Abstract
Sleep difficulties have been implicated in the development and progression of dementia and in all-cause mortality. This study examines the relationship between sleep difficulties, incident dementia and all-cause mortality over 8 years of follow-up among a nationally representative sample of older (≥65yrs) adults in the United States. We used data collected from the National Health and Aging Trends Study (NHATS) from 2011 to 2018, a prospective cohort study of Medicare beneficiaries. At baseline, the NHATS sample was comprised of 6,376 older adults that were representative of 32million older adults. Respondents reported routine difficulty initiating sleep or difficulty falling back asleep “most night” or “every night” in each study year. In each year, dementia was determined by either self-reported diagnosis or performance on immediate and the delayed recall word and clock drawing tests, while all-cause mortality was determined by proxy. We conducted Cox proportional hazards modeling,adjusting for age, sex, marital status, and chronic conditions. In models predicting all-cause mortality, we also control for dementia. Among respondents at baseline, 19% were 65–75 years of age; 71% identified as non-Hispanic white; 59% were female. Difficulty initiating sleep (HR=1.49,95%CI:1.25–1.77), difficulty falling back asleep (HR=1.39,95%CI:1.14–1.70), and concurrent sleep difficulties (HR=1.58,95%CI: 1.25–1.99) were associated with greater risk of dementia. Difficulty initiating sleep (HR=1.44,95%CI:1.20–1.72), difficulty falling back asleep (HR=1.56,95%CI:1.29–1.89), and concurrent sleep difficulties (HR=1.80,95%CI:1.44–2.24) were associated with greater risk of all-cause mortality. Our findings demonstrate that reported difficulties are prospectively associated with an increased risk of dementia and all-cause mortality among older people.
Keywords: Sleep medicine, insomnia, sleep difficulties, gerontology, geriatric medicine
INTRODUCTION
According to the Centers for Disease Control, Alzheimer’s Disease (AD), the most common cause of dementia among older adults, is currently the 6th leading cause of death in the United States (US) (Centers for Disease Control and Prevention, 2018). Moreover, in 2019 it was estimated that 5.8 million Americans were living with AD and related dementia and 14 million are expected to be living with the condition by 2050 (Alzheimer’s Association, 2019). With the rising threat posed by AD and related dementia, it is vital to identify the precipitating factors to its development and progression; one possible contributing factor is sleep difficulty.
Sleep difficulties, such as difficulty initiating sleep or difficulty falling back asleep, are associated with a host of serious, adverse consequences, ranging from poor mental health to diabetes and cardiovascular disease when left untreated (Anothaisintawee et al., 2016; Gangwisch et al., 2010; Li et al., 2014). According to epidemiological research, sleep difficulties are significantly more common among older adults (65 years of age and above) compared to younger adults (35 years of age and below). For instance, sleep difficulties are reported by 47% of older adults compared to only 27% of younger adults (Ohayon & Reynolds, 2009). Sleep difficulties are experienced with greater prevalence among older as compared to younger adults. A nationally representative study found that difficulty initiating sleep is reported by 15% of older adults and only 9% of younger adults, while early morning awakenings are reported by 17% of older adults and 9% of young adults. The most common sleep difficulties according to this study are difficulty falling back asleep, which are reported by 39% of older adults versus 12% of younger adults (Ohayon & Reynolds, 2009).
Research on a variety of sleep-related measures and cognition among older adults has been the subject of a number of studies. The majority of studies focused on this topic have shown that markers of poor sleep (e.g., self-reported poor sleep quality and daytime sleepiness) are associated with subsequent cognitive decline, including AD and dementia (Bubu et al., 2017; de Almondes et al., 2016; Lim et al., 2013; Lobo et al., 2008; Virta et al., 2013). For instance, in a study conducted among older Spanish adults, self-reported “sleep problems” at baseline were associated with a 2-fold greater risk for mild cognitive impairment, dementia, or AD 2 years later (Lobo et al., 2008). In a study conducted among Finnish individuals, poor self-reported sleep quality at baseline was significantly associated with cognitive decline at follow-up 22 years later (Virta et al., 2013). In a smaller cohort, objectively measured fragmented sleep among older adults was associated with a nearly 1.5 times greater risk for AD at 3-year follow-up (Lim et al., 2013). However, not all studies have demonstrated a linkage between markers of poor sleep and reduced cognition in older adults (Elwood et al., 2011; Foley et al., 2001; Potvin et al., 2012). For instance, one study conducted among older men did not find self-reported sleep issues relating to an insomnia diagnosis to be associated with either vascular or non-vascular dementia over a 10-year follow-up interval, but authors did find daytime sleepiness associated with vascular dementia over time (Elwood et al., 2011). One study measuring specific sleep difficulties (i.e., falling asleep and waking too early) at baseline among a sample of older Japanese American individuals did not find either measure to be associated with cognitive decline or dementia at 3-year follow-up Foley et al., 2001).
One limitation of studies focused on sleep difficulties and dementia is the use of an aggregate score of sleep difficulties, a clinical insomnia diagnosis, or a sum score of all difficulties. However, each difficulty (e.g., difficulty initiating sleep) has unique challenges and treatment recommendations, and difficulties are reported at varying intensities across the population of older adults, lending support for exploring each difficulty distinctly (Ohayon & Reynolds, 2009). For instance, difficulty initiating sleep is typically treated with stimulus control therapy, or the instruction to use bed for sleep as opposed to watching television or other activities, and leaving bed when one experiences difficulty initiating sleep (Morin et al., 2006).
Prospective studies examining sleep difficulties and all-cause mortality, on the other hand, have explored the relationships between specific difficulties and all-cause mortality. However, not all studies show a clear relationship between sleep difficulties and mortality. According to a meta-analysis of studies with a median of 10 years of follow-up (Ge et al., 2019), difficulty initiating sleep was associated with all-cause mortality (e.g., Li et al., 2014), yet nighttime awakenings and early morning awakenings were not (e.g., Foley et al., 1995; Lallukka et al., 2016). According to another meta-analysis of studies with a mean of 11 years of follow-up (Lovato & Lack, 2019), there was not an association between insomnia and all-cause mortality (e.g., Choi et al., 2017), yet one study with a 20 year follow-up interval found that, after adjusting for covariates, adult participants (ages 21 to 70) with persistent insomnia (i.e., in most years) were at significantly higher risk for all-cause mortality, while those with transient insomnia (i.e., only some years) were not at greater risk (Parthasarathy et al., 2015). Little attention has been paid to the relationships among sleep difficulties and the competing risks of incident dementia and all-cause mortality in the same population.
To address the limitations in the extant literature, the primary purpose of our study is to examine two specific sleep difficulties, including difficulty initiating sleep and waking from sleep and experiencing difficulty falling back asleep (hereafter termed “difficulty falling back asleep”), captured in the National Health and Aging Trends Study (NHATS), a nationally representative study among adults 65 years and above in the US, and their relationship to incident dementia over an 8-year time interval. The secondary aim is to examine difficulty initiating sleep and difficulty falling back asleep and their relationships to incidence of all-cause mortality over the same interval in the NHATS sample. Finally, we create a new variable indicating individuals who experienced both sleep difficulties (i.e., difficulty initiating sleep and difficulty falling back asleep) in the same year and the relationship between these concurrent sleep difficulties and incident dementia and all-cause mortality.
Methods
Participants
Data for this study were obtained from the National Health and Aging Trends Study (NHATS), an annual in-home, computer-assisted, longitudinal, nationally representative survey of Medicare beneficiaries 65 years and older drawn from the Medicare enrollment database (Montaquila et al., 2012). In order to recruit a nationally representative sample, the NHATS study made use of quota sampling techniques to reach target sample sizes by age groups (65–69, 70–74, 75–79, 80–84, 85–89, and 90+) and by race/ethnicity (non-Hispanic Black and White/Other). Complete details on the sample design and selection can be found elsewhere (Montaquila et al., 2012).
We analyzed eight years of prospectively-collected data (2011 to 2018). A core interview was administered annually to adults, aged 65 and older, randomly sampled from the Medicare enrollment file. NHATS also used proxy respondents for those individuals who were unwilling or unable to complete an interview, which has been shown to reduce attrition bias in longitudinal studies with older adults (Weir et al., 2011). Previous studies recruiting older adult-proxy pairs have demonstrated high agreement between responses from the older adult and their proxy, and more than 80% sensitivity of proxy responses to those of the older adult (Layde et al., 1995). Proxy responses in the current study represented a small proportion of participants (Year 1: n=583, 8%; Year 2: n=964, 15%; Year 3: 897, 17%; Year 4: n=722, 16%; Year 5: n=779, 10%; Year 6: n=894, 13%; Year 7: n=759, 13%; Year 8: n=664, 12%).
The baseline sample comprised 8,245 individuals, which represents a 71% response rate. Four hundred sixty-eight nursing home residents who lacked information on sleep difficulties were excluded. Of the 7,777 remaining respondents, 168 were excluded due to missing outcome information. We further excluded those who screened positive for dementia at baseline (1,236), leaving a sample of 6,373 respondents, which was representative of 33,151,098 older adults in the US. Our analysis of publicly available, de-identified data was considered exempt from IRB review.
Measures
Demographic variables.
In each of the 8 years, participants report demographic variables. Specifically, participants are asked to report age in categoires from 65–69, 70–74, 75–79, 80–84, 85–89, and 90 years of age and older. Participants were asked to report their race/ethnicity by selecting one of the following categories: White, Black, American Indian, Hispanic/Latino, or Other. Gender was measured by asking participants to select male or female. Participants reported marital status as married, living w/ partner, separated, divorced, widowed, or never married. Education was reported by particiapnts as their highest degree, ranging from high school, some college, college, or graduate degree. Participants were asked to report clinical diagnosis received, including history of a heart attack, depression, hypertension, stroke, and diabetes.
Sleep difficulties.
Difficulty initiating sleep was assessed annually with the question “In the last month, how often has it taken more than 30 minutes to fall asleep at night?” Participants recorded their responses on a scale from “every night: 7 nights a week” (1) to “most nights: 5–6 nights a week” (2), “some nights: 2–4 nights a week” (3), “rarely: once a week or less” (4), and “never” (5). Difficulty falling back asleep were measured with the question “In the last month, on the nights you woke up before you wanted, how often did you have trouble falling back asleep?” Participants recorded their responses on a scale from “every night” (1) to “most nights” (2), “some nights” (3), “rarely” (4), and “never” (5). We reverse-coded all sleep responses so that higher values indicated greater difficulties. In accordance with literature showing that sleep difficulty is most problematic when the difficulty is experienced several nights per week,(Edinger et al., 2004) we dichotomized responses so that a value of 1 indicated responses of “most nights” or “every night” as compared to a value of 0 which indicated responses of “never,” “rarely,” or “some nights.”
Finally, we created a variable to indicate concurrent sleep difficulties in the same year. Specifically, those individuals who reported both difficulty falling asleep and waking too early “most nights” or “every night” were assigned a value of 1 whereas those who reported “never,” “rarely, or “some nights” for both difficulties were assigned a value of 0, as were those individuals who reported either difficulty falling asleep or waking too early “most nights” or “every night,” but not both.
Dementia.
Dementia was determined by one of several methods. A person was identified as having dementia if either they or a proxy reported a physician-administered dementia or an AD diagnosis. Participants also rated their memory and then performed a memory-related activity (immediate and delayed 10-word recall). Next, participants responded to items related to orientation and performed a clock drawing test to assess executive function. For proxy interviews, the Alzheimer’s Dementia Questionnaire (AD8), an 8-item informant screener for dementia, was administered (Galvin et al., 2006). Based on their responses to the above validated instruments, participants were classified as having either no dementia, possible dementia, or probable dementia (Kasper et al., 2012). Previous research that has compared screening results using the above validated instruments to clinical diagnoses has shown this approach to perform well in terms of sensitivity (66%) and specificity (87%) (Hunt et al., 2015; Plassman et al., 2007). In our primary analysis, we created a new variable whereby a value of “0” was assigned to those who screened either no dementia or possible dementia and “1” to those who screened probable dementia. Over the follow-up interval, 1,807 individuals met the criteria for probable dementia, hereafter termed incident dementia.
All-cause mortality.
In situations where the participant did not respond to requests for the annual interviews, proxies or informants were contacted for information on the status of the participant. These individuals confirmed all-cause mortality. Over the follow-up interval, 2,690 individuals met the criteria for all-cause mortality. If a proxy was unavailable or did not provide mortality information, the older adult was treated as missing and not included in the analysis.
Statistical Analyses
We computed descriptive statistics to summarize the sleep difficulty variables. Next, in order to explore the stability of sleep difficulties reported by participants across study years, we examined the proportion of participants who either reported no sleep difficulties across the 8 years, those who reported the difficulty in 1 year alone, and those who reported the difficulty in two or more years.
Next, we use Cox proportional hazards modeling, the most widely used time to event analysis, to model the relationship between covariates and survival, or censored outcomes, which include incident dementia and all-cause mortality in this study (Cox, 1972; Crowley & Hu, 1977). A significant strength of this study is the availability of annual reports of sleep difficulties; therefore, we employed a time varying covariance process to model the relationship between time-varying sleep difficulties the outcomes of incident dementia and all-cause mortality. This allowed us to examine the relationship between the survival outcomes (i.e., incident dementia and all-cause mortality) as a function of the change of the time-varying covariates (i.e., sleep difficulties). By way of example, we use Xi for the time-varying covariance process. The model computes a single hazard for each individual i as follows (Cox, 1972; Therneau & Grambsch, 2000):
In the model, each participant was included until the first probable dementia screening result, a proxy reported all-cause mortality, or they were censored at the end of study, whichever occurred first. Since sleep difficulties changed over time, we treated the annually reported sleep difficulties as a binary time-varying predictor of time to either incident dementia or all-cause mortality that was updated each year. In the case of all-cause mortality, if death occurred in the survey year prior to survey administration, all variables from the year prior to all-cause mortality (i.e., sleep difficulties, demographic details, health conditions, and dementia diagnoses) were carried forward to the year in which the outcome occurred. We constructed the Cox proportional hazard models using the population weights provided by the original study (Montaquila et al., 2012). As participants are followed prospectively, the population weights from the first study year are used in the Cox models.
We performed the aforementioned Cox models to examine the relationships between each time-varying sleep difficulty and either incident dementia or all-cause mortality or both. The Cox proportional hazards models were performed both without (“unadjusted”) and with potentially confounding factors (“adjusted”), such as age, sex, and education at baseline. Time varying covariates included marital status and total number of chronic conditions. Each health condition (e.g., myocardial infarction, hypertension) was added for a summary total number of all diagnoses reported (referent=0 for no conditions). Meta-analysis has shown that dementia and cognitive impairment are associated with increased risk of all-cause mortality (Dewey & Saz, 2001; Meller et al., 1999). Therefore, in the adjusted models predicting all-cause mortality, incident dementia was included as a time-varying covariate.
Finally, we graphed the non-parametric estimates from each hazard function for those with and without each difficulty, at each point in time, for the outcome in question so that we can visually compare the probability of the event (i.e., incident dementia or all-cause mortality) and time-varying predictors (i.e., sleep difficulties) over time.
As a sensitivity analysis, we performed the Cox regression examining sleep difficulties and incident dementia without participants for whom proxy responses were provided. We found no significant difference in coefficients, and therefore retained proxies in the incident dementia analyses (see Supplemental information B). All tests were two-sided with alpha set at 0.05. All analyses were performed in Stata (Version 16, College Station, TX).
Results
Demographic characteristics of the sample at baseline are outlined in Table 1. The 6373 participants are representative of 31 million older adults living in the US. Among respondents at baseline, 21% were 70–74 years of age; 71% were white non-Hispanic; 59% were female, and 53% were either married or living with a partner.
Table 1.
Baseline Characteristics of the Study Sample (unweighted N=6,373 respondents; weighted N= 33,151,098).
| Unweighted | Weighted | ||||
|---|---|---|---|---|---|
| Variable | N | % | N | % | |
| Age | 65–69 | 1,345 | 19% | 9,282,307 | 28% |
| 70–74 | 1,503 | 21% | 9,407,049 | 28% | |
| 75–79 | 1,370 | 20% | 6,062,638 | 18% | |
| 80–84 | 1,334 | 19% | 4,471,604 | 14% | |
| 85–89 | 845 | 12% | 2,571,775 | 8% | |
| 90+ | 612 | 9% | 1,178,381 | 4% | |
| Race | White | 4,986 | 71% | 26,404,422 | 80% |
| Black | 1,446 | 21% | 2,495,632 | 8% | |
| American Indian | 169 | 2% | 1,232,367 | 4% | |
| Hispanic/Latino | 344 | 1% | 1,877,678 | 6% | |
| Other | 93 | 12% | 940,316 | 3% | |
| Sex | Male | 2,840 | 41% | 4,602,232 | 42% |
| Female | 4,169 | 59% | 6,367,287 | 58% | |
| Marital Status | Married | 3,217 | 51% | 17,529,676 | 57% |
| Living w/ partner | 138 | 2% | 753,856 | 2% | |
| Separated | 103 | 2% | 376,053 | 1% | |
| Divorced | 710 | 11% | 3,536,771 | 11% | |
| Widowed | 1,980 | 31% | 7,719,918 | 25% | |
| Never married | 110 | 3% | 1,011,352 | 3% | |
| Education | High school | 31 | 1% | 333,148 | 1% |
| Some college | 3,186 | 53% | 16,924,830 | 53% | |
| College | 2,435 | 43% | 13,698,795 | 43% | |
| Grad. degree | 669 | 3% | 953,188 | 3% | |
| Conditions | Heart attack | 911 | 14% | 4,110,744 | 12% |
| Depression | 2,713 | 43% | 12,658,748 | 38% | |
| Hypertension | 4,275 | 67% | 19,685,326 | 59% | |
| Stroke | 616 | 10% | 2,613,432 | 8% | |
| Diabetes | 1,594 | 25% | 7,181,002 | 22% | |
Across the 8-year time interval, difficulty initiating sleep was reported by 19% (year 3) to 22% (year 1) of participants. Difficulty falling back asleep were reported each year by approximately 15% of the population. Difficulty initiating sleep was consistently reported by approximately 20% of the sample each year. Concurrent sleep difficulties were reported by approximately 10% of the sample each year (see full details in Supplemental Information).
Regarding the stability of difficulties across study years, 61% of participants reported no difficulty with initiating sleep in any study year, 19% reported the difficult in 1 study year, and 20% reported the difficulty in 2 or more study years. Regarding difficulty falling back asleep, 68% reported no difficulty in any study year, 18% reported difficulty in 1 study year, and 14% reported the difficulty in 2 or more study years. Regarding concurrent difficulties, 79% reported no issue with concurrent difficulties in any study year, 13% reported concurrent difficulties in 1 year, and 9% reported concurrent difficulties in 2 or more years.
Sleep difficulties and incident dementia
Table 2 displays the results of the Cox proportional hazard models examining the relationships between time-varying sleep difficulties and incident dementia. Difficulty initiating sleep was associated with increased dementia risk (HR=1.49, 95%CI: 1.25–1.77) after adjusting for confounders. Similarly, difficulty falling back asleep were associated with greater risk of dementia (HR=1.39, 95%CI:1.14–1.70) after adjusting for confounders. Concurrent difficulties were also associated with a greater risk of dementia (HR=1.58, 95%CI: 1.25–1.99) after adjusting for confounders. Estimated survival curves display the results of the covariate-adjusted cox models (Figures 1a–1c).
Table 2.
Hazard Models Examining the Relationship Between Sleep Difficulties and Risk for Incident Dementia (unweighted N=6,373 respondents; weighted N= 33,151,098).
| Incident Dementia | ||
|---|---|---|
| OR (95%CI) | ||
| Unadjusteda | Adjustedb | |
| Variable | ||
| Difficulty Initiating Sleep | ||
| Never/rarely/some nights | 1 [Reference] | 1 [Reference] |
| Most nights/every night | 1.59 (1.34–1.88) | 1.49 (1.25–1.77) |
| Difficulty Falling Back Asleep | ||
| Never/rarely/some nights | 1 [Reference] | 1 [Reference] |
| Most nights/every night | 1.63 (1.35–1.96) | 1.39 (1.14–1.70) |
| Concurrent Sleep Difficulties (Difficulty Falling Asleep and Difficulty Falling Back Asleep) | ||
| Never/rarely/some nights | 1 [Reference] | 1 [Reference] |
| Most nights/every night | 1.89 (1.52–2.35) | 1.58 (1.25–1.99) |
The unadjusted model includes no covariates.
The adjusted model controls for age, sex, marital status, education, and chronic conditions.
Figure 1a. Estimated survival curves from adjusted cox models for incident dementia in the sleep difficulty or comparison conditions, controlling for covariates.
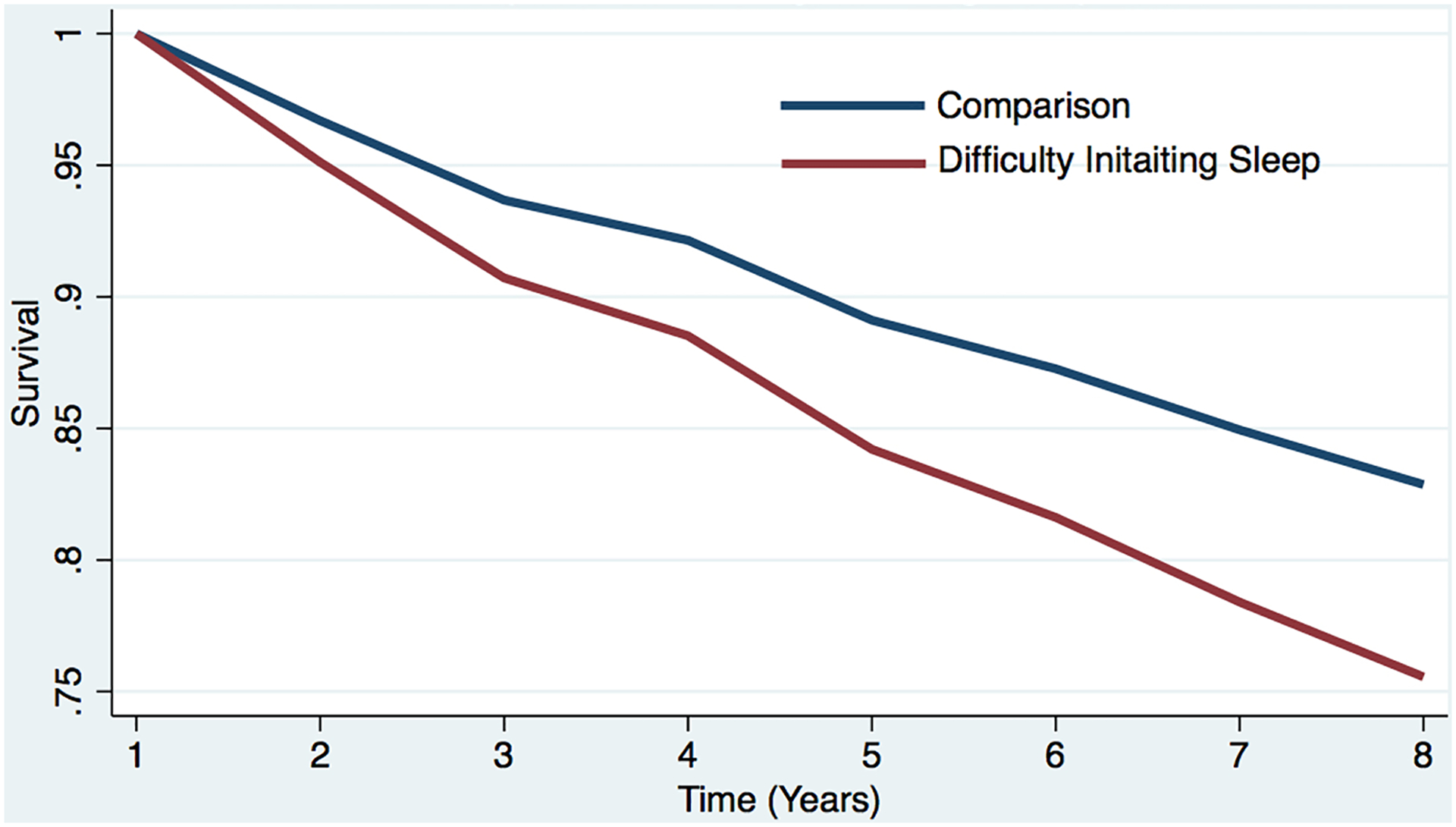
Estimated survival curve from adjusted cox model for incident dementia in the difficulty initiating sleep and comparison conditions, controlling for covariates.
a The red line represents the respondents who reported each sleep difficulty “most nights” or “every night.” In the case of concurrent difficulties, the red line represents the respondents who reported both sleep difficulties “most nights” or “every night.”
b The blue line represents respondents who did not report difficulty (i.e., reported “never,” “rarely,” or “some nights”). In the case of concurrent difficulties, the blue line represents the respondents who reported both sleep difficulties “never,” “rarely,” or “some nights.”
c Respondents who screened positive for dementia at baseline were removed.
d The curves represent covariate-adjusted analyses (i.e., age, sex, education, marital status, and chronic conditions).
Figure 1c. Estimated survival curves from adjusted cox models for incident dementia in the sleep difficulty or comparison conditions, controlling for covariates.
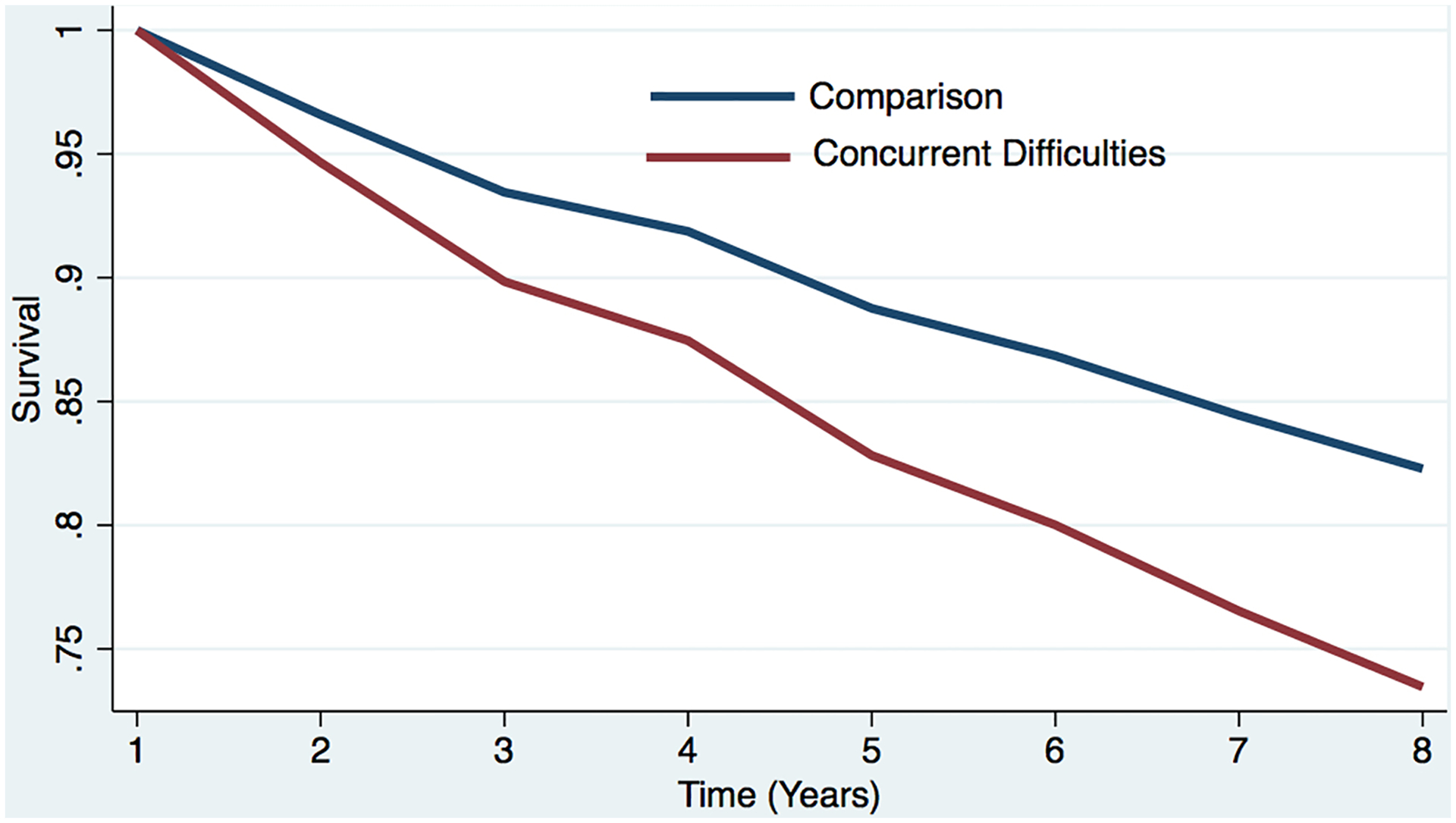
Estimated survival curve from adjusted cox model for incident dementia in the concurrent sleep difficulty and comparison conditions, controlling for covariates.
a The red line represents the respondents who reported each sleep difficulty “most nights” or “every night.” In the case of concurrent difficulties, the red line represents the respondents who reported both sleep difficulties “most nights” or “every night.”
b The blue line represents respondents who did not report difficulty (i.e., reported “never,” “rarely,” or “some nights”). In the case of concurrent difficulties, the blue line represents the respondents who reported both sleep difficulties “never,” “rarely,” or “some nights.”
c Respondents who screened positive for dementia at baseline were removed.
d The curves represent covariate-adjusted analyses (i.e., age, sex, education, marital status, and chronic conditions).
Sleep difficulties and all-cause mortality
Table 3 displays the results of the Cox proportional hazard models examining the relationships between time-varying sleep difficulties and all-cause mortality. Difficulty initiating sleep was associated all-cause mortality (HR=1.44, 95%CI: 1.20–1.72) after adjusting for confounders as were difficulty falling back asleep (HR=1.56, 95%CI:1.29–1.89). Concurrent difficulties were associated with a greater risk for all-cause mortality (HR=1.80, 95%CI: 1.44–2.24) after adjusting for confounders. Estimated survival curves are shown in Figures 2a–2c.
Table 3.
Hazard models examining the relationship between sleep difficulties and all-cause mortality (unweighted N=6,373 respondents; weighted N= 33,151,098).
| All-cause mortality | ||
|---|---|---|
| OR (95%CI) | ||
| Unadjusteda | Adjustedb | |
| Variable | ||
| Difficulty Initiating Sleep | ||
| Never/rarely/some nights | 1 [Reference] | 1 [Reference] |
| Most nights/every night | 1.56 (1.35–1.81) | 1.44 (1.20–1.72) |
| Difficulty Falling Back Asleep | ||
| Never/rarely/some nights | 1 [Reference] | 1 [Reference] |
| Most nights/every night | 1.65 (1.41–1.95) | 1.56 (1.29–1.89) |
| Concurrent Sleep Difficulties (Difficulty Falling Asleep and Difficulty Falling Back Asleep) | ||
| Never/rarely/some nights | 1 [Reference] | 1 [Reference] |
| Most nights/every night | 1.91 (1.58–2.31) | 1.80 (1.44–2.24) |
The unadjusted model includes no covariates.
The adjusted model controls for age, sex, marital status, education, chronic conditions, and dementia.
Figure 2a. Estimated survival curves from adjusted cox models for all-cause mortality in the sleep difficulty or comparison conditions, controlling for covariates.
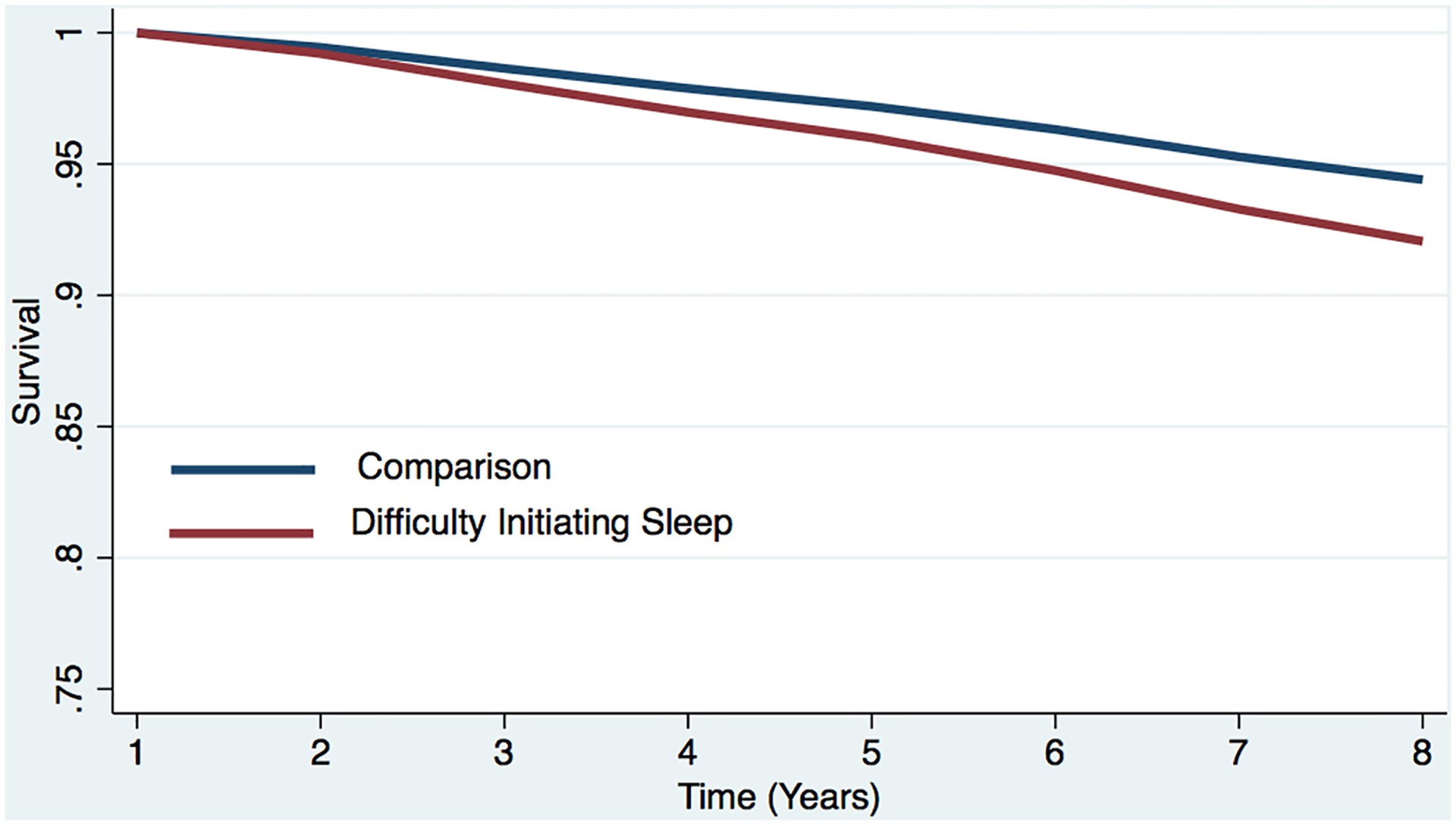
Estimated survival curve from adjusted cox model for all-cause mortality in the difficulty initiating sleep and comparison conditions, controlling for covariates.
a The red line represents the respondents who reported each sleep difficulty “most nights” or “every night.” In the case of concurrent difficulties, the red line represents the respondents who reported both sleep difficulties “most nights” or “every night.”
b The blue line represents respondents who did not report difficulty initiating sleep (i.e., reported “never,” “rarely,” or “some nights”). In the case of concurrent difficulties, the blue line represents the respondents who reported both sleep difficulties “never,” “rarely,” or “some nights.”
c Respondents who screened positive for dementia at baseline were removed.
d The curves represent covariate-adjusted analyses (i.e., age, sex, marital status, education, chronic conditions, and dementia).
Figure 2c. Estimated survival curves from adjusted cox models for all-cause mortality in the sleep difficulty or comparison conditions, controlling for covariates.
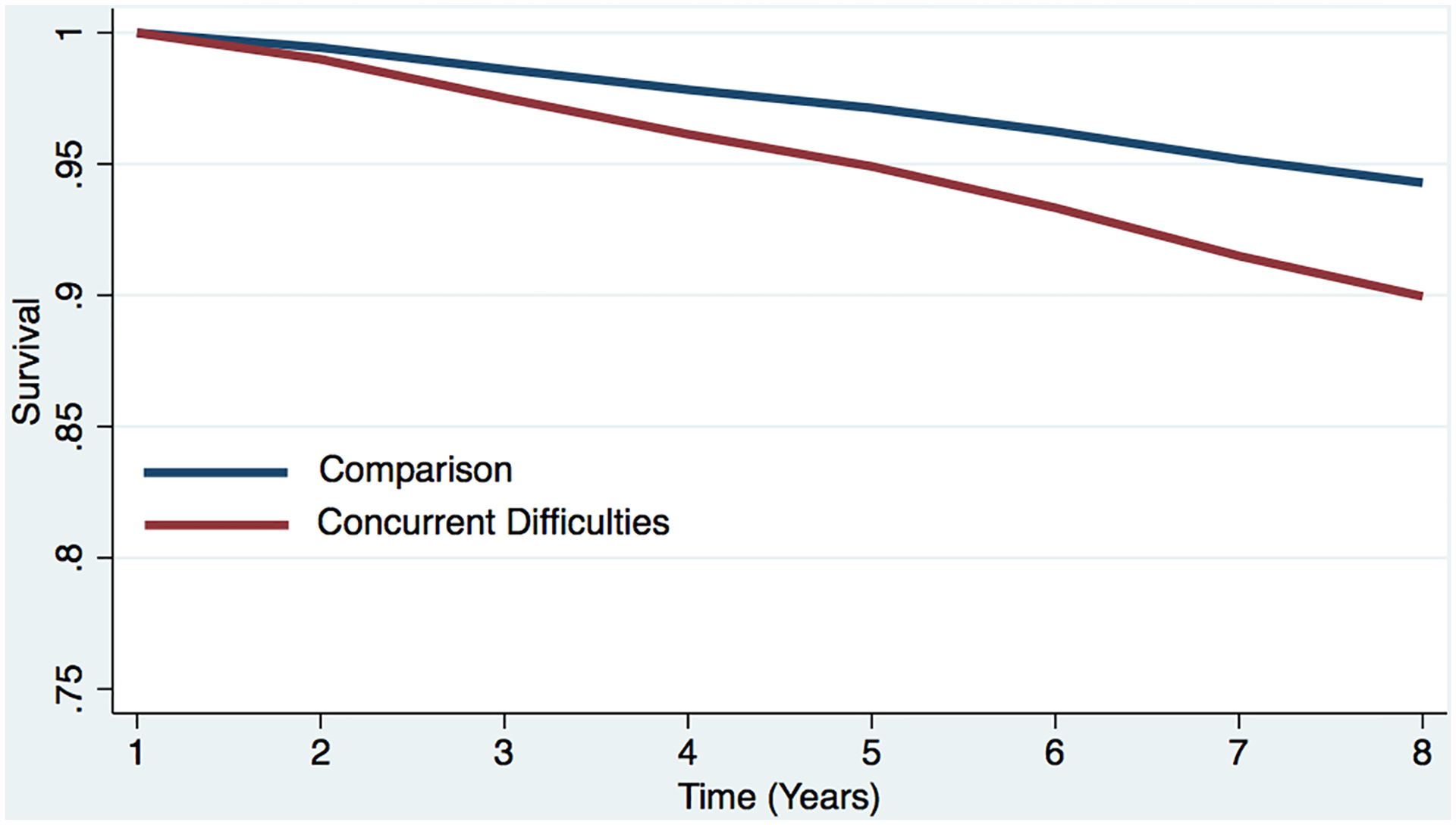
Estimated survival curve from adjusted cox model for all-cause mortality in the concurrent sleep difficulty and comparison conditions, controlling for covariates.
a The red line represents the respondents who reported each sleep difficulty “most nights” or “every night.” In the case of concurrent difficulties, the red line represents the respondents who reported both sleep difficulties “most nights” or “every night.”
b The blue line represents respondents who did not report difficulty initiating sleep (i.e., reported “never,” “rarely,” or “some nights”). In the case of concurrent difficulties, the blue line represents the respondents who reported both sleep difficulties “never,” “rarely,” or “some nights.”
c Respondents who screened positive for dementia at baseline were removed.
d The curves represent covariate-adjusted analyses (i.e., age, sex, marital status, education, chronic conditions, and dementia).
Discussion
We demonstrate a consistent, strong relationship between sleep difficulties and incident dementia over an 8-year follow-up time interval collected from a national sample representative of 32 million older adults in the US. Specifically, self-reported difficulty with initiating asleep was associated with a 49% increased risk of incident dementia over an 8-year study interval. Risk of incident dementia was increased 39% in those who reported difficulty falling back asleep. Difficulty initiating sleep concurrent with difficulty falling back asleep was associated with a 58% greater risk of incident dementia.
Our results also demonstrate a strong association between sleep difficulties and all-cause mortality risk. Specifically, difficulty initiating sleep was associated with a 44% greater risk for all-cause mortality. Difficulty falling back asleep was associated with a 56% greater risk of all-cause mortality, and concurrent sleep difficulties were associated with a 80% greater risk of all-cause mortality after adjustment for variables that could potentially confound this relationship.
Research has demonstrated a long disease trajectory and protracted “pre-clinical” phase for those who ultimately develop dementia (Dubois et al., 2016). The development of dementia likely commences before – in some cases many years prior to – the presentation of symptoms. In an attempt to control for the directionality of the association between sleep difficulties and dementia, we removed those who screened positive for dementia at baseline. After removing those individuals who screened positive for dementia at baseline and controlling for potentially confounding factors, including demographic and health characteristics, our findings show independent relationships between each sleep difficulty and incident dementia. Nevertheless, it is possible that the association observed in our study between sleep difficulties and all-cause dementia is a symptomatic manifestation of the disease that is not necessarily on the causal pathway. Minimally invasive, cost-effective, plasma biomarkers able to to provide pre-clinical markers of neurodegeenation may provide insight into disease mechanisms, such as sleep difficulties, that either predict or protect against dementia (Bateman et al., 2019).
These results contribute to the literature in several ways. First, a large body of evidence (e.g., Blackwell et al., 2006; Lim et al., 2013) and two meta analyses summarizing this literature demonstrate an association between sleep difficulties and cognitive impairment (Bubu et al., 2017; Shi et al., 2018), however, these studies typically used summary measures of sleep difficulties, when the nature of sleep difficulties can vary widely. We evaluated the frequency of three hallmark symptoms of insomnia (i.e., difficulty initiating sleep, difficulty falling back asleep, and concurrent sleep difficulties) and their relationship to dementia. This is important, for each difficulty has nuanced treatment recommendations (Ohayon & Reynolds, 2009). Second, while research has examined clinical insomnia diagnoses across similar follow-up periods as we examined in this study, the results have been somewhat mixed. Two studies, one with 3 years of follow-up and one with 10 years of follow-up, did not find an association between sleep difficulties and dementia (Elwood et al., 2011; D. Foley et al., 2001). Similarly, in the literature examining sleep difficulties and all-cause mortality, studies have examined individual sleep difficulties, but results have been mixed. One meta-analysis found a strong association between difficulty initiating sleep and all-cause mortality, but not other sleep difficulties, including nighttime awakenings or falling back asleep after waking (Ge et al., 2019), yet another meta-analysis that aggregated sleep difficulties did not find a relationship with all-cause mortality (Lovato & Lack, 2019), whereas other research has found associations between specific sleep difficulties and all-cause mortality (Ge et al., 2019). Our findings show strong associations between individual and concurrent sleep difficulties and dementia and all-cause mortality across 8 years of data.
There are several plausible mechanisms for our primary findings on the relationship between sleep difficulties and both dementia and all-cause mortality. First, previous research has shown that, during sleep, there is increased interstitial fluid volume and greater clearance of toxins implicated in the pathogenesis of dementia and AD (Xie et al., 2013). Further, extended wakefulness has been associated with the accumulation, as well as possibly the impaired clearance, of toxic metabolites in the brain (Lucey et al., 2017, 2018). Thus, those reporting sleep difficulties such the ones measured in this study, may not benefit from the sleep-related clearance of toxins and/or wake-related toxic accumulation, thereby increasing their risk for dementia over time. Second, routine sleep difficulties, such as difficulty initiating sleep or waking from sleep, may disrupt sleep architecture leading to a reduction in slow wave sleep (SWS). Lower levels of SWS have been associated with higher levels of cerebral spinal fluid amyloid beta which may lead to brain amyloid plague deposition (Varga et al., 2016). Also, inflammatory biomarkers have been shown to increase more rapidly among those with insomnia compared to those without (Parthasarathy et al., 2015). It could be that the greater risk for dementia and all-cause mortality are due in part, or perhaps exacerbated, by inflammation.
Also, it is important to note that insomnia is a heterogenous condition. For this reasont, recent research has called for a broader list of psychometric characteristics of insomnia that potentially extend beyond sleep and include such additional factors as childhood trauma, life events, and coping (Benjamins et al., 2017). This view of insomnia also highlights the possibility that there are novel contributing factors to each sleep difficulty measured in this study. For instance, it could be that nighttime awakenings are a different phenotype than difficulty falling back asleep, with different precipitating factors, the study of which may uncover novel treatments for the specific sleep difficulties.
Our study contributes to the growing body of evidence regarding sleep difficulties and both cognitive decline and all-cause mortality by prospectively documenting the relationship between specific sleep difficulties (i.e., difficulty initiating sleep, difficulty falling back asleep) and concurrent difficulties and risk for incident dementia and all-cause mortality prospectively across 8 years among a cohort of nationally representative older adults.
Future Research
These findings illuminate a number of opportunities for future research. First, given the long trajectory of AD (Dubois et al., 2016), future research studies should include older adults not expressing symptoms and examine their sleep and risk for dementia over time. Given the association we found between sleep difficulties and incident dementia and all-cause mortality, education and behavioral change programs targeting specific sleep difficulties in older adults should be developed and evaluated on improvements in sleep and downstream impact on dementia and all-cause mortality risk. Further, as our study identified relationships between specific difficulties and dementia, researchers and practitioners may consider designing targeted interventions for these specific sleep issues and their specific behavioral treatment (Ohayon & Reynolds, 2009). For instance, an intervention to address difficulty initiating sleep may offer stimulus control therapy, or coaching to reduce the anxiety or conditioned arousal individuals may feel when attempting to go to bed (Bootzin et al., 1991). Further, it would be interesting to examine the potential causes of the different sleep difficulties examined in this study, which may uncover potential contributing mechanisms that resulted in the different rates of incident dementia and all-cause mortality observed for difficulty initiating sleep, difficulty falling back asleep, and concurrent difficulties in this study.
Limitations
The strengths of our study include the large, nationally representative cohort and the prospective nature of these data across 8 years. Notwithstanding these strengths, there are several limitations. First, insomnia is traditionally characterized by experience of one or more of the following three sleep difficulties: difficulty initiating sleep, difficulty falling back asleep, and early morning awakenings. Unfortunately, we did not have access to data on early morning awakenings, the third characteristic of insomnia. Future research may explore this difficulty and its relationship to incident dementia and mortality. Second, although we identify incident dementia using either reported physician-administered diagnoses or performance on validated memory and cognitive measures of dementia, the sensitivity and specificity of these measures is 66% and 87% respectively, with relation to clinical diagnoses (Hunt et al., 2015; Plassman et al., 2007). Access to actual clinical data on dementia diagnoses would have been beneifical. Finally, although we attempt to control for the directionality of the association between sleep difficulty and dementia by removing those who screened positive for dementia at baseline, research has shown that the AD disease process commences well before the development of symptoms. Therefore, it is possible that sleep difficulties are a symptomatic manifestation of, as opposed to a risk factor for, cognitive decline.
Conclusion
Using nationally representative, prospective data collected among older adults living in the US, we examine sleep difficulties and both incident dementia and all-cause mortality over 8 years. After excluding those who screened positive for dementia at baseline and controlling for relevant covariates, we found sleep difficulties are associated with a significantly greater risk of incident dementia and all-cause mortality over the following 8 years. With an aging population, it is vital to identify the precipitating factors to the development and progression of dementia and ultimately, all-cause mortality. Our data support the role of sleep difficulties as an important factor in the development of dementia and all-cause mortality.
Supplementary Material
Figure 1b. Estimated survival curves from adjusted cox models for incident dementia in the sleep difficulty or comparison conditions, controlling for covariates.
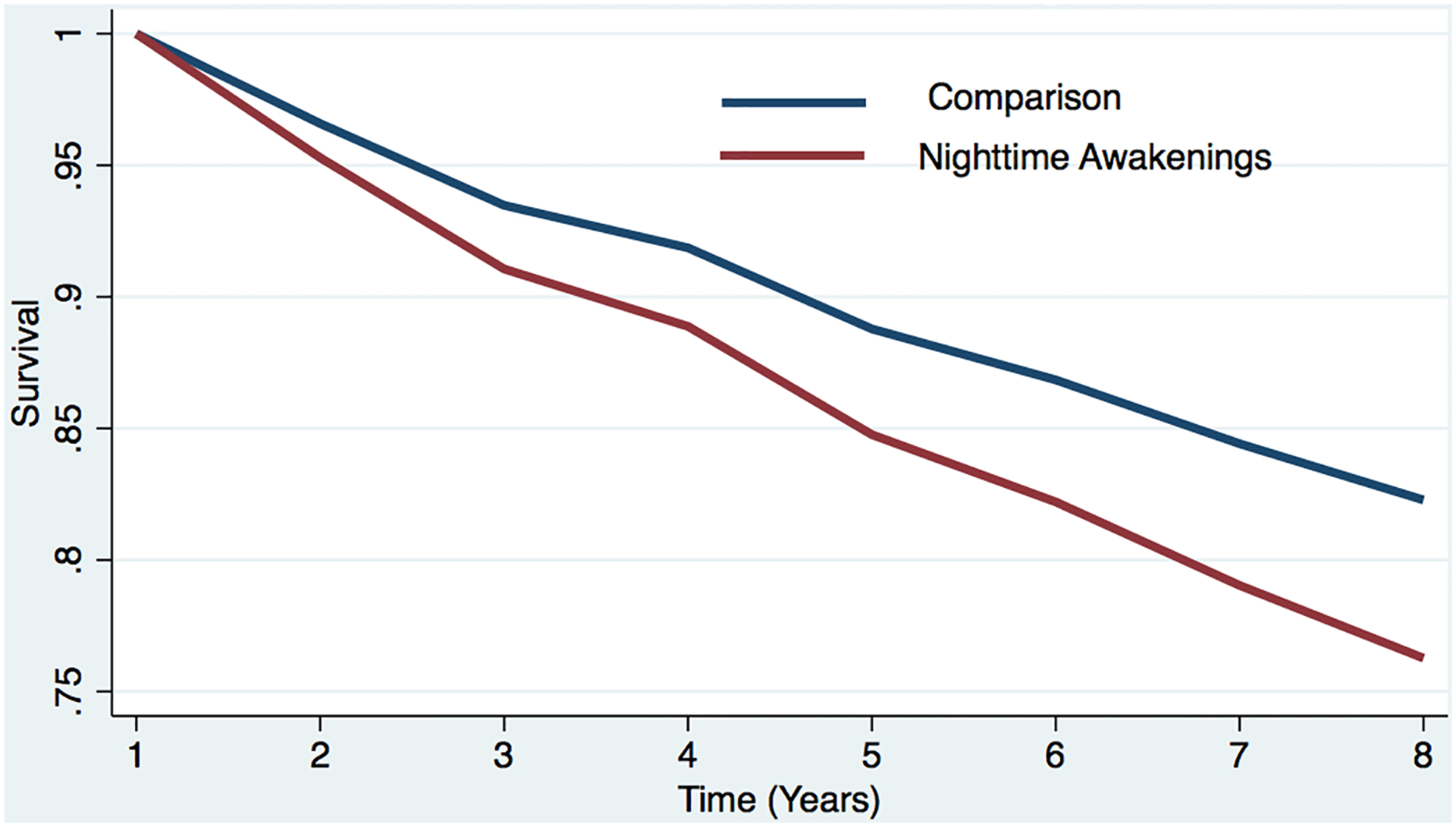
Estimated survival curve from adjusted cox model for incident dementia in the nighttime awakening and comparison conditions, controlling for covariates.
a The red line represents the respondents who reported each sleep difficulty “most nights” or “every night.” In the case of concurrent difficulties, the red line represents the respondents who reported both sleep difficulties “most nights” or “every night.”
b The blue line represents respondents who did not report difficulty (i.e., reported “never,” “rarely,” or “some nights”). In the case of concurrent difficulties, the blue line represents the respondents who reported both sleep difficulties “never,” “rarely,” or “some nights.”
c Respondents who screened positive for dementia at baseline were removed.
d The curves represent covariate-adjusted analyses (i.e., age, sex, education, marital status, and chronic conditions).
Figure 2b. Estimated survival curves from adjusted cox models for all-cause mortality in the sleep difficulty or comparison conditions, controlling for covariates.
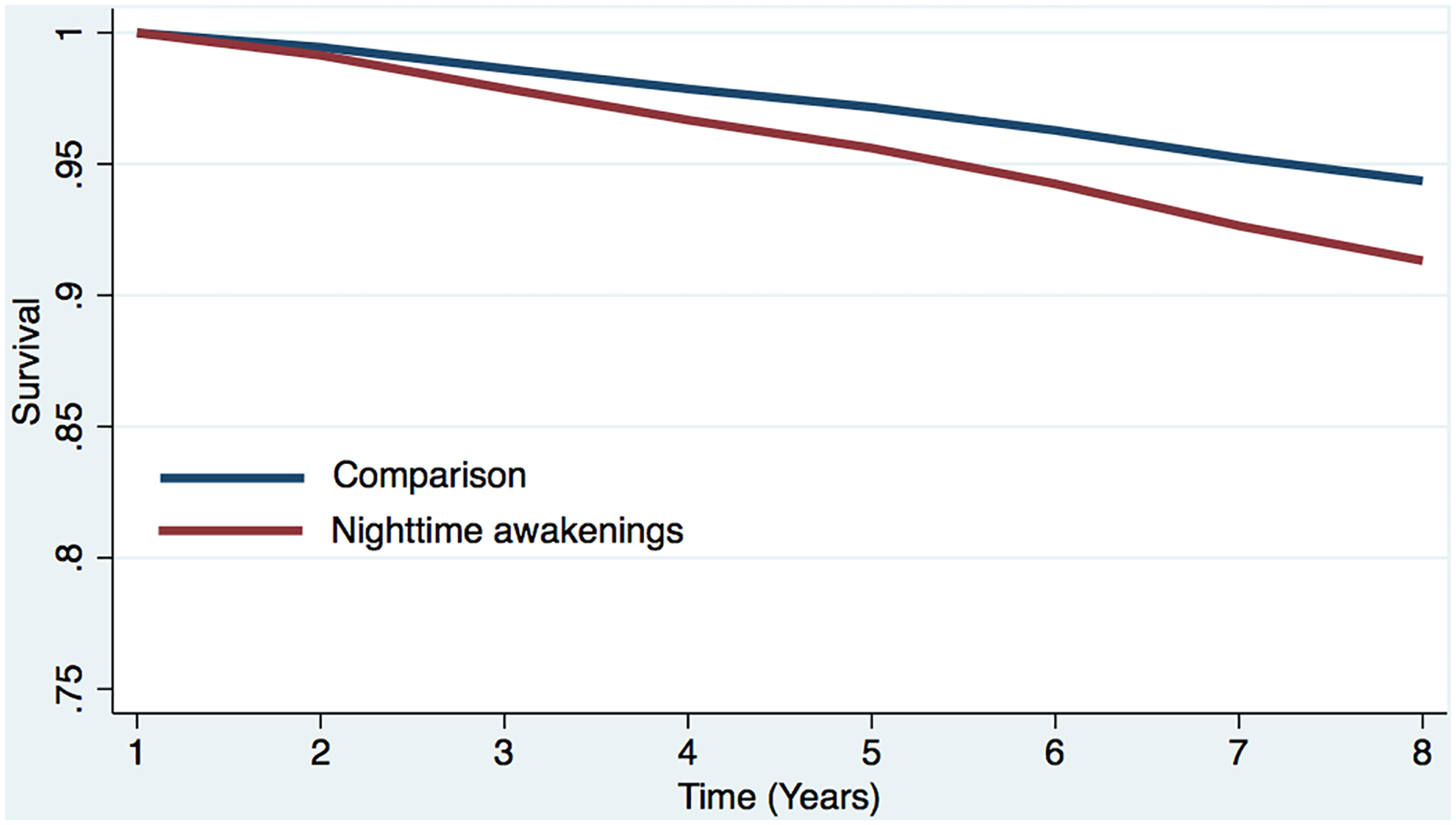
Estimated survival curve from adjusted cox model for all-cause mortality in the nighttime awakening and comparison conditions, controlling for covariates.
a The red line represents the respondents who reported each sleep difficulty “most nights” or “every night.” In the case of concurrent difficulties, the red line represents the respondents who reported both sleep difficulties “most nights” or “every night.”
b The blue line represents respondents who did not report difficulty initiating sleep (i.e., reported “never,” “rarely,” or “some nights”). In the case of concurrent difficulties, the blue line represents the respondents who reported both sleep difficulties “never,” “rarely,” or “some nights.”
c Respondents who screened positive for dementia at baseline were removed.
d The curves represent covariate-adjusted analyses (i.e., age, sex, marital status, education, chronic conditions, and dementia).
Funding:
This work was supported by the NIH grant numbers: K01HL150339 (RR), R01OH011773 (MDW, LKB), and R56HL151637 (MDW).
Conflict of Interest:
SQ serves as a consultant for Jazz Pharmaceuticals, Whispersom, Amerisleep and Best Doctors. RR has received consulting fees from Denihan Hospitality, Rituals Cosmetics, Deep, Wave Sleep, and SleepCycle. CAC reports grants to BWH from FAA, NHLBI, NIA, NIOSH, NASA, and DOD; is/was a paid consultant, Emory University, Inselspital Bern, UCLA, Institute of Digital Media and Child Development, Klarman Family Foundation, Physician’s Seal, Sleep Research Society Foundation, Tencent Holdings Ltd, Teva Pharma Australia, and Vanda Pharmaceuticals Inc, in which CAC also holds an equity interest; received travel support from Bloomage International Investment Group, Inc., UK Biotechnology and Biological Sciences Research Council, Bouley Botanical, Dr. Stanley Ho Medical Development Foundation, European Biological Rhythms Society, German National Academy of Sciences (Leopoldina), National Safey Council, National Sleep Foundation, Stanford Medical School Alumni Association, Tencent Holdings Ltd, and Vanda Pharmaceuticals Inc; receives research/education support through BWH from Cephalon, Mary Ann & Stanley Snider via Combined Jewish Philanthropies, Harmony Biosciences LLC, Jazz Pharmaceuticals PLC Inc, Johnson & Johnson, NeuroCare, Inc., Philips Respironics Inc/Philips Homecare Solutions, Regeneron Pharmaceuticals, Regional Home Care, Teva Pharmaceuticals Industries Ltd, Sanofi SA, Optum, ResMed, San Francisco Bar Pilots, Sanofi, Schneider, Simmons, Sysco, Philips, Vanda Pharmaceuticals; is/was an expert witness in legal cases, including those involving Advanced Power Technologies, Aegis Chemical Solutions LLC, Amtrak; Casper Sleep Inc, C&J Energy Services, Catapult Energy Services Group, LLC, Covenant Testing Technologies, LLC, Dallas Police Association, Enterprise Rent-A-Car, Espinal Trucking/Eagle Transport Group LLC/Steel Warehouse Inc, FedEx, Greyhound Lines Inc/Motor Coach Industries/FirstGroup America, PAR Electrical Contractors Inc, Product & Logistics Services LLC/Schlumberger Technology Corp/Gelco Fleet Trust, Puckett Emergency Medical Services LLC, Union Pacific Railroad, and Vanda Pharmaceuticals; serves as the incumbent of an endowed professorship provided to Harvard University by Cephalon, Inc.; and receives royalties from McGraw Hill, and Philips Respironics for the Actiwatch-2 and Actiwatch Spectrum devices. CAC’s interests were reviewed and are managed by the Brigham and Women’s Hospital and Mass General Brigham in accordance with their conflict of interest policies.
References
- Alzheimer’s Association. (2019). 2019 Alzheimer’s disease facts and figures. Alzheimer’s & Dementia, 15(3), 321–387. [DOI] [PubMed] [Google Scholar]
- Anothaisintawee T, Reutrakul S, Van Cauter E, & Thakkinstian A (2016). Sleep disturbances compared to traditional risk factors for diabetes development: Systematic review and meta-analysis. Sleep Medicine Reviews, 30, 11–24. 10.1016/j.smrv.2015.10.002 [DOI] [PubMed] [Google Scholar]
- Bateman RJ, Blennow K, Doody R, Hendrix S, Lovestone S, Salloway S, Schindler R, Weiner M, Zetterberg H, Aisen P, & Vellas B (2019). Plasma Biomarkers of AD Emerging as Essential Tools for Drug Development: An EU/US CTAD Task Force Report. The Journal of Prevention of Alzheimer’s Disease, 6(3), 169–173. 10.14283/jpad.2019.21 [DOI] [PubMed] [Google Scholar]
- Benjamins JS, Migliorati F, Dekker K, Wassing R, Moens S, Blanken TF, Te Lindert BHW, Sjauw Mook J, & Van Someren EJW (2017). Insomnia heterogeneity: Characteristics to consider for data-driven multivariate subtyping. Sleep Medicine Reviews, 36, 71–81. 10.1016/j.smrv.2016.10.005 [DOI] [PubMed] [Google Scholar]
- Blackwell T, Yaffe K, Ancoli-Israel S, Schneider JL, Cauley JA, Hillier TA, Fink HA, Stone KL, & Study of Osteoporotic Fractures Group. (2006). Poor sleep is associated with impaired cognitive function in older women: The study of osteoporotic fractures. The Journals of Gerontology. Series A, Biological Sciences and Medical Sciences, 61(4), 405–410. [DOI] [PubMed] [Google Scholar]
- Bootzin RR, Epstein D, & Wood JM (1991). Stimulus control instructions. In Case studies in insomnia (pp. 19–28). Springer. http://link.springer.com/chapter/10.1007/978-1-4757-9586-8_2 [Google Scholar]
- Bubu OM, Brannick M, Mortimer J, Umasabor-Bubu O, Sebastião YV, Wen Y, Schwartz S, Borenstein AR, Wu Y, Morgan D, & Anderson WM (2017). Sleep, Cognitive impairment, and Alzheimer’s disease: A Systematic Review and Meta-Analysis. Sleep, 40(1). 10.1093/sleep/zsw032 [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. (2018). Leading causes of death and numbers of deaths, by sex, race, and Hispanic origin: United States, 1980 and 2017. https://www.cdc.gov/nchs/data/hus/2018/006.pdf [Google Scholar]
- Choi J-W, Song JS, Lee YJ, Won T-B, & Jeong D-U (2017). Increased Mortality in Relation to Insomnia and Obstructive Sleep Apnea in Korean Patients Studied with Nocturnal Polysomnography. Journal of Clinical Sleep Medicine : JCSM : Official Publication of the American Academy of Sleep Medicine, 13(1), 49–56. 10.5664/jcsm.6386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox DR (1972). Regression models and life-tables. Journal of the Royal Statistical Society: Series B (Methodological), 34(2), 187–202. [Google Scholar]
- Crowley J, & Hu M (1977). Covariance Analysis of Heart Transplant Survival Data. Journal of the American Statistical Association, 72(357), 27–36. 10.2307/2286902 [DOI] [Google Scholar]
- de Almondes K, Costa MV, Malloy-Diniz LF, & Diniz BS (2016). Insomnia and risk of dementia in older adults: Systematic review and meta-analysis. Journal of Psychiatric Research, 77, 109–115. 10.1016/j.jpsychires.2016.02.021 [DOI] [PubMed] [Google Scholar]
- Dewey ME, & Saz P (2001). Dementia, cognitive impairment and mortality in persons aged 65 and over living in the community: A systematic review of the literature. International Journal of Geriatric Psychiatry, 16(8), 751–761. 10.1002/gps.397 [DOI] [PubMed] [Google Scholar]
- Dubois B, Hampel H, Feldman HH, Scheltens P, Aisen P, Andrieu S, Bakardjian H, Benali H, Bertram L, Blennow K, Broich K, Cavedo E, Crutch S, Dartigues J-F, Duyckaerts C, Epelbaum S, Frisoni GB, Gauthier S, Genthon R, … Proceedings of the Meeting of the International Working Group (IWG) and the American Alzheimer’s Association on “The Preclinical State of AD”; July 23, 2015; Washington DC, USA. (2016). Preclinical Alzheimer’s disease: Definition, natural history, and diagnostic criteria. Alzheimer’s & Dementia: The Journal of the Alzheimer’s Association, 12(3), 292–323. 10.1016/j.jalz.2016.02.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edinger JD, Bonnet MH, Bootzin RR, Doghramji K, Dorsey CM, Espie CA, Jamieson AO, McCall WV, Morin CM, Stepanski EJ, & American Academy of Sleep Medicine Work Group. (2004). Derivation of research diagnostic criteria for insomnia: Report of an American Academy of Sleep Medicine Work Group. Sleep, 27(8), 1567–1596. [DOI] [PubMed] [Google Scholar]
- Elwood PC, Bayer AJ, Fish M, Pickering J, Mitchell C, & Gallacher JEJ (2011). Sleep disturbance and daytime sleepiness predict vascular dementia. Journal of Epidemiology & Community Health, 65(9), 820–824. 10.1136/jech.2009.100503 [DOI] [PubMed] [Google Scholar]
- Foley DJ, Monjan AA, Brown SL, Simonsick EM, Wallace RB, & Blazer DG (1995). Sleep complaints among elderly persons: An epidemiologic study of three communities. Sleep, 18(6), 425–432. [DOI] [PubMed] [Google Scholar]
- Foley D, Monjan A, Masaki K, Ross W, Havlik R, White L, & Launer L (2001). Daytime sleepiness is associated with 3-year incident dementia and cognitive decline in older Japanese-American men. Journal of the American Geriatrics Society, 49(12), 1628–1632. 10.1046/j.1532-5415.2001.t01-1-49271.x [DOI] [PubMed] [Google Scholar]
- Galvin J, Roe C, Xiong C, & Morris J (2006). Validity and reliability of the AD8 informant interview in dementia | Neurology. 67(11), 1942–1948. 10.1212/01.wnl.0000247042.15547.eb [DOI] [PubMed] [Google Scholar]
- Gangwisch JE, Malaspina D, Posner K, Babiss LA, Heymsfield SB, Turner JB, Zammit GK, & Pickering TG (2010). Insomnia and sleep duration as mediators of the relationship between depression and hypertension incidence. American Journal of Hypertension, 23(1), 62–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge L, Guyatt G, Tian J, Pan B, Chang Y, Chen Y, Li H, Zhang J, Li Y, Ling J, & Yang K (2019). Insomnia and risk of mortality from all-cause, cardiovascular disease, and cancer: Systematic review and meta-analysis of prospective cohort studies. Sleep Medicine Reviews, 48, 101215. 10.1016/j.smrv.2019.101215 [DOI] [PubMed] [Google Scholar]
- Hunt LJ, Covinsky KE, Yaffe K, Stephens CE, Miao Y, Boscardin WJ, & Smith AK (2015). Pain in Community-Dwelling Older Adults with Dementia: Results from the National Health and Aging Trends Study. Journal of the American Geriatrics Society, 63(8), 1503–1511. 10.1111/jgs.13536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasper J, Freedman V, & Spillman B (2012). Classification of Persons by Dementia Status in the National Health and Aging Trends Study. In Technical Paper #5. Johns Hopkins University School of Public Health. NHATS.org [Google Scholar]
- Lallukka T, Podlipskytė A, Sivertsen B, Andruškienė J, Varoneckas G, Lahelma E, Ursin R, Tell GS, & Rahkonen O (2016). Insomnia symptoms and mortality: A register-linked study among women and men from Finland, Norway and Lithuania. Journal of Sleep Research, 25(1), 96–103. 10.1111/jsr.12343 [DOI] [PubMed] [Google Scholar]
- Layde PM, Beam CA, Broste SK, Connors AF, Desbiens N, Lynn J, Phillips RS, Reding D, Teno J, & Vidaillet H (1995). Surrogates’ predictions of seriously ill patients’ resuscitation preferences. Archives of Family Medicine, 4(6), 518–523. 10.1001/archfami.4.6.518 [DOI] [PubMed] [Google Scholar]
- Li Y, Zhang X, Winkelman JW, Redline S, Hu FB, Stampfer M, Ma J, & Gao X (2014). Association between insomnia symptoms and mortality: A prospective study of U.S. men. Circulation, 129(7), 737–746. 10.1161/CIRCULATIONAHA.113.004500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim ASP, Kowgier M, Yu L, Buchman AS, & Bennett DA (2013). Sleep Fragmentation and the Risk of Incident Alzheimer’s Disease and Cognitive Decline in Older Persons. Sleep, 36(7), 1027–1032. 10.5665/sleep.2802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobo A, López-Antón R, de-la-Cámara C, Quintanilla MA, Campayo A, Saz P, & ZARADEMP Workgroup. (2008). Non-cognitive psychopathological symptoms associated with incident mild cognitive impairment and dementia, Alzheimer’s type. Neurotoxicity Research, 14(2–3), 263–272. 10.1007/BF03033815 [DOI] [PubMed] [Google Scholar]
- Lovato N, & Lack L (2019). Insomnia and mortality: A meta-analysis. Sleep Medicine Reviews, 43, 71–83. 10.1016/j.smrv.2018.10.004 [DOI] [PubMed] [Google Scholar]
- Lucey BP, Hicks TJ, McLeland JS, Toedebusch CD, Boyd J, Elbert DL, Patterson BW, Baty J, Morris JC, Ovod V, Mawuenyega KG, & Bateman RJ (2018). Effect of sleep on overnight CSF amyloid-β kinetics. Annals of Neurology, 83(1), 197–204. 10.1002/ana.25117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucey BP, Mawuenyega KG, Patterson BW, Elbert DL, Ovod V, Kasten T, Morris JC, & Bateman RJ (2017). Associations Between β-Amyloid Kinetics and the β-Amyloid Diurnal Pattern in the Central Nervous System. JAMA Neurology, 74(2), 207–215. 10.1001/jamaneurol.2016.4202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meller I, Fichter MM, & Schröppel H (1999). Mortality risk in the octo- and nonagenerians: Longitudinal results of an epidemiological follow-up community study. European Archives of Psychiatry and Clinical Neuroscience, 249(4), 180–189. 10.1007/s004060050085 [DOI] [PubMed] [Google Scholar]
- Montaquila J, Freedman VA, Edwards B, & Kasper JD (2012). National Health and Aging Trends Study round 1 sample design and selection (pp. 1–8). www.NHATS.org
- Morin CM, Bootzin RR, Buysse DJ, Edinger JD, Espie CA, & Lichstein KL (2006). Psychological and behavioral treatment of insomnia:update of the recent evidence (1998–2004). Sleep, 29(11), 1398–1414. [DOI] [PubMed] [Google Scholar]
- Ohayon MM, & Reynolds CF (2009). Epidemiological and clinical relevance of insomnia diagnosis algorithms according to the DSM-IV and the International Classification of Sleep Disorders (ICSD). Sleep Medicine, 10(9), 952–960. 10.1016/j.sleep.2009.07.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parthasarathy S, Vasquez MM, Halonen M, Bootzin R, Quan SF, Martinez FD, & Guerra S (2015). Persistent insomnia is associated with mortality risk. The American Journal of Medicine, 128(3), 268–275.e2. 10.1016/j.amjmed.2014.10.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plassman BL, Langa KM, Fisher GG, Heeringa SG, Weir DR, Ofstedal MB, Burke JR, Hurd MD, Potter GG, Rodgers WL, Steffens DC, Willis RJ, & Wallace RB (2007). Prevalence of Dementia in the United States: The Aging, Demographics, and Memory Study. Neuroepidemiology, 29(1–2), 125–132. 10.1159/000109998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potvin O, Lorrain D, Forget H, Dubé M, Grenier S, Préville M, & Hudon C (2012). Sleep quality and 1-year incident cognitive impairment in community-dwelling older adults. Sleep, 35(4), 491–499. 10.5665/sleep.1732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi L, Chen S-J, Ma M-Y, Bao Y-P, Han Y, Wang Y-M, Shi J, Vitiello MV, & Lu L (2018). Sleep disturbances increase the risk of dementia: A systematic review and meta-analysis. Sleep Medicine Reviews, 40, 4–16. 10.1016/j.smrv.2017.06.010 [DOI] [PubMed] [Google Scholar]
- Therneau TM, & Grambsch PM (2000). The cox model. In Modeling survival data: Extending the Cox model (pp. 39–77). Springer. [Google Scholar]
- Varga AW, Wohlleber ME, Giménez S, Romero S, Alonso JF, Ducca EL, Kam K, Lewis C, Tanzi EB, Tweardy S, Kishi A, Parekh A, Fischer E, Gumb T, Alcolea D, Fortea J, Lleó A, Blennow K, Zetterberg H, … Osorio RS (2016). Reduced Slow-Wave Sleep Is Associated with High Cerebrospinal Fluid Aβ42 Levels in Cognitively Normal Elderly. Sleep, 39(11), 2041–2048. 10.5665/sleep.6240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virta JJ, Heikkilä K, Perola M, Koskenvuo M, Räihä I, Rinne JO, & Kaprio J (2013). Midlife sleep characteristics associated with late life cognitive function. Sleep, 36(10), 1533–1541, 1541A. 10.5665/sleep.3052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weir D, Faul J, & Langa K (2011). Proxy interviews and bias in the distribution of cognitive abilities due to non-response in longitudinal studies: A comparison of HRS and ELSA. Longitudinal and Life Course Studies, 2(2), 170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie L, Kang H, Xu Q, Chen MJ, Liao Y, Thiyagarajan M, O’Donnell J, Christensen DJ, Nicholson C, & Iliff JJ (2013). Sleep drives metabolite clearance from the adult brain. Science, 342(6156), 373–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


