Key Points
Question
Is exposure to general anesthesia during childhood associated with deficits in specific neurodevelopmental domains?
Findings
In this systematic review and meta-analysis of 31 studies, childhood exposure to general anesthesia was associated with statistically significantly more behavioral problems and neurodevelopmental disorder diagnoses, as well as deficits in executive function, nonverbal reasoning, motor function, and, to a lesser extent, language, general development, and academics. Cognition score differences, while statistically significant, had the weakest association.
Meaning
These findings suggest that the associations between anesthetic exposure during childhood and subsequent neurodevelopmental deficits differ based on neurodevelopmental domain.
This systematic review and meta-analysis assesses associations between exposure to general anesthesia and domain-specific neurodevelopmental outcomes in children.
Abstract
Importance
Clinical studies of neurodevelopmental outcomes after anesthetic exposure have evaluated a range of outcomes with mixed results.
Objective
To examine via meta-analyses the associations between exposure to general anesthesia and domain-specific neurodevelopmental outcomes in children.
Data Sources
PubMed/MEDLINE, Embase, CINAHL, Web of Science and the Cochrane Library were searched from inception to August 31, 2021.
Study Selection
Inclusion criteria were exposures to procedures requiring general anesthesia at younger than 18 years and evaluation of long-term neurodevelopmental function after exposure. Studies lacking unexposed controls or focused on children with major underlying comorbidities were excluded.
Data Extraction and Synthesis
Extracted variables included effect size; hazard, risk, or odds ratio; number of exposures; procedure type; major comorbidities; age of exposure and assessment; presence of unexposed controls; and study design. Studies were independently reviewed by 2 coders, and review was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines. Data were pooled using a random-effects model.
Main Outcomes and Measures
The main outcomes were standardized mean differences (SMD) for scores in the neurodevelopmental domains of academics, behavioral problems, cognition, executive function, general development, language, motor function, nonverbal reasoning, social cognition, and hazard and risk of neurodevelopmental disorder diagnoses.
Results
A total of 31 studies contributed data for meta-analysis. For each of the assessed neurodevelopmental domains, the numbers of children evaluated ranged from 571 to 63 315 exposed and 802 to 311 610 unexposed. Children with any exposure (single or multiple) had significantly worse behavioral problems scores, indicating more behavioral problems (SMD, −0.10; 95% CI, −0.18 to −0.02; P = .02), and worse scores in academics (SMD, −0.07; 95% CI −0.12 to −0.01; P = .02), cognition (SMD, −0.03; 95% CI, −0.05 to 0.00; P = .03), executive function (SMD, −0.20; 95% CI, −0.32 to −0.09; P < .001), general development (SMD, −0.08; 95% CI, −0.13 to −0.02; P = .01), language (SMD, −0.08; 95% CI, −0.14 to −0.02; P = .01), motor function (SMD, −0.11; 95% CI, −0.21 to −0.02; P = .02), and nonverbal reasoning (SMD, −0.15; 95% CI, −0.27 to −0.02; P = .02). Higher incidences of neurodevelopmental disorder diagnoses were also reported (hazard ratio, 1.19; 95% CI, 1.09 to 1.30; P < .001; risk ratio, 1.81; 95% CI, 1.25 to 2.61; P = .002).
Conclusions and Relevance
These findings support the hypothesis that associations between anesthetic exposure during childhood and subsequent neurodevelopmental deficits differ based on neurodevelopmental domain.
Introduction
Millions of children are exposed to anesthesia for surgical and diagnostic procedures each year.1,2 Clinical studies have evaluated neurodevelopmental outcomes after exposure to anesthesia with mixed results. Methodological variability contributes to the difficulty in interpreting these studies, including heterogeneity in patient populations (eg, underlying comorbid illnesses), doses and durations of anesthetic exposure, and even the outcomes used to evaluate children.3 As anesthetic neurotoxic effects were first identified in rodents,4 and then other animal models prior to the identification of an obvious human phenotype of injury,5 a wide range of outcomes have been evaluated in human studies, including academic performance, general intelligence, language, behavior, and mental disorder diagnoses.3,6 In some meta-analyses, early anesthetic exposure has been associated with overall neurodevelopmental impairment, but these studies pooled data from all outcome types.7,8,9 A 2021 meta-analysis10 only pooled data from the same prospectively collected neuropsychological tests, finding more behavioral problems in children with a single anesthetic exposure but no differences in general intelligence. While the restrictive criteria of that analysis by Ing et al10 only allowed the inclusion of 3 studies, those results suggest that statistically significant differences in children exposed to general anesthesia may be found in meta-analyses pooling data from multiple studies, and that the association between anesthesia exposure and neurodevelopmental deficit may differ based on which neurodevelopmental domain was evaluated.
This study systematically reviewed all studies of neurodevelopmental outcomes after exposure to surgical procedures and anesthesia to map reported outcomes into formal domains. A domain specific meta-analysis was then performed on studies of children without major underlying comorbidities to evaluate the hypothesis that anesthetic exposure is preferentially associated with deficits in specific neurodevelopmental domains.
Methods
Search Strategy
This systematic review and meta-analysis was approved by the institutional review board at Columbia University Medical Center. Systematic review with meta-analysis was performed adhering to the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) reporting guideline and Meta-analysis of Observational Studies in Epidemiology (MOOSE) reporting guideline.11 The review protocol was not registered in an online database.
All studies evaluating neurodevelopmental outcomes in children who were exposed to a procedure requiring general anesthesia at younger than 18 years were identified. Search algorithms were applied to PubMed/MEDLINE, Embase, CINAHL, Web of Science, and the Cochrane Library. Search algorithms were previously published by Clausen and colleagues,3 identifying 67 English-language studies published before June 17, 2017. In this study, the same algorithms were used, save for minor modifications in search term formatting to identify additional studies published June 17, 2017, to August 31, 2021 (eMethods in the Supplement).10 This method of using results from previously published systematic reviews has been advocated as a method for a more efficient review of new evidence.12 Reference lists of included studies were also reviewed to identify additional studies that were missed.
Identification of All Studies of Adverse Outcomes Associated With General Anesthetic
The inclusion criteria for systematic reviews were exposure to procedures requiring general anesthesia at younger than 18 years and any evaluation of neurodevelopmental function after exposure. Exclusion criteria were nonresearch review articles, studies only involving animals, and studies measuring short term outcomes (eg, postoperative delirium, postoperative pain, and outcomes measured within 30 days of exposure). Studies that met criteria were reviewed using Covidence systematic review software (Veritas Health Innovation) with characteristics and outcomes from each study independently reviewed and extracted by 2 of the reviewers (C.R., S.J., W.M.J., A.A., and C.I.), with conflicts resolved through consensus and consultation with a third reviewer.
Grouping Neurodevelopmental Outcomes Into Domains in All Studies
All neurodevelopmental outcomes were categorized by 2 research psychologists, with A.W. initially reviewing all outcomes and placing each into a specific neurodevelopmental domain and subdomain based on standard classifications.13 H.W. subsequently reviewed these domain and subdomain categories and the mapping of each outcome, with any conflicts resolved by discussion and consensus.
Inclusion and Exclusion Criteria for Meta-analysis
Studies eligible for domain specific meta-analysis were required to include children exposed to general anesthesia, as well as an unexposed comparison group, and report outcomes from at least 1 of the psychologist-identified domains. Studies that focused primarily on children with major preexisting comorbidities or congenital anomalies were excluded. This process was adopted owing to the difficulty of determining the contribution of the anesthetic to neurodevelopment in children with significant baseline comorbidity and major perioperative physiological insult, such as children who required cardiopulmonary bypass for heart surgery.
Where duplicate reports evaluating the same population were found, only the study with the largest sample size was chosen. For studies that reported outcomes of the same cases (eg, at different ages), selection was determined by longest follow-up interval. For studies evaluating the same cohorts but reporting different outcomes (ie, scores from different domains) outcomes from all different domains were evaluated.
Evaluation of Specific Neurodevelopmental Domains Using Meta-analysis
Given prior evidence that neurodevelopmental deficits in children exposed to general anesthesia differed by domain,10 meta-analyses were performed evaluating each domain independently. Most studies reported scores that reflected overall function in a given neurodevelopmental domain. Owing to the uncertain validity of comparing overall domain scores with subdomain scores within a specific domain, the overall scores were chosen for analysis. If 2 overall domain scores were available, the more comprehensive overall score was chosen based on consensus by the research psychologists (A.W. and H.W.). For language, evaluations of verbal IQ were pooled with evaluations of overall language. For academics, while most studies reported an overall score, some studies only reported individual subject scores (eg, reading, math). To include results from these studies, an overall academic score was calculated by combining math and reading scores into a synthetic score14 generated by mean math and reading scores and calculating variance using a correlation of 0.55.15 For some domains in which specific subdomains are commonly evaluated, subdomain analysis was also performed. These subdomains included internalizing and externalizing behavioral problems and fine and gross motor function.
For the clinical diagnoses and symptoms domain, outcomes like blindness were not conceptualized as neurodevelopmental disorders and were excluded. Some studies evaluated the presence of any neurodevelopmental disorder diagnoses, while others focused on specific categories. When overall neurodevelopmental disorder diagnoses were not available, learning disability was preferentially chosen since it was the primary outcome of many studies evaluating neurodevelopmental disorder diagnoses, followed by attention-deficit/hyperactivity disorder (ADHD). A subanalysis specifically evaluating ADHD was also performed.
Statistical Analysis
To evaluate all domains on the same scale, the standardized mean differences (SMDs) between exposed and unexposed children were calculated for scores from each study. Negative SMDs indicated worse scores in the exposed group while positive SMDs indicated worse scores in the unexposed group. When studies only reported odds ratios (ORs) or risk ratios (RRs) of crossing a score threshold for deficit, the ORs or RRs were converted to SMDs.16 ORs were natural log–transformed, and SEs were calculated. Each natural log OR and corresponding SE were then converted to effect size and its SE by dividing by π / √3. In studies reporting RRs, RRs were first converted to ORs. For the clinical diagnoses and symptoms domain, studies reporting hazard ratios (HRs) for neurodevelopmental disorder diagnosis were analyzed separately from studies reporting RRs and ORs, which were converted to and reported as RRs.17 Only 1 case-control study was identified for inclusion, and given that RR could not be calculated, the data from this study could not be pooled with the other studies and was therefore excluded. Consistency between studies was evaluated using Cochrane Q and I2 statistics. An overall meta-analysis for each domain was performed by pooling data from eligible studies using random-effects models.
Domain-specific analyses assessed children with any exposure (single or multiple) compared with unexposed children. Given the potential for increased risk of Type I error when fewer than 3 studies are included,18 meta-analyses were only performed if data were available from at least 3 studies in a specific domain. Publication bias was evaluated using a funnel plot. Analyses were performed using RevMan version 5 (Cochrane Collaboration),19 and statistical significance was determined at the 2-sided P < .05 level for all outcomes.
The potential differences associated with the number of anesthetic exposures were explored in additional sensitivity analyses, specifically evaluating children with single exposure with possible multiple exposure, single exposure only, and multiple exposure only. The single with possible multiple exposure classification allowed for single exposure as well as studies designed to identify children who had a single exposure but did not exclude those who had additional exposure prior to outcome assessment. A combined studies classification referred to studies designed to include children with single and multiple exposures but did not report independent results from each group. Combined studies and those in which exposure number was not specified were excluded from these sensitivity analyses.
Critical appraisals were conducted using the Cochrane risk of bias tool20 for randomized clinical trials (RCTs) and the Risk Of Bias in Nonrandomised Studies of Interventions (ROBINS-I)21,22 for nonrandomized studies. Two reviewers (S.J., W.M.J., and C.I.), independently assessed each study, with conflicts resolved through consensus and consultation with a third reviewer.
Results
Systematic Review and Identification of Neurodevelopmental Domains
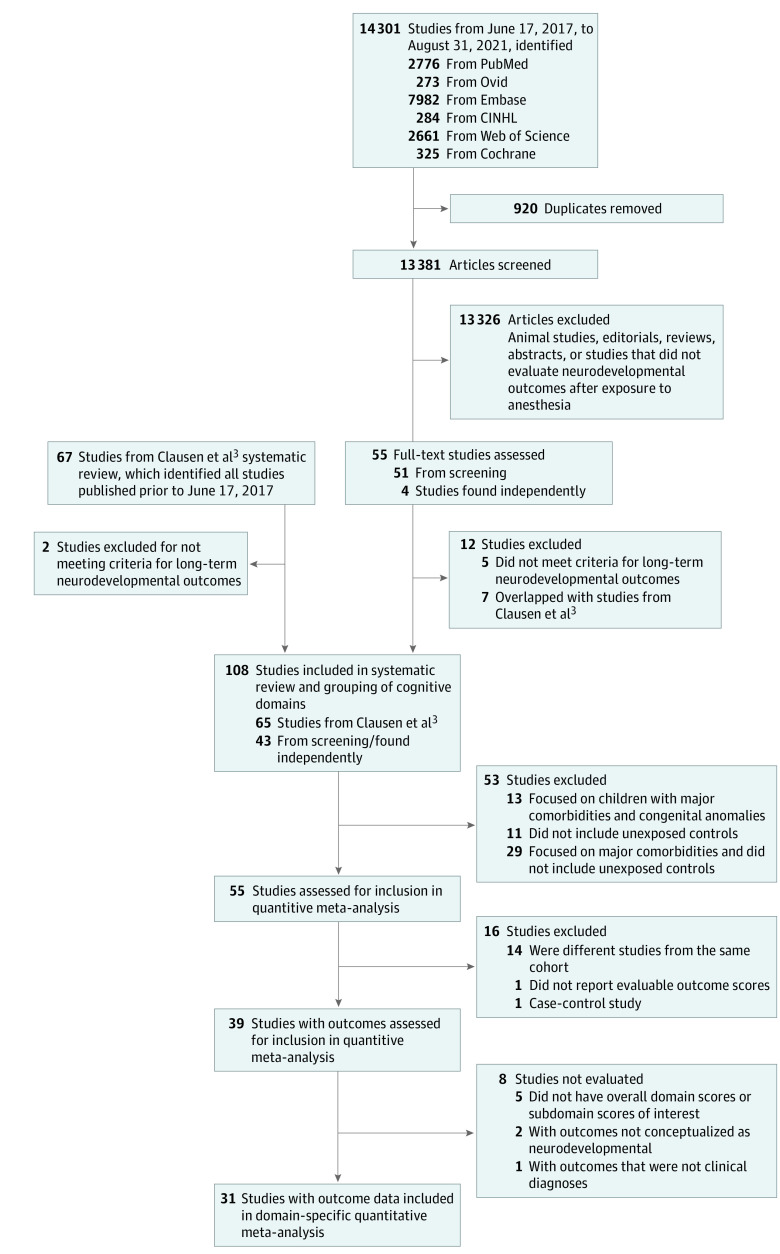
The systemic review identified 14 301 studies published between June 17, 2017, and August 31, 2021, after removal of duplicates. By combining 65 studies identified by Clausen and colleagues,3 and 43 studies (4 were identified by reviewing reference lists of included studies) in the present review, a total of 108 studies23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94,95,96,97,98,99,100,101,102,103,104,105,106,107,108,109,110,111,112,113,114,115,116,117,118,119,120,121,122,123,124,125,126,127,128,129,130 met criteria for inclusion for systematic review and grouping of cognitive domains (Figure 1). Outcomes from studies were classified into 13 domains: academics, adaptive behavior, behavioral problems, cognition, clinical diagnoses and symptoms (only neurodevelopmental disorder diagnoses were evaluated in the meta-analysis), executive function, general development, general health and well-being, language, motor function, nonverbal reasoning, sensory, and social cognition (eTable 1 in the Supplement), with some outcomes also mapping to subdomains. The assigned domains and subdomains of each of the 422 different outcomes are reported sorted by outcome type (eTable 2 in the Supplement) and sorted by domain and subdomain (eTable 3 in the Supplement), as well as the outcomes reported in each study (eTable 4 in the Supplement).
Figure 1. Flowchart of Included Studies.
Study characteristics were also evaluated, including comorbidities and ages of exposure and assessment. Of 108 studies considered for meta-analysis, 53 studies were excluded for lacking an unexposed population or focusing on children with major comorbidities. The major comorbidities justifying exclusion for each study are described in eTable 5 in the Supplement and include congenital heart disease, extreme prematurity, or medulloblastoma. A further 16 studies were excluded for using cohorts that overlapped with other studies or not reporting outcome scores that could be evaluated (eTable 6 in the Supplement), and 8 studies were excluded for not reporting overall domain scores, leaving 31 studies27,31,33,35,36,40,45,48,56,60,61,63,64,72,76,79,82,87,88,90,91,95,102,103,104,105,107,108,120,122,130 contributing data for meta-analysis (Table). Owing to a limited number of eligible studies (less than 3 studies) evaluating adaptive behavior, general health and well-being, sensory, and social cognition, these domains could not be evaluated.
Table. All Outcomes Used From 31 Studies Included in the Domain-Specific Meta-analysisa .
| Study | Outcomes used | Outcomes not used | ||
|---|---|---|---|---|
| Overall | Subdomains | Outcome | Reason for not using | |
| Academics | ||||
| Bartels et al,27 2009 | National standardized test | NA | NA | NA |
| Flick et al,35 2011 | California achievement math and reading | NA | NA | NA |
| Hansen et al,36 2011 | Test score (standardized test and teacher rating) | NA | Test nonattainment | Used test scores |
| Bong et al,48 2013 | PSLE | NA | NA | NA |
| Ing et al al,56 2014 | WAMSE numeracy and reading | NA | WAMSE spelling and writing | Used numeracy and reading outcomes |
| Williams et al,61 2014 | State achievement math and reading | NA | NA | NA |
| Glatz et al,88 2017 | School grades | NA | NA | NA |
| Hu et al, 201790 | OLSAT | NA | NA | NA |
| Schneuer et al,103 2018 | NAPLAN numeracy and reading | NA | NA | NA |
| Warner et al,105 2018 | CLDQ math and reading | NA | NA | NA |
| McCann et al,108 2019 | WIAT-II numerical and reading | NA | WIAT-II spelling | Not overall outcome |
| Walkden et al,122 2020 | Key stage 4 total points | NA | Key stage 2 English, Math, and Science; Key stage 3 English Math, and Science; Number of Key Stage 4 examination entries; Key Stage 2 nonattainment; Key Stage 3 nonattainment, Key stage 4 English and Math A, A, B, or C grade; and Key stage 4 Science 2 “good” passes (≥C grade) | Used overall outcome at oldest age |
| Adaptive behavior | ||||
| Sun et al,82 2016 | NA | NA | ABAS-II composite | Inadequate number of studies for meta-analysis |
| McCann et al,108 2019 | NA | NA | ABAS-II composite | Inadequate number of studies for meta-analysis |
| Kobayashi et al,120 2020 | NA | NA | J-ASQ-3 problem solving, personal-social | Not overall outcome |
| Behavioral problems | ||||
| Bartels et al,27 2009 | NA | CTRS-R | NA | NA |
| Ing et al,40 2012 | CBCL total problems | CBCL internalizing and externalizing | NA | NA |
| Stratmann et al,60 2014 | CBCL total problems | NA | NA | NA |
| Bakri et al,64 2015 | NA | CBCL internalizing and externalizing | CBCL aggressive behavior, anxious depression, delinquent behavior, emotionally reactive, somatic complaints, or withdrawn | Subdomains, not overall scores |
| Sun et al,82 2016 | CBCL total problems | CBCL internalizing and externalizing | NA | NA |
| Warner et al,105 2018 | CBCL total problems | CBCL internalizing and externalizing | NA | NA |
| Khochfe et al,107 2019 | NA | ECBI | NA | NA |
| McCann et al,108 2019 | CBCL total problems | CBCL internalizing and externalizing | NA | NA |
| Walkden et al,122 2020 | SDQ | NA | Skuse sociocognitive dysfunction score | Not overall score |
| Cognition | ||||
| Walker et al,33 2010 | BSID-III cognition | NA | NA | NA |
| Flick et al,35 2011 | TCS total cognitive | NA | NA | NA |
| Stratmann et al,60 2014 | WASI FSIQ | NA | NA | NA |
| Backeljauw et al,63 2015 | WISC-III NL | NA | NA | NA |
| Sun et al,82 2016 | WASI FSIQ | NA | NA | NA |
| Glatz et al,88 2017 | Conscription IQ | NA | NA | NA |
| Lv et al,102 2018 | BSID-II MDI | NA | NA | NA |
| Warner et al,105 2018 | WASI FSIQ | NA | NA | NA |
| McCann et al,108 2019 | WPPSI-III FSIQ | NA | NA | NA |
| Walkden et al,122 2020 | WISC-III GIQ | NA | WASI GIQ | Less comprehensive measure of cognition |
| Zhou et al,130 2021 | WPPSI-IV CR FSIQ | NA | NA | NA |
| Clinical diagnoses and symptoms | ||||
| Wilder et al,31 2009 | LD diagnosis | NA | Reading, written, and math LD | Used overall LD |
| Flick et al,35 2011 | LD diagnosis | NA | Reading, written, and math LD, IEP speech/language, and IEP emotion/behavior | Used overall LD |
| Sprung et al,45 2012 | ADHD diagnosis | ADHD diagnosis | NA | NA |
| Bong et al,48 2013 | LD diagnosis | NA | NA | NA |
| Minutillo et al,51 2013 | NA | NA | Blindness, cerebral palsy, and hearing | Not conceptualized as neurodevelopmental disorder |
| Ing et al a,56 2014 | Mental disorder diagnosis | NA | NA | NA |
| Bakri et al,64 2015 | ADHD diagnosis | ADHD diagnosis | Affective, anxiety, pervasive developmental, and oppositional defiant problems | Used ADHD diagnoses |
| Hu et al,90 2017 | LD diagnosis | ADHD diagnosis | Reading, written, and math LD; IEP speech/language; and IEP emotion/behavior | Used overall LD |
| Ing et al a,91 2017 | Mental disorder diagnoses | ADHD diagnosis | DD diagnosis | Used mental disorder and ADHD diagnosis |
| Nestor et al,95 2017 | Psychiatric diagnoses | NA | DD diagnosis | Used overall outcome |
| Castellheim et al,99 2018 | NA | NA | A-TAC ASD, A-TAC-LD, and A-TAC ADHD | Not clinical diagnoses |
| Kozanhan et al,101 2018 | NA | NA | Cerebral palsy | Not conceptualized as neurodevelopmental disorder |
| Tsai et al,104 2018 | ADHD diagnosis | ADHD diagnosis | NA | NA |
| Warner et al,105 2018 | NA | NA | CBCL ADHD problems | Not clinical diagnoses |
| McCann et al,108 2019 | Behavioral disorder diagnoses | NA | ADHD, ASD, and DD diagnosis; blindness; cerebral palsy; hearing | Used Behavioral disorder diagnoses. ADHD diagnosis could not be evaluated because no other studies reported ADHD using odds or risk ratios |
| Zhou et al,130 2021 | NA | NA | Cerebral palsy, hearing or vision impairment, intervention for neurodevelopmental problem, DD, and language, behavioral, or psychomotor disorder | Not conceptualized as neurodevelopmental disorder, and not formal clinical diagnoses |
| Executive function | ||||
| Flick et al,35 2011 | NA | NA | TCS memory | Not overall score |
| Ing et al,40 2012 | NA | NA | SDMT oral and SDMT written | Not overall scores |
| Fan et al,49 2013 | NA | NA | WPPSI-III Animal House | Not overall score |
| Stratmann et al,60 2014 | NA | NA | Recognition memory | Not overall score |
| Taghon et al,70 2015 | NA | NA | Go task | Not overall score |
| Aun et al,71 2016 | NA | NA | G-TVPS, HKLL, and WJ | Not overall scores |
| Poor Zamany et al,80 2016 | NA | NA | BDS, FDS, PVF, and SVF | Not overall scores |
| Sun et al,82 2016 | BRIEF-GEC | NA | CVLT-C, DKEFS subtests, NEPSY-II (multiple components), WISC-IV coding and digit span, CPT2 commissions, and omissions | Not overall scores |
| Warner et al,105 2018 | BRIEF-GEC | NA | WCST, WRAML-2, CPT2 (any component), DKEFS expressive language composite, category fluency, and Tower Test total | Not overall scores |
| McCann et al,108 2019 | BRIEF-GEC | NA | CMS, NEPSY-II (multiple components), WPPSI-III processing speed | Not overall scores |
| Warner et al,112 2019 | NA | NA | OTB | Not overall score |
| Walkden et al,122 2020 | NA | NA | TEA-Ch sky search and opposite worlds, and counting span | Not overall scores |
| Zhou et al,130 2021 | NA | NA | WPPSI-IV CR working memory, and processing speed | Not overall scores |
| General development | ||||
| Graham et al,76 2016 | EDI total score | NA | NA | NA |
| O’Leary et al,79 2016 | EDI early developmental vulnerability | NA | EDI multiple challenge index and language and cognitive development | Not overall scores |
| Schneuer et al,103 2018 | Developmentally high-risk AVEDI | NA | AVEDI cognitive development | Not overall score |
| General health and well-being | ||||
| Graham et al,76 2016 | NA | NA | EDI physical health and well-being | Not overall score |
| O’Leary et al,79 2016 | NA | NA | EDI physical health and well-being | Not overall score |
| Language | ||||
| Ing et al,40 2012 | CELF Total Score | NA | PPVT | Less comprehensive measure of language than CELF Total Score |
| Stratmann et al,60 2014 | WASI VIQ | NA | NA | NA |
| Backeljauw et al,63 2015 | WISC-III VIQ | NA | OWLS | Not overall score |
| Graham et al,76 2016 | NA | NA | EDI communication | Not overall score |
| O’Leary et al,79 2016 | NA | NA | EDI communication | Not overall score |
| Sun et al,82 2016 | WASI VIQ | NA | NEPSY-II comprehension of instructions and WASI similarities | Not overall score |
| Hu et al,90 2017 | OLSAT language | NA | IEP speech/language | Not overall score |
| Schneuer et al,103 2018 | NA | NA | AVEDI communication health and language | Not overall scores |
| Warner et al,105 2018 | Boston Naming Test | NA | CTOPP and WASI Vocabulary | Not overall score |
| McCann et al,108 2019 | WPPSI-III VIQ | NA | NA | NA |
| Kobayashi et al,120 2020 | J-ASQ-3 Communication | NA | NA | NA |
| Walkden et al,122 2020 | Children’s Communication Checklist | NA | WISC-III VIQ | NA |
| Zhou et al,130 2021 | WPPSI-IV (CR) VCI | NA | NA | NA |
| Motor function | ||||
| Walker et al,33 2010 | NA | BSID-III fine motor and gross motor | NA | NA |
| Ing et al,40 2012 | MAND | NA | NA | NA |
| Davidson et al,72 2016 | BSID-III motor composite | BSID-III fine motor and gross motor | NA | NA |
| Sun et al,82 2016 | NA | GPT dominant hand (fine motor) | NA | NA |
| Lv et al,102 2018 | BSID-II PDI | NA | NA | NA |
| Warner et al,105 2018 | NA | Beery Fine motor composite | Beery Buktenica visual perception, Beery Buktenica VMI, and GPT dominant and nondominant hand | Not overall scores |
| Kobayashi et al,120 2020 | NA | J-ASQ-3 fine motor and gross motor | NA | NA |
| Walkden et al,122 2020 | NA | NA | M-ABC preferred and nonpreferred hand peg, heel-to-toe walking, and and bean bag throwing | Limited assessment of fine and gross motor function |
| Nonverbal reasoning | ||||
| Ing et al,40 2012 | CPM | NA | NA | NA |
| Stratmann et al,60 2014 | WASI-PIQ | NA | NA | NA |
| Backeljauw et al,63 2015 | WISC-III PIQ | NA | NA | NA |
| Sun et al,82 2016 | WASI-PIQ | NA | WASI block design and WASI matrix reasoning | Not overall scores |
| de Heer et al,87 2017 | SON-R | NA | NA | NA |
| McCann et al,108 2019 | WPPSI-III PIQ | NA | NEPSY-II design copy | Not overall score |
| Social cognition | ||||
| Graham et al,76 2016 | NA | NA | EDI emotional health and social knowledge | Not overall scores |
| O’Leary et al,79 2016 | NA | NA | EDI emotional health and social knowledge | Not overall scores |
| McCann et al,108 2019 | NA | NA | NEPSY-II theory of mind and affect recognition | Not overall scores |
Abbreviations: ABAS-II, Adaptive Behavior Assessment System–Second Edition; ADHD, attention-deficit/hyperactivity disorder; ASD, Autism Spectrum Disorder; A-TAC, Autism-Tics, ADHD, and Other Comorbidities Inventory; AVEDI, Early Development Instrument (Australia); BDS, Backward Digit Span Test; BRIEF-GEC, Behavior Rating Inventory of the Executive Functions Global Executive Composite; BSID-II, Bayley Scales of Infant Development–Second Edition; BSID-III, Bayley Scales of Infant Development–Third Edition; CBCL, Child Behavior Checklist; CELF, Clinical Evaluation of Language Fundamentals; CLDQ, Colorado Learning Difficulties Questionnaire; CMS, Children’s Memory Scale; CPM, Raven’s Colored Progressive Matrices; CPT2, Conner Continuous Performance Test II; CTOPP, Comprehensive Test of Phonological Processing; CTRS-R, Conners Teacher Rating Scale–Revised, Short Form; CVLT-C, California Verbal Learning Test–Children; DD, developmental delay; DKEFS, Delis-Kaplan Executive Function System; ECBI, Eyberg Child Behavior Inventory; EDI, Early Development Instrument; FDS, Forward Digit Span Test; FSIQ, Full Scale Intelligence Quotient; GIQ, Global Intelligence Quotient; GPT, Grooved Pegboard Test; G-TVPS, Gardner Test of Visual-Perceptual Skills Revised; HKLL, Hong Kong List Learning; IEP, Individualized Education Plan; J-ASQ-3, Ages and Stages Questionnaire 3 Japanese version; LD, learning disorder or disability; MAND, McCarren Assessment of Neuromuscular Development; MDI, Mental Development Index; NA, not applicable; NAPLAN, National Assessment Program-Literacy and Numeracy; NEPSY-II, Developmental Neuropsychological Assessment Battery–Second Edition; OLSAT, Stanford/Otis-Lennon School Ability Test; OTB, Operant Test Battery; OWLS, Oral and Written Language Scales; PDI, Psychomotor Development Index; PIQ, Performance Intelligence Quotient; PPVT, Peabody Picture Vocabulary Test; PSLE, Primary School Leaving Examination; PVF, Phonemic Verbal Fluency Test; SDMT, Symbol Digit Modality Test; SDQ, Strengths and Difficulties Questionnaire; SON-R, Hogrefe/Snijders-Oomen Nonverbal Intelligence Test–Revised; SVF, Semantic Verbal Fluency Test; TCS, Test of Cognitive Skills; TEA-Ch, Test of Everyday Attention for Children; VCI, Verbal Comprehension Index; VIQ, Verbal Intelligence Quotient; VMI, Visual-motor Integration; WAMSE, Western Australian Literacy and Numeracy Standardized Test; WASI, Wechsler Abbreviated Scale of Intelligence; WCST, Wisconsin Card Sort Test; WIAT-II, Wechsler Individual Achievement Test–Second Edition; WISC-III, Wechsler Intelligence Scale for Children–Third Edition; WISC-IV, Wechsler Intelligence Scale for Children–Fourth Edition; WJ, Woodcock–Johnson Visual Matching Test; WPPSI-III, Wechsler Preschool and Primary Scale of Intelligence–Third Edition; WPPSI-IV CR, Wechsler Preschool & Primary Scale of Intelligence–Fourth Edition, Chinese Version; WRAML-2, Wide Range Assessment of Memory and Learning, Second Edition.
No studies with a sensory outcome were eligible for meta-analysis.
Domain-Specific Meta-analysis
For each of the assessed neurodevelopmental domains, the numbers of children evaluated ranged from 571 to 63 315 children who were exposed and 802 to 311 610 unexposed children, depending on the domain (Figure 2). Any exposure was associated with significantly worse behavioral problems scores, indicating more behavioral problems (SMD, −0.10; 95% CI, −0.18 to −0.02; P = .02) and worse scores in academics (SMD, −0.07; 95% CI −0.12 to −0.01; P = .02), cognition (SMD, −0.03; 95% CI, −0.05 to 0.00; P = .03), executive function (SMD, −0.20; 95% CI, −0.32 to −0.09; P < .001), general development (SMD, −0.08; 95% CI, −0.13 to −0.02; P = .01), language (SMD, −0.08; 95% CI, −0.14 to −0.02; P = .01), motor function (SMD, −0.11; 95% CI, −0.21 to −0.02; P = .02), and nonverbal reasoning (SMD, −0.15; 95% CI, −0.27 to −0.02; P = .02). Any exposure was also associated with a higher incidence of neurodevelopmental disorder diagnoses (HR, 1.19; 95% CI, 1.09 to 1.30; P < .001; RR, 1.81; 95% CI, 1.25 to 2.61; P = .002) (Figure 3).
Figure 2. Domain-Specific Meta-analysis of Scores After Any Exposure to Surgery and Anesthesia.
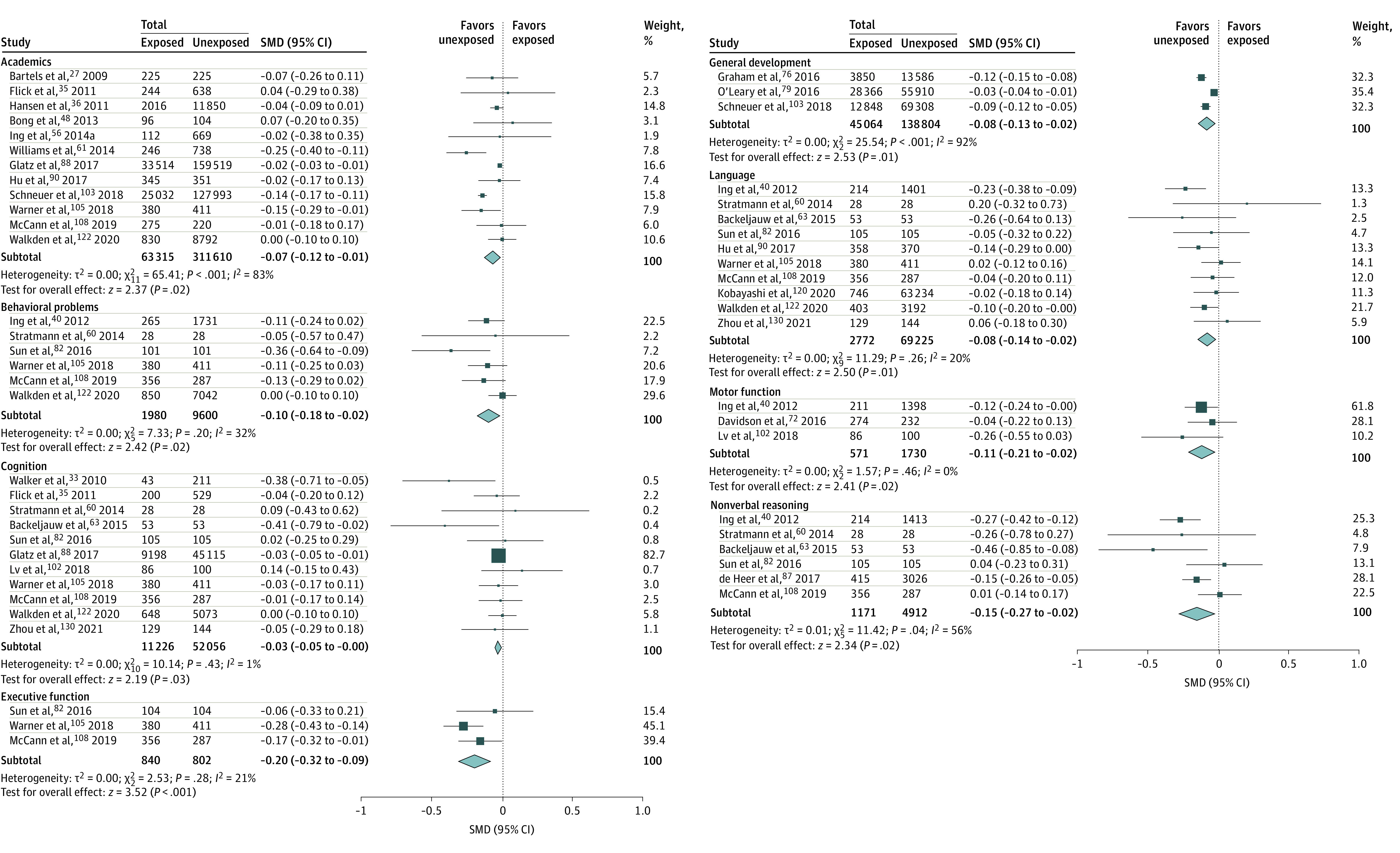
SMD indicates standardized mean difference.
Figure 3. Meta-analysis of Hazard and Risk of Neurodevelopmental Disorder Diagnoses After Any Exposure to Surgery and Anesthesia.
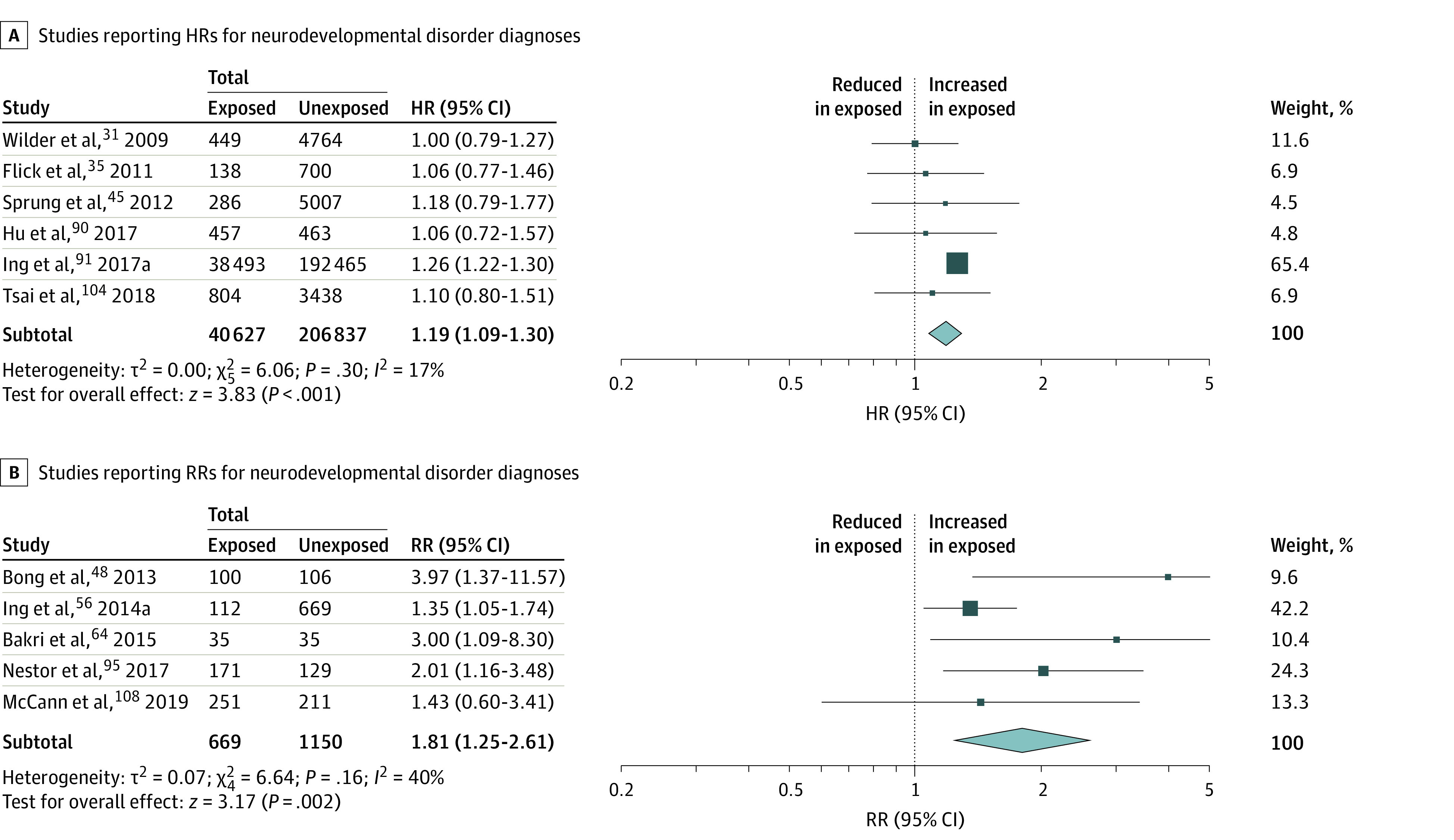
HR indicates hazard ratio; RR, relative risk.
For behavioral problem and motor function subdomains, any exposure was associated with worse internalizing (SMD, −0.14; 95% CI, −0.26 to −0.02; P = .02) and externalizing (SMD, −0.24; 95% CI, −0.43 to −0.06; P = .008) behavioral problem scores, indicating more problems, worse scores in fine (SMD, −0.09; 95% CI, −0.17 to −0.01; P = .02) and gross (SMD, −0.16; 95% CI, −0.27 to −0.04; P = .007) motor function, and a higher incidence of ADHD (HR, 1.30; 95% CI, 1.25 to 1.36; P < .001) (Figure 4). I2 statistics ranged from 0% to 92%, depending on the domain, indicating low between-study inconsistency in some outcomes, such as cognition and motor function domains and the ADHD diagnosis subdomain, but considerable variability in others, such as the academics and general development domains.
Figure 4. Domain-Specific Meta-analysis of Subdomain Scores and Hazard of ADHD After Any Exposure to Surgery and Anesthesia.
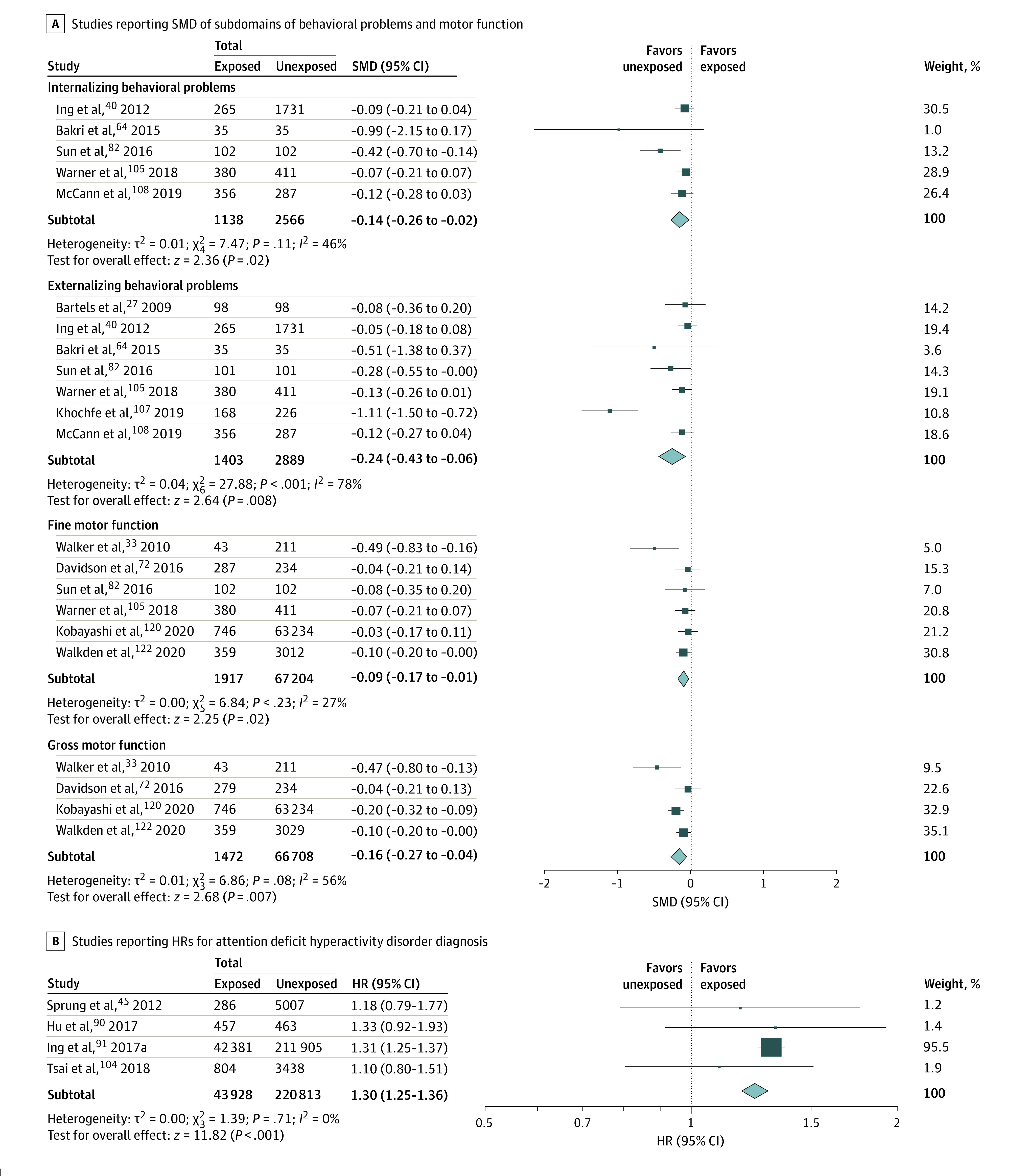
HR indicates hazard ratio; SMD, standardized mean difference.
Sensitivity analyses evaluated the association between exposure number and domain-specific outcomes. Not all studies reported results based on exposure number, and some studies only evaluated 1 exposure type (eg, single or multiple) (eTable 7 in the Supplement). Therefore in exposure number analyses, some domains did not have an adequate number of studies to be evaluated, and in general, fewer domains were evaluated in each of the sensitivity analyses than the any exposure analysis.
In children with single with possible multiple exposure, worse scores were observed in academics (SMD, −0.09; 95% CI, −0.18 to −0.01; P = .03), cognition (SMD, −0.03; 95% CI, −0.05 to −0.01; P = .01), executive function (SMD, −0.20; 95% CI, −0.32 to −0.09; P < .001), and general development (SMD, −0.07; 95% CI, −0.14 to −0.01; P = .02), but no differences in behavioral problems, language, or nonverbal reasoning (eFigure 1 in the Supplement). A higher incidence of neurodevelopmental disorder diagnoses was also observed (HR, 1.15; 95% CI, 1.03 to 1.29; P = .01; RR, 2.07; 95% CI, 1.32 to 3.24; P = .001) (eFigure 2 in the Supplement), worse externalizing behavioral problems (SMD, −0.35; 95% CI, −0.65 to −0.06; P = .02), fine (SMD, −0.09; 95% CI, −0.17 to −0.01; P = .02) and gross (SMD, −0.16; 95% CI, −0.27 to −0.04; P = .007) motor function scores, and a higher incidence of ADHD diagnoses (HR, 1.30; 95% CI, 1.25 to 1.36; P < .001) but no difference in internalizing behavioral problems (eFigure 3 in the Supplement).
In children with single exposures, worse scores were observed in cognition (SMD, −0.03; 95% CI, −0.05 to 0.00; P = .02), general development (SMD, −0.07; 95% CI, −0.14 to −0.01; P = .02), and fine motor function (SMD, −0.08; 95% CI, −0.15 to −0.01; P = .03). There were no differences in academics or language, but we observed an increased incidence of neurodevelopmental disorder diagnoses (HR, 1.15; 95% CI, 1.03 to 1.29; P = .01) and ADHD diagnosis (HR, 1.30; 95% CI, 1.25 to 1.36; P < .001) (eFigure 4 and eFigure 5 in the Supplement).
In children with multiple exposures, worse scores were reported in academics (SMD, −0.16; 95% CI, −0.27 to −0.05; P = .006), general development (SMD, −0.08; 95% CI, −0.16 to −0.01; P = .04), language (SMD, −0.27; 95% CI, −0.45 to −0.09; P = .003), and fine motor function (SMD, −0.33; 95% CI, −0.44 to −0.23; P < .001). There was also an increased incidence of neurodevelopmental disorder diagnoses (HR, 1.86; 95% CI, 1.48 to 2.32; P < .001) and ADHD diagnosis (HR, 2.09; 95% CI, 1.53 to 2.86; P < .001). However, no difference in cognition was observed (eFigure 6 and eFigure 7 in the Supplement).
Bias Assessment and Publication Bias
All studies were at risk of bias, with the highest risks due to confounding, the retrospective determination of intervention status, and presence of cointervention (eg, surgical procedure) with anesthesia exposure (eTable 8, eTable 9, and eFigure 8 in the Supplement). Examination of the funnel plot did not find bias but identified 1 outlier reporting an externalizing behavioral problems outcome from Khochfe et al107 (eFigure 9 in the Supplement). After removing this study, statistically significant differences in externalizing behavioral problems after any exposure persisted (SMD, −0.11; 95% CI, −0.18 to −0.03; P = .03), with an I2 statistic of 0%, and after single with possible multiple exposure (SMD, −0.14; 95% CI, −0.24 to −0.04; P = .004), with an I2 statistic of 0%.
Discussion
In this systematic review and meta-analysis, significant differences were observed in some, but not all, assessed neurodevelopmental domains and subdomains. However, as statistical significance is based in large part on sample size, which varied between domains, a more informative way to interpret this data may be to evaluate the SMD values in addition to the P values.131 Cohen proposed that for interpreting the magnitude of SMD values in the behavioral sciences, an SMD of 0.2 suggests small effect size; 0.5, medium effect size; and 0.8, large effect sizes.132 Based on this interpretation, all outcomes in children with any exposure were associated with no more than small effect sizes. However, domain-specific effect size differences can be appreciated with the largest differences found in behavioral problems, executive function, nonverbal reasoning, and motor function, followed by language, general development, and academics. The smallest effect sizes were in cognition, which was measured primarily using full-scale IQ. Of these, in sensitivity analyses, differences persisted in academics, cognition, and executive function in single with possible multiple exposure, although several domains could not be evaluated owing to an inadequate number of available studies. Regarding multiple exposures, compared with unexposed children, statistically significant differences in academics and language were seen in SMDs that were approximately 2-fold as large as those found in any exposure, single with possible multiple exposure, or single exposure. No differences were found in cognition after multiple exposure.
A higher incidence of neurodevelopmental disorder diagnosis was found in children with any exposure, including a 30% increased hazard of ADHD, with differences persisting even when evaluating single with possible multiple exposure and single exposure. Compared with unexposed children, in children with multiple exposures, incidences of neurodevelopmental disorder diagnoses and ADHD were more than 2-fold as high as in children with any exposure.
Most studies included in these meta-analyses were observational, so anesthetic exposure cannot be causally linked to differences in scores and neurodevelopmental disorder diagnoses using our results. However, these results help define a pattern of deficit to explore in future studies. Diffuse distributions of deficits, such as those we observed in this study, have been reported in studies of other neurotoxic exposures, with the possibility of some clustering around certain domains.133,134 In particular, in this study, the outcome with the largest SMD was the subdomain of externalizing behavioral problems, which are commonly found in children with ADHD.135 The increased rates of neurodevelopmental disorder and ADHD diagnoses is also consistent with studies of other neurotoxic exposures, specifically, children exposed to pesticides have reported behavioral problems, with 50% to 142% increased risks of ADHD and symptoms of hyperactivity.136,137
Limitations
This study has a number of important limitations. First, despite grouping outcomes into domains, there was significant variation in study characteristics, including medical disease in children and adjustment for confounding, as well as some differences in the outcomes combined within domains, which may have contributed to significant between-study heterogeneity, with large I2 statistics seen in several domains. Second, nearly all included studies were observational and therefore likely subject to unmeasured confounding, including underlying medical issues, perioperative physiological disturbances, or psychological trauma due to hospitalization. However, the purpose of this study was not to establish a causal relationship between anesthetic exposure and a given neurodevelopmental domain, but to suggest appropriate neurodevelopmental domains to evaluate in future studies. Third, neurodevelopmental disorders in the clinical diagnoses and symptoms domain are inherently related to scores in other domains (eg, learning disability diagnosis is related to academic scores). Fourth, the analyses were performed without P value adjustment for multiple comparisons. As a result, we are primarily interpreting the calculated effect sizes and CIs of the outcomes to evaluate domain-specific differences and inform the design of future studies.
Conclusions
The results from this systematic review and meta-analysis help identify patterns of deficits in specific domains, with comparatively larger effect sizes seen in the executive function, nonverbal reasoning, motor function, and behavioral problems, particularly externalizing behavioral problems, domains, coupled with an increased incidence of neurodevelopmental disorder diagnoses, particularly ADHD diagnosis. However, the cognition domain was found to have the weakest association with anesthetic exposure. The effect sizes in children with multiple exposures were also found to be 2-fold as large as those with single exposures. Many of the individual published studies may not have adequate power to identify small effect size differences. By using meta-analyses to pool data from individual studies, potential phenotypes of neurodevelopmental deficit that are associated with general anesthesia exposure have been identified. Based on these results, further studies are needed to determine the mechanisms behind these reported associations and whether these differences in specific neurodevelopmental domains can be attributed to childhood exposure to anesthetic medications.
eMethods. Literature Search Strategies
eTable 1. All Domains and Subdomains Evaluated in Studies of Potential Neurotoxic Effects of Anesthetic
eTable 2. All Outcomes Evaluated in Studies of Potential Neurotoxic Effects of Anesthetic and Their Associated Neurodevelopmental Domain and Subdomain Classifications
eTable 3. All Neurodevelopmental Domain and Subdomains and the Classification of Outcomes into These Domains and Subdomains
eTable 4. Outcomes From Each of the 108 Reviewed Studies
eTable 5. Characteristics of All 108 Reviewed Studies
eTable 6. Outcomes of Duplicate Studies or Studies That Did Not Report Outcome Scores That Could Be Evaluated
eTable 7. Exposure Data Used From Each of the 31 Included Studies
eTable 8. Cochrane Risk of Bias Assessment in Randomized Trial
eTable 9. Risk of Bias In Nonrandomized Studies or Interventions (ROBINS-I) Assessment of Eligible Nonrandomized Studies
eFigure 1. Domain-Specific Meta-analysis of Scores After Single With Possible Multiple Exposure to Surgery and Anesthesia
eFigure 2. Meta-analysis of Hazard and Risk of Clinical Diagnoses and Symptoms After Single With Possible Multiple Exposure to Surgery and Anesthesia
eFigure 3. Domain-Specific Meta-analysis of Subdomain Scores and Hazard of ADHD After Single With Possible Multiple Exposure to Surgery and Anesthesia
eFigure 4. Domain-Specific Meta-analysis of Scores and Hazard of Clinical Diagnoses and Symptoms After Single Exposure to Surgery and Anesthesia
eFigure 5. Domain-Specific Meta-analysis of Subdomain Scores and Hazard of ADHD After Single Exposure to Surgery and Anesthesia
eFigure 6. Domain-Specific Meta-analysis of Scores and Hazard of Clinical Diagnoses and Symptoms After Multiple Exposure to Surgery and Anesthesia
eFigure 7. Domain-Specific Meta-analysis of Subdomain Scores and Hazard of ADHD After Multiple Exposure to Surgery and Anesthesia
eFigure 8. ROBINS-I Risk of Bias Assessment Figure
eFigure 9. Funnel Plot for Studies of Any Exposure to Surgery and Anesthesia
References
- 1.Rabbitts JA, Groenewald CB, Moriarty JP, Flick R. Epidemiology of ambulatory anesthesia for children in the United States: 2006 and 1996. Anesth Analg. 2010;111(4):1011-1015. doi: 10.1213/ANE.0b013e3181ee8479 [DOI] [PubMed] [Google Scholar]
- 2.Tzong KY, Han S, Roh A, Ing C. Epidemiology of pediatric surgical admissions in US children: data from the HCUP kids inpatient database. J Neurosurg Anesthesiol. 2012;24(4):391-395. doi: 10.1097/ANA.0b013e31826a0345 [DOI] [PubMed] [Google Scholar]
- 3.Clausen NG, Kähler S, Hansen TG. Systematic review of the neurocognitive outcomes used in studies of paediatric anaesthesia neurotoxicity. Br J Anaesth. 2018;120(6):1255-1273. doi: 10.1016/j.bja.2017.11.107 [DOI] [PubMed] [Google Scholar]
- 4.Jevtovic-Todorovic V, Hartman RE, Izumi Y, et al. Early exposure to common anesthetic agents causes widespread neurodegeneration in the developing rat brain and persistent learning deficits. J Neurosci. 2003;23(3):876-882. doi: 10.1523/JNEUROSCI.23-03-00876.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vutskits L, Xie Z. Lasting impact of general anaesthesia on the brain: mechanisms and relevance. Nat Rev Neurosci. 2016;17(11):705-717. doi: 10.1038/nrn.2016.128 [DOI] [PubMed] [Google Scholar]
- 6.Walkden GJ, Pickering AE, Gill H. Assessing long-term neurodevelopmental outcome following general anesthesia in early childhood: challenges and opportunities. Anesth Analg. 2019;128(4):681-694. doi: 10.1213/ANE.0000000000004052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.DiMaggio C, Sun LS, Ing C, Li G. Pediatric anesthesia and neurodevelopmental impairments: a Bayesian meta-analysis. J Neurosurg Anesthesiol. 2012;24(4):376-381. doi: 10.1097/ANA.0b013e31826a038d [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang X, Xu Z, Miao CH. Current clinical evidence on the effect of general anesthesia on neurodevelopment in children: an updated systematic review with meta-regression. PLoS One. 2014;9(1):e85760. doi: 10.1371/journal.pone.0085760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang H, Du L, Du Z, Jiang H, Han D, Li Q. Association between childhood exposure to single general anesthesia and neurodevelopment: a systematic review and meta-analysis of cohort study. J Anesth. 2015;29(5):749-757. doi: 10.1007/s00540-015-2030-z [DOI] [PubMed] [Google Scholar]
- 10.Ing C, Jackson WM, Zaccariello MJ, et al. Prospectively assessed neurodevelopmental outcomes in studies of anaesthetic neurotoxicity in children: a systematic review and meta-analysis. Br J Anaesth. 2021;126(2):433-444. doi: 10.1016/j.bja.2020.10.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting: meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283(15):2008-2012. doi: 10.1001/jama.283.15.2008 [DOI] [PubMed] [Google Scholar]
- 12.Garner P, Hopewell S, Chandler J, et al. ; Panel for updating guidance for systematic reviews (PUGs) . When and how to update systematic reviews: consensus and checklist. BMJ. 2016;354:i3507. doi: 10.1136/bmj.i3507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Baron IS. Neuropsychological Evaluation of the Child. Oxford University Press; 2004. [Google Scholar]
- 14.Borenstein M. Introduction to Meta-analysis. John Wiley & Sons; 2009. doi: 10.1002/9780470743386 [DOI] [Google Scholar]
- 15.Singer V, Strasser K. The association between arithmetic and reading performance in school: a meta-analytic study. Sch Psychol Q. 2017;32(4):435-448. doi: 10.1037/spq0000197 [DOI] [PubMed] [Google Scholar]
- 16.Chinn S. A simple method for converting an odds ratio to effect size for use in meta-analysis. Stat Med. 2000;19(22):3127-3131. doi: [DOI] [PubMed] [Google Scholar]
- 17.Higgins JPT, Thomas J, Chandler J, et al. , eds. Cochrane Handbook for Systematic Reviews of Interventions. Cochrane; 2021. [Google Scholar]
- 18.Gonnermann A, Framke T, Großhennig A, Koch A. No solution yet for combining two independent studies in the presence of heterogeneity. Stat Med. 2015;34(16):2476-2480. doi: 10.1002/sim.6473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cochrane RevMan. Version 5.42020. Accessed May 11, 2022. https://training.cochrane.org/online-learning/core-software/revman
- 20.Higgins JP, Altman DG, Gøtzsche PC, et al. ; Cochrane Bias Methods Group; Cochrane Statistical Methods Group . The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. doi: 10.1136/bmj.d5928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sterne JA, Hernán MA, Reeves BC, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 2016;355:i4919. doi: 10.1136/bmj.i4919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sterne JAC, Savović J, Page MJ, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898. doi: 10.1136/bmj.l4898 [DOI] [PubMed] [Google Scholar]
- 23.Ludman L, Spitz L, Lansdown R. Developmental progress of newborns undergoing neonatal surgery. J Pediatr Surg. 1990;25(5):469-471. doi: 10.1016/0022-3468(90)90552-K [DOI] [PubMed] [Google Scholar]
- 24.Ludman L, Spitz L, Lansdown R. Intellectual development at 3 years of age of children who underwent major neonatal surgery. J Pediatr Surg. 1993;28(2):130-134. doi: 10.1016/S0022-3468(05)80257-X [DOI] [PubMed] [Google Scholar]
- 25.The Victorian Infant Collaborative Study Group . Surgery and the tiny baby: sensorineural outcome at 5 years of age. J Paediatr Child Health. 1996;32(2):167-172. doi: 10.1111/j.1440-1754.1996.tb00916.x [DOI] [PubMed] [Google Scholar]
- 26.Kayaalp L, Bozkurt P, Odabasi G, et al. Psychological effects of repeated general anesthesia in children. Paediatr Anaesth. 2006;16(8):822-827. doi: 10.1111/j.1460-9592.2006.01867.x [DOI] [PubMed] [Google Scholar]
- 27.Bartels M, Althoff RR, Boomsma DI. Anesthesia and cognitive performance in children: no evidence for a causal relationship. Twin Res Hum Genet. 2009;12(3):246-253. doi: 10.1375/twin.12.3.246 [DOI] [PubMed] [Google Scholar]
- 28.DiMaggio C, Sun LS, Kakavouli A, Byrne MW, Li G. A retrospective cohort study of the association of anesthesia and hernia repair surgery with behavioral and developmental disorders in young children. J Neurosurg Anesthesiol. 2009;21(4):286-291. doi: 10.1097/ANA.0b013e3181a71f11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kalkman CJ, Peelen L, Moons KG, et al. Behavior and development in children and age at the time of first anesthetic exposure. Anesthesiology. 2009;110(4):805-812. doi: 10.1097/ALN.0b013e31819c7124 [DOI] [PubMed] [Google Scholar]
- 30.Majnemer A, Limperopoulos C, Shevell MI, Rohlicek C, Rosenblatt B, Tchervenkov C. A new look at outcomes of infants with congenital heart disease. Pediatr Neurol. 2009;40(3):197-204. doi: 10.1016/j.pediatrneurol.2008.09.014 [DOI] [PubMed] [Google Scholar]
- 31.Wilder RT, Flick RP, Sprung J, et al. Early exposure to anesthesia and learning disabilities in a population-based birth cohort. Anesthesiology. 2009;110(4):796-804. doi: 10.1097/01.anes.0000344728.34332.5d [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fan X-C, Ye M, Li D-Z, Shi Y, Xu Y. Cognitive function in congenital heart disease after cardiac surgery with extracorporeal circulation. World J Pediatr. 2010;6(3):268-270. doi: 10.1007/s12519-010-0017-2 [DOI] [PubMed] [Google Scholar]
- 33.Walker K, Halliday R, Holland AJ, Karskens C, Badawi N. Early developmental outcome of infants with infantile hypertrophic pyloric stenosis. J Pediatr Surg. 2010;45(12):2369-2372. doi: 10.1016/j.jpedsurg.2010.08.035 [DOI] [PubMed] [Google Scholar]
- 34.DiMaggio C, Sun LS, Li G. Early childhood exposure to anesthesia and risk of developmental and behavioral disorders in a sibling birth cohort. Anesth Analg. 2011;113(5):1143-1151. doi: 10.1213/ANE.0b013e3182147f42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Flick RP, Katusic SK, Colligan RC, et al. Cognitive and behavioral outcomes after early exposure to anesthesia and surgery. Pediatrics. 2011;128(5):e1053-e1061. doi: 10.1542/peds.2011-0351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hansen TG, Pedersen JK, Henneberg SW, et al. Academic performance in adolescence after inguinal hernia repair in infancy: a nationwide cohort study. Anesthesiology. 2011;114(5):1076-1085. doi: 10.1097/ALN.0b013e31820e77a0 [DOI] [PubMed] [Google Scholar]
- 37.Andropoulos DB, Easley RB, Brady K, et al. Changing expectations for neurological outcomes after the neonatal arterial switch operation. Ann Thorac Surg. 2012;94(4):1250-1255. doi: 10.1016/j.athoracsur.2012.04.050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Block RI, Thomas JJ, Bayman EO, Choi JY, Kimble KK, Todd MM. Are anesthesia and surgery during infancy associated with altered academic performance during childhood? Anesthesiology. 2012;117(3):494-503. doi: 10.1097/ALN.0b013e3182644684 [DOI] [PubMed] [Google Scholar]
- 39.Filan PM, Hunt RW, Anderson PJ, Doyle LW, Inder TE. Neurologic outcomes in very preterm infants undergoing surgery. J Pediatr. 2012;160(3):409-414. doi: 10.1016/j.jpeds.2011.09.009 [DOI] [PubMed] [Google Scholar]
- 40.Ing C, DiMaggio C, Whitehouse A, et al. Long-term differences in language and cognitive function after childhood exposure to anesthesia. Pediatrics. 2012;130(3):e476-e485. doi: 10.1542/peds.2011-3822 [DOI] [PubMed] [Google Scholar]
- 41.Long SH, Harris SR, Eldridge BJ, Galea MP. Gross motor development is delayed following early cardiac surgery. Cardiol Young. 2012;22(5):574-582. doi: 10.1017/S1047951112000121 [DOI] [PubMed] [Google Scholar]
- 42.Long SH, Galea MP, Eldridge BJ, Harris SR. Performance of 2-year-old children after early surgery for congenital heart disease on the Bayley Scales of Infant and Toddler Development, Third Edition. Early Hum Dev. 2012;88(8):603-607. doi: 10.1016/j.earlhumdev.2012.01.007 [DOI] [PubMed] [Google Scholar]
- 43.Rocha G, Azevedo I, Pinto JC, Guimarães H. Follow-up of the survivors of congenital diaphragmatic hernia. Early Hum Dev. 2012;88(4):255-258. doi: 10.1016/j.earlhumdev.2011.08.025 [DOI] [PubMed] [Google Scholar]
- 44.Sananes R, Manlhiot C, Kelly E, et al. Neurodevelopmental outcomes after open heart operations before 3 months of age. Ann Thorac Surg. 2012;93(5):1577-1583. doi: 10.1016/j.athoracsur.2012.02.011 [DOI] [PubMed] [Google Scholar]
- 45.Sprung J, Flick RP, Katusic SK, et al. Attention-deficit/hyperactivity disorder after early exposure to procedures requiring general anesthesia. Mayo Clin Proc. 2012;87(2):120-129. doi: 10.1016/j.mayocp.2011.11.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Walker K, Badawi N, Halliday R, et al. Early developmental outcomes following major noncardiac and cardiac surgery in term infants: a population-based study. J Pediatr. 2012;161(4):748-752.e1. doi: 10.1016/j.jpeds.2012.03.044 [DOI] [PubMed] [Google Scholar]
- 47.Yang HK, Chungh DS, Hwang J-M. The effect of general anesthesia and strabismus surgery on the intellectual abilities of children: a pilot study. Am J Ophthalmol. 2012;153(4):609-613. doi: 10.1016/j.ajo.2011.09.014 [DOI] [PubMed] [Google Scholar]
- 48.Bong CL, Allen JC, Kim JT. The effects of exposure to general anesthesia in infancy on academic performance at age 12. Anesth Analg. 2013;117(6):1419-1428. doi: 10.1213/ANE.0b013e318299a7c2 [DOI] [PubMed] [Google Scholar]
- 49.Fan Q, Cai Y, Chen K, Li W. Prognostic study of sevoflurane-based general anesthesia on cognitive function in children. J Anesth. 2013;27(4):493-499. doi: 10.1007/s00540-013-1566-z [DOI] [PubMed] [Google Scholar]
- 50.Hansen TG, Pedersen JK, Henneberg SW, Morton NS, Christensen K. Educational outcome in adolescence following pyloric stenosis repair before 3 months of age: a nationwide cohort study. Paediatr Anaesth. 2013;23(10):883-890. doi: 10.1111/pan.12225 [DOI] [PubMed] [Google Scholar]
- 51.Minutillo C, Rao SC, Pirie S, McMichael J, Dickinson JE. Growth and developmental outcomes of infants with gastroschisis at one year of age: a retrospective study. J Pediatr Surg. 2013;48(8):1688-1696. doi: 10.1016/j.jpedsurg.2012.11.046 [DOI] [PubMed] [Google Scholar]
- 52.Andropoulos DB, Ahmad HB, Haq T, et al. The association between brain injury, perioperative anesthetic exposure, and 12-month neurodevelopmental outcomes after neonatal cardiac surgery: a retrospective cohort study. Paediatr Anaesth. 2014;24(3):266-274. doi: 10.1111/pan.12350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cheng HH, Wypij D, Laussen PC, et al. Cerebral blood flow velocity and neurodevelopmental outcome in infants undergoing surgery for congenital heart disease. Ann Thorac Surg. 2014;98(1):125-132. doi: 10.1016/j.athoracsur.2014.03.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Garcia Guerra G, Robertson CM, Alton GY, et al. ; Western Canadian Complex Pediatric Therapies Follow-up Group . Neurotoxicity of sedative and analgesia drugs in young infants with congenital heart disease: 4-year follow-up. Paediatr Anaesth. 2014;24(3):257-265. doi: 10.1111/pan.12257 [DOI] [PubMed] [Google Scholar]
- 55.Gaynor JW, Ittenbach RF, Gerdes M, et al. Neurodevelopmental outcomes in preschool survivors of the Fontan procedure. J Thorac Cardiovasc Surg. 2014;147(4):1276-1282. doi: 10.1016/j.jtcvs.2013.12.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ing CH, DiMaggio CJ, Malacova E, et al. Comparative analysis of outcome measures used in examining neurodevelopmental effects of early childhood anesthesia exposure. Anesthesiology. 2014;120(6):1319-1332. doi: 10.1097/ALN.0000000000000248 [DOI] [PubMed] [Google Scholar]
- 57.Ing CH, DiMaggio CJ, Whitehouse AJ, et al. Neurodevelopmental outcomes after initial childhood anesthetic exposure between ages 3 and 10 years. J Neurosurg Anesthesiol. 2014;26(4):377-386. doi: 10.1097/ANA.0000000000000121 [DOI] [PubMed] [Google Scholar]
- 58.Ko WR, Liaw YP, Huang JY, et al. Exposure to general anesthesia in early life and the risk of attention deficit/hyperactivity disorder development: a nationwide, retrospective matched-cohort study. Paediatr Anaesth. 2014;24(7):741-748. doi: 10.1111/pan.12371 [DOI] [PubMed] [Google Scholar]
- 59.Morriss FH Jr, Saha S, Bell EF, et al. ; Eunice Kennedy Shriver National Institute of Child Health and Human Development Neonatal Research Network . Surgery and neurodevelopmental outcome of very low-birth-weight infants. JAMA Pediatr. 2014;168(8):746-754. doi: 10.1001/jamapediatrics.2014.307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Stratmann G, Lee J, Sall JW, et al. Effect of general anesthesia in infancy on long-term recognition memory in humans and rats. Neuropsychopharmacology. 2014;39(10):2275-2287. doi: 10.1038/npp.2014.134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Williams RK, Black IH, Howard DB, et al. Cognitive outcome after spinal anesthesia and surgery during infancy. Anesth Analg. 2014;119(3):651-660. doi: 10.1213/ANE.0000000000000288 [DOI] [PubMed] [Google Scholar]
- 62.Yin J, Wang S-L, Liu X-B. The effects of general anaesthesia on memory in children: a comparison between propofol and sevoflurane. Anaesthesia. 2014;69(2):118-123. doi: 10.1111/anae.12504 [DOI] [PubMed] [Google Scholar]
- 63.Backeljauw B, Holland SK, Altaye M, Loepke AW. Cognition and brain structure following early childhood surgery with anesthesia. Pediatrics. 2015;136(1):e1-e12. doi: 10.1542/peds.2014-3526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bakri MH, Ismail EA, Ali MS, Elsedfy GO, Sayed TA, Ibrahim A. Behavioral and emotional effects of repeated general anesthesia in young children. Saudi J Anaesth. 2015;9(2):161-166. doi: 10.4103/1658-354X.152843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gano D, Andersen SK, Glass HC, et al. Impaired cognitive performance in premature newborns with two or more surgeries prior to term-equivalent age. Pediatr Res. 2015;78(3):323-329. doi: 10.1038/pr.2015.106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hansen TG, Pedersen JK, Henneberg SW, Morton NS, Christensen K. Neurosurgical conditions and procedures in infancy are associated with mortality and academic performances in adolescence: a nationwide cohort study. Paediatr Anaesth. 2015;25(2):186-192. doi: 10.1111/pan.12533 [DOI] [PubMed] [Google Scholar]
- 67.Ko WR, Huang JY, Chiang YC, et al. Risk of autistic disorder after exposure to general anaesthesia and surgery: a nationwide, retrospective matched cohort study. Eur J Anaesthesiol. 2015;32(5):303-310. doi: 10.1097/EJA.0000000000000130 [DOI] [PubMed] [Google Scholar]
- 68.Naguib AN, Winch PD, Tobias JD, et al. Neurodevelopmental outcome after cardiac surgery utilizing cardiopulmonary bypass in children. Saudi J Anaesth. 2015;9(1):12-18. doi: 10.4103/1658-354X.146255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Petráčková I, Zach J, Borský J, et al. Early and late operation of cleft lip and intelligence quotient and psychosocial development in 3-7 years. Early Hum Dev. 2015;91(2):149-152. doi: 10.1016/j.earlhumdev.2014.12.015 [DOI] [PubMed] [Google Scholar]
- 70.Taghon TA, Masunga AN, Small RH, Kashou NH. A comparison of functional magnetic resonance imaging findings in children with and without a history of early exposure to general anesthesia. Paediatr Anaesth. 2015;25(3):239-246. doi: 10.1111/pan.12606 [DOI] [PubMed] [Google Scholar]
- 71.Aun CST, McBride C, Lee A, et al. Short-term changes in postoperative cognitive function in children aged 5 to 12 years undergoing general anesthesia: a cohort study. Medicine (Baltimore). 2016;95(14):e3250. doi: 10.1097/MD.0000000000003250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Davidson AJ, Disma N, de Graaff JC, et al. ; GAS consortium . Neurodevelopmental outcome at 2 years of age after general anaesthesia and awake-regional anaesthesia in infancy (GAS): an international multicentre, randomised controlled trial. Lancet. 2016;387(10015):239-250. doi: 10.1016/S0140-6736(15)00608-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Diaz LK, Gaynor JW, Koh SJ, et al. Increasing cumulative exposure to volatile anesthetic agents is associated with poorer neurodevelopmental outcomes in children with hypoplastic left heart syndrome. J Thorac Cardiovasc Surg. 2016;152(2):482-489. doi: 10.1016/j.jtcvs.2016.03.095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Djurhuus BD, Hansen TG, Pedersen JK, Faber CE, Christensen K. School performance in cholesteatoma-operated children in Denmark: a nationwide population-based register-study. Acta Otolaryngol. 2016;136(7):663-668. doi: 10.3109/00016489.2016.1146412 [DOI] [PubMed] [Google Scholar]
- 75.Doberschuetz N, Dewitz R, Rolle U, Schlösser R, Allendorf A. Follow-Up of Children with Gastrointestinal Malformations and postnatal surgery and anesthesia: evaluation at two years of age. Neonatology. 2016;110(1):8-13. doi: 10.1159/000443873 [DOI] [PubMed] [Google Scholar]
- 76.Graham MR, Brownell M, Chateau DG, Dragan RD, Burchill C, Fransoo RR. neurodevelopmental assessment in kindergarten in children exposed to general anesthesia before the age of 4 years: a retrospective matched cohort study. Anesthesiology. 2016;125(4):667-677. doi: 10.1097/ALN.0000000000001245 [DOI] [PubMed] [Google Scholar]
- 77.Hansen JH, Rotermann I, Logoteta J, et al. Neurodevelopmental outcome in hypoplastic left heart syndrome: impact of perioperative cerebral tissue oxygenation of the Norwood procedure. J Thorac Cardiovasc Surg. 2016;151(5):1358-1366. doi: 10.1016/j.jtcvs.2016.02.035 [DOI] [PubMed] [Google Scholar]
- 78.Hoffman GM, Brosig CL, Bear LM, Tweddell JS, Mussatto KA. Effect of intercurrent operation and cerebral oxygenation on developmental trajectory in congenital heart disease. Ann Thorac Surg. 2016;101(2):708-716. doi: 10.1016/j.athoracsur.2015.08.059 [DOI] [PubMed] [Google Scholar]
- 79.O’Leary JD, Janus M, Duku E, et al. A population-based study evaluating the association between surgery in early life and child development at primary school entry. Anesthesiology. 2016;125(2):272-279. doi: 10.1097/ALN.0000000000001200 [DOI] [PubMed] [Google Scholar]
- 80.Poor Zamany Nejat Kermany M, Roodneshin F, Ahmadi Dizgah N, Gerami E, Riahi E. Early childhood exposure to short periods of sevoflurane is not associated with later, lasting cognitive deficits. Paediatr Anaesth. 2016;26(10):1018-1025. doi: 10.1111/pan.12969 [DOI] [PubMed] [Google Scholar]
- 81.Seltzer L, Swartz MF, Kwon J, et al. Neurodevelopmental outcomes after neonatal cardiac surgery: role of cortical isoelectric activity. J Thorac Cardiovasc Surg. 2016;151(4):1137-1142. doi: 10.1016/j.jtcvs.2015.10.065 [DOI] [PubMed] [Google Scholar]
- 82.Sun LS, Li G, Miller TL, et al. Association between a single general anesthesia exposure before age 36 months and neurocognitive outcomes in later childhood. JAMA. 2016;315(21):2312-2320. doi: 10.1001/jama.2016.6967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Aly SA, Zurakowski D, Glass P, Skurow-Todd K, Jonas RA, Donofrio MT. Cerebral tissue oxygenation index and lactate at 24 hours postoperative predict survival and neurodevelopmental outcome after neonatal cardiac surgery. Congenit Heart Dis. 2017;12(2):188-195. doi: 10.1111/chd.12426 [DOI] [PubMed] [Google Scholar]
- 84.Birajdar S, Rao S, McMichael J. Neurodevelopmental outcomes of neonates undergoing surgery under general anesthesia for malrotation of intestines. Early Hum Dev. 2017;109:32-36. doi: 10.1016/j.earlhumdev.2017.04.003 [DOI] [PubMed] [Google Scholar]
- 85.Clausen NG, Pedersen DA, Pedersen JK, et al. Oral clefts and academic performance in adolescence: the impact of anesthesia-related neurotoxicity, timing of surgery, and type of oral clefts. Cleft Palate Craniofac J. 2017;54(4):371-380. doi: 10.1597/15-185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Conrad AL, Goodwin JW, Choi J, Block RI, Nopoulos P. The relationship of exposure to anesthesia on outcomes in children with isolated oral clefts. J Child Neurol. 2017;32(3):308-315. doi: 10.1177/0883073816681257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.de Heer IJ, Tiemeier H, Hoeks SE, Weber F. Intelligence quotient scores at the age of 6 years in children anaesthetised before the age of 5 years. Anaesthesia. 2017;72(1):57-62. doi: 10.1111/anae.13687 [DOI] [PubMed] [Google Scholar]
- 88.Glatz P, Sandin RH, Pedersen NL, Bonamy AK, Eriksson LI, Granath F. Association of anesthesia and surgery during childhood with long-term academic performance. JAMA Pediatr. 2017;171(1):e163470. doi: 10.1001/jamapediatrics.2016.3470 [DOI] [PubMed] [Google Scholar]
- 89.Harmsen WJ, Aarsen FJ, van der Cammen-van Zijp MHM, et al. Developmental problems in patients with oesophageal atresia: a longitudinal follow-up study. Arch Dis Child Fetal Neonatal Ed. 2017;102(3):F214-F219. doi: 10.1136/archdischild-2015-309976 [DOI] [PubMed] [Google Scholar]
- 90.Hu D, Flick RP, Zaccariello MJ, et al. Association between exposure of young children to procedures requiring general anesthesia and learning and behavioral outcomes in a population-based birth cohort. Anesthesiology. 2017;127(2):227-240. doi: 10.1097/ALN.0000000000001735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ing C, Sun M, Olfson M, et al. Age at exposure to surgery and anesthesia in children and association with mental disorder diagnosis. Anesth Analg. 2017;125(6):1988-1998. doi: 10.1213/ANE.0000000000002423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ing C, Hegarty MK, Perkins JW, et al. Duration of general anaesthetic exposure in early childhood and long-term language and cognitive ability. Br J Anaesth. 2017;119(3):532-540. doi: 10.1093/bja/aew413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ing C, Wall MM, DiMaggio CJ, et al. Latent class analysis of neurodevelopmental deficit after exposure to anesthesia in early childhood. J Neurosurg Anesthesiol. 2017;29(3):264-273. doi: 10.1097/ANA.0000000000000303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lap CCMM, Bolhuis SW, Van Braeckel KNJA, et al. Functional outcome at school age of children born with gastroschisis. Early Hum Dev. 2017;106-107:47-52. doi: 10.1016/j.earlhumdev.2017.01.005 [DOI] [PubMed] [Google Scholar]
- 95.Nestor KA, Zeidan M, Boncore E, et al. Neurodevelopmental outcomes in infants undergoing general anesthesia. J Pediatr Surg. 2017;52(6):895-900. doi: 10.1016/j.jpedsurg.2017.03.008 [DOI] [PubMed] [Google Scholar]
- 96.Terushkin V, Brauer J, Bernstein L, Geronemus R. Effect of general anesthesia on neurodevelopmental abnormalities in children undergoing treatment of vascular anomalies with laser surgery: a retrospective review. Dermatol Surg. 2017;43(4):534-540. doi: 10.1097/DSS.0000000000001003 [DOI] [PubMed] [Google Scholar]
- 97.Zhang Q, Peng Y, Wang Y. Long-duration general anesthesia influences the intelligence of school age children. BMC Anesthesiol. 2017;17(1):170. doi: 10.1186/s12871-017-0462-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Berghmans JM, Poley MJ, van der Ende J, et al. Changes in sensory processing after anesthesia in toddlers. Minerva Anestesiol. 2018;84(8):919-928. doi: 10.23736/S0375-9393.18.12132-8 [DOI] [PubMed] [Google Scholar]
- 99.Castellheim A, Lundström S, Molin M, Kuja-Halkola R, Gillberg C, Gillberg C. The role of general anesthesia on traits of neurodevelopmental disorders in a Swedish cohort of twins. J Child Psychol Psychiatry. 2018;59(9):966-972. doi: 10.1111/jcpp.12885 [DOI] [PubMed] [Google Scholar]
- 100.Hunt RW, Hickey LM, Burnett AC, Anderson PJ, Cheong JLY, Doyle LW; Victorian Infant Collaborative Study group . Early surgery and neurodevelopmental outcomes of children born extremely preterm. Arch Dis Child Fetal Neonatal Ed. 2018;103(3):F227-F232. doi: 10.1136/archdischild-2017-313161 [DOI] [PubMed] [Google Scholar]
- 101.Kozanhan B, Kocaoğlu C, Gündüz M, Akça ÖF. Posttraumatic stress disorder symptoms in children exposed to circumcision under general or local anesthesia. Turk J Pediatr. 2018;60(6):718-725. doi: 10.24953/turkjped.2018.06.013 [DOI] [PubMed] [Google Scholar]
- 102.Lv X, Lu Y, Tang Y, Jiang J, Yan J, Jiang H. Neurodevelopmental outcomes after a brief exposure to the inhaled anesthetic sevoflurane in young children undergoing palatoplasty. Int J Clin Exp Med. 2018;11(3):2240-2247. [Google Scholar]
- 103.Schneuer FJ, Bentley JP, Davidson AJ, et al. The impact of general anesthesia on child development and school performance: a population-based study. Paediatr Anaesth. 2018;28(6):528-536. doi: 10.1111/pan.13390 [DOI] [PubMed] [Google Scholar]
- 104.Tsai CJ, Lee CT, Liang SH, Tsai PJ, Chen VC, Gossop M. Risk of ADHD after multiple exposures to general anesthesia: a nationwide retrospective cohort study. J Atten Disord. 2018;22(3):229-239. doi: 10.1177/1087054715587094 [DOI] [PubMed] [Google Scholar]
- 105.Warner DO, Zaccariello MJ, Katusic SK, et al. Neuropsychological and behavioral outcomes after exposure of young children to procedures requiring general anesthesia: the Mayo Anesthesia Safety in Kids (MASK) study. Anesthesiology. 2018;129(1):89-105. doi: 10.1097/ALN.0000000000002232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Banerjee P, Rossi MG, Anghelescu DL, et al. Association between anesthesia exposure and neurocognitive and neuroimaging outcomes in long-term survivors of childhood acute lymphoblastic leukemia. JAMA Oncol. 2019;5(10):1456-1463. doi: 10.1001/jamaoncol.2019.1094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Khochfe AR, Rajab M, Ziade F, Naja ZZ, Naja AS, Naja ZM. The effect of regional anaesthesia versus general anaesthesia on behavioural functions in children. Anaesth Crit Care Pain Med. 2019;38(4):357-361. doi: 10.1016/j.accpm.2018.03.012 [DOI] [PubMed] [Google Scholar]
- 108.McCann ME, de Graaff JC, Dorris L, et al. ; GAS Consortium . Neurodevelopmental outcome at 5 years of age after general anaesthesia or awake-regional anaesthesia in infancy (GAS): an international, multicentre, randomised, controlled equivalence trial. Lancet. 2019;393(10172):664-677. doi: 10.1016/S0140-6736(18)32485-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.O’Leary JD, Janus M, Duku E, et al. Influence of surgical procedures and general anesthesia on child development before primary school entry among matched sibling pairs. JAMA Pediatr. 2019;173(1):29-36. doi: 10.1001/jamapediatrics.2018.3662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Sun Y, Jia L, Yu H, Zhu M, Sheng M, Yu W. The effect of pediatric living donor liver transplantation on neurocognitive outcomes in children. Ann Transplant. 2019;24:446-453. doi: 10.12659/AOT.914164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Vedovelli L, Cogo P, Cainelli E, et al. Pre-surgery urine metabolomics may predict late neurodevelopmental outcome in children with congenital heart disease. Heliyon. 2019;5(10):e02547. doi: 10.1016/j.heliyon.2019.e02547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Warner DO, Chelonis JJ, Paule MG, et al. Performance on the Operant Test Battery in young children exposed to procedures requiring general anaesthesia: the MASK study. Br J Anaesth. 2019;122(4):470-479. doi: 10.1016/j.bja.2018.12.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Zaccariello MJ, Frank RD, Lee M, et al. Patterns of neuropsychological changes after general anaesthesia in young children: secondary analysis of the Mayo Anesthesia Safety in Kids study. Br J Anaesth. 2019;122(5):671-681. doi: 10.1016/j.bja.2019.01.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Han SM, Knell J, Henry O, et al. Long-term outcomes of severe surgical necrotizing enterocolitis. J Pediatr Surg. 2020;55(5):848-851. doi: 10.1016/j.jpedsurg.2020.01.019 [DOI] [PubMed] [Google Scholar]
- 115.Batta V, Rao S, Wagh D, et al. Early neurodevelopmental outcomes of congenital gastrointestinal surgical conditions: a single-centre retrospective study. BMJ Paediatr Open. 2020;4(1):e000736. doi: 10.1136/bmjpo-2020-000736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Feng Y-P, Yang T-S, Chung C-H, Chien W-C, Wong C-S. Early childhood general anesthesia exposure associated with later developmental delay: a national population-based cohort study. PLoS One. 2020;15(9):e0238289. doi: 10.1371/journal.pone.0238289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Arana Håkanson C, Fredriksson F, Engstrand Lilja H. Attention deficit hyperactivity disorder and educational level in adolescent and adult individuals after anesthesia and abdominal surgery during infancy. PLoS One. 2020;15(10):e0240891. doi: 10.1371/journal.pone.0240891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Ing C, Ma X, Sun M, et al. Exposure to surgery and anesthesia in early childhood and subsequent use of attention deficit hyperactivity disorder medications. Anesth Analg. 2020;131(3):723-733. doi: 10.1213/ANE.0000000000004619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Jacola LM, Anghelescu DL, Hall L, et al. Anesthesia exposure during therapy predicts neurocognitive outcomes in survivors of childhood medulloblastoma. J Pediatr. 2020;223:141-147.e4. doi: 10.1016/j.jpeds.2020.04.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Kobayashi Y, Tokuda N, Adachi S, Takeshima Y, Hirose M, Shima M; Japan Environment and Children’s Study (JECS) Group . Association between surgical procedures under general anesthesia in infancy and developmental outcomes at 1 year: the Japan Environment and Children’s Study. Environ Health Prev Med. 2020;25(1):32. doi: 10.1186/s12199-020-00873-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Sedighnejad A, Soltanipour S, Saberi A, et al. Risk of attention deficit hyper activity disorder after early exposure to general anesthesia; a case control study. Iran J Pediatr. 2020;30(3):e99976. doi: 10.5812/ijp.99976 [DOI] [Google Scholar]
- 122.Walkden GJ, Gill H, Davies NM, Peters AE, Wright I, Pickering AE. Early childhood general anesthesia and neurodevelopmental outcomes in the Avon Longitudinal Study of Parents and Children birth cohort. Anesthesiology. 2020;133(5):1007-1020. doi: 10.1097/ALN.0000000000003522 [DOI] [PubMed] [Google Scholar]
- 123.Gleich SJ, Shi Y, Flick R, et al. Hypotension and adverse neurodevelopmental outcomes among children with multiple exposures to general anesthesia: subanalysis of the Mayo Anesthesia Safety in Kids (MASK) Study. Paediatr Anaesth. 2021;31(3):282-289. doi: 10.1111/pan.14106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Ing C, Landau R, DeStephano D, et al. Prenatal exposure to general anesthesia and childhood behavioral deficit. Anesth Analg. 2021;133(3):595-605. doi: 10.1213/ANE.0000000000005389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Lauritzen DJ, Asschenfeldt B, Evald L, Hjortdal VE, Heiberg J. Long-term neurodevelopmental effects of intraoperative blood pressure during surgical closure of a septal defect in infancy or early childhood. Cardiol Young. 2021;31(12):2002-2008. doi: 10.1017/S1047951121001414 [DOI] [PubMed] [Google Scholar]
- 126.Partanen M, Anghelescu DL, Hall L, et al. Longitudinal associations between exposure to anesthesia and neurocognitive functioning in pediatric medulloblastoma. Eur J Cancer. 2021;148:103-111. doi: 10.1016/j.ejca.2021.02.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.van Hoorn CE, van der Cammen-van Zijp MHM, Stolker RJ, van Rosmalen J, Wijnen RMH, de Graaff JC. Associations of perioperative characteristics with motor function in preschool children born with esophageal atresia. Paediatr Anaesth. 2021;31(8):854-862. doi: 10.1111/pan.14204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Walsh BH, Paul RA, Inder TE, Shimony JS, Smyser CD, Rogers CE. Surgery requiring general anesthesia in preterm infants is associated with altered brain volumes at term equivalent age and neurodevelopmental impairment. Pediatr Res. 2021;89(5):1200-1207. doi: 10.1038/s41390-020-1030-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Warner DO, Hu D, Zaccariello MJ, et al. Association between behavioral and learning outcomes and single exposures to procedures requiring general anesthesia before age 3: secondary analysis of data from Olmsted County, MN. Anesth Analg. 2021;133(1):160-167. doi: 10.1213/ANE.0000000000005180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Zhou P, Zhang C, Huang G, Hu Y, Ma W, Yu C. The effect of sevoflurane anesthesia for dental procedure on neurocognition in children: a prospective, equivalence, controlled trial. BMC Pediatr. 2021;21(1):177. doi: 10.1186/s12887-021-02649-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Amrhein V, Greenland S, McShane B. Scientists rise up against statistical significance. Nature. 2019;567(7748):305-307. doi: 10.1038/d41586-019-00857-9 [DOI] [PubMed] [Google Scholar]
- 132.Cohen J. Statistical Power Analysis for the Behavioral Sciences. 2nd ed. L. Erlbaum Associates; 1988. [Google Scholar]
- 133.González-Alzaga B, Lacasaña M, Aguilar-Garduño C, et al. A systematic review of neurodevelopmental effects of prenatal and postnatal organophosphate pesticide exposure. Toxicol Lett. 2014;230(2):104-121. doi: 10.1016/j.toxlet.2013.11.019 [DOI] [PubMed] [Google Scholar]
- 134.Shang L, Yang L, Yang W, et al. Effects of prenatal exposure to no2 on children’s neurodevelopment: a systematic review and meta-analysis. Environ Sci Pollut Res Int. 2020;27(20):24786-24798. doi: 10.1007/s11356-020-08832-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Kuja-Halkola R, Lichtenstein P, D’Onofrio BM, Larsson H. Codevelopment of ADHD and externalizing behavior from childhood to adulthood. J Child Psychol Psychiatry. 2015;56(6):640-647. doi: 10.1111/jcpp.12340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Bouchard MF, Bellinger DC, Wright RO, Weisskopf MG. Attention-deficit/hyperactivity disorder and urinary metabolites of organophosphate pesticides. Pediatrics. 2010;125(6):e1270-e1277. doi: 10.1542/peds.2009-3058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Wagner-Schuman M, Richardson JR, Auinger P, et al. Association of pyrethroid pesticide exposure with attention-deficit/hyperactivity disorder in a nationally representative sample of U.S. children. Environ Health. 2015;14:44. doi: 10.1186/s12940-015-0030-y [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods. Literature Search Strategies
eTable 1. All Domains and Subdomains Evaluated in Studies of Potential Neurotoxic Effects of Anesthetic
eTable 2. All Outcomes Evaluated in Studies of Potential Neurotoxic Effects of Anesthetic and Their Associated Neurodevelopmental Domain and Subdomain Classifications
eTable 3. All Neurodevelopmental Domain and Subdomains and the Classification of Outcomes into These Domains and Subdomains
eTable 4. Outcomes From Each of the 108 Reviewed Studies
eTable 5. Characteristics of All 108 Reviewed Studies
eTable 6. Outcomes of Duplicate Studies or Studies That Did Not Report Outcome Scores That Could Be Evaluated
eTable 7. Exposure Data Used From Each of the 31 Included Studies
eTable 8. Cochrane Risk of Bias Assessment in Randomized Trial
eTable 9. Risk of Bias In Nonrandomized Studies or Interventions (ROBINS-I) Assessment of Eligible Nonrandomized Studies
eFigure 1. Domain-Specific Meta-analysis of Scores After Single With Possible Multiple Exposure to Surgery and Anesthesia
eFigure 2. Meta-analysis of Hazard and Risk of Clinical Diagnoses and Symptoms After Single With Possible Multiple Exposure to Surgery and Anesthesia
eFigure 3. Domain-Specific Meta-analysis of Subdomain Scores and Hazard of ADHD After Single With Possible Multiple Exposure to Surgery and Anesthesia
eFigure 4. Domain-Specific Meta-analysis of Scores and Hazard of Clinical Diagnoses and Symptoms After Single Exposure to Surgery and Anesthesia
eFigure 5. Domain-Specific Meta-analysis of Subdomain Scores and Hazard of ADHD After Single Exposure to Surgery and Anesthesia
eFigure 6. Domain-Specific Meta-analysis of Scores and Hazard of Clinical Diagnoses and Symptoms After Multiple Exposure to Surgery and Anesthesia
eFigure 7. Domain-Specific Meta-analysis of Subdomain Scores and Hazard of ADHD After Multiple Exposure to Surgery and Anesthesia
eFigure 8. ROBINS-I Risk of Bias Assessment Figure
eFigure 9. Funnel Plot for Studies of Any Exposure to Surgery and Anesthesia