Abstract
Physical exercise in rodents has repeatedly been shown to trigger positive effects on brain function including increased neurotrophic factors and improved learning and memory. However, most of this work has focused on the adult hippocampus and hippocampal-dependent behavior. Here we examined the effect of running wheel exercise in adult and adolescent male rats on ABA renewal of extinguished instrumental conditioning, in which acquisition occurs in context A, extinction in context B, and renewal testing occurs back in context A. In the first experiment, rats were given unlocked (exercise) or locked (no exercise) running wheel access in their home cages beginning at postnatal day 30 (adolescent) or postnatal day 56 (adult). Rats underwent lever-press acquisition in context A and extinction in context B. ABA renewal testing took place two weeks after the start of running wheel exposure. Non-exercising adolescent rats showed greater ABA renewal than non-exercising adult rats and exercise reduced ABA renewal in adolescents but not adults. ABA renewal depends on medial prefrontal cortex function. In a second experiment, we compared adolescent and adult apical dendrite branch length, branch number, and spine density of medial prefrontal cortex pyramidal neurons after two weeks of unlocked or locked running wheel access. The results revealed a higher density of dendritic spines and a lower dendritic branch length in adolescent exercisers than adolescent non-exercisers. Adult exercisers and non-exercisers did not differ. Collectively, these experiments suggest that exercise may have particularly strong effects on adolescent medial prefrontal cortex function and structure.
In 1953, Jerry Morris and his colleagues first empirically demonstrated the link between physical activity and health by showing that London bus conductors, who climbed hundreds of steps each day, had a lower incidence of heart disease than drivers, who were mainly sedentary (Morris, Heady, Raffle, Roberts, & Parks, 1953). In the sixty-plus years since that finding, the body of research on physical exercise has expanded immensely, encompassing links between physical activity and benefits ranging from increases in the number of new neurons to improved mental health.
Indeed, research on the neurobiological effects of exercise has consistently shown that humans and laboratory rodents experience various cognitive benefits from physical activity. While these benefits are wide ranging both in terms of brain areas affected and mechanisms, some of the most reliably shown changes take place in the rodent hippocampus in the form of increased neurogenesis, cell survival, and improved performance on hippocampus-reliant tasks, such as spatial learning and contextual fear conditioning (B. J. Anderson et al., 2000; Baruch, Swain, & Helmstetter, 2004; Michael E. Hopkins & Bucci, 2010; Molteni et al., 2004; van Praag, Christie, Sejnowski, & Gage, 1999; Vaynman, Ying, & Gomez-Pinilla, 2003). However, in humans, exercise-induced improvements are generally seen in “higher order” cognitive abilities along with reduction of normal age-related decline, improvement following injury, and mitigation of some diseases, including Alzheimer’s and Parkinson’s disease (Abe, 2012; Ahlskog, 2011; Norton, Matthews, Barnes, Yaffee, & Brayne, 2014). For example, improvements in prefrontal cortex (PFC) mediated executive function (encompassing working memory, planning, decision making, and cognitive flexibility) (Funahashi, 2001) have been observed following acute and chronic physical exercise (Colcombe et al., 2006; Hillman, Kramer, Beloposky, & Smith, 2006; Kramer et al., 1999).
Conditions associated with PFC dysfunction including ADHD, schizophrenia, and drug abuse (Gamo & Arnsten, 2011) often emerge during adolescence and early adulthood, a period marked by increased impulsivity and risk-taking behavior in both humans and rodents (Brenhouse & Andersen, 2011; Gardner & Steinberg, 2005; Kessler et al., 2005). As maturation of the PFC is relatively late compared to other cortical regions, with full maturity not achieved until around 20-25 years of age in humans and at least 42-50 days old in rodents (Spear, 2000), this relative incompleteness is often assumed to underlie risky and impulsive behaviors. Regions of the rat medial PFC (mPFC) show an increase in volume between 6 and 24 days of age followed by a decrease between 24 and 30 days of age (van Eden & Uylings, 1985). Some regions, such as the prelimbic cortex, continue to show a volume decrease up until at least 90 day of age (van Eden & Uylings, 1985). Between 35 and 90 days of age in male and female rats, neurons are lost from ventral mPFC (J. A. Markham, Morris, & Juraska, 2007). In male rats, there is an increase in the number of synaptophysin boutons, a measure of synaptic number, in layer V/VI of ventral mPFC between 35 and 45 days of age (Drzewiecki, Willing, & Juraska, 2016). However, males that reached puberty by 45 days of age had significantly fewer synapses than males who had not (Drzewiecki et al., 2016). In female rats, there is an increase in layer I synapses between 25 and 35 days of age followed by a decrease between 35 and 45 days of age (Drzewiecki et al., 2016).
Because the PFC reaches final maturity so late in development, this may permit environmental factors to strongly modulate its function. How the developing rodent PFC is affected by exercise, a type of environmental enrichment, has until this point largely gone unstudied. In adult rats, Brockett et al. showed that home cage running wheel access improved several prefrontal-dependent tasks, including set-shifting, accompanied by increased dendritic spine and synapse density in the PFC (Brockett, LaMarca, & Gould, 2015). Here we have examined the effects of exercise, in adolescent (wheel running at 30-44 days of age) and adult (wheel running at 56-70 days of age) rats, on an especially well-understood model of voluntary behavior, ABA renewal of extinguished instrumental behavior (Bouton, Todd, Vurbic, & Winterbauer, 2011; Crombag & Shaham, 2002; Nakajima, Tanaka, Urishihara, & Imada, 2000; Todd, 2013; Willcocks & McNally, 2014). ABA renewal of extinguished instrumental behavior has mPFC underpinnings (Bossert et al., 2011; Bossert et al., 2012; Eddy, Todd, Bouton, & Green, 2016; Fuchs, Eaddy, Su, & Bell, 2007; Fuchs et al., 2005; Peters, LaLumiere, & Kalivas, 2008; S. Trask, Shipman, Green, & Bouton, 2017; Willcocks & McNally, 2013). Wheel running has been shown to reduce renewal (“ABC” renewal) of extinguished Pavlovian fear conditioning in adult male rats (Bouchet et al., 2017; Mika et al., 2015). In the current study, we also examined the effects of wheel running on mPFC pyramidal neuron morphology (apical dendritic spine density, branch number, and branch length).
Experiment 1 was designed to examine how exercise during adolescence affects extinction and ABA renewal in an appetitive instrumental conditioning paradigm (Bouton et al., 2011). Experiment 1 used the same procedure as in one of our previous experiments showing the involvement of mPFC in ABA renewal of extinguished instrumental behavior (Eddy et al., 2016).
Plasticity (e.g., alterations in dendritic spines) of the mPFC varies across development (R. M. Anderson, Birnie, Koblesky, Romig-Martin, & Radley, 2014; Bloss et al., 2011; Dickstein, Weaver, Luebke, & Hof, 2013), though whether the effects of exercise vary at different developmental time points remains unknown. While there are data showing increases in dendritic spines and synapses in the PFC of adult rats after running (Brockett et al., 2015), the effects of exercise on PFC plasticity during adolescence has not been investigated. Experiment 2 examined apical dendrite spine density, number of branches, and dendrite branch length of mPFC pyramidal neurons of adult and adolescent rats following two weeks of exercise.
Methods
Experiment 1
Subjects.
Male Wistar rats obtained from Charles River Canada were used. Adolescent rats were 21 days old upon delivery, and adults 47 days old. All rats were initially housed in groups of 2-4. Animals were housed in a colony room that was temperature and humidity controlled, and kept on a 12/12 hr light/dark schedule. All rats were given at least 7 days of acclimation in the colony following their arrival, during which time they had ad libitum access to food and water. At the start of the experiment (young rats: PD 30; adult rats: PD 56) rats were individually housed in cages equipped with a locked or unlocked running wheel (described below). Prior to magazine training, rats were food-restricted to approximately 85-90% of their free feeding weight. Target weights for young rats were determined by taking 85-90% of the projected normal weight at the appropriate point in the growth curve for non-restricted male Wistar rats. All subject procedures were approved by the University of Vermont Institutional Animal Care and Use Committee (protocol 11-036).
Voluntary Exercise.
Rats assigned to the exercise group were given unlocked running wheels following colony acclimation. Animals in the no exercise group were given identical wheels that were locked in place to control for environmental enrichment effects. Rats had ad libitum access to running wheels for 14 days total, beginning two days prior to magazine training. Rats had wheel access throughout the experiment, including testing. The running wheels (Med Associates Inc., St Albans, VT) were 36 cm in diameter and had an automatic counter attached that recorded every quarter revolution. Wheel counts were taken at approximately the same time each day.
Apparatus.
Two sets of four operant chambers were used in these experiments, which served as context A and context B (counterbalanced across groups). The chambers were slightly modified versions of Med Associates (St. Albans, VT) model ENV-008-VP chambers. They measured 30.5 x 24.1 x 21.0 cm (l x w x h) and were individually housed in sound attenuation chambers.
In one set of boxes the sidewalls and ceiling were made of clear acrylic plastic, while the front and rear walls were made of brushed aluminum. The floor was made of stainless steel grids (0.48 cm diameter) staggered such that odd- and even-numbered grids were mounted in two separate planes, one 0.5 cm above the other. A recessed 5.1 x 5.1 cm food cup was centered in the front wall approximately 2.5 cm above the level of the floor. Retractable levers were positioned to the left and right of the food cup. These levers were 4.8 cm long and were positioned 6.2 cm above the grid floor. The left lever protruded 1.9 cm when extended (the right lever remained retracted over the course of the experiment). A pair of 28-V panel lights (2.5 cm in diameter) was attached to the wall 10.8 cm above the floor and 6.4 cm to both the left and right of the food cup. These chambers were illuminated by two 7.5-W incandescent bulbs mounted to the ceiling of the sound attenuation chamber, approximately 34.9 cm from the grid floor at the front wall of the chamber. Ventilation fans provided background noise of 65 dB. This set of boxes had no distinctive visual cues. A glass dish containing lemon scented cleaner was placed outside of each chamber near the front wall.
The second set of boxes was similar to the lemon-scented boxes (also model ENV-008-VP, with similar placements of levers and panel lights), except for the following features. In the second set of boxes, one sidewall had black diagonal stripes (rather than clear acrylic plastic), 3.8 cm wide and 3.8 cm apart. The ceiling had similarly spaced stripes oriented in the same direction (rather than clear acrylic plastic). The grids of the floor were mounted on the same plane (rather than separate planes) and were spaced 1.6 cm apart (center to center). These chambers were illuminated by one 7.5-W incandescent bulb (rather than two) mounted to the ceiling of the sound attenuation chamber, near the back wall of the chamber. A distinct odor was continuously presented by placing pine scented cleaner in a glass dish outside the chamber (rather than lemon scented cleaner). Both sets of boxes delivered a 45-mg sucrose pellet (TestDiet, Richmond, IN, USA).
Procedure.
On day 1 of training (day 3 of wheel access), rats learned to retrieve sucrose pellets from the food cup in both contexts. Pellets were delivered on average every 30 seconds. Rats underwent two separate 30-minute sessions, one in context A and one in context B (contexts were counterbalanced such that half of the rats in each group were assigned the lemon-scented boxes as context A and the other half were assigned the pine-scented boxes). During these sessions there was no lever present. Following magazine training in both contexts, rats underwent 6 daily sessions of lever training (acquisition) in context A. During these sessions, following a two-minute interval, the lever to the left of the magazine was inserted and a variable interval (VI) 30-s reinforcement schedule was in effect. Each session lasted 30 minutes. No additional response shaping was necessary.
Following acquisition, extinction occurred in the opposite context (context B). All parameters were identical except that a lever press no longer produced a sucrose pellet. Extinction took place over 4 daily sessions. On the day after the final extinction session, all animals were tested in a within-subject manner. Each rat was tested in two separate 10-minute sessions, one in context A and one in context B, in a counterbalanced order. As in extinction sessions, after 2 minutes the left lever was extended but lever presses did not result in a sucrose pellet. The two test sessions were separated by approximately 30 minutes. In all sessions, lever presses, magazine entries, and pellets obtained were counted. Following each daily session rats were returned to the home cage and fed approximately 10-20g of food.
Data Analysis.
Responses per minute were analyzed with IBM SPSS Statistics version 24.0. Analysis of variance (ANOVA) and independent samples t-tests were used. An alpha level of 0.05 was the null hypothesis rejection criterion.
Experiment 2
Subjects.
32 male Wistar rats obtained from Charles River Canada were used, as described above. Adults were given access to a locked or unlocked running wheel starting at PD 56 and adolescents at PD 30. Animals were not food restricted. After 14 days of wheel access, all rats were euthanized by an overdose of sodium pentobarbital and transcardially perfused with 0.9% saline. All subject procedures were approved by the University of Vermont Institutional Animal Care and Use Committee (protocol 11-036).
Histology and Dendritic Analysis.
Golgi-Cox solution consisted of 5% potassium dichromate and 5% mercuric chloride mixed 1:1. 5% potassium chromate was then diluted 4:10 with the potassium dichromate/mercuric chloride solution. The solution was stored in the dark for 5 days and then filtered. Saline perfused brains were placed whole into Golgi-Cox solution and stored for 12-15 days in the dark at room temperature. Solution was changed every other day for the first 4 days. Whole brains were then transferred to a 30% sucrose solution for cryoprotection for approximately 48 hours. Brains were then blocked, flash frozen using isopentane and dry ice, and then mounted onto a cryostat chuck using distilled water. Sections were taken at −30°C through prefrontal cortex at a thickness of 150 μm and mounted directly onto slides. The following day, tissue was processed through a series of distilled water washes, followed by ammonium hydroxide, Kodak D19 developer and Ilford rapid fix (diluted 1:4 with distilled water). After several distilled water washes, the tissue was then dehydrated through an increasing series of ethanol (50%, 70%, 90%, 100%), cleared in Histoclear, and coverslipped.
Tracings were done at 600x magnification using Neurolucida (MBF Technologies, Williston, VT). Selection criteria were based on methods used in previous work examining Golgi-Cox stained PFC neurons (Wellman, 2001). Neurons were from mPFC, from both prelimbic and infralimbic areas. Layer II/III pyramidal neurons were identified as having a single apical dendritic tree, two or more basilar dendritic trees, and with no broken/incomplete branches, or obstruction by surrounding neurons. Of the neurons that met criteria, 9-10 per rat were randomly chosen (half left hemisphere, half right). Data on average apical dendritic branch (i.e., branch off the apical dendrite) length, branch number, and spine density were collected from apical dendrites. Final analyses included 8 rats per group, with each rat’s average coming from 9-10 traced neurons. Mean within-rat SEM was 40.3 (±2.63) for branch length, 1.7 (±0.08) for branch number, and 0.7 (±0.03) for spine density.
Data Analysis.
Data were analyzed with IBM SPSS version 24.0. ANOVA and independent-samples t-tests were used. For post-hoc tests of significant interaction effects, a Bonferroni adjustment for multiple comparisons was used. An alpha level of 0.05 was the null hypothesis rejection criterion.
Results
Experiment 1
Adolescent non-exercising rats showed greater ABA renewal of extinguished instrumental responding than adult non-exercising rats and two weeks of exercise in adolescent, but not adult, rats reduced ABA renewal (Figure 1). Adult rats emitted more lever-presses during acquisition sessions 5 and 6 than adolescent rats but exercise status did not interact with age (i.e., adolescent exercisers and non-exercisers did not differ from each other, nor did adult exercisers and non-exercisers).
Figure 1.
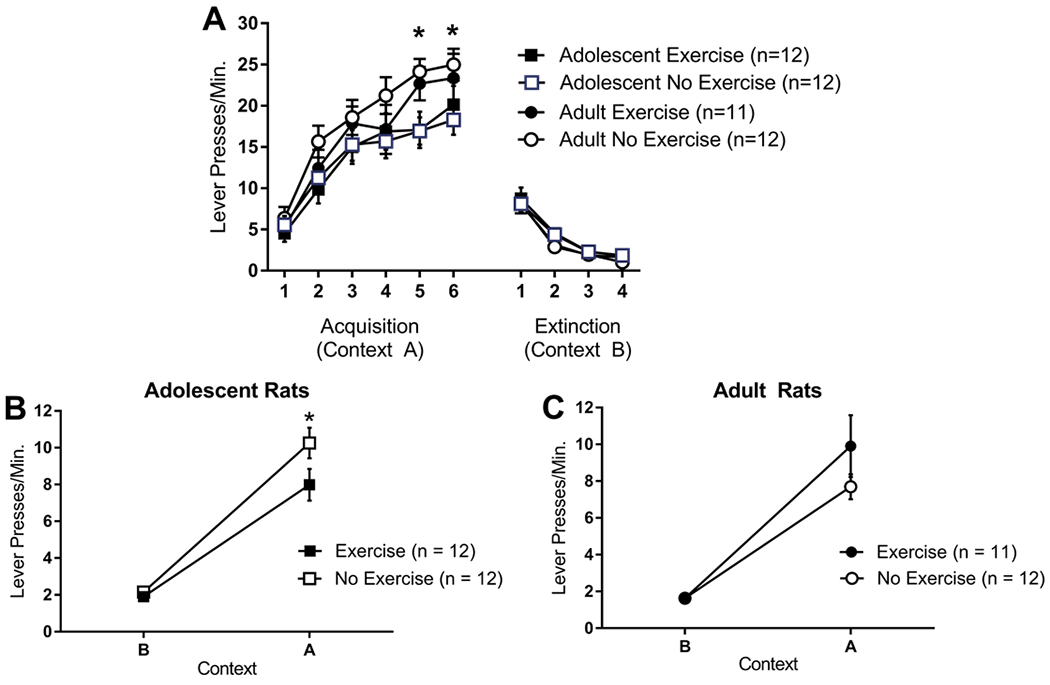
Lever press rates for adolescent and adult rats, with access to an unlocked (exercise) or locked (no exercise) running wheel in the home cage, in (A) acquisition in context A, (B) extinction in context B, and (C) testing in contexts A and B. Adult rats emitted more lever presses per minute than adolescent rats in acquisition sessions 5 and 6, regardless of exercise status. There were no differences in extinction. During the test phase, all groups showed ABA renewal of responding (increased responding in context A compared to context B). Adolescent exercising rats showed less ABA renewal than adolescent non-exercising rats. There was no difference in ABA renewal between exercising adults and non-exercising adults. Adolescent non-exercising rats showed greater ABA renewal than adult non-exercising rats, while exercising adolescent and adult rats did not differ. These results were the unchanged when performance in acquisition sessions 5 and 6 was used as a covariate. * = p < 0.05.
For acquisition, a 2 (adolescent, adult) x 2 (exercise, no exercise) x 6 (acquisition session) repeated measures ANOVA revealed a significant main effect of session, F(5, 215) = 126.26, p < 0.01. There was also a significant main effect of age, F(1, 43) = 4.38, p = 0.04, but no main effect of exercise, F < 1. There was a significant session x age interaction effect, F(1,43) = 4.70, p < 0.04 but none of the other interaction effects was significant, F’s < 1. Independent sample t-tests comparing adolescents and adults in each session revealed that adults exhibited higher lever-pressing per minute in sessions 5 and 6 (p’s < 0.03) of acquisition with a trend towards higher lever-pressing per minute in session 2 (p = 0.06) (Figure 1A).
For extinction, a 2 (adolescent, adult) x 2 (exercise, no exercise) x 4 (extinction session) repeated measures ANOVA revealed a significant main effect of session, F(3, 129) = 146.93, p < 0.01. No other main effects or interactions were significant, F’s < 2.1 (Figure 1A).
Adolescent exercisers (Figure 1B), but not adult exercisers (Figure 1C), showed attenuation of ABA renewal of extinguished instrumental responding. A 2 (adolescent, adult) x 2 (exercise, no exercise) x 2 (test context: acquisition context A, extinction context B) repeated measures ANOVA revealed a significant main effect of test context, F(1,43) = 321.73, p < 0.01, a significant age x exercise interaction effect, F(1,43) = 8.35, p < 0.01, and, importantly, a significant context x age x exercise interaction effect, F(1,43) = 4.55, p < 0.04.
Exercise attenuated ABA renewal in adolescents (Figure 1B). A 2 (exercise, no exercise) x 2 (test context: acquisition context A, extinction context B) repeated measures ANOVA examining adolescents revealed a significant main effect of test context, F(1,22) = 189.08, p < 0.01, a significant main effect of exercise, F(1,22) = 9.41, p < 0.01, and a significant test context x exercise interaction effect, F(1,22) = 7.60, p < 0.02. However, exercise did not affect ABA renewal in adults (Figure 1C). A 2 (exercise, no exercise) x 2 (test context: acquisition context A, extinction context B) repeated measures ANOVA examining adults revealed a significant main effect of test context, F(1,21) = 137.38, p < 0.01, indicating ABA renewal, but no effect of exercise and no test context x exercise interaction, F’s < 1. The same pattern of results was obtained when the test session analyses were conducted with performance in acquisition sessions 5 and 6 entered as covariates; exercise significantly attenuated ABA renewal in adolescent exercisers but not adult exercisers. This suggests that the somewhat greater conditioning in adults than adolescents was unrelated to the differential exercise effects on ABA renewal.
Additionally, when lever pressing for adolescents in context A during test was divided into 30 second bins, it was clear that exercising adolescent rats began suppressing lever pressing earlier in the test session than non-exercising adolescents (Figure 2). This was confirmed by a 2 (exercise, no exercise) x 20 (30-sec test session bin) repeated measures ANOVA, which revealed a significant main effect of exercise, F(1,14) = 6.40, p < 0.03, a significant main effect of test session bin, F(19, 266) = 5.97, p < 0.01 and significant bin x exercise interaction, F(19, 266) = 1.74, p = 0.03. Post hoc tests confirmed that exercising and non-exercising adolescent rats differed on the first seven 30-second bins (i.e., the first 3.5 minutes of the session).
Figure 2.

Lever press rates for adolescent rats in Context A as a function of 30-second bin during the test phase. Exercising adolescent rats emitted fewer lever-presses than non-exercising juvniles in the first 7 bins (3.5 minutes) of the Context A test phase. * = p < 0.05.
When comparing by age, we observed that ABA renewal was greater in adolescent non-exercisers compared to adult non-exercisers but did not differ between adolescent exercisers and adult exercisers. This suggests that the effect of exercise in adolescent rats was to bring ABA renewal down to an adult-like level. Because of the difference in performance between adolescent rats and adult rats at the end of acquisition (sessions 5 and 6), we conducted these analyses using lever-pressing in acquisition sessions 5 and 6 as covariates. A 2 (adolescent non-exercisers, adult non-exercisers) x 2 (test context: acquisition context A, extinction context B) repeated measures ANCOVA revealed a significant main effect of test context, F(1,20) = 5.24, p < 0.04, a significant main effect of age, F(1,20) = 24.77, p < 0.01, and a significant test context x age interaction effect, F(1,20) = 5.46, p = 0.03. The significant interaction effect was caused by more lever-presses per minute in context A (the renewal context) in adolescent non-exercisers than adult non-exercisers, F(1,20) = 8.48, p < 0.01 but no difference in context B (the extinction context), F(1,20) = 0.79, p > 0.38. A 2 (adolescent exercisers, adult exercisers) x 2 (test context: acquisition context A, extinction context B) repeated measures ANCOVA revealed a significant main effect of test context, F(1,19) = 9.43, p < 0.01 but no main effect of age, F(1,19) = 0.85, p > 0.36 and no test context x age interaction effect, F(1,19) = 2.49, p > 0.13.
Finally, a 2 (adolescent, adult) x 14 (day of wheel access) repeated measures ANOVA showed no difference between adolescents and adults in running distance (p > 0.20) (Figure 3).
Figure 3.

Average daily distance run by adolescent and adult rats in Experiment 1. There were no differences between adolescents and adults.
Experiment 2
In a separate, naïve group of adolescent and adult rats, density of apical dendritic spines, number of apical dendritic branches, and length of apical branches were analyzed in the mPFC following two weeks of home cage running wheel exposure (unlocked or locked). Exercise had an effect on adolescent rats only; there were no differences between adult exercisers and adult non-exercisers in these measures. Adolescent exercisers had a greater density of apical dendritic spines than adolescent non-exercisers, and apical dendrites were shorter in adolescent exercisers than non-exercisers (Figure 4).
Figure 4.

Medial prefrontal cortex pyramidal neuron apical dendrite morphology as a function of exercise and age. (A) apical dendritic spine density (number of spines per 10 μm), (B) number of apical dendritic branches, and (C) length of apical dendritic branches. Exercising adolescent rats had significantly more dendritic spines, and significantly shorter dendritic branches than adolescents that did not exercise. Non-exercising adolescents had significantly longer dendritic branches than non-exercising adults. (D) An example Golgi-Cox stained pyramidal neuron from medial prefrontal cortex. * = p < 0.05. Scale bar = 100 μm.
For density of apical dendritic spines, a 2 (adolescent, adult) x 2 (exercise, no exercise) ANOVA revealed a main effect of exercise, F(1,28) = 4.19, p = 0.05 and a significant age x exercise interaction effect, F(1,28) = 7.64, p = 0.01 (Figure 4A). There was no effect of age, F < 1. Further analysis of the significant interaction effect revealed that adolescent exercisers had greater apical dendritic spine density than adolescent non-exercisers, F(1,14) = 9.57, p = 0.01 while adult exercisers and adult non-exercisers did not differ, F < 1. Comparisons based on age revealed a trend towards lower apical spine density in adolescent non-exercisers than adult non-exercisers (p = 0.08) and a trend towards greater apical spine density in adolescent exercisers than adult exercisers (p = 0.06).
Number of apical dendritic branches was not affected by exercise or age, F’s < 1. (Figure 4B). For apical dendritic branch length, a 2 (adolescent, adult) x 2 (exercise, no exercise) ANOVA revealed no main effect of exercise, F(1,28) = 1.77, p > 0.19, but a main effect of age F(1,28) = 10.05, p < 0.01, and an exercise x age interaction effect right at the threshold for a significant effect, F(1,28) = 4.20, p = 0.05 (Figure 4C). Further analysis of the interaction effect revealed lower dendritic branch length in adolescent exercisers than adolescent non-exercisers, F(1,14) = 9.55, p = 0.01 while adult exercisers and adult non-exercisers did not differ, F < 1. Comparisons based on age revealed greater dendritic branch length in adolescent non-exercisers than adult non-exercisers (p < 0.01) but no difference between adolescent exercisers than adult exercisers (p > 0.38).
Finally, we estimated spine number by multiplying the number of dendritic spines by dendritic branch length for each rat (Figure 4D). A 2 (adolescent, adult) x 2 (exercise, no exercise) ANOVA revealed no differences in spine number based on exercise, F(1,28) = 1.39, p > 0.24 or age, F(1,28) = 2.54, p > 0.12. This suggests that the decreased dendritic branch length observed in non-exercising adolescent rats may have contributed to the increased dendritic spine density also observed in this group.
Finally, there were no significant difference in distance run between adults and adolescents (p > 0.05) (Figure 5).
Figure 5.

Average daily distance run by adolescent and adult rats used in Experiment 2. There were no differences between adolescents and adults.
Discussion
In the current experiments, two weeks of running wheel exercise reduced ABA renewal of extinguished instrumental responding in adolescent (44 day old) but not adult (70 day old) rats. In addition, ABA renewal was greater in non-exercising adolescent rats than non-exercising adult rats, and exercise reduced ABA renewal in adolescents to adult-like levels. We did not observe any exercise- or age-related differences in instrumental extinction or extinction retention; in Pavlovian fear extinction, adolescent rats (approximately 35 days old at the time of extinction) show deficits in retention of extinction (Baker, McNally, & Richardson, 2013; Baker & Richardson, 2015; McCallum, Kim, & Richardson, 2010). Exercise after fear extinction, in adult rats, enhances retention of fear extinction (Bouchet et al., 2017).
The behavioral effects of exercise may be underpinned by exercise-induced morphological changes in the mPFC; we have shown here (in a separate group of naïve rats) that exercise during adolescence, but not adulthood, was associated with greater apical dendrite spine density and lower apical dendrite branch length of pyramidal neurons in the mPFC. Finally, it is worth noting that our study also revealed that not only adult rats but also adolescent rats show ABA renewal of extinguished instrumental behavior. While ABA renewal of extinguished Pavlovian fear conditioning has been demonstrated in pre-adolescent (22 days old at the time of extinction) (Yap & Richardson, 2007) and adolescent (approximately 35 days old at the time of extinction) (Baker et al., 2013) rats by Richardson and colleagues, to our knowledge this is the first demonstration of ABA renewal of extinguished instrumental conditioning in adolescent rats.
All forms of renewal of extinguished instrumental responding (ABA, AAB, ABC) are due at least partly to the removal of context-specific response inhibition when testing occurs outside of the extinction context (Bouton et al., 2011; Todd, 2013). ABA renewal tends to be larger than AAB or ABC renewal (Bouton et al., 2011), unless the reinforcement histories of the contexts are equalized (Todd, 2013), because excitation from conditioning only partially transfers from context A to context B (AAB renewal) or from context A to context C (ABC renewal) (Bouton et al., 2011; Thrailkill & Bouton, 2015; Todd, 2013; S. Trask et al., 2017). We did not observe any exercise-related effects on instrumental responding during acquisition, nor any exercise-related effects on the transfer of this responding from the acquisition context, context A, to the extinction context, context B. Exercise in adolescents may have promoted the transfer of inhibition, developed during extinction, from the extinction context to the acquisition context, reducing ABA renewal. Exercise may have made extinction learning easier to retrieve for adolescent rats. A distinctive cue that is a feature of extinction attenuates both Pavlovian (Brooks & Bouton, 1994) and instrumental (Nieto, Uengoer, & Bernal-Gamboa, 2017; Sydney Trask & Bouton, 2016; Willcocks & McNally, 2014) ABA renewal; a cue that is not a feature of extinction has no effect on ABA renewal (Brooks & Bouton, 1994; Sydney Trask & Bouton, 2016; Willcocks & McNally, 2014).
Although it seems less likely given the lack of effects of exercise on transfer of excitation from the acquisition context to the extinction context, it is also possible that exercise in adolescents reduced ABA renewal by promoting a reduction in retrieval of the original response. Unlike AAB or ABC renewal, ABA renewal involves a return to the acquisition context. We have recently shown that inactivation of the prelimbic cortex attenuates expression of instrumental responding in the acquisition context but not in a neutral context (S. Trask et al., 2017). Thus, the prelimbic cortex may play a role in expression of responding that is learned during acquisition. The current results do not allow us to distinguish between exercise effects on memory of responding learned during acquisition versus inhibition learned during extinction. If exercise in adolescent rats attenuates ABA renewal of extinguished instrumental responding through strengthened memory of inhibition learned during extinction, then exercise in adolescents should also attenuate AAB and ABC renewal of extinguished instrumental responding, since renewal in these paradigms occurs in a neutral context.
In a separate experiment using non-trained rats, pyramidal neuron morphology in the mPFC was analyzed in adult (70 days old) and adolescent (44 days old) male rats following exercise. Exercising adolescent rats exhibited an increased dendritic spine density and reduced dendritic branch length of layer II/III pyramidal neurons in the mPFC compared to non-exercising adolescent rats. The greater spine density in exercising adolescent rats is likely a function of the shorter branch length of apical dendrites. Dendritic spine density and branch length of mPFC pyramidal neurons from exercising adolescent rats were similar to those of adult rats, regardless of whether adults were exercisers or not, suggesting that the effect of exercise in adolescent rats was to bring apical dendrite morphology of pyramidal neurons in mPFC to an adult-like level earlier than what occurs for non-exercising adolescent rats. The ages of rats that we compared (44 days vs. 70 days old) are within a window of pyramidal neuron apical dendrite plasticity in the rat mPFC; for example, apical dendrites of pyramidal neurons in layer 3 of rat prelimbic cortex exhibit an increase in complexity (as measured with Sholl ring analysis) between 20 and 56 days of age in male rats, and between 20 and 90 days of age in female rats (Julie A. Markham, Mullins, & Koenig, 2013). In addition, there is an increase in spine density in layer 3 and layer 5 pyramidal neurons for both sexes between 20 and 30-35 days of age (Koss, Belden, Hristov, & Juraska, 2014; Julie A. Markham et al., 2013) while layer 5 pyramidal neurons show a decrease in spine density between 35 and 90 days of age in both sexes (Koss et al., 2014).
Exercising adolescent rats, and both exercising and non-exercising adult rats, exhibited shorter apical dendrite branches of pyramidal neurons in mPFC than non-exercising adolescent rats. Shorter apical dendrites may increase excitability to weak inputs by changing passive properties of the cell membrane. For example, reduction in the length of the apical dendrite of layer 5 pyramidal neurons by mechanical dendrotomy or pinching off of the main apical trunk increases cell excitability to weak inputs through an increase in input resistance and a decrease in membrane capacitance (Bekkers & Hausser, 2007). Laser dendrotomy of first order dendrites also increased layer 5 pyramidal neuron input resistance and increased firing rate (Go et al., 2016). We also observed greater dendritic spine density of pyramidal neurons from mPFC in exercising adolescent rats than non-exercising adolescent rats, which would also be consistent with an increase in excitability of these neurons. However, another effect of a reduction in the length of the apical dendrite in pyramidal neurons is to reduce burst firing to strong inputs, likely through a reduction in the contribution of voltage-gated ion channels in the dendrites (Bekkers & Hausser, 2007; Mainen & Sejnowski, 1996; van Elburg & van Ooyen, 2010). Thus, a simple picture of increased excitability of pyramidal neurons in adolescent mPFC as a result of exercise is not so easy to draw. In addition, we did not examine the basilar dendritic tree. Further complicating the picture is the fact that exercise very likely affected pyramidal neurons in other cortical regions. Nevertheless, the observation of a selective effect of exercise in adolescent rats on mPFC pyramidal neuron dendrites is interesting, particularly when combined with a similarly selective effect on ABA renewal of extinguished instrumental responding.
In the current study, exercise-related differences in ABA renewal and exercise-related differences in dendritic morphology in mPFC were studied in separate groups of rats. The rats in which we measured dendritic morphology in mPFC did not undergo either food restriction or behavioral testing. Studying dendritic morphology in mPFC in rats that have undergone only exercise or not has the advantage of allowing us to conclude that exercise-related differences in ABA renewal were not the cause of exercise-related differences in dendritic morphology in mPFC. An important next step would be to examine dendritic morphology in mPFC in rats immediately before and/or after ABA renewal testing.
An intriguing idea is that exercise may have accelerated the timing of puberty in adolescent rats. In the current study, we compared male rats that had access to a home cage running wheel between 30 and 44 days of age to male rats that had access to a home cage running wheel between 56 and 70 days of age. Juraska and colleagues have shown that 45 day old male rats that had reached puberty had fewer synaptophysin boutons (a measure of synapse number) in mPFC than 45 day old male rats that had not yet reached puberty (Drzewiecki et al., 2016). In females, a similar effect was seen when comparing pre-pubertal 35 day old female rats to post-pubertal 45 day old female rats (Drzewiecki et al., 2016). In addition to synaptic pruning in the developing mPFC, neuronal pruning in the developing mPFC also appears to be at least partially regulated by puberty, at least in females; pre-pubertal (20-22 days of age) gonadectomy was associated with a greater number of mPFC neurons measured in female (but not male) adult rats. Furthermore, both male and female pre-pubertal rats show a deficit in water maze reversal learning (Willing, Drzewiecki, Cuenod, Cortes, & Juraska, 2016). A future study could specifically examine the timing of visible anatomical markers of puberty (Drzewiecki et al., 2016) in exercising and non-exercising rats, including possible sex differences.
One key aspect that should be studied is the longevity of the effects of exercise on instrumental learning. We tested rats while they had access to a running wheel in their home cage, and did not explore whether or not these effects would persist if exercise was stopped. Exercise effects may be more persistent in adolescents than in adults. For example, it has been shown that adolescent rats that exercised for four weeks could better discriminate in a novel object recognition task and had increased brain-derived neurotrophic factor (BDNF) in the perirhinal cortex. Both of these effects remained when rats were tested four weeks later. However, in adult rats, the behavioral improvement was only observed immediately after exercise had taken place; no benefit was seen two weeks later (M. E. Hopkins, Nitecki, & Bucci, 2011).
Finally, understanding the factors that modulate renewal of extinguished voluntary behavior has important translational value. An inability to inhibit unwanted behaviors underlies many neuropsychiatric diseases, including ADHD, PTSD, problem gambling, overeating, and drug addiction (V. A. Anderson, Anderson, Northam, Jacobs, & Catroppa, 2001; Gardner & Steinberg, 2005; Hauser et al., 2014). These disorders are also associated with sub-optimal PFC function, and tend to manifest around the time of puberty (V. A. Anderson et al., 2001; Gardner & Steinberg, 2005; Hauser et al., 2014). Considering this, research examining the PFC and its functions, particularly when it may be most vulnerable to environmental influence, is critical for not only the basic understanding of how these mechanisms work, but also for clinical application.
Acknowledgements
The authors wish to thank Travis P. Todd for help with data collection and Sydney Trask and Mark E. Bouton for comments on an earlier version of this manuscript. Experiment 1 was presented at the 2015 meeting of the Society for Neuroscience (program/poster 32.26/A87). Funding for this work was provided by NIMH R01 MH082893 to JTG.
References Cited
- Abe K (2012). Total daily physical activity and the risk of AD and cognitive decline in older adults. Neurology, 79, 1071. [DOI] [PubMed] [Google Scholar]
- Ahlskog JE (2011). Does vigorous exercise have a neuroprotective effect in Parkinson disease? Neurology, 77, 288–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson BJ, Rapp DN, Baek DH, McCloskey DP, Coburn-Litvak PS, & Robinson JK (2000). Exercise influences spatial learning in the radial arm maze. Physiology & Behavior, 70, 425–429. [DOI] [PubMed] [Google Scholar]
- Anderson RM, Birnie AK, Koblesky NK, Romig-Martin SA, & Radley JJ (2014). Adrenocortical status predicts the degree of age-related deficits in prefrontal structural plasticity and working memory. Journal of Neuroscience, 34, 8387–8397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson VA, Anderson P, Northam E, Jacobs R, & Catroppa C (2001). Development of executive functions through late childhood and adolescence in an Australian sample. Developmental Neuropsychology, 20, 385–406. [DOI] [PubMed] [Google Scholar]
- Baker KD, McNally GP, & Richardson R (2013). Memory retrieval before or after extinction reduces recovery of fear in adolescent rats. Learning & Memory, 20, 467–473. [DOI] [PubMed] [Google Scholar]
- Baker KD, & Richardson R (2015). Forming competing fear learning and extinction memories in adolescence makes fear difficult to inhibit. Learning & Memory, 22, 537–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baruch DE, Swain RA, & Helmstetter FJ (2004). Effects of exercise on Pavlovian fear conditioning. Behavioral Neuroscience, 118, 1123–1127. [DOI] [PubMed] [Google Scholar]
- Bekkers JM, & Hausser M (2007). Targeted dendrotomy reveals active and passive contributions of the dendritic tree to synaptic integration and neuronal output. Proceedings of the National Academy of Science, 104, 11447–11452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloss EB, Janssen WG, Ohm DT, Yuk FJ, Wadsworth S, Saardi KM, … Morrison JH. (2011). Evidence for reduced experience-dependent dendritic spine plasticity in the aging prefrontal cortex. Journal of Neuroscience, 31, 7831–7839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bossert JM, Stern AL, Theberge FR, Cifani C, Koya E, Hope BT, & Shaham Y (2011). Ventral medial prefrontal cortex neuronal ensembles mediate context-induced relapse to heroin. Nature Neuroscience, 14, 420–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bossert JM, Stern AL, Theberge FR, Marchant NJ, Wang HL, Morales M, & Shaham Y (2012). Role of projections from ventral medial prefrontal cortex to nucleus accumbens shell in context-induced reinstatement of heroin seeking. Journal of Neuroscience, 32, 4982–4991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouchet CA, Lloyd BA, Loetz EC, Farmer CE, Ostrovskyy M, Haddad N, … Greenwood BN. (2017). Acute exercise enhances consolidation of fear extinction memroy and reduces conditioned fear relapse in a sex-dependent manner. Learning & Memory, 24, 358–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouton ME, Todd TP, Vurbic D, & Winterbauer NE (2011). Renewal after the extinction of free operant behavior. Learning & Behavior, 39, 57–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenhouse HC, & Andersen SL (2011). Developmental trajectories during adolescence in males and females: A cross-species understanding of underlying brain changes. Neuroscience and Biobehavioral Reviews, 35, 1687–1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brockett AT, LaMarca EA, & Gould E (2015). Physical exercise enhances cognitive flexibility as well as astrocytic and synaptic markers in the medial prefrontal cortex. PLoS ONE, 10, e0124859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks DC, & Bouton ME (1994). A retrieval cue for extinction attenuates response recovery (renewal) caused by a return to the conditioning context. Journal of Experimental Psychology: Animal Behavior Processes, 20, 366–379. [DOI] [PubMed] [Google Scholar]
- Colcombe SJ, Erickson KI, Scalf PE, Kim JS, Prakash R, McAuley E, … Kramer AF. (2006). Aerobic exercise training increases brain volume in aging humans. Journal of Gerontology: Medical Sciences, 61A, 1166–1170. [DOI] [PubMed] [Google Scholar]
- Crombag HS, & Shaham Y (2002). Renewal of drug seeking by contextual cues after prolonged extinction in rats. Behavioral Neuroscience, 116, 169–173. [DOI] [PubMed] [Google Scholar]
- Dickstein DL, Weaver CM, Luebke JI, & Hof PR (2013). Dendritic spine changes associated with normal aging. Neuroscience, 251, 21–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drzewiecki CM, Willing J, & Juraska JM (2016). Synaptic number changes in the medial prefrontal cortex across adolescence in male and female rats: A role for pubertal onset. Synapse, 70, 361–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eddy MC, Todd TP, Bouton ME, & Green JT (2016). Medial prefrontal cortex involvement in the expression of extinction and ABA renewal of instrumental behavior for a food reinforcer. Neurobiology of Learning and Memory, 128, 33–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs RA, Eaddy JL, Su ZI, & Bell GH (2007). Interactions of the basolateral amygdala with the dorsal hippocampus and dorsomedial prefrontal cortex regulate drug context-induced reinstatement of cocaine-seeking in rats. European Journal of Neuroscience, 26, 487–498. [DOI] [PubMed] [Google Scholar]
- Fuchs RA, Evans KA, Ledford CC, Parker MP, Case JM, Mehta RH, & See RE (2005). The role of the dorsomedial prefrontal cortex, basolateral amygdala, and dorsal hippocampus in contextual reinstatement of cocaine seeking in rats. Neuropsychopharmacology, 30, 296–309. [DOI] [PubMed] [Google Scholar]
- Funahashi S (2001). Neuronal mechanisms of executive control by the prefrontal cortex. Neuroscience Research, 39, 147–165. [DOI] [PubMed] [Google Scholar]
- Gamo NJ, & Arnsten AF (2011). Molecular modulation of prefrontal cortex: Rational development of treatments for psychiatric disorders. Behavioral Neuroscience, 125, 282–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner M, & Steinberg L (2005). Peer influence on risk taking, risk preference, and risky decision making in adolescence and adulthood: An experimental study. Developmental Psychology, 41, 625–635. [DOI] [PubMed] [Google Scholar]
- Go MA, Choy JMC, Colibaba AS, Redman S, Bachor H-A, Stricker C, & Daria VR (2016). Targeted pruning of a neuron’s dendritic tree via femtosecond laser dendrotomy. Scientific Reports, 6, 19078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser TU, Iannaccone R, Ball J, Mathys C, Brandeis D, Walitza S, & Brem S (2014). Role of the medial prefrontal cortex in impaired decision making in juvenile attention-deficit/hyperactivity disorder. JAMA Psychiatry, 71, 1165–1173. [DOI] [PubMed] [Google Scholar]
- Hillman CH, Kramer AF, Beloposky AV, & Smith DP (2006). A cross-sectional examination of age and physical activity on performance and event-related brain potentials in a task switching paradigm. International Journal of Psychophysiology, 59, 30–39. [DOI] [PubMed] [Google Scholar]
- Hopkins ME, & Bucci DJ (2010). BDNF expression in perirhinal cortex is associated with exercise-induced improvement in object recognition memory. Neurobiology of Learning and Memory, 94, 278–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins ME, Nitecki R, & Bucci DJ (2011). Physical exercise during adolescence versus adulthood: Differential effects on object recognition memory and brain-derived neurotrophic factor levels. Neuroscience, 194, 84–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, & Walters EE (2005). Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Archives of General Psychiatry, 62, 593–602. [DOI] [PubMed] [Google Scholar]
- Koss WA, Belden CE, Hristov AD, & Juraska JM (2014). Dendritic remodeling in the adolescent medial prefrontal cortex and the basolateral amygdala of male and female rats. Synapse, 68, 61–72. [DOI] [PubMed] [Google Scholar]
- Kramer AF, Hahn S, Cohen NJ, Banich MT, McAuley E, Harrison CR, . . . Colcombe A. (1999). Ageing, fitness and neurocognitive function. Nature, 400, 418–419. [DOI] [PubMed] [Google Scholar]
- Mainen ZF, & Sejnowski TJ (1996). Influence of dendritic structure on firing pattern in model neocortical neurons. Nature, 382, 363–366. [DOI] [PubMed] [Google Scholar]
- Markham JA, Morris JR, & Juraska JM (2007). Neuron number decreases in the rat ventral, but not dorsal, medial prefrontal cortex between adolescence and adulthood. Neuroscience, 144, 961–968. [DOI] [PubMed] [Google Scholar]
- Markham JA, Mullins SE, & Koenig JI (2013). Periadolescent maturation of the prefrontal cortex is sex-specific and is disrupted by prenatal stress. Journal of Comparative Neurology, 521, 1828–1843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCallum J, Kim JH, & Richardson R (2010). Impaired extinction retention in adolescent rats: Effects of D-Cycloserine. Neuropsychopharmacology, 35, 2134–2142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mika A, Bouchet CA, Bunker P, Hellwinkel JE, Spence KG, Day HEW, . . . Greenwood BN. (2015). Voluntary exercise during extinction of auditory fear conditioning reduces the relapse of fear associated with potentiated activity of striatal direct pathway neurons. Neurobiology of Learning and Memory, 125, 224–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molteni R, Wu A, Vaynman S, Ying Z, Barnard RJ, & Gomez-Pinilla F (2004). Exercise reverses the harmful effects of consumption of a high-fat diet on synaptic and behavioral plasticity associated to the action of brain-derived neurotrophic factor. Neuroscience, 123, 429–440. [DOI] [PubMed] [Google Scholar]
- Morris JN, Heady JA, Raffle PA, Roberts CG, & Parks JW (1953). Coronary heart-disease and physical activity of work. Lancet, 265, 1111–1120. [DOI] [PubMed] [Google Scholar]
- Nakajima S, Tanaka S, Urishihara K, & Imada H (2000). Renewal of extinguished lever-press responses upon return to the training context. Learning and Motivation, 31, 416–431. [Google Scholar]
- Nieto J, Uengoer M, & Bernal-Gamboa R (2017). A reminder of extinction reduces relapse in an animal model of voluntary behavior. Learning & Memory, 24, 76–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norton S, Matthews FE, Barnes DE, Yaffee K, & Brayne C (2014). Potential for primary prevention of Alzheimer’s disease: An analysis of population-based data. Lancet Neurology, 13, 788–794. [DOI] [PubMed] [Google Scholar]
- Peters J, LaLumiere RT, & Kalivas PW (2008). Infralimbic prefrontal cortex is responsible for inhibiting cocaine seeking in extinguished rats. Journal of Neuroscience, 28, 6046–6053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spear LP (2000). The adolescent brain and age-related behavioral manifestations. Neuroscience and Biobehavioral Reviews, 24, 417–463. [DOI] [PubMed] [Google Scholar]
- Thrailkill EA, & Bouton ME (2015). Contextual control of instrumental actions and habits. Journal of Experimental Psychology: Animal Learning and Cognition, 41, 69–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todd TP (2013). Mechanisms of renewal after the extinction of instrumental behavior. Journal of Experimental Psychology: Animal Behavior Processes, 39, 193–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trask S, & Bouton ME (2016). Discriminative properties of the reinforcer can be used to attenuate the renewal of extinguished operant behavior. Learning & Behavior, 44, 151–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trask S, Shipman ML, Green JT, & Bouton ME (2017). Inactivation of the prelimbic cortex attenuates context-dependent operant responding. Journal of Neuroscience, 37, 2317–2324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Eden CG, & Uylings HB (1985). Postnatal volumetric development of the prefrontal cortex in the rat. Journal of Comparative Neurology, 241, 268–274. [DOI] [PubMed] [Google Scholar]
- van Elburg RAJ, & van Ooyen A (2010). Impact of dendritic size and dendritic topology on burst firing in pyramidal cells. PLoS Computational Biology, 6, e100781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Praag H, Christie BR, Sejnowski TJ, & Gage FH (1999). Running enhances neurogenesis, learning, and long-term potentiation in mice. Proceedings of the National Academy of Sciences, 96, 13427–13431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaynman S, Ying Z, & Gomez-Pinilla F (2003). Interplay between brain-derived neurotrophic factor and signal transduction modulators in the regulation of the effects of exercise on synaptic-plasticity. Neuroscience, 122, 647–657. [DOI] [PubMed] [Google Scholar]
- Wellman CL (2001). Dendritic reorganization in pyramidal neurons in medial prefrontal cortex after chronic corticosterone administration. Journal of Neurobiology, 49, 245–253. [DOI] [PubMed] [Google Scholar]
- Willcocks AL, & McNally G (2013). The role of the medial prefrontal cortex in extinction and reinstatement of alcohol-seeking in rats. European Journal of Neuroscience, 37, 259–268. [DOI] [PubMed] [Google Scholar]
- Willcocks AL, & McNally GP (2014). An extinction retrieval cue attenuates renewal but not reacquisition of alcohol seeking. Behavioral Neuroscience, 128, 83–91. [DOI] [PubMed] [Google Scholar]
- Willing J, Drzewiecki CM, Cuenod BA, Cortes LR, & Juraska JM (2016). A role for puberty in water maze performance in male and female rats. Behavioral Neuroscience, 130, 422–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yap CSL, & Richardson R (2007). Extinction in the developing rat: An examination of renewal effects. Developmental Psychobiology, 49, 565–575. [DOI] [PubMed] [Google Scholar]


