Key Points
Question
In critically ill adult patients undergoing tracheal intubation, does intravenous infusion of a crystalloid solution as a 500-mL fluid bolus decrease the incidence of severely low blood pressure, cardiac arrest, or death (referred to as cardiovascular collapse) during or shortly after the procedure?
Findings
In this randomized clinical trial that included 1065 critically ill adults, the incidence of cardiovascular collapse was 21.0% with administration of a fluid bolus vs 18.2% without administration of a fluid bolus, a difference that was not statistically significant.
Meaning
Among critically ill adults undergoing tracheal intubation, administration of a fluid bolus did not significantly decrease the incidence of cardiovascular collapse.
Abstract
Importance
Hypotension is common during tracheal intubation of critically ill adults and increases the risk of cardiac arrest and death. Whether administering an intravenous fluid bolus to critically ill adults undergoing tracheal intubation prevents severe hypotension, cardiac arrest, or death remains uncertain.
Objective
To determine the effect of fluid bolus administration on the incidence of severe hypotension, cardiac arrest, and death.
Design, Setting, and Participants
This randomized clinical trial enrolled 1067 critically ill adults undergoing tracheal intubation with sedation and positive pressure ventilation at 11 intensive care units in the US between February 1, 2019, and May 24, 2021. The date of final follow-up was June 21, 2021.
Interventions
Patients were randomly assigned to receive either a 500-mL intravenous fluid bolus (n = 538) or no fluid bolus (n = 527).
Main Outcomes and Measures
The primary outcome was cardiovascular collapse (defined as new or increased receipt of vasopressors or a systolic blood pressure <65 mm Hg between induction of anesthesia and 2 minutes after tracheal intubation, or cardiac arrest or death between induction of anesthesia and 1 hour after tracheal intubation). The secondary outcome was the incidence of death prior to day 28, which was censored at hospital discharge.
Results
Among 1067 patients randomized, 1065 (99.8%) completed the trial and were included in the primary analysis (median age, 62 years [IQR, 51-70 years]; 42.1% were women). Cardiovascular collapse occurred in 113 patients (21.0%) in the fluid bolus group and in 96 patients (18.2%) in the no fluid bolus group (absolute difference, 2.8% [95% CI, −2.2% to 7.7%]; P = .25). New or increased receipt of vasopressors occurred in 20.6% of patients in the fluid bolus group compared with 17.6% of patients in the no fluid bolus group, a systolic blood pressure of less than 65 mm Hg occurred in 3.9% vs 4.2%, respectively, cardiac arrest occurred in 1.7% vs 1.5%, and death occurred in 0.7% vs 0.6%. Death prior to day 28 (censored at hospital discharge) occurred in 218 patients (40.5%) in the fluid bolus group compared with 223 patients (42.3%) in the no fluid bolus group (absolute difference, −1.8% [95% CI, −7.9% to 4.3%]; P = .55).
Conclusions and Relevance
Among critically ill adults undergoing tracheal intubation, administration of an intravenous fluid bolus compared with no fluid bolus did not significantly decrease the incidence of cardiovascular collapse.
Trial Registration
ClinicalTrials.gov Identifier: NCT03787732
This randomized clinical trial compares the effect of fluid bolus administration vs no fluid bolus on the incidence of severe hypotension, cardiac arrest, and death in critically ill adults undergoing tracheal intubation with sedation and positive pressure ventilation in the intensive care unit.
Introduction
From 2014 to 2018, approximately 2 million critically ill adults underwent tracheal intubation each year in the US.1 Hypotension occurs during 25% to 40% of tracheal intubations in the intensive care unit (ICU)2,3 and can lead to cardiac arrest and death.2,4,5,6
Hypotension during tracheal intubation results, in part, from medication-induced vasodilation and decreased return of venous blood to the heart due to increased intrathoracic pressure from positive pressure ventilation.7,8 The intravenous administration of a crystalloid solution (referred to as a fluid bolus) might counteract these effects by transiently increasing intravascular volume. Current international guidelines9,10,11 and expert recommendations12 suggest that critically ill adults undergoing tracheal intubation receive a fluid bolus. A fluid bolus is administered during approximately 40% to 50% of emergency tracheal intubations in current clinical practice.2
One prior randomized clinical trial examined whether administration of a fluid bolus to critically ill adults undergoing tracheal intubation prevents severe hypotension, cardiac arrest, and death (referred to as cardiovascular collapse).5 The trial found that administration of a fluid bolus did not affect the risk of cardiovascular collapse overall, but appeared beneficial among patients who received positive pressure ventilation with a bag-mask device or a noninvasive ventilator during the tracheal intubation procedure. This suggested a plausible link between reduced cardiac venous return from positive pressure ventilation and a beneficial effect of a fluid bolus.8,13 Positive pressure ventilation has been demonstrated to prevent hypoxemia14,15 and is received by the majority of ICU patients undergoing tracheal intubation.2
The Preventing Cardiovascular Collapse With Administration of Fluid Resuscitation During Induction and Intubation (PREPARE II) trial examined the effect of intravenous fluid bolus administration on cardiovascular collapse among critically ill adults undergoing tracheal intubation with positive pressure ventilation. The hypothesis was that administration of an intravenous fluid bolus would decrease the incidence of cardiovascular collapse.
Methods
Trial Design and Oversight
This multicenter, parallel-group, unblinded, pragmatic randomized clinical trial compared administration of an intravenous fluid bolus vs no fluid bolus for critically ill adults undergoing tracheal intubation. The trial was approved with a waiver of informed consent by the central institutional review board at Vanderbilt University Medical Center and the local institutional review board at each trial site through reliance agreement or primary review.
The trial was registered prior to enrollment and was overseen by an independent data and safety monitoring board. Enrollment began on February 1, 2019, was paused from February 28, 2020, until August 24, 2020, during the COVID-19 pandemic, and concluded on May 24, 2021. The trial protocol and statistical analysis plan were published16 before enrollment concluded and appear in Supplement 1.
Trial Sites and Patient Population
The trial was conducted in 11 ICUs across the US. Adult patients (aged ≥18 years) were eligible if they were undergoing tracheal intubation with the planned use of (1) medications to induce anesthesia and (2) positive pressure ventilation with either a bag-mask device or a noninvasive ventilator between induction of anesthesia and laryngoscopy. Limiting eligibility to patients receiving positive pressure ventilation was a predictive enrichment strategy17,18 intended to selectively enroll patients more likely to benefit from administration of a fluid bolus.
Patients were excluded if they were pregnant, were incarcerated, had an immediate need for tracheal intubation that precluded randomization, or if the clinician performing the tracheal intubation procedure (referred to as the operator) determined that administration of a fluid bolus during tracheal intubation was either required or contraindicated. Details of the trial sites and complete lists of the inclusion and exclusion criteria appear in the eMethods in Supplement 2.
Randomization
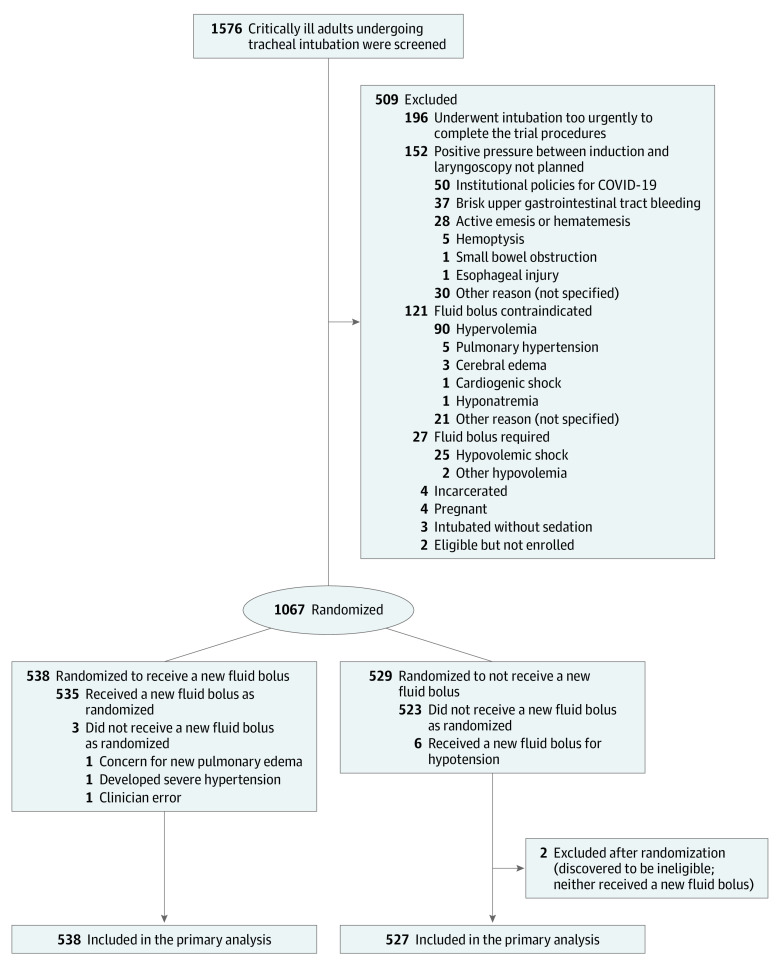
Patients were randomized using a 1:1 ratio to receive or not receive an intravenous fluid bolus (Figure 1) according to a computer-generated list that used randomly permuted block sizes of 2, 4, and 6 and stratified patients according to trial site. Trial group assignments were placed in sequentially numbered opaque envelopes and remained concealed until after enrollment. Given the nature of the intervention, operators and research personnel were aware of trial group assignments after randomization.
Figure 1. Flow of Participants Through the PREPARE II Trial.
PREPARE II indicates Preventing Cardiovascular Collapse With Administration of Fluid Resuscitation During Induction and Intubation.
Trial Interventions
For patients assigned to the fluid bolus group, operators were instructed to intravenously infuse 500 mL of an isotonic crystalloid solution of the operator’s choice. Operators were instructed to: (1) infuse the fluid from above the level of the intravenous or intraosseous access by gravity, manual pressure, or bag pressure; (2) infuse as much of the 500-mL solution before induction of anesthesia could be achieved without delaying the tracheal intubation procedure; and (3) infuse any of the 500-mL solution that remained after induction of anesthesia during the tracheal intubation procedure.
For patients assigned to the no fluid bolus group, initiation of a new intravenous fluid bolus was not permitted except as treatment for hypotension or if the operator determined that an intravenous fluid bolus was necessary for the safety of the patient.
As a pragmatic trial, delivery of the assigned intervention occurred within routine clinical care, and trial group assignment determined only whether a fluid bolus was initiated between enrollment and induction of anesthesia. In either trial group, patients could continue to receive intravenous fluid infusions that had been initiated prior to enrollment. All other aspects of the tracheal intubation procedure were deferred to the operator, including the choice of induction agents, the use of vasopressors to prevent or treat hypotension, and the use of a fluid bolus to treat hypotension after induction of anesthesia.
Data Collection
Trial personnel or a clinician trained according to the trial protocol, but who was not involved in the performance of the tracheal intubation, collected data during the procedure, including the volume of intravenous fluid administered, the lowest levels of systolic blood pressure and oxygen saturation, and initiation of or increased dose of vasopressors. Immediately after tracheal intubation, the operator recorded the induction agent used, the devices used to provide supplemental oxygen or ventilation before the induction of anesthesia and between the induction of anesthesia and laryngoscopy, and the occurrence of complications, including cardiac arrest.
Research personnel collected data on baseline characteristics, patient management before and after laryngoscopy, and clinical outcomes from the electronic health record. Race and ethnicity were self-reported by patients or reported by their surrogates as part of clinical care. Race and ethnicity were collected from the electronic health record by research personnel using fixed categories to facilitate assessment of the representativeness of the trial population and the generalizability of the trial results.
Outcomes
The prespecified primary outcome was cardiovascular collapse,3,5,19 which was defined as the occurrence of 1 or more of the following: new or increased receipt of vasopressors (bolus or infusion) between induction of anesthesia and 2 minutes after tracheal intubation; a systolic blood pressure of less than 65 mm Hg between induction of anesthesia and 2 minutes after tracheal intubation; cardiac arrest between induction of anesthesia and 1 hour after tracheal intubation; or death between induction of anesthesia and 1 hour after tracheal intubation. The single prespecified secondary outcome was the incidence of death prior to day 28, which was censored at hospital discharge. Exploratory procedural, safety, and clinical outcomes are described in the eMethods in Supplement 2.
Sample Size Calculation
Details regarding the determination of the sample size have been reported previously.16 Assuming that 25% of patients in the no fluid bolus group would experience cardiovascular collapse and anticipating less than 5% of missing data for the primary outcome, it was determined that enrollment of 750 patients would provide 80% power at a 2-sided α level of .05 to detect a between-group absolute difference of 8.75% (a relative risk difference of 35%), which is smaller than the absolute difference of 10.2% (a relative risk difference of 40%) that was observed among patients receiving positive pressure ventilation in a prior trial.5
As specified in the initial trial protocol (Supplement 1), the data and safety monitoring board examined the observed incidence of cardiovascular collapse in the no fluid bolus group during the interim analysis after enrollment of 375 patients. Because the observed incidence of cardiovascular collapse was lower than expected, the sample size was increased to 1065 patients based on the recommendation of the data and safety monitoring board to maintain 80% statistical power to detect a between-group relative risk difference of 35%. Study personnel remained blinded to the results of the interim analysis and trial outcomes until enrollment was completed and the database was locked (additional details of the sample size calculation appear in Supplement 1 and in the eMethods in Supplement 2).
Statistical Analysis
The primary analysis was an unadjusted comparison of the primary outcome between patients assigned to the 2 trial groups using the χ2 test, with the results reported as an absolute risk difference and 95% CI. The primary analysis included all randomized patients; however, 2 patients were withdrawn from the study after randomization for incarcerated status, which was identified after tracheal intubation. Patients were analyzed according to the group to which they were randomly assigned. The sensitivity analyses used alternate definitions of the components of the primary outcome or alternative methods of analysis, including an ordinal regression analysis that ranked the components of the primary outcome from least severe (new or increased receipt of vasopressors) to most severe (death) (additional details appear in the eMethods in Supplement 2).
Additional analyses included (1) a generalized, linear mixed-effects model using a logit link function with a random effect for trial site and a fixed effect for trial group and (2) a logistic regression model adjusting for the following prespecified baseline covariates: age, Acute Physiology and Chronic Health Evaluation II score,20 sepsis or septic shock, receipt of vasopressors, and receipt of intravenous fluids prior to enrollment. In the adjusted analyses, missing data for baseline covariates were imputed using multiple imputations. Effect modification was assessed using unadjusted logistic regression models that included study group assignment, the prespecified effect modifier of interest, and the interaction between the 2. Further details appear in the eMethods in Supplement 2 and in the statistical analysis plan in Supplement 1.16
After enrollment of 375 patients, the data and safety monitoring board conducted a single, planned interim analysis comparing the incidence of cardiovascular collapse between groups using a Haybittle-Peto stopping boundary of P < .001 for efficacy. For the final analysis of the primary outcome, a 2-sided P < .05 was considered to indicate statistical significance. Secondary and exploratory outcomes were compared between groups using the χ2 test for categorical outcomes and the Mann-Whitney test for continuous variables using complete case analysis. Between-group differences were reported as point estimates and 2-sided 95% CIs. The widths of the 95% CIs were not adjusted for multiplicity. Findings for these analyses should be considered exploratory. All analyses were performed using R version 4.1.0 (R Foundation for Statistical Computing).
Results
Patients
Of 1576 patients screened, 196 were excluded due to the urgency of the tracheal intubation procedure, 152 were excluded because positive pressure ventilation during tracheal intubation was not planned, 121 were excluded because a fluid bolus was contraindicated, 27 were excluded because a fluid bolus was required, and 13 were excluded for other reasons, leaving 1067 who were randomized and enrolled in the trial (Figure 1). Two patients were determined to be incarcerated after enrollment and were excluded from subsequent data collection and analysis. The remaining 1065 patients were included in the primary analysis (Figure 1). The median age was 62 years (IQR, 51-70 years), 448 (42.1%) were women, and approximately 60% of patients had acute respiratory failure as an indication for tracheal intubation. Approximately 20% of patients were receiving vasopressors and 10% were receiving intravenous fluid at the time of enrollment. A total of 538 patients (50.5%) were assigned to the fluid bolus group and 527 patients (49.5%) were assigned to the no fluid bolus group (Table 1 and eTables 1-4 in Supplement 2). The date of final follow-up was June 21, 2021.
Table 1. Baseline Characteristics.
| Characteristic | Fluid bolus (n = 538) | No fluid bolus (n = 527) |
|---|---|---|
| Age, median (IQR), y | 61 (51-70) | 62 (51-71) |
| Sex, No. (%) | ||
| Female | 220 (40.9) | 228 (43.3) |
| Male | 318 (59.1) | 299 (56.7) |
| Race and ethnicity, No. (%)a | (n = 536) | (n = 525) |
| American Indian/Alaska Native | 4 (0.7) | 3 (0.6) |
| Asian | 7 (1.3) | 11 (2.1) |
| Hispanic | 24 (4.5) | 23 (4.4) |
| Native Hawaiian/Other Pacific Islander | 1 (0.2) | 0 |
| Non-Hispanic Black | 135 (25.2) | 104 (19.8) |
| Non-Hispanic White | 355 (66.2) | 383 (73.0) |
| Otherb | 10 (1.9) | 2 (0.4) |
| Weight, median (IQR), kg | 81.2 (65.8-99.8) | 80.7 (67.6-99.3) |
| Body mass index, median (IQR)c | (n = 537) 27.6 (23.6-33.3) |
(n = 526) 27.7 (23.5-32.7) |
| Chronic condition, No. (%)d | ||
| Obesity | 199 (37.0) | 196 (37.2) |
| Hypertension | 181 (33.6) | 180 (34.2) |
| Diabetes | 141 (26.2) | 151 (28.7) |
| Malignancy (hematologic and solid tumor) | 106 (19.7) | 106 (20.1) |
| Chronic obstructive pulmonary disease | 101 (18.8) | 90 (17.1) |
| Congestive heart failure | 83 (15.4) | 73 (13.9) |
| Kidney failure | 22 (4.1) | 32 (6.1) |
| Active conditions, No. (%)d | ||
| Sepsis or septic shock | 312 (58.0) | 318 (60.3) |
| Sepsise | 217 (40.3) | 210 (39.8) |
| Septic shockf | 95 (17.7) | 108 (20.5) |
| Pneumonia | 133 (24.7) | 123 (23.3) |
| Acute respiratory distress syndrome | 58 (10.8) | 71 (13.5) |
| Gastrointestinal tract hemorrhage | 59 (11.0) | 50 (9.5) |
| COVID-19 | 31 (5.8) | 30 (5.7) |
| Indication for tracheal intubation, No. (%)d | ||
| Respiratory failure characterized by hypoxia | 222 (41.3) | 226 (42.9) |
| Altered mental status | 110 (20.4) | 106 (20.1) |
| Respiratory failure characterized by hypoxia and hypercarbia | 61 (11.3) | 56 (10.6) |
| Facilitation of urgent procedure | 43 (8.0) | 37 (7.0) |
| Respiratory failure characterized by hypercarbia | 37 (6.9) | 42 (8.0) |
| Other | 65 (12.1) | 60 (11.4) |
| APACHE II score, median (IQR)g | 20 (14-25) | 18 (14-25) |
| Use of vasopressor within prior 1 h, No. (%) | (n = 536) 107 (20.0) |
(n = 527) 102 (19.4) |
| Receiving intravenous fluid at enrollment, No. (%)h | 55 (10.2) | 52 (9.9) |
Self-reported or reported by a surrogate and collected from the electronic health record by research personnel using these fixed categories. More than 1 race and ethnicity could be reported.
Recorded when not represented by any of the fixed categories.
Calculated as weight in kilograms divided by height in meters squared.
Could have more than 1. The complete lists of chronic conditions, active conditions, and indication for tracheal intubation appear in eTables 1-3 in Supplement 2.
Defined as life-threatening organ dysfunction caused by a dysregulated host response to infection.
Defined as sepsis plus requirement of vasopressors to maintain a mean arterial pressure of 65 mm Hg or greater and a serum lactate level greater than 2 mmol/L in the absence of hypovolemia.
Scores on the Acute Physiology and Chronic Health Evaluation (APACHE) II range from 0 to 71; higher scores indicate a greater severity of illness.20 The scores were calculated using the most extreme (worst) values during the 24 hours preceding enrollment. Severe organ insufficiency was defined as any of the following: cirrhosis with portal hypertension; New York Heart Association class IV heart failure; chronic restrictive, obstructive, or vascular disease resulting in severe exercise restriction or documented chronic hypoxia, hypercapnia, or pulmonary hypertension; undergoing chronic dialysis; or immunocompromised (defined as immunosuppression; receiving chemotherapy, radiation, or steroids; or having leukemia, lymphoma, or AIDS).
Defined as ongoing infusion of a crystalloid or colloid solution.
Receipt of Intravenous Fluid
An intravenous fluid bolus was initiated between enrollment and induction of anesthesia in 535 patients (99.4%) in the fluid bolus group and in 6 patients (1.1%) in the no fluid bolus group (Table 2 and eTable 5 in Supplement 2). The median volume of intravenous fluid received between randomization and 2 minutes after tracheal intubation was 500 mL (IQR, 300-500 mL) in the fluid bolus group and 0 mL (IQR, 0-0 mL) in the no fluid bolus group. In the fluid bolus group, the majority of the fluid bolus (median, 300 mL; IQR, 150-450 mL) was administered prior to the induction of anesthesia (eFigure 1 in Supplement 2).
Table 2. Characteristics of the Tracheal Intubation Procedures.
| Characteristic | Fluid bolus (n = 538) | No fluid bolus (n = 527) | Difference (95% CI)a |
|---|---|---|---|
| Intravenous fluids | |||
| Fluid bolus initiated prior to induction of anesthesia, No. (%) | 535 (99.4) | 6 (1.1) | Absolute, 98.3 (97.0 to 99.6) |
| Volume of fluid infused between enrollment and 2 minutes after tracheal intubation | (n = 536) | (n = 526) | |
| Median (IQR), mL | 500 (300 to 500) | 0 (0 to 0) | Median, 500 (450 to 500) |
| Per body weight, median (IQR), mL/kg | 5.0 (3.5 to 6.8) | 0 (0 to 0) | Median, 5.0 (4.8 to 5.3) |
| New or additional fluid bolus initiated after induction of anesthesia for the treatment of hypotension, No. (%) | 20 (3.7) | 31 (5.9) | Absolute, −2.2 (−4.9 to 0.6) |
| Management of tracheal intubation | |||
| Preoxygenation method, No. (%)b | |||
| Bilevel positive airway pressure | 161 (29.9) | 148 (28.1) | Absolute, 1.8 (−3.8 to 7.5) |
| Bag-mask device | 151 (28.1) | 173 (32.8) | Absolute, −4.8 (−10.5 to 0.9) |
| Nonrebreather mask | 133 (24.7) | 129 (24.5) | Absolute, 0.2 (−5.1 to 5.6) |
| High-flow nasal cannula | 102 (19.0) | 95 (18.0) | Absolute, 0.9 (−3.9 to 5.8) |
| Standard nasal cannula | 55 (10.2) | 43 (8.2) | Absolute, 2.1 (−1.6 to 5.7) |
| Did not receive preoxygenation | 4 (0.7) | 2 (0.4) | Absolute, 0.4 (−0.7 to 1.4) |
| Vasopressor bolus or infusion administered between enrollment and induction of anesthesia for prevention of hypotension, No. (%)c | (n = 538) 66 (12.3) |
(n = 526) 62 (11.8) |
Absolute, 0.5 (−3.6 to 4.6) |
| Oxygen saturation at induction of anesthesia, median (IQR), % | (n = 531) 99 (96 to 100) |
(n = 521) 99 (96 to 100) |
Median, 0 (−0 to 1.0) |
| Systolic blood pressure at induction of anesthesia, median (IQR), mm Hg | (n = 538) 128 (110 to 147) |
(n = 526) 126 (110 to 145) |
Median, 2.0 (−2.0 to 6.5) |
| Induction agent, No. (%)d | 536 (99.6) | 526 (99.8) | Absolute, −0.2 (−1.0 to 0.6) |
| Etomidate | 413 (76.8) | 416 (78.9) | Absolute, −2.2 (−7.3 to 3.0) |
| Ketamine | 66 (12.3) | 55 (10.4) | Absolute, 1.8 (−2.2 to 5.8) |
| Propofol | 53 (9.9) | 57 (10.8) | Absolute, −1.0 (−4.8 to 2.9) |
| Neuromuscular blocking agent, No. (%)e | 509 (94.6) | 492 (93.4) | Absolute, 1.3 (−1.8 to 4.3) |
| Rocuronium | 402 (74.7) | 378 (71.7) | Absolute, 3.0 (−2.5 to 8.5) |
| Succinylcholine | 105 (19.5) | 109 (20.7) | Absolute, −1.2 (−6.2 to 3.8) |
| Positive pressure ventilation delivered between induction of anesthesia and laryngoscopy, No. (%)f | 526 (97.8) | 513 (97.3) | Absolute, 0.4 (−1.6 to 2.5) |
The absolute differences are expressed as percentages.
Patients could receive more than 1. Preoxygenation via bag mask with concurrent ventilation and noninvasive ventilation were considered positive pressure ventilation mechanisms, whereas provision of bag-mask preoxygenation without ventilation was not (eFigure 4 in Supplement 2).
As either a 1-time bolus, a new infusion, or an increased rate of infusion for the purpose of preventing procedural hypotension.
Patients could receive more than 1. A complete list and the dosages appear in eTable 6 in Supplement 2.
A complete list and the dosages appear in eTable 6 in Supplement 2.
Included ventilation provided through a bag-mask device or a noninvasive ventilator.
A total of 20 patients (3.7%) in the fluid bolus group and 31 patients (5.9%) in the no fluid bolus group received a new or additional fluid bolus initiated after induction of anesthesia for the treatment of hypotension.
Management of Tracheal Intubation
The approach to preoxygenation, choice of agents for induction of anesthesia and neuromuscular blockade, and levels of systolic blood pressure and oxygen saturation at induction of anesthesia were not significantly different between groups (Table 2 and eTables 6-7 in Supplement 2). A total of 66 patients (12.3%) in the fluid bolus group and 62 patients (11.8%) in the no fluid bolus group had a vasopressor bolus or infusion administered between enrollment and induction of anesthesia for prevention of hypotension. A total of 526 patients (97.8%) in the fluid bolus group and 513 patients (97.3%) in the no fluid bolus group received positive pressure ventilation with a bag-mask device or a noninvasive ventilator between induction of anesthesia and laryngoscopy (eTable 7 in Supplement 2).
Primary Outcome
Data for the primary outcome were available for all patients. Cardiovascular collapse occurred in 113 patients (21.0%) in the fluid bolus group and in 96 patients (18.2%) in the no fluid bolus group (absolute difference, 2.8% [95% CI, −2.2% to 7.7%], P = .25; Table 3, Figure 2, and eFigure 2 in Supplement 2). Cardiovascular collapse did not significantly differ between groups in the sensitivity analyses (eTable 8 in Supplement 2) or in the adjusted analyses (eTable 9 and eFigure 3 in Supplement 2). Administration of a fluid bolus did not significantly decrease the incidence of cardiovascular collapse in any prespecified subgroup (Figure 2 and eFigures 4-7 in Supplement 2).
Table 3. Outcomes of Tracheal Intubation.
| Fluid bolus (n = 538) | No fluid bolus (n = 527) | Difference (95% CI)a | |
|---|---|---|---|
| Primary outcome | |||
| Cardiovascular collapse, No. (%)b | 113 (21.0) | 96 (18.2) | Absolute, 2.8 (−2.2 to 7.7) |
| New or increased receipt of vasopressors | 111 (20.6) | 93 (17.6) | Absolute, 3.0 (−1.9 to 7.9) |
| Systolic blood pressure <65 mm Hgc | (n = 535) 21 (3.9) |
(n = 524) 22 (4.2) |
Absolute, −0.3 (−2.8 to 2.3) |
| Cardiac arrest | 9 (1.7) | 8 (1.5) | Absolute, 0.2 (−1.5 to 1.8) |
| Death | 4 (0.7) | 3 (0.6) | Absolute, 0.2 (−1.0 to 1.3) |
| Secondary outcome | |||
| In-hospital death prior to 28 d, No. (%) | 218 (40.5) | 223 (42.3) | Absolute, −1.8 (−7.9 to 4.3) |
| Exploratory procedural outcomes d | |||
| Systolic blood pressure, median (IQR), mm Hgc | |||
| Lowest level | 116 (93 to 139) | 113 (95 to 134) | Median, 3.0 (−3.0 to 7.0) |
| Change in level | −7 (−26 to 0) | −9 (−27 to 0) | Median, 2.0 (−2.0 to 5.0) |
| Lowest arterial oxygen saturation, median (IQR), mm Hg | 96 (86 to 100) | 96 (88 to 100) | Median, 0 (−2.0 to 1.0) |
| Oxygen saturation <80%, No. (%) | (n = 531) 79 (14.9) |
(n = 518) 71 (13.7) |
Absolute, 1.2 (−3.3 to 5.6) |
| Exploratory clinical outcomes, median (IQR) | |||
| Invasive mechanical ventilation–free days through 28 de | 14 (0 to 25) | 12 (0 to 25) | Median, 2.0 (−10.0 to 15.0) |
| Intensive care unit–free days through 28 df | 9 (0 to 22) | 9 (0 to 22) | Median, −0.5 (−9.0 to 9.5) |
The absolute differences are expressed as percentages.
The between-group difference was not statistically significant (P = .25 with χ2 test). Cardiovascular collapse was defined as the occurrence of 1 or more of the following: a systolic blood pressure of less than 65 mm Hg between induction of anesthesia and 2 minutes after tracheal intubation; new or increased receipt of vasopressors between induction of anesthesia and 2 minutes after tracheal intubation; cardiac arrest within 1 hour of tracheal intubation; or death within 1 hour of tracheal intubation. Patients could experience more than 1 component of the composite primary outcome.
Data recorders were instructed to report no value for the lowest systolic blood pressure if no blood pressure reading could be obtained (eg, during cardiac arrest). All 6 patients for whom lowest systolic blood pressure was missing met the primary outcome of cardiovascular collapse; 1 such patient in the fluid bolus group died between induction of anesthesia and 1 hour after the procedure. 4 of these patients (3 in the fluid bolus group and 1 in the no fluid bolus group) experienced cardiac arrest between induction of anesthesia and 1 hour after the procedure, and all 6 had new or increased receipt of vasopressors between induction of anesthesia and 2 minutes after tracheal intubation.
Between induction of anesthesia and 2 minutes after completion of the tracheal intubation procedure.
Defined as the number of calendar days between enrollment and 28 days after enrollment in which the patient was alive and not receiving invasive mechanical ventilation after the patient’s final receipt of invasive mechanical ventilation. Patients who died before day 28 received a value of 0.
Calculated using the same approach as invasive mechanical ventilation–free days. Further details appear in eTable 10 in Supplement 2.
Figure 2. Effect of Fluid Bolus Administration on Cardiovascular Collapse.
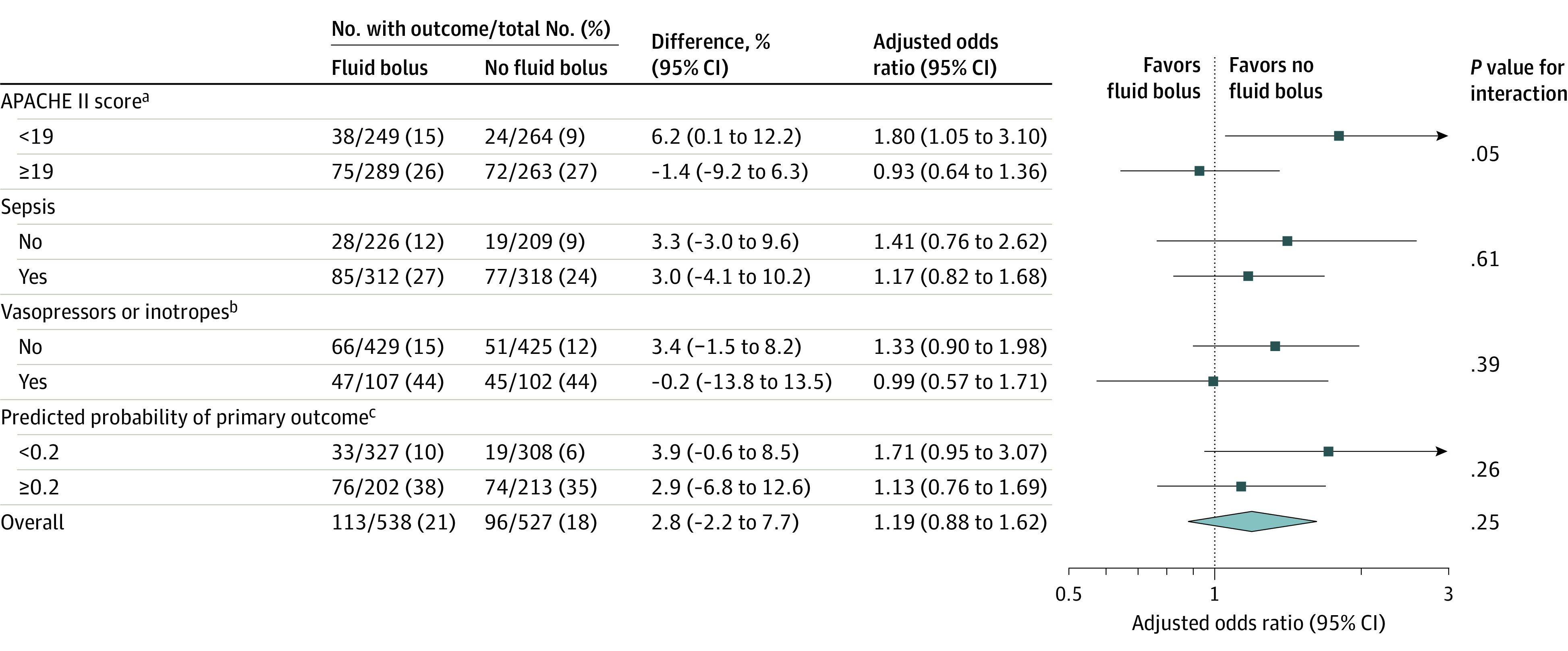
aScores on the Acute Physiology and Chronic Health Evaluation (APACHE) II can range from 0 to 71; higher scores indicate a greater severity of illness.20 Patients were dichotomized at the median APACHE II score of the cohort, which was 19.
bRefers to patients who received vasopressors or inotropes within the hour prior to enrollment.
cPredicted probability of cardiovascular collapse21 was calculated from age, systolic blood pressure at induction of anesthesia, APACHE II score, use of vasopressors, presence of cirrhosis, oxygen saturation at induction of anesthesia, presence or absence of a procedural indication for tracheal intubation, the anesthetic agent used, the neuromuscular blocking agent used, indication for tracheal intubation, presence of chronic kidney disease, and sex (additional details appear in eFigure 6 in Supplement 2).
Secondary Outcome
A total of 218 patients (40.5%) in the fluid bolus group experienced death prior to day 28, which was censored at hospital discharge, compared with 223 patients (42.3%) in the no fluid bolus group (absolute difference, −1.8% [95% CI, −7.9% to 4.3%], P = .55; Table 3).
Exploratory Outcomes
The incidences of each component of the cardiovascular collapse composite outcome did not significantly differ between groups (Table 3 and eFigure 2 in Supplement 2). New or increased receipt of vasopressors occurred in 20.6% of patients in the fluid bolus group vs 17.6% of patients in the no fluid bolus group (absolute difference, 3.0% [95% CI, −1.9% to 7.9%]), a systolic blood pressure of less than 65 mm Hg occurred in 3.9% vs 4.2%, respectively (absolute difference, −0.3% [95% CI, −2.8% to 2.3%]), cardiac arrest occurred in 1.7% vs 1.5% (absolute difference, 0.2% [95% CI, −1.5% to 1.8%]), and death occurred in 0.7% vs 0.6% (absolute difference, 0.2% [95% CI, −1.0% to 1.3%]). The median lowest level of systolic blood pressure from induction of anesthesia to 2 minutes after tracheal intubation was 116 mm Hg (IQR, 93 to 139 mm Hg) in the fluid bolus group and 113 mm Hg (IQR, 95 to 134 mm Hg) in the no fluid bolus group (median difference, 3.0 mm Hg [95% CI, −3.0 to 7.0 mm Hg]). The arterial oxygen saturation, fraction of inspired oxygen, and positive end-expiratory pressure at 24 hours after tracheal intubation did not significantly differ between the groups (eTable 10 in Supplement 2). The number of days alive and free of invasive mechanical ventilation and the number of days alive outside the ICU did not significantly differ between groups (Table 3).
Discussion
In this multicenter, randomized clinical trial, administration of an intravenous fluid bolus to critically ill adults undergoing tracheal intubation with positive pressure ventilation did not significantly decrease the incidence of cardiovascular collapse compared with no fluid bolus.
The effect of administration of a fluid bolus on cardiovascular collapse during tracheal intubation of critically ill adults has been evaluated in 1 prior randomized clinical trial.5 That trial examined the effect of administration of an intravenous fluid bolus compared with no fluid bolus on the incidence of cardiovascular collapse during tracheal intubation of 337 critically ill adults at 9 centers. That trial found that administration of a fluid bolus did not reduce the risk of cardiovascular collapse overall, but appeared to decrease the risk of cardiovascular collapse among patients receiving positive pressure ventilation with a bag-mask device (odds ratio, 0.61 [95% CI, 0.33-1.13]; P = .03 for interaction) or a noninvasive ventilator (odds ratio, 0.51 [95% CI, 0.24-1.09]; P = .008 for interaction).5 Physiologically, the effect of fluid bolus administration on the risk of cardiovascular collapse might be expected to be greatest among patients receiving positive pressure ventilation during induction of anesthesia because positive pressure ventilation decreases return of venous blood to the heart. Because most critically ill adults receive positive pressure ventilation during tracheal intubation,2 the current, larger randomized clinical trial was conducted to definitively evaluate whether administration of a fluid bolus prevents cardiovascular collapse in this population.8,13
Contrary to the hypothesis of the current trial and to 3 national ICU tracheal intubation guidelines9,10,11 that advise the intravenous infusion of fluid bolus to prevent hemodynamic complications, fluid bolus administration did not significantly decrease the risk of cardiovascular collapse overall or in any prespecified subgroup among the 1065 patients in this trial. Together, the results from a prior randomized trial (which found no benefit from fluid bolus administration overall but potential benefit limited to patients receiving positive pressure ventilation) and the current trial (which found no benefit from fluid bolus administration among patients receiving positive pressure ventilation) provide substantial evidence that administration of a 500-mL fluid bolus does not reduce the incidence of cardiovascular collapse for critically ill adults undergoing tracheal intubation.
Tracheal intubation in the ICU is a complex, high-risk, and time-sensitive procedure,2,9 and avoiding the routine administration of a 500-mL fluid bolus for the prevention of cardiovascular collapse may simplify operator decision-making during the tracheal intubation of patients without another indication for a fluid bolus. Future research should evaluate the effectiveness of other interventions to prevent severe hypotension, cardiac arrest, and death during tracheal intubation, such as the administration of vasopressors prior to induction of anesthesia22 or the choice or dose of induction agent.23,24
The strengths of this trial include the use of predictive enrichment to enroll patients likely to benefit based on prior evidence, use of randomization to balance baseline characteristics, concealment of group assignment until enrollment to prevent selection bias, excellent separation between trial groups for receipt of the intervention, pragmatic design conducted at multiple centers to mirror routine clinical practice and increase generalizability, publication of the statistical analysis plan prior to completion of enrollment to improve statistical rigor, and ascertainment of trial end points by an independent observer to minimize observer bias.
Limitations
This trial has several limitations. First, approximately 15% of patients screened were excluded because the urgency of the tracheal intubation did not permit performance of trial procedures. These results may not generalize to the tracheal intubation of patients experiencing cardiac arrest, respiratory arrest, and other highly urgent indications for tracheal intubation.
Second, a volume of 500 mL was selected because this volume of crystalloid solution has been demonstrated to increase cardiac output and blood pressure among critically ill adults,25 is the volume recommended during emergency tracheal intubation in 2 international guidelines,9,10 is the most common volume of fluid bolus administered in clinical practice,26 and was the volume that appeared potentially effective for patients receiving positive pressure ventilation during tracheal intubation in a prior trial.5 Whether the results would have differed if the volume of fluid were greater overall, or personalized to the condition of each patient, is unknown. This trial evaluated only the administration of a 500-mL fluid bolus as part of the tracheal intubation procedure and does not inform the use of fluid in critical illness outside the period immediately surrounding tracheal intubation.
Third, to capture the common hemodynamic complications of tracheal intubation, this trial used a composite outcome. The component of the composite outcome that occurred most commonly was the administration of new or increased vasopressor therapy. Although receipt of vasopressors during tracheal intubation of critically ill adults is independently associated with the risk of death,4 this element of the composite outcome may not be an important patient-centered end point.
Fourth, this trial evaluated the initiation of a fluid bolus prior to induction of anesthesia to prevent cardiovascular collapse, and does not directly inform the use of a fluid bolus to treat hypotension developing during tracheal intubation. Fifth, the trial intervention was not blinded, which raises the potential for bias in administration of co-interventions or outcome ascertainment.
Conclusions
Among critically ill adults undergoing tracheal intubation, administration of an intravenous fluid bolus compared with no fluid bolus did not significantly decrease the incidence of cardiovascular collapse.
Study protocol and statistical analysis plan
eLists. Manuscript authors; PREPARE II Investigators; Pragmatic Critical Care Research Group Members
eMethods. IRB approval and waiver of consent; characteristics of the study intensive care units; inclusion and exclusion criteria; measurement of blood pressure; exploratory outcomes; sample size calculation and re-estimation; modeling of the primary outcome; effect modification (subgroup analyses); sensitivity analyses of the primary outcome; handling of missing data
eTable 1. Chronic comorbidities
eTable 2. Active medical conditions at the time of intubation
eTable 3. Primary indication for tracheal intubation
eTable 4. Operator characteristics
eTable 5. Description of patients who did not receive assigned intervention
eTable 6. Medications administered for the intubation procedure
eTable 7. Additional characteristics of the intubation procedure
eTable 9. Adjusted analyses of the primary outcome
eTable 10. Outcomes of tracheal intubation
eFigure 1. Fluid volumes given during tracheal intubation
eFigure 2. Components of the primary outcome by group
eFigure 3. Calibration of prespecified multivariable model
eFigure 4. Additional analyses of effect modification
eFigure 5. Effect modification by APACHE II Score
eFigure 6. Effect modification by probability of cardiovascular collapse
eFigure 7. Effect modification by systolic blood pressure at induction
eReferences
Nonauthor collaborators
Data sharing statement
References
- 1.Zilberberg MD, Nathanson BH, Ways J, Shorr AF. Characteristics, hospital course, and outcomes of patients requiring prolonged acute versus short-term mechanical ventilation in the United States, 2014-2018. Crit Care Med. 2020;48(11):1587-1594. doi: 10.1097/CCM.0000000000004525 [DOI] [PubMed] [Google Scholar]
- 2.Russotto V, Myatra SN, Laffey JG, et al. ; INTUBE Study Investigators . Intubation practices and adverse peri-intubation events in critically ill patients from 29 countries. JAMA. 2021;325(12):1164-1172. doi: 10.1001/jama.2021.1727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Perbet S, De Jong A, Delmas J, et al. Incidence of and risk factors for severe cardiovascular collapse after endotracheal intubation in the ICU: a multicenter observational study. Crit Care. 2015;19:257. doi: 10.1186/s13054-015-0975-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Smischney NJ, Demirci O, Ricter BD, et al. Vasopressor use as a surrogate for post-intubation hemodynamic instability is associated with in-hospital and 90-day mortality: a retrospective cohort study. BMC Res Notes. 2015;8:445. doi: 10.1186/s13104-015-1410-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Janz DR, Casey JD, Semler MW, et al. ; PrePARE Investigators; Pragmatic Critical Care Research Group . Effect of a fluid bolus on cardiovascular collapse among critically ill adults undergoing tracheal intubation (PrePARE): a randomised controlled trial. Lancet Respir Med. 2019;7(12):1039-1047. doi: 10.1016/S2213-2600(19)30246-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Smischney NJ, Demirci O, Diedrich DA, et al. Incidence of and risk factors for post-intubation hypotension in the critically ill. Med Sci Monit. 2016;22:346-355. doi: 10.12659/MSM.895919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Morris C, Perris A, Klein J, Mahoney P. Anaesthesia in haemodynamically compromised emergency patients: does ketamine represent the best choice of induction agent? Anaesthesia. 2009;64(5):532-539. doi: 10.1111/j.1365-2044.2008.05835.x [DOI] [PubMed] [Google Scholar]
- 8.Shekerdemian L, Bohn D. Cardiovascular effects of mechanical ventilation. Arch Dis Child. 1999;80(5):475-480. doi: 10.1136/adc.80.5.475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Higgs A, McGrath BA, Goddard C, et al. ; Difficult Airway Society; Intensive Care Society; Faculty of Intensive Care Medicine; Royal College of Anaesthetists . Guidelines for the management of tracheal intubation in critically ill adults. Br J Anaesth. 2018;120(2):323-352. doi: 10.1016/j.bja.2017.10.021 [DOI] [PubMed] [Google Scholar]
- 10.Myatra SN, Ahmed SM, Kundra P, et al. The All India Difficult Airway Association 2016 guidelines for tracheal intubation in the intensive care unit. Indian J Anaesth. 2016;60(12):922-930. doi: 10.4103/0019-5049.195481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Quintard H, l’Her E, Pottecher J, et al. Experts’ guidelines of intubation and extubation of the ICU patient of French Society of Anaesthesia and Intensive Care Medicine (SFAR) and French-speaking Intensive Care Society (SRLF): in collaboration with the pediatric Association of French-speaking Anaesthetists and Intensivists (ADARPEF), French-speaking Group of Intensive Care and Paediatric emergencies (GFRUP) and Intensive Care physiotherapy society (SKR). Ann Intensive Care. 2019;9(1):13. doi: 10.1186/s13613-019-0483-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Natt B, Mosier J. Airway management in the critically ill patient. Curr Anesthesiol Rep. 2021;11(2):116-127. doi: 10.1007/s40140-021-00448-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhou L, Cai G, Xu Z, Weng Q, Ye Q, Chen C. High positive end expiratory pressure levels affect hemodynamics in elderly patients with hypertension admitted to the intensive care unit: a prospective cohort study. BMC Pulm Med. 2019;19(1):224. doi: 10.1186/s12890-019-0965-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Casey JD, Janz DR, Russell DW, et al. ; PreVent Investigators and the Pragmatic Critical Care Research Group . Bag-mask ventilation during tracheal intubation of critically ill adults. N Engl J Med. 2019;380(9):811-821. doi: 10.1056/NEJMoa1812405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Frat JP, Ricard JD, Quenot JP, et al. ; FLORALI-2 study group; REVA network . Non-invasive ventilation versus high-flow nasal cannula oxygen therapy with apnoeic oxygenation for preoxygenation before intubation of patients with acute hypoxaemic respiratory failure: a randomised, multicentre, open-label trial. Lancet Respir Med. 2019;7(4):303-312. doi: 10.1016/S2213-2600(19)30048-7 [DOI] [PubMed] [Google Scholar]
- 16.Russell DW, Casey JD, Gibbs KW, et al. ; PREPARE II Investigators . Protocol and statistical analysis plan for the PREventing cardiovascular collaPse with Administration of fluid REsuscitation during Induction and Intubation (PREPARE II) randomised clinical trial. BMJ Open. 2020;10(9):e036671. doi: 10.1136/bmjopen-2019-036671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Semler MW, Bernard GR, Aaron SD, et al. Identifying clinical research priorities in adult pulmonary and critical care: NHLBI Working Group Report. Am J Respir Crit Care Med. 2020;202(4):511-523. doi: 10.1164/rccm.201908-1595WS [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.US Food and Drug Administration . Enrichment strategies for clinical trials to support determination of effectiveness of human drugs and biological products: guidance for industry. Accessed October 20, 2021. https://www.fda.gov/media/121320/download
- 19.Jaber S, Jung B, Corne P, et al. An intervention to decrease complications related to endotracheal intubation in the intensive care unit: a prospective, multiple-center study. Intensive Care Med. 2010;36(2):248-255. doi: 10.1007/s00134-009-1717-8 [DOI] [PubMed] [Google Scholar]
- 20.Knaus WA, Draper EA, Wagner DP, Zimmerman JE. APACHE II: a severity of disease classification system. Crit Care Med. 1985;13(10):818-829. doi: 10.1097/00003246-198510000-00009 [DOI] [PubMed] [Google Scholar]
- 21.Halliday SJ, Casey JD, Rice TW, et al. Risk factors for cardiovascular collapse during tracheal intubation of critically ill adults. Ann Am Thorac Soc. 2020;17(8):1021-1024. doi: 10.1513/AnnalsATS.201912-894RL [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Panchal AR, Satyanarayan A, Bahadir JD, Hays D, Mosier J. Efficacy of bolus-dose phenylephrine for peri-intubation hypotension. J Emerg Med. 2015;49(4):488-494. doi: 10.1016/j.jemermed.2015.04.033 [DOI] [PubMed] [Google Scholar]
- 23.Bendel S, Ruokonen E, Pölönen P, Uusaro A. Propofol causes more hypotension than etomidate in patients with severe aortic stenosis: a double-blind, randomized study comparing propofol and etomidate. Acta Anaesthesiol Scand. 2007;51(3):284-289. doi: 10.1111/j.1399-6576.2006.01206.x [DOI] [PubMed] [Google Scholar]
- 24.Van Berkel MA, Exline MC, Cape KM, et al. Increased incidence of clinical hypotension with etomidate compared to ketamine for intubation in septic patients: a propensity matched analysis. J Crit Care. 2017;38:209-214. doi: 10.1016/j.jcrc.2016.11.009 [DOI] [PubMed] [Google Scholar]
- 25.Glassford NJ, Eastwood GM, Bellomo R. Physiological changes after fluid bolus therapy in sepsis: a systematic review of contemporary data. Crit Care. 2014;18(6):696. doi: 10.1186/s13054-014-0696-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cecconi M, Hofer C, Teboul JL, et al. ; FENICE Investigators; ESICM Trial Group . Fluid challenges in intensive care: the FENICE study: a global inception cohort study. Intensive Care Med. 2015;41(9):1529-1537. Published correction appears in Intensive Care Med. 2015;41(9):1737-1738. doi: 10.1007/s00134-015-3850-x [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Study protocol and statistical analysis plan
eLists. Manuscript authors; PREPARE II Investigators; Pragmatic Critical Care Research Group Members
eMethods. IRB approval and waiver of consent; characteristics of the study intensive care units; inclusion and exclusion criteria; measurement of blood pressure; exploratory outcomes; sample size calculation and re-estimation; modeling of the primary outcome; effect modification (subgroup analyses); sensitivity analyses of the primary outcome; handling of missing data
eTable 1. Chronic comorbidities
eTable 2. Active medical conditions at the time of intubation
eTable 3. Primary indication for tracheal intubation
eTable 4. Operator characteristics
eTable 5. Description of patients who did not receive assigned intervention
eTable 6. Medications administered for the intubation procedure
eTable 7. Additional characteristics of the intubation procedure
eTable 9. Adjusted analyses of the primary outcome
eTable 10. Outcomes of tracheal intubation
eFigure 1. Fluid volumes given during tracheal intubation
eFigure 2. Components of the primary outcome by group
eFigure 3. Calibration of prespecified multivariable model
eFigure 4. Additional analyses of effect modification
eFigure 5. Effect modification by APACHE II Score
eFigure 6. Effect modification by probability of cardiovascular collapse
eFigure 7. Effect modification by systolic blood pressure at induction
eReferences
Nonauthor collaborators
Data sharing statement