Abstract
Environmental and patient isolates of Mycobacterium avium were resistant to chlorine, monochloramine, chlorine dioxide, and ozone. For chlorine, the product of the disinfectant concentration (in parts per million) and the time (in minutes) to 99.9% inactivation for five M. avium strains ranged from 51 to 204. Chlorine susceptibility of cells was the same in washed cultures containing aggregates and in reduced aggregate fractions lacking aggregates. Cells of the more slowly growing strains were more resistant to chlorine than were cells of the more rapidly growing strains. Water-grown cells were 10-fold more resistant than medium-grown cells. Disinfectant resistance may be one factor promoting the persistence of M. avium in drinking water.
Mycobacterium avium is an environmental, opportunistic human pathogen (8, 25) that infects between 25 and 50% of advanced-stage AIDS patients in the United States (15). M. avium has been isolated from drinking water and municipal water systems (6, 9, 10, 12, 14, 23) and grows in water (11). M. avium isolates recovered from municipal water systems and local natural water sources have the same DNA fingerprints as those recovered from AIDS patients exposed to the water (24). One reason for the persistence of M. avium in drinking water could be resistance to disinfection methods (e.g., chlorination). A number of environmental, opportunistic mycobacteria, including M. avium, have been shown to be relatively resistant to chlorine or chloramine at concentrations used in municipal water systems for disinfection (3, 4, 7, 14, 19, 20, 21). Unfortunately, those earlier studies were flawed because strains were not completely identified, different colony types were used, cells were grown on different media and to different stages, and aggregates were not excluded from the cell suspensions. Most mycobacterial species, including M. avium, form aggregates or clumps during growth in media (16, 18), and the presence of aggregates can lead to spurious disinfection resistance (22) and variable colony counts due to irregular dispersal of aggregates. The objective of the studies described here was to develop a method to produce M. avium cell suspensions lacking large aggregates and to compare the susceptibility of medium- and water-grown M. avium suspensions to chlorine, monochloramine, chlorine dioxide, and ozone.
The M. avium strains A5 (1), 1508, 1060, 5002, and 5502 (24) were identified by DNA probe (Gen-Probe, San Diego, Calif.). M. avium strain A5 was isolated from an AIDS patient and was received from Marjorie Beggs McClellan Veterans Hospital, Little Rock, Ark. Because A5 was not isolated by Marjorie Beggs, it was undoubtedly subject to numerous transfers before receipt in the Virginia Tech laboratory. Its inclusion in this study is based on the fact that it is one of the few strains of M. avium that have been transformed. Thus, it can serve as a host for genes involved in chlorine resistance. In contrast, strains 1060, 1508, 5002, and 5502 were isolated as part of a study in which the Virginia Tech laboratory participated. Cultures were inoculated from the original slants. All strains had a transparent colony morphology, and the frequency of opaque colony variants was less than 1 in 1,000. The M. avium strains were grown to mid-log phase in either Middlebrook 7H9 broth (BBL Microbiology Systems, Cockeysville, Md.) containing 10% (vol/vol) oleic acid-albumin enrichment and 0.5% (vol/vol) glycerol or in autoclaved water from the East St. Louis Municipal Water system (assimilable organic carbon range, 500 to 750 mg/liter). Escherichia coli strain C was grown in nutrient broth (Difco Laboratories, Detroit, Mich.) to mid-log phase at 37°C. Cultures were then centrifuged (8,000 × g for 15 min at 20°C) and washed in an equal volume of chlorine-demand-free phosphate buffer (CDFPB) (5.3 g of KH2PO4/liter, 8.29 g of K2HPO4/liter [pH 7.0] [13]) three times and finally suspended in an equal volume of CDFPB (i.e., washed cultures). To prepare reduced aggregate fractions (RAFs), the washed cultures were centrifuged at 1,300 × g for 5 min at 20°C and the supernatant, containing single cells and small aggregates, was collected. Single cells (i.e., 1 to 5 cells), small aggregates (i.e., those containing 5 to 25 cells), and large aggregates (i.e., those containing more than 25 cells) of three independent cultures were counted in triplicate by phase-contrast microscopy and reported as percent total cells. Only washed cultures contained cells in large aggregates (Table 1). Aggregates did not form during disinfection (data not shown).
TABLE 1.
Characteristics of M. avium RAFs
| M. avium strain | Suspension | % of total cells in aggregates ofa:
|
||
|---|---|---|---|---|
| 1–5 cells | 6–25 cells | >25 cells | ||
| A5 | Washed culture | 10 | 22 | 68 |
| RAF | 88 | 12 | <1 | |
| 1060 | Washed culture | 5 | 21 | 75 |
| RAF | 84 | 16 | <1 | |
| 1508 | Washed culture | 7 | 17 | 76 |
| RAF | 82 | 18 | <1 | |
| 5002 | Washed culture | 12 | 40 | 48 |
| RAF | 89 | 11 | <1 | |
| 5502 | Washed culture | 8 | 35 | 57 |
| RAF | 85 | 15 | <1 | |
Values are averages from three independent cultures measured in triplicate. Standard deviations ranged from <2 to 5%.
To measure disinfectant susceptibility, 200 ml of CDFPB contained in a 250-ml glass, stoppered Erlenmeyer flask with a Teflon-coated magnetic stir bar was inoculated with 6 × 105 CFU from a washed culture or RAF. Disinfection was performed at 23°C, under reduced light levels and with gentle stirring (60 rpm). Chlorine, monochloramine, and chlorine dioxide were prepared and added to cell suspensions, and their free concentrations were measured by using published methods (13). Ozone was generated by an M-1500 corona discharge ozonator (Clearwater Technology, Inc., San Luis Obispo, Calif.) fed with oxygen gas. Ozone-demand-free glassware and ozone-demand-free phosphate buffer were prepared, and ozone concentrations were measured by using published methods (13). In all disinfection experiments whose results are reported here, the concentration of disinfectant did not vary by more than 75% of the initial value. Surviving M. avium cells were enumerated as CFU following inactivation of disinfectants with 0.1% sodium thiosulfate (13) on Middlebrook 7H10 agar medium (BBL Microbiology Systems) containing 10% (vol/vol) oleic acid-albumin enrichment and 0.5% (vol/vol) glycerol. Surviving E. coli cells were enumerated on plate count agar (Difco Laboratories).
The data presented are the averages of a minimum of three replicates. Linear regressions based on the logarithm of the percent survival with time (in minutes) for each strain were computed and used to calculate the product of disinfectant concentration (in parts per million) and time (in minutes) to reach 3 log units of cell death (CT99.9%) for each strain.
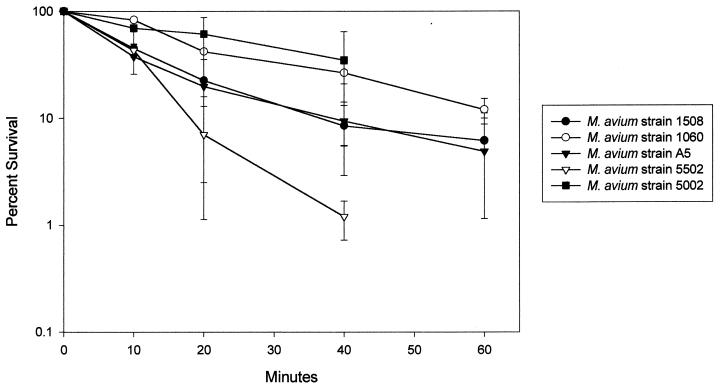
Because chlorine concentrations fell from 1 to 0.15 ppm in 10 min in M. avium suspensions containing more than 5 × 106 CFU/ml (5), disinfection was measured in suspensions of between 104 and 105 CFU/ml. There was no difference in disinfection kinetics over the range of 103 to 106 CFU/ml for M. avium strain A5 (data not shown). M. avium cells in RAFs were significantly more resistant to chlorine, monochloramine, chlorine dioxide, and ozone than E. coli cells (Table 2). The values for E. coli agreed with published data (17). Strains of M. avium had chlorine CT99.9% values ranging from 51 to 204 (Fig. 1). Susceptibility to chlorine correlated with the growth rate in the Middlebrook 7H9 medium. Though the cultures of each M. avium strain were harvested in mid-log phase, the turbidity of the cultures differed because strains grew at different rates. The most chlorine-susceptible strain (i.e., strain 5502) had the highest turbidity, whereas the most chlorine-resistant strain (i.e., strain 1060) had the lowest turbidity. When chlorine CT99.9% values were plotted against turbidity of 9-day, log-phase cultures for each strain, a straight line was obtained with an R value of −0.902 by linear regression. Others have shown that antecedent growth conditions influence susceptibility to chlorine-based disinfectants (2, 17).
TABLE 2.
Calculated disinfection CT99.9% values for M. avium strainsa
| Disinfectant | CT99.9% (mean ± SD)
|
|||||
|---|---|---|---|---|---|---|
|
M. avium strain
|
E. coli strain C | |||||
| A5 | 1060 | 1508 | 5002 | 5502 | ||
| Chlorine | ||||||
| Medium grown | 106 ± 9 | 204 ± 36 | 164 ± 28 | 126 ± 27 | 51 ± 10 | 0.09 ± 0.003 |
| Water grown | 1,552 ± 403 | 1,445 ± 238 | 596 ± 292 | 962 ± 431 | 551 ± 290 | ND |
| Monochloramine | 97 ± 9 | 458 ± 152 | 548 ± 62 | 1,710 ± 814 | 91 ± 34 | 73 ± 28 |
| Chlorine dioxide | NDb | 8 ± 3 | ND | 11 ± 2 | 2 ± 0.1 | 0.02 ± 0.003 |
| Ozone | ND | 0.17 ± 0.14 | ND | 0.12 ± 0.01 | 0.10 ± 0.01 | 0.002 ± 0.002 |
Calculated as described in Materials and Methods.
ND, not done.
FIG. 1.
M. avium disinfection kinetics for a chlorine concentration of 1.0 ± 0.2 ppm (pH 7.0; temperature, 23°C). The error bars indicate the 95% confidence intervals.
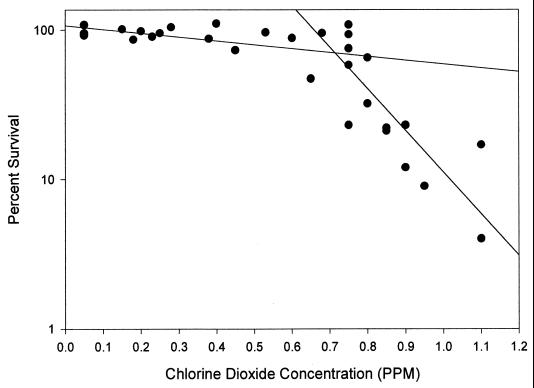
The M. avium strains were also resistant to monochloramine, chlorine dioxide, and ozone (Table 2). The M. avium strains could be divided into monochloramine-resistant and -susceptible groups, though that separation was not carried through for chlorine dioxide or ozone susceptibility (Table 2). The chlorine dioxide (Fig. 2) and ozone disinfection kinetics for M. avium strain 5002 were biphasic, unlike those for the other strains tested.
FIG. 2.
M. avium strain 5002 disinfection kinetics with different concentrations of chlorine dioxide (pH 7.0; temperature, 23°C). The curve is a best fit linear regression line created with the Sigma Plot program (Jandel Scientific, San Rafael, Calif.).
The chlorine susceptibility of RAFs and washed cultures of M. avium strain 1060 were measured to determine whether the presence of large aggregates would increase the resistance to disinfection. This strain was chosen because washed cultures had a high proportion of cells in large aggregates (75% [Table 1]). The average chlorine CT99.9% value for the washed cultures was 207 (standard deviation, ±43), based on triplicate measurements of two independent cultures. Because that value was approximately equal to that for the RAF (Table 1) and the variation was the same, the presence of cells in aggregates of more than 25 cells does not appear to influence chlorine susceptibility for that M. avium strain.
The chlorine susceptibility of water-grown cells was also measured to assess the effect of the growth medium on susceptibility, since M. avium is normally an inhabitant of water (11, 24). Water-grown cells of all five M. avium strains were significantly more chlorine resistant than were cells grown in medium (Table 2). That effect may be related to growth rate, because the growth rate of M. avium in water is much slower than that in medium (11).
This study documents the heretofore suspected disinfectant resistance of M. avium. Most of the M. avium strains were highly resistant to chlorine, monochloramine, chlorine dioxide, and ozone (Table 2). The M. avium strains possessed very high CT99.9% values; for example, chlorine CT99.9% values for the M. avium strains were 580 to 2,300 times greater than those for E. coli (Table 2). Similarly, the CT99.9% values of chlorine dioxide and ozone for the M. avium strains were at least 100- and 50-fold greater (respectively) than those for the E. coli strain (Table 2). In agreement with other studies (4), chlorine dioxide was a better mycobacterial disinfectant than chlorine at equal concentrations (Table 1).
The M. avium strains could be divided into two groups based upon their susceptibility to monochloramine. One of the susceptible M. avium strains (strain 5502) was a water isolate that had the same pulsed-field gel electrophoresis (PFGE) pattern as did an epidemiologically linked AIDS patient isolate, strain 5002 (24). The relative resistance of the AIDS patient isolate M. avium strain 5002 compared to its PFGE-matched and epidemiologically matched water isolate, strain 5502, was due to a difference in growth rate. Though the strains were isolated during the same study at approximately the same time in 1992 and retain the same, shared PFGE pattern (24) and colonial morphology, the water isolate, strain 5502, grew faster than did the patient isolate, strain 5002.
We believe the values reported here accurately document the disinfectant resistance of M. avium strains and are not artifacts of experimental variation. All strains were of the same colony type and grown in the same medium to the same stage of growth. None of the RAF suspensions used for disinfection contained aggregates consisting of more than 25 cells (Table 1). Further, the presence of cells in large aggregates did not increase chlorine CT99.9% values for one strain. All colony counts were obtained by spreading suspensions on the surface of M7H10 agar medium plates that were used 3 days after preparation. The suspensions were spread to dryness to ensure the disruption of aggregates (18). Such manipulations on all CFU measurements ensured the reproducibility of results. In spite of those precautions, there was variation in the results (Table 2). The source of that variation has not been identified. However, the magnitudes of the average CT99.9% values were so high that the relative resistance of M. avium to disinfectants was evident and the differences between M. avium strains could be demonstrated.
Acknowledgments
This work was supported by a grant from the American Water Works Association Research Foundation.
Marjorie Beggs of the McClellan Veterans Hospital generously supplied M. avium strain A5. We acknowledge the expert technical assistance of Myra D. Williams.
REFERENCES
- 1.Beggs M L, Crawford J T, Eisenbach K P. Isolation and sequencing of the replication region of Mycobacterium avium plasmid pLR7. J Bacteriol. 1995;117:4836–4840. doi: 10.1128/jb.177.17.4836-4840.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Berg J D, Matin A, Roberts P V. Effect of antecedent growth conditions on sensitivity of Escherichia coli to chlorine dioxide. Appl Environ Microbiol. 1982;44:814–819. doi: 10.1128/aem.44.4.814-819.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carson L A, Peterson N J, Favero M S, Aguero S M. Growth characteristics of atypical mycobacteria in water and their comparative resistance to disinfectants. Appl Environ Microbiol. 1978;36:839–846. doi: 10.1128/aem.36.6.839-846.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carson L A, Bland L A, Cusick L B, Favero M S. Efficacy of chemical dosing methods for isolating nontuberculous mycobacteria from water supplies of dialysis centers. Appl Environ Microbiol. 1988;54:1756–1760. doi: 10.1128/aem.54.7.1756-1760.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cowan H E. Rapid, quantitative assessment of Mycobacterium avium susceptibility to chlorine based on the firefly luciferase reporter gene. M.S. thesis. Blacksburg, Va: Virginia Polytechnic Institute and State University; 1999. [Google Scholar]
- 6.du Moulin G C, Stottmeier K D. Waterborne mycobacteria: an increasing threat to health. ASM News. 1986;52:525–529. [Google Scholar]
- 7.Englebrecht R S, Severin B F, Masarik M T, Farooq S, Lee S H, Haas C H, Lalchandani A. New microbial indicators of disinfection efficiency. Report EPA 600/2-77-052. U.S. Cincinnati, Ohio: Environmental Protection Agency; 1977. [Google Scholar]
- 8.Falkinham J O., III Epidemiology of infection by nontuberculous mycobacteria. Clin Microbiol Rev. 1996;9:177–215. doi: 10.1128/cmr.9.2.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Falkinham J O, III, Parker B C, Gruft H. Epidemiology of infection by nontuberculous mycobacteria. I. Geographic distribution in the eastern United States. Am Rev Respir Dis. 1980;121:931–937. doi: 10.1164/arrd.1980.121.6.931. [DOI] [PubMed] [Google Scholar]
- 10.Fischeder R, Schulze-Robbecke R, Weber A. Occurrence of mycobacteria in drinking water samples. Zentbl Hyg Umweltmed. 1991;192:154–158. [PubMed] [Google Scholar]
- 11.George K L, Parker B C, Gruft H, Falkinham J O., III Epidemiology of infection by nontuberculous mycobacteria. II. Growth and survival in natural waters. Am Rev Respir Dis. 1980;122:89–94. doi: 10.1164/arrd.1980.122.1.89. [DOI] [PubMed] [Google Scholar]
- 12.Glover N, Holtzman N, Aronson T, Froman S, Berlin O G W, Dominguez P, Kunkel K A, Overturf G, Stelma G, Jr, Smith C, Yakrus M. The isolation of Mycobacterium avium complex (MAC) recovered from Los Angeles potable water, a possible source of infection in AIDS patients. Int J Environ Health Res. 1994;4:63–72. [Google Scholar]
- 13.Greenberg A E, Clesceri L S, Eaton A D, editors. Standard methods for the examination of water and wastewater. 18th ed. Washington, D.C.: American Public Health Association; 1992. [Google Scholar]
- 14.Haas C N, Meyer M A, Paller M S. The ecology of acid-fast organisms in water supply, treatment, and distribution systems. J Am Water Works Assoc. 1983;75:139–144. [Google Scholar]
- 15.Inderlied C B, Kemper C A, Bermudez L E M. The Mycobacterium avium complex. Clin Microbiol Rev. 1993;6:266–310. doi: 10.1128/cmr.6.3.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McCarthy C, Ashbaugh P. Factors that affect the cell cycle of Mycobacterium avium. Rev Infect Dis. 1981;3:914–925. doi: 10.1093/clinids/3.5.914. [DOI] [PubMed] [Google Scholar]
- 17.Millbauer R, Grossowicz N. Effect of growth conditions on chlorine sensitivity of Escherichia coli. Appl Microbiol. 1959;7:71–74. doi: 10.1128/am.7.2.71-74.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Parker B C, Ford M A, Gruft H, Falkinham J O., III Epidemiology of infection by nontuberculous mycobacteria. IV. Preferential aerosolization of Mycobacterium intracellulare from natural waters. Am Rev Respir Dis. 1984;128:652–656. doi: 10.1164/arrd.1983.128.4.652. [DOI] [PubMed] [Google Scholar]
- 19.Pelletier P A, Carney E M, du Moulin G C. Water quality for the new decade. Proceedings of the American Water Works Association. Denver, Colo: American Water Works Association; 1991. Comparative resistance of Mycobacterium avium complex and other nontuberculous mycobacteria to chloramine; pp. 47–58. [Google Scholar]
- 20.Pelletier P A, du Moulin G C, Stottmeier K D. Mycobacteria in public water supplies: comparative resistance to chlorine. Microb Sci. 1988;5:147–148. [PubMed] [Google Scholar]
- 21.Sobsey M D. Inactivation of health-related microorganisms in water by disinfection processes. Water Sci Technol. 1989;21:179–195. [Google Scholar]
- 22.Stewart M H, Olson B H. Physiological studies of chloramine resistance developed by Klebsiella pneumoniae under low-nutrient growth conditions. Appl Environ Microbiol. 1992;58:2918–2927. doi: 10.1128/aem.58.9.2918-2927.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.von Reyn C F, Waddell R D, Eaton T, Arbeit R D, Maslow J N, Barber T W, Brindle R J, Gilks C F, Lumito J, Lahdevirta J, Ranki A, Dawson D, Falkinham J O., III Isolation of Mycobacterium avium complex from water in the United States, Finland, Zaire, and Kenya. J Clin Microbiol. 1993;31:3227–3230. doi: 10.1128/jcm.31.12.3227-3230.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.von Reyn C F, Maslow J N, Barber T W, Falkinham III J O, Arbeit R D. Persistent colonization of potable water as a source of Mycobacterium avium infection in AIDS. Lancet. 1994;343:1137–1141. doi: 10.1016/s0140-6736(94)90239-9. [DOI] [PubMed] [Google Scholar]
- 25.Wolinsky E. Nontuberculous mycobacteria and associated diseases. Am Rev Respir Dis. 1979;119:107–159. doi: 10.1164/arrd.1979.119.1.107. [DOI] [PubMed] [Google Scholar]