This randomized clinical trial compares the efficacy and safety of the DDGP (dexamethasone, cisplatin, gemcitabine, and pegaspargase) regimen with the SMILE (dexamethasone, methotrexate, ifosfamide, L-asparaginase, and etoposide) regimen in newly diagnosed advanced-stage (III/IV) extranodal natural killer/T-cell lymphoma.
Key Points
Question
Which chemotherapy regimen has better safety and efficacy in treating newly diagnosed advanced extranodal natural killer/T-cell lymphoma (ENKL): DDGP (dexamethasone, cisplatin, gemcitabine, and pegaspargase) or SMILE (dexamethasone, methotrexate, ifosfamide, L-asparaginase, and etoposide)?
Findings
In this randomized clinical trial of 80 patients with newly diagnosed ENKL, at a median follow-up of 41.5 months, the median progression-free survival was not reached in the DDGP group vs 6.8 months in the SMILE group, and the median overall survival was not reached in the DDGP group vs 75.2 months in the SMILE group. Grade 3 and 4 hematologic toxic effects such as leukopenia and neutropenia were less frequently reported in the DDGP group than in the SMILE group.
Meaning
The DDGP regimen was well tolerated and showed statistically significant survival benefit over the SMILE regimen in treating newly diagnosed ENKL.
Abstract
Importance
The L-asparaginase–based SMILE (dexamethasone, methotrexate, ifosfamide, L-asparaginase, and etoposide) chemotherapy regimen has shown higher response rates and survival benefit over an anthracycline-containing regimen. However, the safety profile was not satisfied. A well-tolerated regimen with promising efficacy is lacking.
Objective
To compare the efficacy and safety of the DDGP (dexamethasone, cisplatin, gemcitabine, and pegaspargase) regimen with the SMILE regimen in newly diagnosed advanced-stage (III/IV) extranodal natural killer/T-cell lymphoma (ENKL).
Design, Setting, and Participants
This was an open-label, multicenter, randomized clinical trial that took place across 12 participating hospitals in China from January 2011 to February 2019. Patients were eligible if they were 14 to 70 years old with newly diagnosed ENKL in stages III/IV and had an Eastern Cooperative Oncology Group performance status of 0 to 2. Eligible patients were evenly randomized to either the DDGP or SMILE group.
Interventions
Patients in each group were treated with the assigned regimen every 21 days for 6 cycles.
Main Outcomes and Measures
The primary end point was progression-free survival (PFS), and secondary end points included overall response rate and overall survival (OS). The adverse events between the DDGP and SMILE groups were compared.
Results
Among the 87 randomized patients, 80 received treatment (40 in the DDGP group and 40 in the SMILE group); the median (IQR) age was 43 (12) years, and 51 (64%) were male. The baseline characteristics were similar between the groups. At a median follow-up of 41.5 months, the median PFS was not reached in the DDGP group vs 6.8 months in the SMILE group (HR, 0.42; 95% CI, 0.23-0.77; P = .004), and the median OS was not reached in the DDGP group vs 75.2 months in the SMILE group (HR, 0.41; 95% CI, 0.19-0.89, P = .02). The PFS rate at 3 years and OS rate at 5 years were higher in the DDGP group vs the SMILE group (3-year PFS, 56.6% vs 41.8%; 5-year OS, 74.3% vs 51.7%). The overall response rate was higher in the DDGP group than in the SMILE group (90.0% vs 60.0%; P = .002). Grade 3 and 4 hematologic toxic effects were more frequently reported in the SMILE group vs the DDGP group (leukopenia, 85.0% vs 62.5%; neutropenia, 85.0% vs 65.0%).
Conclusions and Relevance
In this randomized clinical trial, the DDGP regimen showed promising preliminary results for patients with newly diagnosed local advanced ENKL. A confirmation trial based on larger population is warranted.
Trial Registration
ClinicalTrials.gov Identifier: NCT01501149
Introduction
Extranodal natural killer/T-cell lymphoma (ENKL), a subtype of mature T-cell and NK-cell lymphomas, is characterized by Epstein-Barr virus infection. It is highly prevalent in Asia and South America, and is more common in men than in women. Clinically, ENKL lesions often invade the nasal cavity, nasopharynx, sinuses, and other midline structures. Some ENKLs may even develop into hemophagocytic syndrome, which leads to poor prognosis. Compared with other types of lymphoid tumors, ENKL is usually associated with poor survival outcomes.1
Limited-stage ENKL is usually sensitive to radiotherapy. However, radiotherapy alone is not effective against occult lesions outside of the radiation field and leads to lymphoma recurrence at distant sites.2 Recently, the 5-year overall survival (OS) of the limited-stage ENKL has increased to 72% to 74% owing to the introduction of a novel strategy of concurrent chemoradiotherapy.3 Compared with limited-stage ENKL, advanced-stage ENKL progresses rapidly, and the prognosis is very poor. Systemic chemotherapy is still the main treatment for advanced ENKL. The conventional anthracycline-based CHOP (cyclophosphamide, doxorubicin hydrochloride, vincristine sulfate, and prednisone) regimen was not effective against ENKL owing to the overexpression of P-glycoprotein in neoplasm cells, which may result in multidrug resistance. The 2-year OS rate of advanced ENKL was only about 30.3% before 2010 but was about 40.5% in 2010 or after.4
Recently, increasing evidence has shown that an asparagine-based regimen had a better effect on ENKL because it was not affected by P-glycoprotein.5 In 2011, SMILE (dexamethasone, methotrexate, ifosfamide, L-asparaginase, and etoposide), an L-asparaginase–based regimen, was designed to tackle the aforementioned problems. The results of a phase 2 study demonstrated that the SMILE regimen achieved higher response rate, prolonged OS, and progression-free survival (PFS) compared with the CHOP-like regimen in patients with newly diagnosed stage IV, relapsed, or refractory ENKL. However, grade 4 neutropenia was observed in 92% of patients, which limited further application of the SMILE regimen.6 Therefore, new chemotherapeutic options with high efficacy and low levels of toxic effects need to be found.
Based on clinical practice and laboratory data, the novel systemic chemotherapy regimen DDGP (dexamethasone, cisplatin, gemcitaline, and pegaspargase) was defined in 2011 by this team of authors for untreated stage III/IV ENKL. More groups explored L-asparaginase–based systemic chemotherapy regimens, such as the AspaMetDex (L-asparaginase, methotrexate, and dexamethasone) regimen,7 IMEP-L-asp (ifosfamide, methotrexate, etoposide, prednisolone, and L-asparaginase) regimen,8 and P-GEMOX (pegaspargase, gemcitabine, and oxaliplatin) regimen.9 The complete remission rate of these regimens ranged between 45% and 66%. In addition, some studies suggested that autologous hematopoietic stem cell transplantation might benefit patients with advanced-stage ENKL. However, selection of an appropriate systemic chemotherapy regimen to achieve a complete remission (CR) status before autologous hematopoietic stem cell transplantation is critical and comprises an independent risk factor for the prognosis of transplantation.10 At present, hematopoietic stem cell transplantation has not been determined for standardized care. Some studies have shown that immune checkpoint inhibitors11 and targeted CD3012 treatment was effective in some patients with recurrent/refractory ENKL, while limited studies were performed in patients with advanced untreated ENKL.
The aforementioned studies provided various options of regimens and strategies for treating ENKL. However, most of them were small-sized, single-arm studies, and patients recruited by the study included not only newly diagnosed patients, but also patients with refractory cancer. Further research was needed to evaluate which combination was the best option for patients with untreated advanced ENKL. Even now, to our knowledge, no randomized clinical trial has been conducted to explore the appropriate treatment for these patients. In 2016, preliminary results demonstrated that the DDGP regimen resulted in encouraging improvement in PFS and OS and had better tolerability compared with SMILE chemotherapy. Herein, we present updated results of this clinical trial.
Methods
Study Design and Patients
This prospective, randomized, multicenter, open-label clinical trial compared the efficacy and safety of the DDGP regimen vs the SMILE regimen in patients with newly diagnosed stage III/IV ENKL (patients with nasal-involved and extranasal-involved ENKL were evaluated by Chinese Southwest Oncology Group criteria and the Cotswolds modification of Ann Arbor staging system, respectively). Patients were randomized 1:1 on study entry into either the DDGP regimen or SMILE regimen based on a computer-generated randomization schedule. The inclusion criteria included (1) age of 14 to 70 years, (2) an Eastern Cooperative Oncology Group performance status of 0 to 2, (3) estimated survival time of more than 3 months, (4) histologically confirmed ENKL (assessed by tissue-based Epstein-Barr encoding region assay), (5) no previous chemotherapy or radiotherapy, (6) no chemotherapy contraindications, (7) at least 1 measurable lesion, (8) no other serious diseases, (9) normal cardiopulmonary function, (10) negative pregnancy test, and (11) no other previous antitumor treatments. Patients were excluded if they had central nervous system involvement or a prior malignant tumor. Withdrawal from the study was considered if patients had disease progression or unacceptable toxic effects, or refusal by patients.
Before treatment, each participant underwent routine biochemical tests and a computed tomography scan or 18F-fludeoxyglucose–positron emission tomography/computed tomography scan to evaluate primary lesions, including in the head, neck, thorax, and abdomen. Bone marrow aspiration and biopsy were applied to assess bone marrow invasion. The clinical stage standard proposed by Lin et al13 was adopted. International Prognostic Index scores were used to evaluate the risk for patients with ENKL. The performance status was evaluated per the Eastern Cooperative Oncology Group scale. The cutoff for a high Epstein-Barr virus DNA titer in the plasma was not well established. Hence, any detection of Epstein-Barr virus DNA was defined as positive.
This study was approved by the ethical committee of Zhengzhou University and the Scientific Council of the Faculty of Medicine. All 12 participating hospitals in China were approved to conduct this clinical trial. The researchers followed Good Clinical Practice Guidelines and the Declaration of Helsinki. All of the participants provided written informed consent. The Consolidated Standards of Reporting Trials (CONSORT) reporting guidelines were followed, and the study protocol can be found in Supplement 1.
Procedures
Patients in the DDGP group received intravenous cisplatin, 20 mg/m2, on days 1 through 4; intravenous dexamethasone, 15 mg/m2, on days 1 through 5; intravenous gemcitabine, 800 mg/m2, on day 1 and day 8; and intramuscular pegaspargase, 2500 IU/m2, on day 1. Patients in the SMILE group received intravenous dexamethasone, 40 mg, on days 2 through 4; intravenous methotrexate, 2 g/m2, on day 1; intravenous ifosfamide, 1.5 g/m2, on days 2 through 4; L-asparaginase, 6000 U/m2, on days 3 through 9; and intravenous etoposide, 100 mg/m2, on days 2 through 4. The DDGP and SMILE regimens were repeated every 21 days for 6 cycles. The detailed treatment regimens are shown in eTable 1 in Supplement 2.
Dose modification was applied when severe grade 3 and 4 adverse events occurred. The dose of subsequent chemotherapy drugs was reduced by 20%, or 10% for patients 65 to 70 years old (excluding pegasparaginase and L-asparaginase). In the SMILE arm, all patients underwent the routine allergy testing 1 hour before L-asparaginase administration to prevent L-asparaginase–induced allergic reactions. This situation was also applicable to pegasparaginase in the DDGP scheme. Furthermore, granulocyte colony-stimulating factor and granulocyte-macrophage colony-stimulating factor were given as the supportive therapy to patients who developed neutropenia and thrombopenia.
End Points and Assessment
The primary end point was PFS, which was defined as the interval from the date of randomization to the date of disease progression or death for any reason. Secondary end points included overall response rate (ORR), OS, and toxic effects. Overall survival was defined as from the date of randomization to the date of death. If a patient was lost to follow-up or withdrew from the study before death was observed, the patient was censored at last follow-up. The ORR was defined as the proportion of patients who achieved CR or partial response. Each 3 weeks comprised a treatment cycle. Efficacy evaluation was conducted every 2 cycles. The tumor responses were assessed by the Central Imaging Review Board according to criteria modified from the World Health Organization response criteria.14
Treatment-related toxic effects were monitored by routine physical examination, biochemistry and hematological tests, urinalysis, and electrocardiography. Adverse events were graded according to the National Cancer Institute’s Common Terminology Criteria for Adverse Events, version 3.0, and monitored continuously throughout the course of chemotherapy and the follow-up period.15
Statistical Analysis
We assumed that the 1-year PFS rate would be 0.4 in the SMILE group and that 71 PFS events would provide at least 80% power to detect a 0.5 hazard ratio (HR) favoring the DDGP group using a 2-sided log-rank test at a significance level of .05. The planned accrual period was 60 months with the total study period planned at 120 months. The total sample size was approximately 85, considering a dropout rate of 15%. The categorical outcomes were compared between the DDGP and SMILE groups using the χ2 test or Fisher exact test. All patients who received at least 1 dose of study treatment were included in the safety analysis. The survival curve was plotted by the Kaplan-Meier method, and PFS and OS were also analyzed by different subgroups. Statistical significance was determined at a level of P = .05. All statistical analyses were performed with SAS, version 9.4 (SAS Institute).
Results
Patients and Treatment
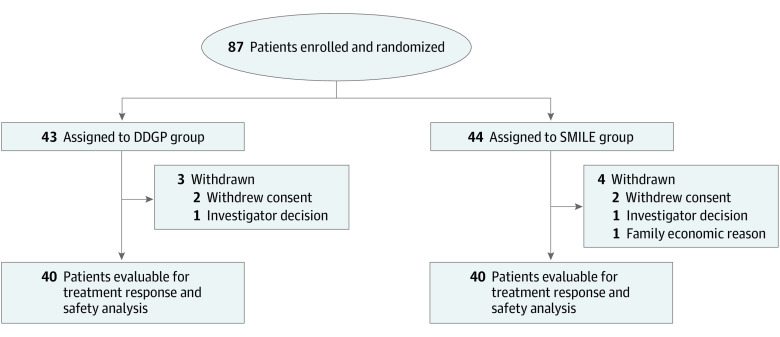
From January 2011 to February 2019, 87 eligible patients with newly diagnosed advanced ENKL were enrolled in 12 centers and randomly assigned to either the DDGP (n = 43) or SMILE (n = 44) group. The data cutoff occurred in February 2019. Seven patients (4 in the SMILE group and 3 in the DDGP group) did not receive the assigned treatment. Of these, 2 patients withdrew from the study owing to the decision of researchers and 5 patients refused or withdrew from the study for various reasons before the start of the study, as shown in Figure 1. The demographic characteristics of the patients and the disease characteristics at baseline were generally well balanced between treatment groups (Table 1).
Figure 1. CONSORT Diagram.
The DDGP regimen includes dexamethasone, cisplatin, gemcitabine, and pegaspargase; SMILE includes dexamethasone, methotrexate, ifosfamide, L-asparaginase, and etoposide.
Table 1. Baseline Characteristics of Patientsa.
| Characteristic | DDGP Group (n = 40) | SMILE Group (n = 40) |
|---|---|---|
| Age, median (IQR), y | 41.0 (19.5) | 42.5 (22.5) |
| Sex | ||
| Female | 17 (42.5) | 12 (30.0) |
| Male | 23 (57.5) | 28 (70.0) |
| Primary site | ||
| Non–upper aerodigestive tract | 2 (5.0) | 5 (12.5) |
| Upper aerodigestive tract | 38 (95.0) | 35 (87.5) |
| Cancer stage | ||
| III | 25 (62.5) | 18 (45.0) |
| IV | 15 (37.5) | 22 (55.0) |
| B Symptoms | ||
| Normal | 21 (52.5) | 19 (47.5) |
| Abnormal | 19 (47.5) | 21 (52.5) |
| Serum lactate dehydrogenase | ||
| Normal | 23 (57.5) | 18 (45.0) |
| Elevated | 17 (42.5) | 22 (55.0) |
| β2 Microglobulin level | ||
| Normal | 20 (50.0) | 20 (50.0) |
| Abnormal | 20 (50.0) | 20 (50.0) |
| International Prognostic Index score | ||
| 0-2 | 14 (35.0) | 13 (32.5) |
| 3-4 | 26 (65.0) | 27 (67.5) |
| ECOG Performance status | ||
| 0 | 6 (15.0) | 4 (10.0) |
| 1 | 24 (60.0) | 23 (57.5) |
| 2 | 10 (25.0) | 13 (32.5) |
| EBV-DNA copy numberb | ||
| Normal | 22 (55.0) | 19 (47.5) |
| Elevated | 18 (45.0) | 21 (52.5) |
Abbreviations: EBV, Epstein-Barr virus; ECOG, Eastern Cooperative Oncology Group.
No statistically significant difference was detected in the presented baseline characteristics (all P > .05).
The EBV-DNA copy number was tested with plasma samples by polymerase chain reaction method.
The average number of chemotherapy cycles was 5.75 (range, 2-6) cycles and 4.68 (range, 1-6) cycles in the DDGP and SMILE groups, respectively. Among the 40 patients in the DDGP group, 35 (88%) completed 6 cycles of the DDGP regimen, and other patients discontinued treatment owing to disease progression (n = 4) and family reasons (n = 1). Among the 40 patients in the SMILE group, 26 (65%) completed 6 cycles of the SMILE regimen, and other patients discontinued treatment owing to disease progression (n = 7), serious adverse events (n = 6), and complication (n = 1 [hemophagocytic syndrome]).
Efficacy
At a median follow-up of 41.5 months, the median PFS was not reached in the DDGP group vs 6.8 months in the SMILE group (HR, 0.42; 95% CI, 0.23-0.77; P = .004; Figure 2A), and the median OS was not reached in the DDGP group vs 75.2 months in the SMILE group (HR, 0.41; 95% CI, 0.19-0.89; P = .02; Figure 2B). The PFS rate at 3 years and OS rate at 5 years were higher in the DDGP group vs the SMILE group (3-year PFS, 56.6% vs 41.8%; 5-year OS, 74.3% vs 51.7%; Figure 2). The DDGP regimen showed consistent PFS and OS benefit over the SMILE regimen in various subgroups of patients except for patients with a primary site of non–upper aerodigestive tract (eFigures 1 and 2 in Supplement 2). The efficacy evaluation after treatment showed no statistically significant difference in the CR rate between the 2 groups, while the ORR was statistically significantly higher in the DDGP group vs the SMILE group (90.0% vs 60.0%; P = .002; Table 2).
Figure 2. Progression-Free Survival and Overall Survival by Treatment Group.
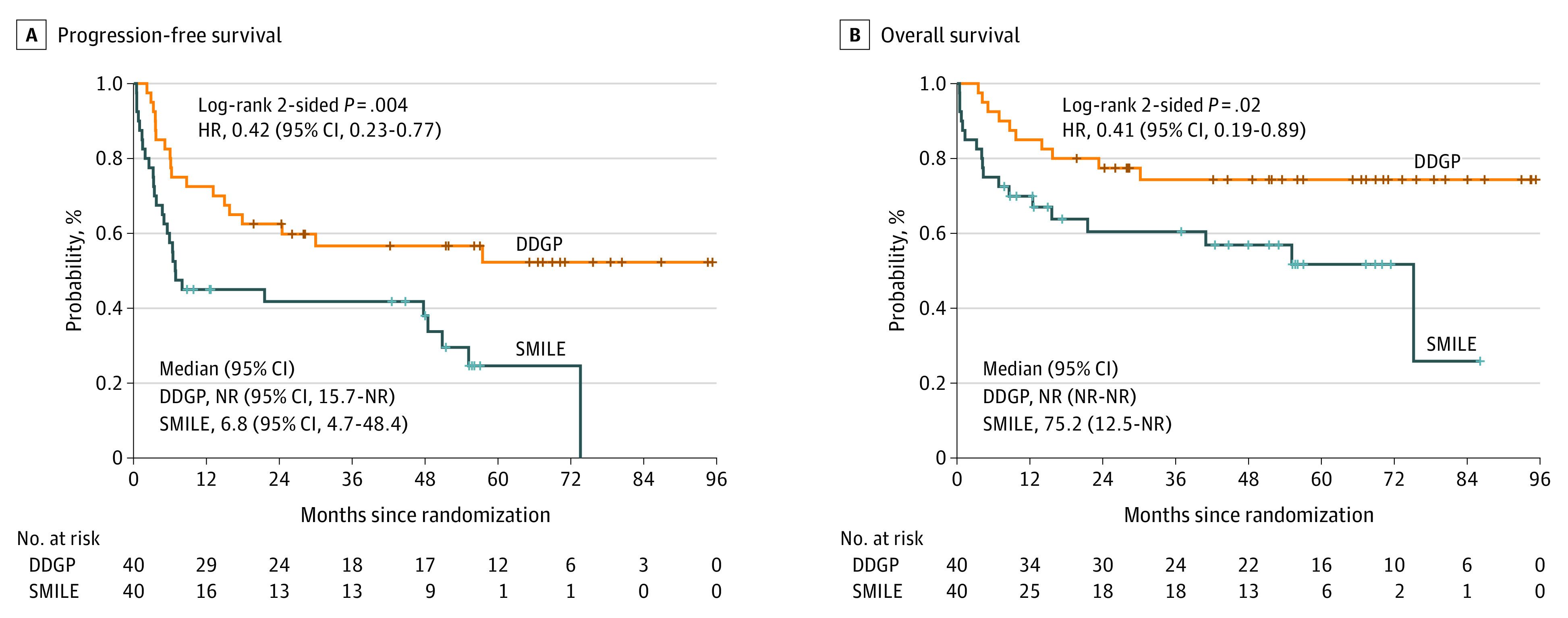
The DDGP regimen includes dexamethasone, cisplatin, gemcitabine, and pegaspargase; SMILE includes dexamethasone, methotrexate, ifosfamide, L-asparaginase, and etoposide. HR indicates hazard ratio; NR, not reached.
Table 2. Response to Treatment Among Groups.
| Response | No. (%) | P valuea | |
|---|---|---|---|
| DDGP Group (n = 40) | SMILE Group (n = 40) | ||
| CR | |||
| Yes | 27 (67.5) | 19 (47.5) | .07 |
| No | 13 (32.5) | 21 (52.5) | |
| ORR (CR + PR) | |||
| Yes | 36 (90.0) | 24 (60.0) | .002 |
| No | 4 (10.0) | 16 (40.0) | |
Abbreviations: CR, complete response; ORR, overall response rate; PR, partial response.
χ2 test.
Safety
Grade 3 and 4 hematologic toxic effects, such as leukopenia and neutropenia, were more frequent in the SMILE group vs the DDGP group (leukopenia, 85.0% vs 62.5%; neutropenia, 85.0% vs 65.0%). The prevalence of nonhematologic toxic effects, including elevated transaminase, mucositis, and allergy, were higher in the SMILE group than in the DDGP group (eTable 2 in Supplement 2). The rate of treatment-related deaths was 17.5% in the SMILE group, which was mainly caused by infection (6 patients with grade 4 neutropenia) and hemorrhage (1 patient with severe thrombopenia). The incidence of death was 2.5% in the DDGP group (1 patient with cerebral hemorrhage), and 1 patient (2.5%) in SMILE group experienced treatment-related grade 3/4 heart failure.
Discussion
In 2006, Yong et al16 reported that L-asparaginase might be a promising salvage regimen for patients with ENKL who were unsuccessful with the CHOP treatment. A French study group implemented a prospective phase 2 trial regimen, including AspaMetDex, in 19 patients with relapsed or refractory disease, and 61% of patients achieved CR.7 In some studies, the P-GEMOX regimen showed tolerable adverse effects and better efficacy for ENKL.5 Another multicenter study in China investigated the efficacy and toxic effects of combined gemcitabine, oxaliplatin, and pegasparaginase in 35 patients with newly diagnosed stage III or IV, relapsed, or refractory ENKL; the ORR was 80.09%, and the 2-year OS rate was 64.78%.17 Subsequently, in the phase 1 study of SMILE for 6 enrolled patients, 67% of the patients had objective response and 50% of the patients had a CR rate at dose level 1. Compared with the CHOP-like regimen, the SMILE regimen at dose level 1 was thought to be a promising regimen, although its safety and efficacy still required further evaluation.18 However, the SMILE regimen caused severe bone marrow suppression and increased the risk of infection, which induced additional adverse reactions such as coagulopathy, allergies, or pancreatitis, thus limiting the further application of the SMILE regimen.19,20
The refined chemotherapeutic DDGP regimen was initiated in 2010, and a patient with relapsed and refractory advanced ENKL, who was again unsuccessful after third-line chemotherapy with the DDGP regimen, was successfully treated. The lesions disappeared quickly, and the patient tolerated the DDGP regimen well. A preliminary cohort study of 12 patients with newly treated ENKL were treated with the DDGP regimen. The results showed that 8 patients achieved CR, 3 achieved partial response, ORR was 91%, and the main adverse reaction included hematologic toxic effects; grade 3 and 4 bone marrow suppression reached 24.8%, but no one discontinued treatment owing to adverse reactions. Another study retrospectively examined 17 patients with relapsed and refractory ENKL treated with the DDGP regimen and demonstrated an ORR of 88.2%; the 1-year OS rate and 1-year PFS rate were 82.4% and 64.7%, respectively.21 These preliminary studies showed that the DDGP regimen was promising and achieved better results for patients with untreated or relapsed and refractory ENKL. At that time, drugs such as L-asparaginase or pegasparaginase had just emerged to be effective against ENKL.4,22,23
However, most of the previous asparaginase-based studies were single arm or had a retrospective design. Because of the different combinations of reagents in different study groups, to our knowledge, no randomized clinical multicenter trials had been conducted to determine which regimen was the optimal treatment for ENKL. The SMILE regimen was chosen as the controlled regimen for this randomized clinical trial because it was effective for treatment of ENKL. This study, initiated in 2011, aimed to provide clinicians with a potential treatment option for newly diagnosed advanced ENKL. In 2016, we reported encouraging preliminary results, which showed that DDGP provided survival benefit in patients with newly diagnosed advanced ENKL compared with SMILE (1-year PFS rate, 86% vs 38%; P = .006; 2-year OS rate, 74% vs 45%; P = .03). The same was true for the CR rate and ORR in the DDGP vs SMILE groups (CR rate, 71% vs 29%; P = .005; ORR, 95% vs 67%; P = .02).24 Now, the final results show that the DDGP regimen had statistically significant survival advantage and better tolerability compared with the SMILE regimen. The median PFS was statistically significantly higher in the DDGP group than in the SMILE group (not reached vs 6.8 months; P = .004), and the median OS was much longer in the DDGP group than in the SMILE group (not reached vs 75.2 months; P = .02). In the present study, the mortality rate was higher in the SMILE group (17.5%) than in the DDGP group (2.5%). The SMILE regimen was administered every 28 days in the phase 2 study, while according to our clinical practice, disease progression occurred in some patients when they approached the next chemotherapy cycle. Additionally, we did not observe new safety signals when we reduced the interval from 4 weeks to 3 weeks in clinical practice. Therefore, in this clinical trial, the SMILE regimen was administrated as a 21-day cycle. Most treatment-related toxic effects were tolerable in both treatment groups. Ten patients died in the DDGP group, and 1 death (cerebral hemorrhage) was considered possibly related to treatment. Eighteen patients died in the SMILE group, with 7 deaths considered possibly treatment related (6 deaths were caused by infection, and 1 was caused by cerebral hemorrhage).
In terms of adverse reactions, although patients in the DDGP group had grade 3 and 4 anemia, it could be controlled without delay of chemotherapy. However, patients in the SMILE group had a higher rate of grade 3 and 4 leukopenia, neutropenia, allergy, and mucositis, which posed a major challenge to the follow-up treatment implementation. Most patients were prone to upper respiratory tract mucosal inflammation and ulceration owing to the damage of nasal ENKL lymphoma itself and the mucosal damage caused by a large dose of methotrexate, which prevented the smooth operation of chemotherapy. Furthermore, given the high treatment-related mortality in the SMILE group, it was particularly important to deal with adverse reactions after chemotherapy (fibrinogen and albumin supplements) and strengthen protective measures (oral care). Adverse reactions should be handled more carefully to reduce infections and protect mucosa.
Limitations
This study has limitations. The median survival time in the DDGP group was not reached owing to the considerable survival benefit in this group. The survival rates in the DDGP and SMILE groups were reported instead. Additionally, the number of patients with ENKL included was relatively small. It might induce some baseline unevenness even after randomization. Larger-scale confirmation trial is warranted.
Conclusions
This randomized clinical trial showed that when compared with the SMILE regimen, the DDGP regimen provided a safer and more effective strategy for patients with advanced-stage (III/IV) ENKL. However, a larger number of patients and more detailed data, such as genetic and molecular analyses, are needed to determine subsets of patients who are more suitable for these treatments in the future.
Trial Protocol
eFigure 1. Subgroup analysis of PFS by key factors
eFigure 2. Subgroup analysis of OS by key factors
eTable 1. Specific treatment regimens
eTable 2. Treatment-related toxicities and adverse reactions
Data Sharing Statement
References
- 1.Makita S, Tobinai K. Clinical features and current optimal management of natural killer/T-cell lymphoma. Hematol Oncol Clin North Am. 2017;31(2):239-253. doi: 10.1016/j.hoc.2016.11.007 [DOI] [PubMed] [Google Scholar]
- 2.Li YX, Yao B, Jin J, et al. Radiotherapy as primary treatment for stage IE and IIE nasal natural killer/T-cell lymphoma. J Clin Oncol. 2006;24(1):181-189. doi: 10.1200/JCO.2005.03.2573 [DOI] [PubMed] [Google Scholar]
- 3.Kwong YL, Kim SJ, Tse E, et al. Sequential chemotherapy/radiotherapy was comparable with concurrent chemoradiotherapy for stage I/II NK/T-cell lymphoma. Ann Oncol. 2018;29(1):256-263. doi: 10.1093/annonc/mdx684 [DOI] [PubMed] [Google Scholar]
- 4.Suzuki R. NK/T Cell lymphoma: updates in therapy. Curr Hematol Malig Rep. 2018;13(1):7-12. doi: 10.1007/s11899-018-0430-5 [DOI] [PubMed] [Google Scholar]
- 5.Jing XM, Zhang ZH, Wu P, et al. Efficacy and tolerance of pegaspargase, gemcitabine and oxaliplatin with sandwiched radiotherapy in the treatment of newly-diagnosed extranodal nature killer (NK)/T cell lymphoma. Leuk Res. 2016;47:26-31. doi: 10.1016/j.leukres.2016.05.004 [DOI] [PubMed] [Google Scholar]
- 6.Yamaguchi M, Kwong YL, Kim WS, et al. Phase II study of SMILE chemotherapy for newly diagnosed stage IV, relapsed, or refractory extranodal natural killer (NK)/T-cell lymphoma, nasal type: the NK-Cell Tumor Study Group study. J Clin Oncol. 2011;29(33):4410-4416. doi: 10.1200/JCO.2011.35.6287 [DOI] [PubMed] [Google Scholar]
- 7.Jaccard A, Gachard N, Marin B, et al. ; GELA and GOELAMS Intergroup . Efficacy of L-asparaginase with methotrexate and dexamethasone (AspaMetDex regimen) in patients with refractory or relapsing extranodal NK/T-cell lymphoma, a phase 2 study. Blood. 2011;117(6):1834-1839. doi: 10.1182/blood-2010-09-307454 [DOI] [PubMed] [Google Scholar]
- 8.Kim M, Kim TM, Kim KH, et al. Ifosfamide, methotrexate, etoposide, and prednisolone (IMEP) plus L-asparaginase as a first-line therapy improves outcomes in stage III/IV NK/T cell-lymphoma, nasal type (NTCL). Ann Hematol. 2015;94(3):437-444. doi: 10.1007/s00277-014-2228-4 [DOI] [PubMed] [Google Scholar]
- 9.Wei W, Wu P, Li L, Zhang ZH. Effectiveness of pegaspargase, gemcitabine, and oxaliplatin (P-GEMOX) chemotherapy combined with radiotherapy in newly diagnosed, stage IE to IIE, nasal-type, extranodal natural killer/T-cell lymphoma. Hematology. 2017;22(6):320-329. doi: 10.1080/10245332.2016.1264163 [DOI] [PubMed] [Google Scholar]
- 10.Jeong SH, Song HN, Park JS, et al. Allogeneic stem cell transplantation for patients with natural killer/T cell lymphoid malignancy: a multicenter analysis comparing upfront and salvage transplantation. Biol Blood Marrow Transplant. 2018;24(12):2471-2478. doi: 10.1016/j.bbmt.2018.07.034 [DOI] [PubMed] [Google Scholar]
- 11.Kwong YL, Chan TSY, Tan D, et al. PD1 blockade with pembrolizumab is highly effective in relapsed or refractory NK/T-cell lymphoma failing l-asparaginase. Blood. 2017;129(17):2437-2442. doi: 10.1182/blood-2016-12-756841 [DOI] [PubMed] [Google Scholar]
- 12.Kawamoto K, Miyoshi H, Suzuki T, et al. Frequent expression of CD30 in extranodal NK/T-cell lymphoma: potential therapeutic target for anti-CD30 antibody-based therapy. Hematol Oncol. 2018;36(1):166-173. doi: 10.1002/hon.2482 [DOI] [PubMed] [Google Scholar]
- 13.Lin T, Hong H, Liang C, et al. Extranodal natural killer T-cell lymphoma, nasal-type—a new staging system from CSWOG—a multicenter study. J Clin Oncol. 2014;32(15):8552. doi: 10.1200/jco.2014.32.15_suppl.8552 [DOI] [Google Scholar]
- 14.Cheson BD, Pfistner B, Juweid ME, et al. ; International Harmonization Project on Lymphoma . Revised response criteria for malignant lymphoma. J Clin Oncol. 2007;25(5):579-586. doi: 10.1200/JCO.2006.09.2403 [DOI] [PubMed] [Google Scholar]
- 15.Trotti A, Colevas AD, Setser A, et al. CTCAE v3.0: development of a comprehensive grading system for the adverse effects of cancer treatment. Semin Radiat Oncol. 2003;13(3):176-181. doi: 10.1016/S1053-4296(03)00031-6 [DOI] [PubMed] [Google Scholar]
- 16.Yong W, Zheng W, Zhu J, et al. Midline NK/T-cell lymphoma nasal-type: treatment outcome, the effect of L-asparaginase based regimen, and prognostic factors. Hematol Oncol. 2006;24(1):28-32. doi: 10.1002/hon.765 [DOI] [PubMed] [Google Scholar]
- 17.Wang JH, Wang L, Liu CC, et al. Efficacy of combined gemcitabine, oxaliplatin and pegaspargase (P-gemox regimen) in patients with newly diagnosed advanced-stage or relapsed/refractory extranodal NK/T-cell lymphoma. Oncotarget. 2016;7(20):29092-29101. doi: 10.18632/oncotarget.8647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yamaguchi M, Suzuki R, Kwong YL, et al. Phase I study of dexamethasone, methotrexate, ifosfamide, L-asparaginase, and etoposide (SMILE) chemotherapy for advanced-stage, relapsed or refractory extranodal natural killer (NK)/T-cell lymphoma and leukemia. Cancer Sci. 2008;99(5):1016-1020. doi: 10.1111/j.1349-7006.2008.00768.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kwong YL, Kim WS, Lim ST, et al. SMILE for natural killer/T-cell lymphoma: analysis of safety and efficacy from the Asia Lymphoma Study Group. Blood. 2012;120(15):2973-2980. doi: 10.1182/blood-2012-05-431460 [DOI] [PubMed] [Google Scholar]
- 20.Yong W. Clinical study of l-asparaginase in the treatment of extranodal NK/T-cell lymphoma, nasal type. Hematol Oncol. 2016;34(2):61-68. doi: 10.1002/hon.2207 [DOI] [PubMed] [Google Scholar]
- 21.Zhou Z, Li X, Chen C, et al. Effectiveness of gemcitabine, pegaspargase, cisplatin, and dexamethasone (DDGP) combination chemotherapy in the treatment of relapsed/refractory extranodal NK/T cell lymphoma: a retrospective study of 17 patients. Ann Hematol. 2014;93(11):1889-1894. doi: 10.1007/s00277-014-2136-7 [DOI] [PubMed] [Google Scholar]
- 22.Kim SJ, Yoon SE, Kim WS. Treatment of localized extranodal NK/T cell lymphoma, nasal type: a systematic review. J Hematol Oncol. 2018;11(1):140. doi: 10.1186/s13045-018-0687-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Allen PB, Lechowicz MJ. Management of NK/T-cell lymphoma, nasal type. J Oncol Pract. 2019;15(10):513-520. doi: 10.1200/JOP.18.00719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li X, Cui Y, Sun Z, et al. DDGP versus SMILE in newly diagnosed advanced natural killer/T-cell lymphoma: a randomized controlled, multicenter, open-label study in China. Clin Cancer Res. 2016;22(21):5223-5228. doi: 10.1158/1078-0432.CCR-16-0153 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol
eFigure 1. Subgroup analysis of PFS by key factors
eFigure 2. Subgroup analysis of OS by key factors
eTable 1. Specific treatment regimens
eTable 2. Treatment-related toxicities and adverse reactions
Data Sharing Statement