Auer rods were first described by john Auer as rod-shaped inclusions in 1906 and confirmed to be formed by the crystallisation of cytoplasmic azurophilic granules hereafter. As the hallmark of acute myeloid leukemia (AML), Auer rods are seen occasionally in patients with myelodysplastic syndrome (MDS). The presence of Auer rods is generally associated with favourable prognosis in AML (Yoshida et al., 2009). In the French-American-British (FAB) (Bennett et al., 1982) and World Health Organisation (WHO) (Arber et al., 2016) systems, patients with MDS and Auer rods are classified as high-risk, regardless of myeloblast percentage in blood or bone marrow (BM). However, the mutation topography and the clinical outcomes of MDS with excess blasts-2 (MDS-EB2) with Auer rods, when treated with chemotherapy or hypo-methylating drugs, are unknown.
We reviewed medical records of 516 consecutive patients with newly diagnosed MDS-EB2 according to the 2016 revised WHO criteria (Arber et al., 2016) at our centre from January 2006 to December 2018. We then studied Wright-Giemsa stained blood (≥100 cells) and BM slides (≥200 cells), obtained at diagnosis for Auer rods. Positivity was defined as ≥1 Auer rods in blood or BM cells. Auer rods were found in 59 patients (11%).
Previous data indicate that the frequency of acquired somatic mutations increases with age (Silva et al., 2017; Li et al., 2018). To adjust for this, we compared clinical and laboratory variables and results of next generation sequencing (NGS) in these 59 patients with 236 age-matched controls with MDS-EB2 without Auer rods. Auer rod-positive patients were divided into two cohorts: (i) patients diagnosed as MDS-EB2 based solely on having Auer rods, regardless of blood or BM myeloblast levels (cohort-1; N = 25); and (ii) patients meeting blood and/or BM blast criteria for MDS-EB2 with Auer rods (cohort-2; N = 34). Patients with MDS-EB2 without Auer rods (N = 236) were designated cohort-3. Patients and controls gave informed consent compliant with the Declaration of Helsinki.
Patients with Auer rods had higher reticulocyte levels (0·0421 × 10E + 12/l vs. 0·0302 × 10E + 12/l; P = 0·011), higher neutrophil levels (0·98 × 10E + 9/l vs. 0·76 × 10E + 9/l, P = 0·041), higher platelet levels (68 × 10E + 9/l vs. 52 × 10E + 9/l; P = 0·02) (Table I). Cohort-2 had higher reticulocyte levels than cohort-3 (P = 0·005) and higher platelet levels than cohort-1 (P = 0·009) and cohort-3 (P = 0·001) (Table S1).
Table I.
Clinical and laboratory characteristics of MDS-EB2 patients with or without Auer rods.
| Characteristics | Auer Rods pos (N = 59) | Auer Rods neg (N = 236) | Total (N = 295) | P value |
|---|---|---|---|---|
| Male | 36 (61·0) | 165 (69·9) | 201 (68·1) | |
| Age, median (range), year | 49 (16–84) | 51 (17–78) | 51 (16–84) | Match |
| Hb, median (range), g/l | 74 (45–127) | 77 (30–157) | 76 (30–157) | 0·696 |
| Rc, median (range), ×1012/l | 0·0421 (0·004–0·1746) | 0·0302 (0·0004–0·2391) | 0·0321 (0·0004–0·2391) | 0·011 |
| WBC, median (range), ×109/l | 2·64 (1·00–13·59) | 2·37 (0·32–52·36) | 2·45 (0·32–52·36) | 0·082 |
| ANC, median (range), ×109/l | 0·98 (0·00–10·26) | 0·76 (0·00–13·18) | 0·82 (0·00–13·18) | 0·041 |
| PLT, median (range), ×109/l | 68 (3–405) | 52 (1–566) | 54 (1–566) | 0·020 |
| IPSS-R karyotype (%), N = 222 | ||||
| Very good | 0 (0) | 2 (1·1) | 2 (0·9) | 0·045 |
| Good | 28 (62·2) | 85 (48·0) | 113 (50·9) | |
| Intermediate | 13 (28·9) | 43 (24·3) | 56 (25·2) | |
| Poor | 2 (4·4) | 8 (4·5) | 10 (4·5) | |
| Very poor | 2 (4·4) | 39 (22·0) | 41 (18·5) | |
| Normal karyotype (%) | 27 (60·0) | 78 (44·1) | 105 (47·3) | 0·056 |
| Complex karyotype (%) | 3 (6·7) | 44 (24·9) | 47 (21·2) | 0·008 |
| MDS specific karyotype (%) | 5 (11·1) | 53 (29·9) | 58 (26·1) | 0·010 |
| IPSS-R risk group (%), N = 222 | ||||
| Low | 1 (2·2) | 0 (0·0) | 1 (0·5) | 0·001 |
| Intermediate | 7 (15·6) | 14 (7·9) | 21 (9·5) | |
| High | 22 (48·9) | 55 (31·1) | 76 (34·4) | |
| Very high | 15 (33·3) | 108 (61·0) | 123 (55·7) | |
| Treatment (%), N = 222 | ||||
| HMAs alone | 12 (24·5) | 30 (17·3) | 42 (18·9) | 0·616 |
| Chemotherapy alone | 8 (16·3) | 35 (20·2) | 43 (19·4) | |
| Chemotherapy combined with HMAs | 6 (12·2) | 26 (15·0) | 32 (14·4) | |
| Low-dose melphalan | 1 (2·0) | 13 (7·5) | 14 (6·3) | |
| Allotransplant | 13 (26·5) | 32 (18·5) | 45 (20·3) | |
| EPO ± G-CSF and RBC and/or PLT transfusions | 9 (18·4) | 37 (21·4) | 46 (20·7) |
MDS-specific karyotype, defined as cytogenetic abnormalities sufficient for diagnosis of AML with MDS-related changes when 20% or more PB (peripheral blood) or BM blasts are present according to WHO criteria, including complex aberrant, −7/del(7q), −5/del(5q), i(17q), −13/del(13q), del(11q), del(12p)/t(12p), del(9q), idic(X)(q13), t(11;16), t(3,21), t(1;3), t(2;11), t(5;12), t(5;7), t(5;17), t(5;10), t(3;5). pos, positive; neg, negative; Hb, hemoglobin; Rc, reticulocyte count; WBC, white blood cell count; ANC, absolute neutrophil count; PLT, platelet count; IPSS-R, Revised International Prognostic Scoring System; HMAs, hypomethylating drugs; EPO, erythropoietin; G-CSF, granulocyte-colony-stimulating factor; RBC, red blood cell.
Patients with Auer rods were more likely to have normal cytogenetics and less likely to have complex karyotype, compared with patients without (P = 0·056; P = 0.008; Table I). Cytogenetic abnormalities sufficient for diagnosing AML with MDS-related changes, according to WHO criteria(Vardiman et al., 2009), were defined as MDS-specific cytogenetic abnormalities and were more common in patients without Auer rods compared to patients with Auer rods (P = 0·01; Table I). Cohorts-1 and −2 had similar frequencies of complex and MDS-specific cytogenetic abnormalities which were lower than cohort-3 (Tables S1 and S2). Patients with Auer rods were less often classified as very-high-risk in IPSS-R (Revised International Prognostic Scoring System) compared with patients without (P = 0·005; Table I). This difference was found mainly in cohort-1 (P < 0·001; Table S1), reflecting the lower blast percentage and more favourable cytogenetics of this cohort.
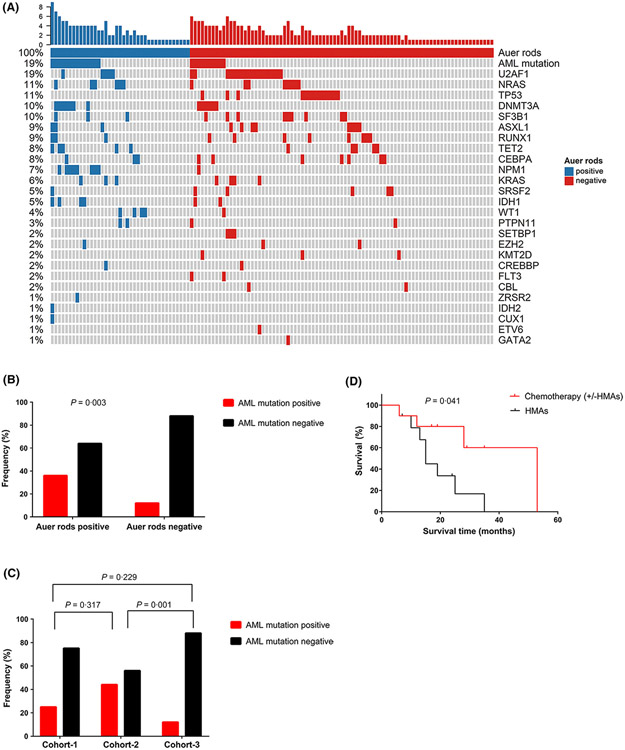
NGS of 112 genes was done on BM samples obtained at diagnosis from 124 patients (Table S3), including 39 patients with Auer rods, and 85 without Auer rods and detected 202 high-confidence mutations (Table S4). There was no significant difference in numbers of mutations (mean, 1·82 vs. 1·54; P = 0·576). Mutation topography is shown in Fig 1A and Figure S1. NPM1 was the most frequently mutated gene in patients with Auer rods [21%, 95% confidence interval (CI), 9, 36%] followed by DNMT3A (18% [8, 34%]), NRAS (15% [6, 31%]), TET2 (13% [4, 27%]), U2AF1 (13% [4, 27%]), WT1 (10% [3, 24%]) and IDH1 (10% [3, 24%]). In patients without Auer rods, the three most frequently mutated genes were U2AF1 (21% [12, 30%]), TP53 (17% [8, 25%]) and SF3B1 (11%, [4, 17%]). NPM1, WT1 and IDH1 were significantly more frequently mutated in Auer rod-positive compared with Auer rod-negative patients (P < 0·001; P = 0·034; P = 0·077). No patient with Auer rods had a TP53 mutation, compared with 17% of patients without Auer rods (P = 0·003).
Fig 1.
The mutation distribution and clinical outcome under different treatment regimens of MDS with excess blast-2 (MDS-EB2) with Auer rods. (A) Mutation topography of MDS-EB2 patients with or without Auer rods. (B) Patients with Auer rods had a higher incidence of AML-related mutation (FLT3, NPM1, DNMT3A, IDH1, IDH2) than patients without Auer rods. (C) Patients in cohort-2 had a significantly higher incidence of AML-related mutation than patients in cohort-3. (D) Kaplan–Meier curves of overall survival in the Auer rod-positive group stratified by different treatment regimens, including chemotherapy [with or without hypo-methylating drugs (HMAs)] and HMAs alone.
Previous data indicate that recurrently mutated genes in high-risk MDS (TP53, etc.) differ from those in de novo AML (FLT3, NPM1, DNMT3A, IDH1, IDH2) (Walter et al., 2013). Patients with Auer rods were more likely to have these AML mutations compared to those without Auer rods [36% (21, 53%) vs. 12% (5, 19%); P = 0·003; Fig 1B]. This was especially so for patients in cohort-2 [44% (23, 66%); P = 0·001; Fig 1C]. Similarly, according to the categorising molecular mutations in MDS and AML as reported by Munich Leukemia Laboratory (Rose et al., 2015), the AML mutations (NPM1, CEBPA, FLT3, IDH1, IDH2 and CBFB) showed more frequency in patients with Auer rods (33%), both in cohort-1 (31%) and −2 (35%), than those without (13%; P = 0·013; Figure S2). Lindsley et al. (2015) defined NPM1 mutations, MLL/11q23 rearrangements, and CBF rearrangements as de novo-type alterations for AML, and found that NPM1 and TP53 mutations were mutually exclusive. In our cohort, the NPM1 mutation was significantly associated with Auer rods while the TP53 mutation was inversely associated. These data suggest MDS with Auer rods is biologically the same as de novo AML.
Ninety-five patients receiving hypo-methylating drugs and/or chemotherapy in our centre (Table S5) could be evaluated for response using criteria of the MDS International Working Group (Cheson et al., 2006). Patients with Auer rods receiving chemotherapy (with or without hypo-methylating drugs) had a higher frequency of complete remission compared to patients received hypo-methylating drugs only [64% (31, 89%) vs. 20% (3, 56%); P = 0·08; Table S6]. Previous studies reported that patients with MDS and Auer rods responded better to intensive AML-like chemotherapy than patients without Auer rods (Seymour & Estey, 1995). Conversely, we detected no difference in complete response rates with these regimens in patients without Auer rods (25% vs. 23%; P = 0·835; Table S6). There was also no difference in the overall response rate between these cohorts (P > 0·1, Table S6).
In patients with Auer rods, those receiving chemotherapy (with or without hypo-methylating drugs) had a longer median survival compared to those patients with Auer rods receiving only hypo-methylating drugs (53 vs. 15 months; P = 0·041; Fig 1D). This difference was not detected in patients without Auer rods (18 vs. 21 months; P = 0·642; Figure S3A). Amongst patients receiving chemotherapy (with or without hypo-methylating drugs), those with Auer rods had a longer median survival compared to patients without (53 vs. 18 months; P = 0·254; Figure S3B).
MDS was previously called preleukemia, smoldering leukemia, or oligoblastic leukemia (Heaney & Golde, 1999). Albitar et al. (2002) reported MDS as biologically and clinically distinct from AML. Others think some cases are similar (Gale & Bennett, 2009). In the past decade, distinction of the mutation spectrum between MDS and AML (as discovered by NGS) indicate that MDS is distinct from AML in many respects, just as splicing gene mutations are more common in people with MDS, and transcription gene abnormalities are more common in people with AML (Walter et al., 2013).
Our data indicate that patients with MDS and Auer rods have a high frequency of AML-related mutations. We suggest that MDS with Auer rods is a special subgroup which is biologically more akin to AML, regardless of blasts percentage, and may benefit from chemotherapy. Because our study was retrospective, our conclusions require validation.
Supplementary Material
Table S1. Clinical and laboratory characteristics of MDS patients in three cohorts.
Table S2. Cytogenetic analysis of MDS patients in three cohorts.
Table S3. Gene list of the 112-gene panel.
Table S4. 202 high-confidence mutations of 124 MDS patients with genetic mutations.
Table S5. Treatment regimens of patients with or without Auer rods who received chemotherapy and/or HMAs in our centre and can be evaluated for response.
Table S6. Response outcomes of patients with or without Auer rods, based on the type of therapy.
Fig S1. Mutation topography of patients between three cohorts.
Fig S2. (A) Patients with Auer rods had higher incidence of AML-related mutation (NPM1, CEBPA, FLT3, IDH1, IDH2 and CBFB) than patients without Auer rods. (B) Patients in cohort-1 and cohort-2 had a higher incidence of AML-related mutation than patients in cohort-3.
Fig S3. (A) In the Auer rod-negative cohort, we observed no difference of median survival between patients receiving chemotherapy (with or without HMAs) and HMAs alone. (B) In patients receiving chemotherapy (with or without HMAs) those with Auer rods had a longer median survival compared to patients without Auer rods.
Acknowledgements
This study is supported in part by National Natural Science Funds (No. 81870104, No. 81530008, No. 81470297), Tianjin Natural Science Funds (18JCZDJC34900, 16JCQNJC11400, 19JCQNJC09400), and CAMS Initiative Fund for Medical Sciences (No. 2016-I2M-1-001). R.P.G. acknowledges support from the National Institute of Health Research (NIHR) Biomedical Research Centre funding scheme.
Footnotes
Conflict of Interest
R.P.G. is a part-time employee of Celgene Corp.
Supporting Information
Additional supporting information may be found online in the Supporting Information section at the end of the article.
References
- Albitar M, Manshouri T, Shen Y, Liu D, Beran M, Kantarjian HM, Rogers A, Jilani I, Lin CW, Pierce S, Freireich EJ & Estey EH (2002) Myelodysplastic syndrome is not merely "preleukemia". Blood, 100, 791–798. [DOI] [PubMed] [Google Scholar]
- Arber DA, Orazi A, Hasserjian R, Thiele J, Borowitz MJ, Le Beau MM, Bloomfield CD, Cazzola M & Vardiman JW (2016) The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood, 127, 2391–2405. [DOI] [PubMed] [Google Scholar]
- Bennett JM, Catovsky D, Daniel MT, Flandrin G, Galton DA, Gralnick HR & Sultan C (1982) Proposals for the classification of the myelodysplastic syndromes. British Journal of Haematology, 51, 189–199. [PubMed] [Google Scholar]
- Cheson BD, Greenberg PL, Bennett JM, Lowenberg B, Wijermans PW, Nimer SD, Pinto A, Beran M, de Witte TM, Stone RM, Mittelman M, Sanz GF, Gore SD, Schiffer CA & Kantarjian H (2006) Clinical application and proposal for modification of the International Working Group (IWG) response criteria in myelodysplasia. Blood, 108, 419–425. [DOI] [PubMed] [Google Scholar]
- Gale RP & Bennett JM (2009) Are myelodysplastic syndromes and acute myeloid leukemia one disease? Leukemia Research, 33, 351–354. [DOI] [PubMed] [Google Scholar]
- Heaney ML & Golde DW (1999) Myelodysplasia. New England Journal of Medicine, 340, 1649–1660. [DOI] [PubMed] [Google Scholar]
- Li B, Liu J, Jia Y, Wang J, Xu Z, Qin T, Shi Z, Song Z, Peng S, Huang H, Fang L, Zhang H, Pan L, Hu N, Qu S, Zhang Y, Wu J, Liu N, Ru K, Huang G & Xiao Z (2018) Clinical features and biological implications of different U2AF1 mutation types in myelodysplastic syndromes. Genes, Chromosomes & Cancer, 57, 80–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindsley RC, Mar BG, Mazzola E, Grauman PV, Shareef S, Allen SL, Pigneux A, Wetzler M, Stuart RK, Erba HP, Damon LE, Powell BL, Lindeman N, Steensma DP, Wadleigh M, DeAngelo DJ, Neuberg D, Stone RM & Ebert BL (2015) Acute myeloid leukemia ontogeny is defined by distinct somatic mutations. Blood, 125, 1367–1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose D, Haferlach T, Kern W & Haferlach C (2015) Categorizing molecular mutations in MDS and AML. Blood, 126, 5222. [Google Scholar]
- Seymour JF & Estey EH (1995) The contribution of Auer rods to the classification and prognosis of myelodysplastic syndromes. Leukemia & Lymphoma, 17, 79–85. [DOI] [PubMed] [Google Scholar]
- Silva P, Neumann M, Schroeder MP, Vosberg S, Schlee C, Isaakidis K, Ortiz-Tanchez J, Fransecky LR, Hartung T, Turkmen S, Graf A, Krebs S, Blum H, Muller-Tidow C, Thiede C, Ehninger G, Serve H, Hecht J, Berdel WE, Greif PA, Rollig C & Baldus CD (2017) Acute myeloid leukemia in the elderly is characterized by a distinct genetic and epigenetic landscape. Leukemia, 31, 1640–1644. [DOI] [PubMed] [Google Scholar]
- Vardiman JW, Thiele J, Arber DA, Brunning RD, Borowitz MJ, Porwit A, Harris NL, Le Beau MM, Hellstrom-Lindberg E, Tefferi A & Bloomfield CD (2009) The 2008 revision of the World Health Organization (WHO) classification of myeloid neoplasms and acute leukemia: rationale and important changes. Blood, 114, 937–951. [DOI] [PubMed] [Google Scholar]
- Walter MJ, Shen D, Shao J, Ding L, White BS, Kandoth C, Miller CA, Niu B, McLellan MD, Dees ND, Fulton R, Elliot K, Heath S, Grillot M, Westervelt P, Link DC, DiPersio JF, Mardis E, Ley TJ, Wilson RK & Graubert TA (2013) Clonal diversity of recurrently mutated genes in myelodysplastic syndromes. Leukemia, 27, 1275–1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida Y, Oguma S & Ohno H (2009) John Auer and Auer rods; controversies revisited. Leukemia Research, 33, 614–616. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Clinical and laboratory characteristics of MDS patients in three cohorts.
Table S2. Cytogenetic analysis of MDS patients in three cohorts.
Table S3. Gene list of the 112-gene panel.
Table S4. 202 high-confidence mutations of 124 MDS patients with genetic mutations.
Table S5. Treatment regimens of patients with or without Auer rods who received chemotherapy and/or HMAs in our centre and can be evaluated for response.
Table S6. Response outcomes of patients with or without Auer rods, based on the type of therapy.
Fig S1. Mutation topography of patients between three cohorts.
Fig S2. (A) Patients with Auer rods had higher incidence of AML-related mutation (NPM1, CEBPA, FLT3, IDH1, IDH2 and CBFB) than patients without Auer rods. (B) Patients in cohort-1 and cohort-2 had a higher incidence of AML-related mutation than patients in cohort-3.
Fig S3. (A) In the Auer rod-negative cohort, we observed no difference of median survival between patients receiving chemotherapy (with or without HMAs) and HMAs alone. (B) In patients receiving chemotherapy (with or without HMAs) those with Auer rods had a longer median survival compared to patients without Auer rods.