopulmonary parameters, but this is predictable as these outcome tools are non-specific to ILO.22 36
Effectiveness
The high risk of bias across the studies means true effectiveness of intervention is unknown. Specifically, issues with confounding was common meaning external factors influenced data reported. Halevi-Katz demonstrated a significant improvement in outcome measures across several parameters.36 However, participants within the study all had ILO with hyperventilation syndrome. Therefore, the positive effectiveness achieved maybe reflective of the intervention targeting and improving an underlying breathing pattern disorder, rather than ILO. Similarly, co-concurrent changes to medication targeting asthma and reflux during the intervention period occurred in several studies, with inherent implications for the true effectiveness of data relating to the target intervention.18 33 39
Despite this, there was an overall trend towards a positive direction of effect across all studies for interventions used to treat ILO in adults. Acknowledging the limitations in methodological approach, the data signals a potential likelihood of effectiveness.
Limitations to study
The synthesis approach used within this review was mandated due to limitations on data retrieved and high bias in the evidence. The inability to perform meta-analysis precludes precise estimates of the effect size of interventions and therefore generalisability for contemporary clinical care is not possible.
Inclusion of conference abstracts within the studies synthesised is problematic. The four abstracts are not subject to the same robust peer review process, when compared with the other included data sets. The study team felt inclusion was important as those abstracts identified were not summaries of full reports included and therefore there was no risk of double counting of data. Despite this, inherent to abstract protocols, there was a clear lack of detailed information regarding intervention components and study methodology. Similarly, inclusion of case reports only provides anecdotal insight meaning data presented should not be overinterpreted due to their inherent bias. However, those included informed on potential novel components of non-pharmacological intervention (ie, electromyography and inspiratory muscle training) justifying their inclusion.
Conclusions
This review identifies the evidence for non-pharmacological interventions used to treat adults with ILO. The literature eludes to several intervention components but due to the quality of research methodology no meaningful conclusions on efficacy of these is possible. However, signals in the synthesis performed supports the opinion that non-pharmacological interventions may be an effective ILO treatment and warrant further development and investigation, with robust processes applied, to evaluate effectiveness.
Acknowledgments
The authors would like to acknowledge Mrs Nicola Pargeter, Dr Julia Selby and Mrs Claire Slinger for their helpful contributions in peer reviewing the search terms.
Footnotes
Contributors: JH, JY, JAS and SF conceptualised the study and proof outline. JH designed the study in detail, performed and led the analysis and drafted the manuscript (with input from all authors). SF, JY and JAS were involved in planning and supervision throughout the study. JW-D and JK piloted screening tools, data extraction forms and screened abstracts for inclusion with JH. JW-D and JH performed quality assessment and risk of bias appraisal for all included studies. All authors discussed the results and contributed to the manuscript.
Funding: This work was supported by the National Institute of Heath Research Manchester Biomedical Research Centre.
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
No data are available. No new data sets generated. Data analysis sets of review available upon reasonable request.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
Not applicable.
References
- 1.Christensen PM, Heimdal J-H, Christopher KL, et al. ERS/ELS/ACCP 2013 international consensus conference nomenclature on inducible laryngeal obstructions. Eur Respir Rev 2015;24:445–50. 10.1183/16000617.00006513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Christopher KL, Wood RP, Eckert RC, et al. Vocal-Cord dysfunction presenting as asthma. N Engl J Med 1983;308:1566–70. 10.1056/NEJM198306303082605 [DOI] [PubMed] [Google Scholar]
- 3.Halvorsen T, Walsted ES, Bucca C, et al. Inducible laryngeal obstruction: an official joint European respiratory Society and European Laryngological Society statement. Eur Respir J 2017;50:09. 10.1183/13993003.02221-2016 [DOI] [PubMed] [Google Scholar]
- 4.Maillard I, Schweizer V, Broccard A, et al. Use of botulinum toxin type A to avoid tracheal intubation or tracheostomy in severe paradoxical vocal cord movement. Chest 2000;118:874–7. 10.1378/chest.118.3.874 [DOI] [PubMed] [Google Scholar]
- 5.Fowler SJ, Thurston A, Chesworth B, et al. The VCDQ--a Questionnaire for symptom monitoring in vocal cord dysfunction. Clin Exp Allergy 2015;45:1406–11. 10.1111/cea.12550 [DOI] [PubMed] [Google Scholar]
- 6.Haines J, Chua SHK, Smith J, et al. Triggers of breathlessness in inducible laryngeal obstruction and asthma. Clin Exp Allergy 2020;50:1230–7. 10.1111/cea.13715 [DOI] [PubMed] [Google Scholar]
- 7.Christensen PM, Thomsen SF, Rasmussen N, et al. Exercise-Induced laryngeal obstructions: prevalence and symptoms in the general public. Eur Arch Otorhinolaryngol 2011;268:1313–9. 10.1007/s00405-011-1612-0 [DOI] [PubMed] [Google Scholar]
- 8.Hull JH, Backer V, Gibson PG, et al. Laryngeal dysfunction: assessment and management for the clinician. Am J Respir Crit Care Med 2016;194:1062–72. 10.1164/rccm.201606-1249CI [DOI] [PubMed] [Google Scholar]
- 9.Haines J, Hull JH, Fowler SJ. Clinical presentation, assessment, and management of inducible laryngeal obstruction. Curr Opin Otolaryngol Head Neck Surg 2018;26:174–9. 10.1097/MOO.0000000000000452 [DOI] [PubMed] [Google Scholar]
- 10.Newman KB, Mason UG, Schmaling KB. Clinical features of vocal cord dysfunction. Am J Respir Crit Care Med 1995;152:1382–6. 10.1164/ajrccm.152.4.7551399 [DOI] [PubMed] [Google Scholar]
- 11.Morris MJ, Christopher KL. Diagnostic criteria for the classification of vocal cord dysfunction. Chest 2010;138:1213–23. 10.1378/chest.09-2944 [DOI] [PubMed] [Google Scholar]
- 12.Newman K, Dubester S. Vocal cord dysfunction: Masquerader of asthma. Semin Respir Crit Care Med 1994;15:161–7. 10.1055/s-2007-1006358 [DOI] [Google Scholar]
- 13.Mikita J, Parker J. High levels of medical utilization by ambulatory patients with vocal cord dysfunction as compared to age- and gender-matched asthmatics. Chest 2006;129:905–8. 10.1378/chest.129.4.905 [DOI] [PubMed] [Google Scholar]
- 14.Traister RS, Fajt ML, Petrov AA. The morbidity and cost of vocal cord dysfunction misdiagnosed as asthma. Allergy Asthma Proc 2016;37:25–31. 10.2500/aap.2016.37.3936 [DOI] [PubMed] [Google Scholar]
- 15.Vertigan AE, Bone SL, Gibson PG. Laryngeal sensory dysfunction in laryngeal hypersensitivity syndrome. Respirology 2013;18:948–56. 10.1111/resp.12103 [DOI] [PubMed] [Google Scholar]
- 16.Cukier-Blaj S, Bewley A, Aviv JE, et al. Paradoxical vocal fold motion: a sensory-motor laryngeal disorder. Laryngoscope 2008;118:367–70. 10.1097/MLG.0b013e31815988b0 [DOI] [PubMed] [Google Scholar]
- 17.Kenn K, Balkissoon R. Vocal cord dysfunction: what do we know? Eur Respir J 2011;37:194–200. 10.1183/09031936.00192809 [DOI] [PubMed] [Google Scholar]
- 18.Marcinow AM, Thompson J, Forrest LA, et al. Irritant-Induced paradoxical vocal fold motion disorder: diagnosis and management. Otolaryngol Head Neck Surg 2015;153:996–1000. 10.1177/0194599815600144 [DOI] [PubMed] [Google Scholar]
- 19.Patel RR, Venediktov R, Schooling T, et al. Evidence-Based systematic review: effects of speech-language pathology treatment for individuals with paradoxical vocal fold motion. Am J Speech Lang Pathol 2015;24:566–84. 10.1044/2015_AJSLP-14-0120 [DOI] [PubMed] [Google Scholar]
- 20.Mahoney J, Hew M, Vertigan A, et al. Treatment effectiveness for vocal cord dysfunction in adults and adolescents: a systematic review. Clin Exp Allergy 2022;52:387–404. 10.1111/cea.14036 [DOI] [PubMed] [Google Scholar]
- 21.Craig P, Dieppe P, Macintyre S, et al. Developing and evaluating complex interventions: the new medical Research Council guidance. BMJ 2008;337:a1655. 10.1136/bmj.a1655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mathers-Schmidt BA, Brilla LR. Inspiratory muscle training in exercise-induced paradoxical vocal fold motion. J Voice 2005;19:635–44. 10.1016/j.jvoice.2005.03.005 [DOI] [PubMed] [Google Scholar]
- 23.Sandnes A, Andersen T, Clemm HH, et al. Exercise-Induced laryngeal obstruction in athletes treated with inspiratory muscle training. BMJ Open Sport Exerc Med 2019;5:e000436. 10.1136/bmjsem-2018-000436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Higgins JPT, Thomas J, Chandler J. Cochrane Handbook for systematic reviews of interventions version 6.3, 2022. Available: www.training.cochrane.org/handbook
- 25.Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg 2010;8:336–41. 10.1016/j.ijsu.2010.02.007 [DOI] [PubMed] [Google Scholar]
- 26.Schardt C, Adams MB, Owens T, et al. Utilization of the PICO framework to improve searching PubMed for clinical questions. BMC Med Inform Decis Mak 2007;7:1–6. 10.1186/1472-6947-7-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Traister RS, Fajt ML, Landsittel D, et al. A novel scoring system to distinguish vocal cord dysfunction from asthma. J Allergy Clin Immunol Pract 2014;2:65–9. 10.1016/j.jaip.2013.09.002 [DOI] [PubMed] [Google Scholar]
- 28.Campbell M, McKenzie JE, Sowden A, et al. Synthesis without meta-analysis (SWiM) in systematic reviews: reporting guideline. BMJ 2020;368:l6890. 10.1136/bmj.l6890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Aromataris E, Fernandez R, Godfrey CM, et al. Summarizing systematic reviews: methodological development, conduct and reporting of an umbrella review approach. Int J Evid Based Healthc 2015;13:132–40. 10.1097/XEB.0000000000000055 [DOI] [PubMed] [Google Scholar]
- 30.Aboudara M, Mikita J W, et al. Change in exercise capacity after speech therapy in patients with vocal cord dysfunction. in B70. sleep disordered breathing pathophysiology. American Thoracic Society 2012:A3622. [Google Scholar]
- 31.Ryan NM, Vertigan AE, Gibson PG. Chronic cough and laryngeal dysfunction improve with specific treatment of cough and paradoxical vocal fold movement. Cough 2009;5:4–8. 10.1186/1745-9974-5-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee JW, Tay TR, Paddle P, et al. Diagnosis of concomitant inducible laryngeal obstruction and asthma. Clin Exp Allergy 2018;48:1622–30. 10.1111/cea.13185 [DOI] [PubMed] [Google Scholar]
- 33.Kramer S, deSilva B, Forrest LA, et al. Does treatment of paradoxical vocal fold movement disorder decrease asthma medication use? Laryngoscope 2017;127:1531–7. 10.1002/lary.26416 [DOI] [PubMed] [Google Scholar]
- 34.Baxter M, Ruane L, Phyland D, et al. Multidisciplinary team clinic for vocal cord dysfunction directs therapy and significantly reduces healthcare utilization. Respirology 2019;24:758–64. 10.1111/resp.13520 [DOI] [PubMed] [Google Scholar]
- 35.Haines J, Slinger C, Vyas A. Impact of respiratory speech and language therapy on symptoms in vocal cord dysfunction. Eur Respiratory Soc 2016;48. [Google Scholar]
- 36.Halevi-Katz D, Sella O, Golan H, et al. Buteyko breathing technique for exertion-induced paradoxical vocal fold motion (EI-PVFM). J Voice 2021;35:40–51. 10.1016/j.jvoice.2019.07.017 [DOI] [PubMed] [Google Scholar]
- 37.Hatzelis V, Murry T. Paradoxical vocal fold motion: respiratory retraining to manage long-term symptoms. J Soc Bras Fonoaudiol 2012;24:80–5. 10.1590/S2179-64912012000100014 [DOI] [PubMed] [Google Scholar]
- 38.Murry T, Branski RC, Yu K, et al. Laryngeal sensory deficits in patients with chronic cough and paradoxical vocal fold movement disorder. Laryngoscope 2010;120:1576–81. 10.1002/lary.20985 [DOI] [PubMed] [Google Scholar]
- 39.Nacci A, Fattori B, Segnini G, et al. Respiratory retraining therapy in long-term treatment of paradoxical vocal fold dysfunction. Folia Phoniatr Logop 2011;63:134–41. 10.1159/000316405 [DOI] [PubMed] [Google Scholar]
- 40.Olley A, Johnston R M-G, et al. Physiotherapy intervention improves symptoms in patients with exercise induced laryngeal obstruction[abstract]. Eur Respiratory Soc 2013. [Google Scholar]
- 41.Pargeter N, Mansur AH. P226 Vocal cord dysfunction; clinical outcomes of speech & language therapy intervention. Thorax 2016;71:A209.2–10. 10.1136/thoraxjnl-2016-209333.369 [DOI] [Google Scholar]
- 42.Pinho SM, Tsuji DH, Sennes L, et al. Paradoxical vocal fold movement: a case report. J Voice 1997;11:368–72. 10.1016/s0892-1997(97)80017-3 [DOI] [PubMed] [Google Scholar]
- 43.Shin TD, Matrka LA. Does laryngeal control therapy provide benefit in patients with paradoxical vocal fold motion disorder and comorbid chronic obstructive pulmonary disease? Otolaryngology - Head and Neck 2018. [Google Scholar]
- 44.Warnes E, Allen KD. Biofeedback treatment of paradoxical vocal fold motion and respiratory distress in an adolescent girl. J Appl Behav Anal 2005;38:529–3. 10.1901/jaba.2005.26-05 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Baz M, Haji GS, Menzies-Gow A, et al. Dynamic laryngeal narrowing during exercise: a mechanism for generating intrinsic PEEP in COPD? Thorax 2015;70:251–7. 10.1136/thoraxjnl-2014-205940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Walsted ES, Faisal A, Jolley CJ, et al. Increased respiratory neural drive and work of breathing in exercise-induced laryngeal obstruction. J Appl Physiol 2018;124:356–63. 10.1152/japplphysiol.00691.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Collett PW, Brancatisano T, Engel LA. Changes in the glottic aperture during bronchial asthma. Am Rev Respir Dis 1983;128:719–23. 10.1164/arrd.1983.128.4.719 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjresp-2022-001199supp001.pdf (14.9KB, pdf)
bmjresp-2022-001199supp002.pdf (196.3KB, pdf)
bmjresp-2022-001199supp003.pdf (261.8KB, pdf)
bmjresp-2022-001199supp004.pdf (267.3KB, pdf)
Data Availability Statement
No data are available. No new data sets generated. Data analysis sets of review available upon reasonable request.