Abstract
Background
Neonatal pain not only has negative impact on the acute physiological parameters of the neonate but also has got the potential to cause long-term neurodevelopmental disabilities. However, neonatal pain especially related to procedures is not well recognised and often poorly managed in neonatal intensive care unit (NICU).
Local problem
Oral sucrose solution became available commercially in late 2017 and this provided us the opportunity to alleviate some of the procedural pain in neonates admitted in our NICU.
Methods
Point of care quality improvement method (POCQI) was leveraged to identify root causes, change ideas and solutions were tested using PDSA cycles. Four procedures were selected by team for sucrose analgesia namely intravenous cannula insertion, tracheal suctioning, removal of tapes and phlebotomy. Change ideas tested included training of staff and doctors, providing dosage chart in NICU, method of administration of sucrose, affixing sucrose vial to baby bed, using prefilled sucrose syringe and bedside availability of sucrose and checklist for documentation. The study was conducted over a period of 8 weeks from 15 June 2017 on all eligible babies getting admitted.
AIM statement
We aim to increase compliance to administration of sucrose analgesia to all eligible newborns (undergoing 4 selected procedures intravenous cannula insertion, tracheal suctioning, removal of tapes and phlebotomy) in NICU prior to painful procedure from current 0% to >80% by 8 weeks.
Results
This quality improvement study implementing the use of evidence-based sucrose analgesia using PDSA cycles found that percentage of babies getting sucrose analgesia has increased from baseline 0% to 96.27% in the study period and is sustained at >80% for 4 years.
Conclusions
POCQI methodology can be used effectively to implement a new simple strategy of administering oral sucrose solution to address procedural pain in care pathway of neonates admitted in NICU. Sustaining the gains achieved by POCQI needs active leadership involvement and addressing adaptive or behavioural challenges with solutions like team huddles.
Keywords: Quality improvement, Pain Management, Pain
What is already known on this topic?
Non-pharmacologic methods of pain reduction are often underutilized. These methods can be easily used by healthcare providers in neonatal intensive care unit (NICU). They are less dangerous & as effective as pharmacologic therapy.
What this study adds?
Point of care quality improvement methodology can be effectively used to introduce & implement a care strategy by adapting it to the local context. Sustaining the gains achieved by point of care quality improvement needs active leadership involvement & addressing adaptive or behavioral challenges with solutions like team huddles.
How this study might affect research, practice or policy?
This study might provide impetus for further research in the area of neonatal pain management. It can encourage neonatal caregivers to effectively use sucrose analgesia in the care of neonates. This study has implications to stimulate further research in developmentally supportive care in neonatal intensive care for effective pain and stress management by combining sucrose analgesia with other non-pharmacologic measures such as facilitated tuck, swaddling and kangaroo mother care.
Introduction
Only few studies have evaluated the effect of quality initiatives in improving neonatal procedural pain management. Agnelica et al1 have focused on nurses’ beliefs, knowledge and practices regarding neonatal pain to decrease pain related to venipuncture. Following a preeducational and posteducational questionnaire and improving access to sucrose, they noticed increased utilisation of sucrose as non-pharmacological analgesia from 15% to 90%. Lago et al2 have noted that training staff in pain assessment, entering pain scores in medical records and developing a pain protocol could increase the use of pain relief measures from 28% to 76%.
Spence and Henderson-Smart3 have shown that use of breast feeding or sucrose analgesia has improved after an initiative which included audit and feedback, benchmarking, educational workshops on critical appraisal and audit of family awareness of pain.
Anne et al4 in their quality improvement initiative to improve management of procedural pain in preterm neonates concluded that targeted interventions can improve neonatal procedural pain management by improving use of analgesic measures, decreasing the number of procedures and educating and training healthcare personnel. Nana et al5 have reported that educational interventions like training have modest effect 9.7% (5.5%–21.3%) on improving processes of care in 2018. Hall in,6 anaesthesia and analgesia in the neonatal intensive care unit (NICU), clinics in perinatology, as cited by Barker and Rutter7 mentioned that neonates less than 32 weeks’ gestation were exposed to 10–15 painful procedures per day, up to 22 procedures per day in the first 2 weeks of life, and most of these procedures were untreated. A recent study by Carbajal et al8 have documented the increased occurrence and lack of treatment of neonatal pain in almost 80% of newborns in intensive care. Current medical evidence concludes that there are long-term hampering effects of repeated pain experienced by the neonates in NICU. Many studies have shown that repeated pain experience by the neonates in NICU during routine procedures has dampened the bio behavioural responses to pain and is an indicator of interrupted development of heightened peripheral sensitivity to pain and altered hypothalamic pituitary and adrenal axis reactivity as cited by Grunau et al.9
Physiological responses to painful stimuli are manifested as acute increase in heart rate, blood pressure, heart rate variability, intracranial pressure and decreased arterial oxygen saturations. These physiological changes are of significant magnitude and rapidity to produce reperfusion injury and venous congestion leading to intraventricular haemorrhage and/or periventricular leukomalacia.9
Thus, untreated pain has the potential to lead to significant nuerodevelopmental derangements in the neonate directly as well as indirectly.
Non-pharmacological pain treatment in neonates has been clearly demonstrated to relieve mild to moderate pain.6 10–13
The best studied techniques include nonnutritive sucking (with and without sucrose); breast feeding; swaddling; kangaroo care (skin-to-skin contact); and massage therapy. Non-nutritive sucking and sucrose work by increasing endogenous endorphins. Although sucrose has been shown to enhance effectiveness, they have both been shown to decrease crying time and improve pain scores after acute mild pain, such as heel-stick pain.10 Sucrose is efficacious in reducing the pain from single events, such as retinopathy of prematurity screening,11 oral gastric tube insertion12 and heel lance.13 However, sucrose is controversial when given repeatedly, possibly leading to adverse long-term outcomes.6
In our NICU, we were following various developmentally supportive care interventions like nesting, therapeutic positioning, facilitated tuck, swaddling, kangaroo mother care and non-nutritive sucking to reduce stress and pain in stable neonates. Once sucrose 24% solution was available, it was decided to take further measures to reduce pain in neonates as it is a simple, relatively safe intervention suitable for administration to most of the neonates in NICU undergoing mild to moderate painful procedure. Accordingly, a quality improvement project was planned for administration of sucrose for selected four painful procedures (because of cumulative toxicity concern1) which the neonate in NICU undergoes frequently namely:
Insertion of intravenous cannula.
Phlebotomy.
Tracheal suctioning.
Removal of tapes.
In Cochrane review done by Steven et al in July 2015,14 it was concluded that sucrose is effective in reducing procedural pain in neonates without any serious side effects.
There are many published practice guidelines for use in NICUs to decrease pain in neonates. However, the practice is variable across neonatal units. A number of procedural guidelines for pain relief in neonates during clinical procedures were developed to fit local and regional practices.15 A cross sectional study in 2008 found a modest increase in measures to prevent neonatal pain in the UK since a survey in 2000, but there was no pain guideline in nearly 25% of the neonatal units and no guidelines for routine painful procedures in the majority of neonatal hospital care.16 Lack of enforcement also rendered the guideline ineffective, as observed in a study involving eight Australian states and territories which found that only 39% of neonatal units implemented a procedural guideline to control pain in neonates during routine procedures.15 16
We decided to use point of care quality improvement methodology (POCQI) for implementing use of sucrose analgesia in eligible neonates in our unit.
We aim to increase compliance to administration of sucrose analgesia to all eligible newborns (undergoing four selected procedures intravenous cannula insertion, tracheal suctioning, removal of tapes and phlebotomy) in NICU prior to painful procedure from current 0% to >80% by 8 weeks.
Methods
This was a quality improvement initiative to provide sucrose analgesia to eligible neonates in our 20-bed level 3 private sector standalone NICU with an average bed occupancy of about 14 and more than 50% neonates admitted having birth weight less than 1500 g and many requiring respiratory support in the form CPAP (Continuous Positive Airway Pressure) or invasive ventilation. Neonates who underwent four selected procedures namely—insertion of intravenous cannula, tracheal suctioning, phlebotomy, removal of tapes were administered sucrose analgesia as these were common procedures causing pain. Project was undertaken from 15 June 2017.
We aim to increase compliance to administration of sucrose analgesia to all eligible newborns in NICU prior to painful procedure from current 0% to >80% by 8 weeks.
QI (Quality improvement) team was formed consisting of consultant, duty doctors including fellow, staff nurses, administration office staff and pharmacist. The team was led by senior nurse staff. Documentation and literature review were done by a consultant and fellow.
We used fish bone analysis to identify the root causes. Fish bone analysis (online supplemental file 1) yielded lack of awareness regarding neonatal pain, busy in work, lack of knowledge regarding pain management, non-availability of sucrose in NICU, sucrose vials kept away in drug trolley and therefore staff could not get it during painful procedure, no supply by pharmacy, no knowledge of dose of sucrose, lack of knowledge regarding method of administration of sucrose, no policy on documentation of pain score, no policy on administration of sucrose.
bmjoq-2022-001830supp001.pdf (35.2KB, pdf)
Based on these causes, team identified certain changes which were subsequently tested, contextualised and adopted accordingly to achieve the set aim.
Intervention measures
As the emphasis was mainly on improving the process of administration of sucrose, only process measure was targeted and no specific outcome measure was followed.
Process measures
Per cent of times babies receiving sucrose analgesia—for the four procedures selected calculated as no of times sucrose analgesia provided for each procedure divided by no of such procedures done in a day. Data source was bedside sheet/bedside chart.
Per cent of babies having sucrose availability at bedside—no. of baby warmers with sucrose vials or prefilled syringe affixed divided by total no. of babies. Data source was handover chart and clinician check during round.
Per cent of babies with bedside charts—number of baby warmers having bedside chart divided by total number of babies. As per the daily check by designated staff.
Outcome measure
No specific outcome measure like pain score was targeted as the emphasis was on improving the process of sucrose analgesia although pain relief using pain score was used during training period.
Balancing measure
% of staff expressing concern about work overload. A survey was carried out which included 3 questions contributing to work overload namely, preparing & affixing sucrose at bedside, administering sucrose solution for selected procedures, documentation of sucrose administration. Survey results indicated major concern regarding documentation work overload (80%)
Change ideas tested and details of PDSA’s done are described below.
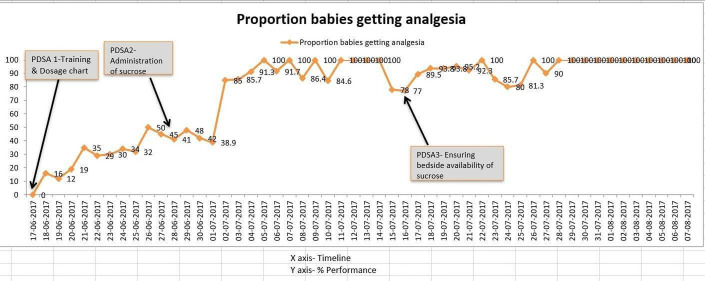
PDSA 1—training of staff and doctors (figure 1):
Figure 1.
PDSA 1/2/3.
Plan—consultant/fellow will train all the staff and doctors to enhance their knowledge about the use of sucrose analgesia.
Do—doctors and staff were trained regarding use of sucrose, dosage by fellow under guidance of consultant after literature review.
Study—this change was carried out over 2 weeks. Staff requested for dosage chart in NICU which was displayed. A questionary was used to assess the impact of training on knowledge change in the form of pretest and post test which demonstrated twofold increase in knowledge from 40% to >80%, respectively.
Act—this change was adapted. Compliance to sucrose administration increased to about 40% as documented in patient case sheets, not enough to reach our aim. On enquiry, staff expressed concern regarding method of sucrose administration.
PDSA 2—Administration of sucrose (figure 1):
2a-
Plan—different methods of sucrose administration will be tested through small PDSA’s by staff and doctors in NICU.
Do—administration of sucrose was tested by staff using different methods—using sterile gauze, nipple coated with sucrose solution.
Study—staff was not comfortable with administration and expressed concern regarding contamination.
Act—this change idea was abandoned. Staff who tested it suggested that we can use prefilled sucrose syringe and fix it to warmer for ready use.
2b-
Plan—use of prefilled sucrose syringe affixed to warmer (baby bed) will be tested in a shift by staff nurse in the NICU.
Do—prefilled sucrose syringe 1 mL was fixed to warmer of babies and sucrose was administered using it.
Study—this idea worked well but staff identified wastage as some babies on full feeds did not require sucrose use and prefilled syringe had to be discarded after 24 hours.
Act—therefore, this change was modified with prefill syringe for babies who need frequent painful procedures and fixing of vials to stable babies who need it less likely.
2c-
Plan—using prefilled syringes and vials.
Do—prefilled sucrose syringes were affixed to warmers of babies who were likely to undergo frequent painful procedures and only vials were affixed to warmers of those babies who were less likely to need sucrose analgesia.
Study—this modified change resulted in less wastage of sucrose vials.
Act—this change was adapted with a modification that all warmers will have sucrose vials affixed and prefilled syringes can be prepared after opening the sucrose vial for the first use for each baby who needs it after transferring solution to syringe which was then affixed to warmer after first use for subsequent use. This idea improved compliance to sucrose administration to around 80% for few days. But compliance decreased to <80% as bedside sucrose was not available because staff forgot to take replacement.
PDSA 3—improving bedside availability of sucrose (figure 1):
Plan—documenting bedside sucrose vial availability in the staff handover sheet. Staff had forgotten to take replacement so could not administer sucrose analgesia.
Do—one line indicating bedside sucrose availability or need for replacement was added to the nurses’ bedside handover sheet.
Study—this change worked well in ensuring sucrose availability at bedside and administration.
Act—this idea was adopted. Compliance to sucrose administration again dropped to <80% as sucrose vials were not available in the hospital pharmacy.
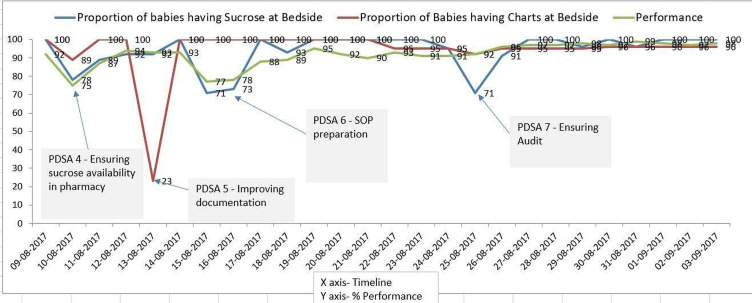
PDSA 4—ensuring availability of sucrose in the pharmacy (figure 2):
Figure 2.
PDSA 4/5/6/7.
Plan—keeping enough stock of sucrose with the hospital pharmacy as sucrose vials were out of stock in the pharmacy due to lack of stock with the supplier as only our NICU was using it in the region.
Do—pharmacist was requested to ensure additional stock in the hospital pharmacy by ordering to the supplier keeping in mind the average consumption per month.
Syudy—this was a special cause variation as sucrose was new product in the market.
Act—pharmacist started ordering enough additional stock. But staff forgot to document (figure 2) administration despite doing so resulting in false documentation which was corrected after interviewing concerned staff.
PDSA 5—improving documentation of sucrose administration:
Plan—bedside checklist for documentation of sucrose administration.
Do—bedside checklist with four selected procedures for sucrose analgesia was made available by the administration department staff who ensured it during their daily round.
Study—improved documentation of sucrose administration.
Act—proper recording of data was done. This idea was adopted. Newly recruited staff did not administer sucrose analgesia resulting poor compliance.
PDSA 6—preparation of standard operating procedure (SOP) (figure 2):
Plan—SOP for training new recruited staff.
Study—SOP was prepared by fellow under guidance of consultant and new staff was trained.
Act—orientation of new staff ensured compliance with sucrose administration to >80%.
PDSA 7—ensuring audit of administration of sucrose:
After staff expressed concern regarding documentation work overload, formal documentation was stopped. But it was noted by clinicians on round that bedside sucrose was not available for some babies. So, consultant check during rounds was suggested.
Plan—consultant check during rounds as documentation of administration checklist was discontinued after survey indicated staff work overload (balancing measure).
Do—consultant audited sucrose availability by random checks during round.
Study—consultant check ensured audit of sucrose availability and administration.
Act—this idea was adapted with a modification of surprise check in sustenance phase (figure 2).
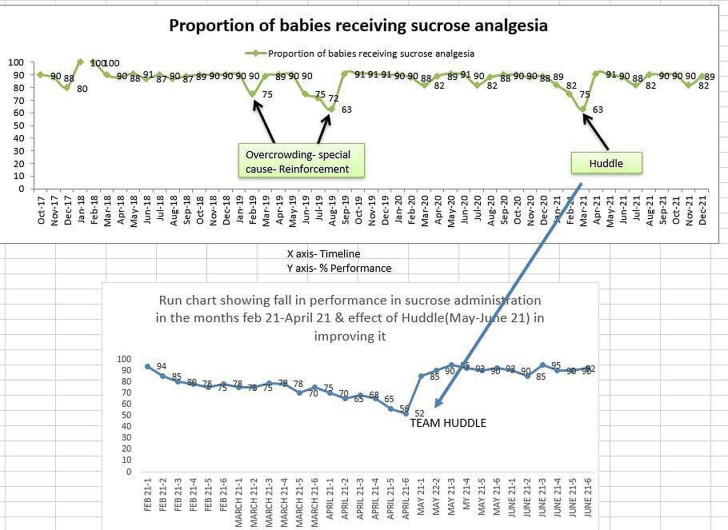
PDSA 8—ensuring sustenance using team huddle (figure 3):
Figure 3.
Huddle effect.
Plan—daily huddle: a point of discussion regarding bedside sucrose availability and administration was added in daily team huddle in the sustenance phase as there was a tendency to forget administration or ensuring bedside availability of sucrose especially when nursery was very busy and during COVID-19 times.
Do—sucrose availability and administration were added in daily huddle discussion.
Study—team huddle motivated staff and ensured compliance to many processes including sucrose administration.
Act—adding a point regarding sucrose availability and administration in daily huddle was adopted.
Although formal documentation of data regarding sucrose administration was stopped, surprise checks are being conducted by consultants once or twice a week to ascertain the compliance and it was found that compliance regarding administration of sucrose was >80%. Incorporating sucrose availability and administration in daily team huddle ensured sustained compliance by motivating the team.
Results
This quality improvement study implementing the use of evidence-based sucrose analgesia using PDSA cycles found that percentage of babies getting sucrose analgesia was increased to 96.27%. The study was conducted over a period of 8 weeks from 15 June 2017 on all eligible babies getting admitted. In the preintervention period before starting the improvement project, zero number of babies received sucrose analgesia for the four selected painful procedures which increased up to 40% after training and after iterative PDSA cycles 96.27% babies undergoing painful procedure received sucrose analgesia. It was seen that 97.5% babies undergoing intravenous cannulation, 94% babies undergoing endotracheal suction, 100% undergoing phlebotomy and 93.6% babies undergoing sticking removal got sucrose analgesia prior to these procedures during study period.
In the sustenance phase over last 4 years, it was found that there were dips in administration of sucrose analgesia for eligible babies because of factors like non-availability at bedside, new staff forgetting to administer sucrose analgesia and overcrowding. To reduce the burden of documentation on staff, administration checklist was removed from bedside and weekly audit for administration was done by consultants during rounds. Later a discussion point during daily team huddle regarding provision of sucrose analgesia was added to reinforce its sustained use as suggested by a consultant. Huddle acted as a motivating factor to sustain the improvement. SOP and educational videos as part of induction training are shown to staff for various procedures which included use of sucrose analgesia. All these interventions have resulted in sustained use of sucrose analgesia in >80% of eligible neonates over last 4 years.
Discussion
This was a first QI project in a standalone level 3 NICU in a small hospital. As the intervention of use of sucrose analgesia was new introduction in care pathway of the NICU team, educational intervention in the form of training was needed initially. Questionnaire was used to assess the knowledge change and scoring was done which showed improvement in average score from 40% to >80%. But this change had a modest effect with sucrose administration reaching around 40% (figure 1) by week 2, not enough to achieve our aim.
It needed contextual solutions (figure 1) suggested by frontline staff to get the desired results. Findings of our quality improvement project indicate that simple contextual interventions at the point of care can result in achievement of aim of use of sucrose analgesia.
It also brings out the fact that there is always a tendency to fall back to previous status which needs to be addressed by not only applying technical solutions offered by tools used in methodology but also by addressing the adaptive challenges, for example, by using team huddles (figure 3) as a means of sustaining the gains achieved by tools used in POCQI methodology. Active participation of clinical leadership has paved the way for sustaining the improvement.
In our study, we noticed that impact of educational intervention was not enough, 10%–40% to achieve our aim and needed contextual solutions offered by the point of care team, that is, frontline staff to provide solutions with effective use of tools of POCQI,17 18 active participation of clinical leaders as well to achieve the aim and by addressing adaptive challenges with solutions like team huddle19 to sustain the results.
Strengths and limitations
Strengths of our study include empowerment of frontline staff while using the tools of POCQI methodology, active involvement of leadership and use of solutions in the form of team huddles to address adaptive (behavioural) challenges.
Limitations of our study are that we did not target any specific outcome like pain score, although we did demonstrate effective reduction in pain scores using premature infant pain profile scale with sucrose analgesia during training sessions. As we are using multiple non-pharmacological methods to address neonatal pain and stress, we are definitely looking forward to assess the impact of all these measures on pain by documenting pain scores as an outcome measure.
We feel that contextual solutions applied in our study using POCQI can easily be adapted by other units for providing sucrose analgesia.
For sustaining the results, we have introduced documenting bedside sucrose availability in the nurses’ bedside handover sheet, audiovisual aids in the form of training videos along with SOP, audit by the consultants during rounds and adding a discussion point regarding sucrose analgesia in the daily morning team huddles.
Conclusion
This quality improvement study implementing the use of evidence-based sucrose analgesia using PDSA cycles found that percentage of babies getting sucrose analgesia has increased from baseline 0% to 96.27% in the study period and is sustained at >80% for 4 years.
POCQI methodology can be effectively used to introduce and implement a care strategy by adapting it to the local context. Sustaining the gains achieved by POCQI needs empowerment of frontline staff, active participation of clinical leadership and addressing adaptive challenges with solutions like team huddles.
Footnotes
Twitter: @kedarpriya1, @DrMahtabSingh1
Contributors: KS, SL and RC conceptualised study, conducted research, data collection and team meetings. MS provided mentoring support for the use of POCQI methodology. RB was involved in literature review and SOP formation. KS is responsible for overall content.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors. Publication of this article is made Open Access with funding from the Nationwide Quality of Care Network.
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
All data relevant to the study are included in the article or uploaded as supplementary information.
Ethics statements
Patient consent for publication
Not applicable.
References
- 1.Angelica M, Lagunas DNP, et al. Improving pain management in neonates exposed to intravenous insertions: a quality improvement initiative. J Neonatal Nurs 2016;22. 10.1016/j.jnn.2016.06.001 [DOI] [Google Scholar]
- 2.Lago P, Allegro A, Heun N. Improving newborn pain management: systematic pain assessment and operators' compliance with potentially better practices. J Clin Nurs 2014;23:596–9. 10.1111/jocn.12036 [DOI] [PubMed] [Google Scholar]
- 3.Spence K, Henderson-Smart D. Closing the evidence-practice gap for newborn pain using clinical networks. J Paediatr Child Health 2011;47:92–8. 10.1111/j.1440-1754.2010.01895.x [DOI] [PubMed] [Google Scholar]
- 4.Anne RP, Deshabhotla S, Ahmed SW, et al. A quality improvement initiative to improve management of procedural pain in preterm neonates. Paediatr Anaesth 2021;31:221–9. 10.1111/pan.14075 [DOI] [PubMed] [Google Scholar]
- 5.Nana AY, Gage AD, Arsenault C, et al. High-Quality health systems in the sustainable development goals. Lancet Glob Health 2018. 10.1016/S2214-109X(18)30386-3 [DOI] [Google Scholar]
- 6.Hall RW. Anesthesia and analgesia in the NICU. Clin Perinatol 2012;39:239–54. 10.1016/j.clp.2011.12.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barker DP, Rutter N. Exposure to invasive procedures in neonatal intensive care unit admissions. Arch Dis Child Fetal Neonatal Ed 1995;72:F47–8. 10.1136/fn.72.1.F47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carbajal R, Rousset A, Danan C, et al. Epidemiology and treatment of painful procedures in neonates in intensive care units. JAMA 2008;300:60–70. 10.1001/jama.300.1.60 [DOI] [PubMed] [Google Scholar]
- 9.Grunau R, MT T. Long term consequences of pain in human neonates. In: Pain in neonates & infants. 3rd edition. Philadelphia: Elsevier Science B.V, 2007: 45–55. [Google Scholar]
- 10.Mitchell A, Waltman PA. Oral sucrose and pain relief for preterm infants. Pain Manag Nurs 2003;4:62–9. 10.1016/S1524-9042(02)54201-6 [DOI] [PubMed] [Google Scholar]
- 11.O'Sullivan A, O'Connor M, Brosnahan D, et al. Sweeten, soother and swaddle for retinopathy of prematurity screening: a randomised placebo controlled trial. Arch Dis Child Fetal Neonatal Ed 2010;95:F419–22. 10.1136/adc.2009.180943 [DOI] [PubMed] [Google Scholar]
- 12.Kristoffersen L, Skogvoll E, Hafström M. Pain reduction on insertion of a feeding tube in preterm infants: a randomized controlled trial. Pediatrics 2011;127:e1449–54. 10.1542/peds.2010-3438 [DOI] [PubMed] [Google Scholar]
- 13.Johnston CC, Filion F, Campbell-Yeo M, et al. Enhanced kangaroo mother care for heel lance in preterm neonates: a crossover trial. J Perinatol 2009;29:51–6. 10.1038/jp.2008.113 [DOI] [PubMed] [Google Scholar]
- 14.Gray L, Garza E, Zageris D, et al. Sucrose and warmth for analgesia in healthy newborns: an RCT. Pediatrics 2015;135:e607–14. 10.1542/peds.2014-1073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McKechnie L, Levene M. Procedural pain guidelines for the newborn in the United Kingdom. J Perinatol 2008;28:107–11. 10.1038/sj.jp.7211822 [DOI] [PubMed] [Google Scholar]
- 16.Lee GY, Yamada J, Kyololo O'Brien, et al. Pediatric clinical practice guidelines for acute procedural pain: a systematic review. Pediatrics 2014;133:500–15. 10.1542/peds.2013-2744 [DOI] [PubMed] [Google Scholar]
- 17.Sachan R, Srivastava H, Srivastava S, et al. Use of point of care quality improvement methodology to improve newborn care, immediately after birth, at a tertiary care teaching Hospital, in a resource constraint setting. BMJ Open Qual 2021;10:e001445. 10.1136/bmjoq-2021-001445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mallick A, Banerjee M, Mondal B, et al. A quality improvement initiative for early initiation of emergency management for sick neonates. Indian Pediatr 2018;55:768–72. 10.1007/s13312-018-1378-1 [DOI] [PubMed] [Google Scholar]
- 19.Silver SA, McQuillan R, Harel Z, et al. How to sustain change and support continuous quality improvement. Clin J Am Soc Nephrol 2016;11:916–24. 10.2215/CJN.11501015 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjoq-2022-001830supp001.pdf (35.2KB, pdf)
Data Availability Statement
All data relevant to the study are included in the article or uploaded as supplementary information.