Key Points
Question
How does the efficacy and safety of treatment with enzalutamide monotherapy compare with active surveillance in patients with clinically localized low-risk or intermediate-risk prostate cancer?
Findings
In this phase 2, open-label, randomized clinical trial of 227 patients with low-risk or intermediate-risk localized prostate cancer, treatment with enzalutamide was well tolerated. Compared with active surveillance alone, enzalutamide significantly reduced the risk of pathological or therapeutic prostate cancer progression by 46%.
Meaning
The trial results suggest that enzalutamide monotherapy may offer a potential treatment option for this patient population.
Abstract
Importance
There are few published studies prospectively assessing pharmacological interventions that may delay prostate cancer progression in patients undergoing active surveillance (AS).
Objective
To compare the efficacy and safety of enzalutamide monotherapy plus AS vs AS alone in patients with low-risk or intermediate-risk prostate cancer.
Design, Setting, and Participants
The ENACT study was a phase 2, open-label, randomized clinical trial conducted from June 2016 to August 2020 at 66 US and Canadian sites. Eligible patients were 18 years or older, had received a diagnosis of histologically proven low-risk or intermediate-risk localized prostate cancer within 6 months of screening, and were undergoing AS. Patients were monitored during 1 year of treatment and up to 2 years of follow-up. Data analysis was conducted in February 2021.
Interventions
Randomized 1:1 to enzalutamide, 160 mg, monotherapy for 1 year or continued AS, as stratified by cancer risk and follow-up biopsy type.
Main Outcomes and Measures
The primary end point was time to pathological or therapeutic prostate cancer progression (pathological, ≥1 increase in primary or secondary Gleason pattern or ≥15% increased cancer-positive cores; therapeutic, earliest occurrence of primary therapy for prostate cancer). Secondary end points included incidence of a negative biopsy result, percentage of cancer-positive cores, and incidence of a secondary rise in serum prostate-specific antigen (PSA) levels at 1 and 2 years, as well as time to PSA progression. Adverse events were monitored to assess safety.
Results
A total of 114 patients were randomized to treatment with enzalutamide plus AS and 113 to AS alone; baseline characteristics were similar between treatment arms (mean [SD] age, 66.1 [7.8] years; 1 Asian individual [0.4%], 21 Black or African American individuals [9.3%], 1 Hispanic individual [0.4%], and 204 White individuals [89.9%]). Enzalutamide significantly reduced the risk of prostate cancer progression by 46% vs AS (hazard ratio, 0.54; 95% CI, 0.33-0.89; P = .02). Compared with AS, odds of a negative biopsy result were 3.5 times higher; there was a significant reduction in the percentage of cancer-positive cores and the odds of a secondary rise in serum PSA levels at 1 year with treatment with enzalutamide; no significant difference was observed at 2 years. Treatment with enzalutamide also significantly delayed PSA progression by 6 months vs AS (hazard ratio, 0.71; 95% CI, 0.53-0.97; P = .03). The most commonly reported adverse events during enzalutamide treatment were fatigue (62 [55.4%]) and gynecomastia (41 [36.6%]). Three patients in the enzalutamide arm died; none were receiving the study drug at the time of death. No deaths were considered treatment-related.
Conclusions and Relevance
The results of this randomized clinical trial suggest that enzalutamide monotherapy was well-tolerated and demonstrated a significant treatment response in patients with low-risk or intermediate-risk localized prostate cancer. Enzalutamide may provide an alternative treatment option for patients undergoing AS.
Trial Registration
ClinicalTrials.gov Identifier: NCT02799745
This randomized clinical trial examines the efficacy and safety of enzalutamide monotherapy vs active surveillance in patients with low-risk or intermediate-risk prostate cancer.
Introduction
Prostate cancer is the most commonly diagnosed cancer in men in the US,1 with approximately 70% of patients with a new diagnosis having localized disease.2 Based on National Comprehensive Cancer Network treatment guidelines, active surveillance (AS) is a recommended management option for patients with clinically localized very low-risk, low-risk, or intermediate-risk prostate cancer.3 Patients who are undergoing or are eligible to undergo AS who opt for treatment with a definitive therapy, such as radical prostatectomy, external beam radiation therapy, or brachytherapy, may experience adverse effects, including sexual and voiding dysfunction.3 The decision for treatment intervention is influenced by various factors, including pathological upgrading of disease, grade reclassification, and patient well-being. Biopsy type may influence disease assessment, with some studies showing an exclusion of men from AS protocols because of disease reclassification following a multiparametric magnetic resonance imaging (mpMRI)–targeted biopsy.4
To our knowledge, there are few publications prospectively assessing pharmacological interventions to delay prostate cancer progression in the AS population. Results from the REDEEM trial demonstrated that dutasteride, a 5α-reductase inhibitor, is a beneficial adjunct to AS in men with low-risk prostate cancer, significantly reducing the risk of progression by 38%.5 However, additional pharmacological approaches that may reduce the risks associated with disease progression and interventional treatment are needed. Enzalutamide is a potent oral androgen receptor inhibitor with demonstrated efficacy in patients with localized6 and more advanced stages of prostate cancer,7,8,9,10,11,12 potentially providing a treatment option for patients undergoing AS. The ENACT randomized clinical trial compared the efficacy and safety of treatment with enzalutamide monotherapy plus AS vs AS alone in patients with clinically localized low-risk or intermediate-risk prostate cancer and to our knowledge is the first trial to assess a known anticancer agent in an AS population.
Methods
Study Design
The ENACT study (NCT02799745) was a multicenter, randomized, open-label, phase 2, exploratory clinical trial conducted in patients with low-risk or intermediate-risk localized prostate cancer in the US and Canada from June 2016 to August 2020 (Supplement 1 and Supplement 2). The study was conducted in accordance with International Council for Harmonization guidelines, applicable local laws, regulations, and guidelines governing clinical study conduct, as well as ethical principles derived from the Declaration of Helsinki. The study was approved by the independent ethics committee or institutional review board at each study site. This article was prepared in accordance with Consolidated Standards of Reporting Trials (CONSORT) guidelines.
Patients were randomized 1:1 via interactive response technology to receive 1 year of treatment with enzalutamide, 160 mg, monotherapy plus AS or AS alone, as stratified by cancer risk (low vs intermediate) and type of follow-up biopsy (transrectal ultrasonography-guided prostate biopsy [mpMRI-targeted vs non–mpMRI-targeted]) (eFigure 1 in Supplement 3). Enrollment of patients with low-risk prostate cancer was capped to not exceed 80% of the study population. Treatment with oral enzalutamide was administered once daily as 4 × 40-mg capsules per the approved product label. Patients randomized to AS did not receive the study drug. Monitoring continued until the last patient completed the 24-month posttreatment visit (1 year of follow-up and 1 year of continued follow-up).
Treatment adjustment was permitted in the event of a National Cancer Institute Common Terminology Criteria for Adverse Event (NCI-CTCAE) of grade 3 or higher, for which enzalutamide treatment was paused for 1 week or until the adverse event (AE) reduced in severity to grade 2 or less. Treatment then resumed at the original or reduced dose (120 mg or 80 mg) at the investigator’s discretion. Dose reduction was permitted to manage gynecomastia or breast complications at the investigator’s discretion.
Patients and Procedures
Men 18 years or older who received a diagnosis of histologically proven low-risk or intermediate-risk (defined per National Comprehensive Cancer Network Guidelines3) clinically localized adenocarcinoma of the prostate (with ≥10 core biopsies) within 6 months of screening and who were undergoing AS were eligible for inclusion. Enrollment required an Eastern Cooperative Oncology Group status score of 2 or less and estimated life expectancy of more than 5 years. All eligible men were required to provide written informed consent. Information on race was collected to assess differences in baseline demographic characteristics and treatment effects; race was self-identified by the patients.
Patients who had received prior local or systemic prostate cancer therapy were excluded, as were those who had received treatment with oral glucocorticoids within 1 month of screening or a 5α-reductase inhibitor either within 1 month of screening or for more than 3 months within the previous 2 years. Patients with very low-risk disease (T1c, prostate-specific antigen [PSA] level of <10 ng/mL [to convert to μg/L, multiply by 1]; Gleason score [GS] of ≤6; <3 cancer-positive cores; ≤50% cancer in any core; and a PSA density of <0.15 ng/mL/g) were not eligible.
Transrectal ultrasonography-guided prostate biopsies (with or without mpMRI targeting) were performed at the 12-month and 24-month visits. Each site was required to be consistent with its method of biopsy throughout the trial, and all biopsies were evaluated by masked central pathology. For mpMRI-targeted biopsies, 2 biopsies were required from each target site, plus 12 systematic biopsies. Serum PSA samples were collected at all visits and analyzed in a central laboratory.
End Points
The primary end point was time to pathological or therapeutic prostate cancer progression. Pathological progression was defined as an increase in primary or secondary Gleason pattern by 1 or more or a higher proportion of cancer-positive cores (≥15% increase). Therapeutic progression was defined as the earliest occurrence of primary therapy for prostate cancer (prostatectomy, radiation, focal therapy, or any systemic therapy). Incidence of pathological or therapeutic prostate cancer progression at 1 and 2 years was also assessed.
Secondary end points included incidence of negative biopsy results at 1 and 2 years; the percentage of cancer-positive cores at 1 and 2 years; time to PSA progression; and incidence of a secondary rise in serum PSA levels at 1 and 2 years. Prostate-specific antigen progression was defined as a secondary rise in serum PSA levels of 25% or more of the baseline, an increase of 25% or more than the nadir, or an absolute increase of 2 or more ng/mL. Patient-reported outcomes (PROs) were assessed as secondary end points via questionnaires, including the Brief Fatigue Inventory (assessing the severity and effect of cancer-related fatigue), 12-item Short Form Survey (measuring health-related quality of life), Expanded Prostate Cancer Index Composite (sexual, urinary, and hormonal domains; measuring function following treatment), and Memorial Anxiety Scale for Prostate Cancer (measuring anxiety).
Additional analyses of the primary end point, time to prostate cancer progression, were performed by pathological vs therapeutic progression and low (6) vs high (7) GS. Additional analyses of the incidence of prostate cancer progression (secondary analysis of the primary end point) were performed by pathological vs therapeutic progression and by low vs intermediate risk. Additional analyses of all primary and secondary end points were performed for patients with a consistent biopsy type (at screening, month 12, and month 24; screening and month 12; screening and month 24; or just a screening biopsy with no follow-up biopsy).
Adverse events were monitored from enrollment to study completion to assess safety. All AEs were followed up until they resolved, were no longer considered clinically significant, or were deemed chronic.
Statistical Analyses
Sample size was calculated based on an assumed study duration of 3 years with 16% loss to follow-up and a 3-year median time to progression for the AS group (0.23 rate), with an underlying hazard ratio (HR) of 0.52.5 Therefore, 72 events were required to provide a 2-sided type 1 error rate of .05 and power of 80%, resulting in a total sample size of 222 men randomized 1:1, accrued during 1 year.
Efficacy analyses were performed on the full analysis set (FAS), defined as all randomized patients. Safety analyses were performed on the safety analysis set, defined as all randomized patients who received study treatment.
Data were summarized using descriptive statistics for continuous end points and frequency and percentage for categorical end points. There was no imputation for missing data, with the exception of start/stop dates for AEs. Data were analyzed using SAS, version 9.3 or higher (SAS Institute), and all statistical analyses were performed using 2-sided tests with a significance level of .05.
Time to prostate cancer progression and time to PSA progression were calculated using the Kaplan-Meier method. Patients were censored at the last assessment date in the absence of progression at the time of study completion, discontinuation, or death. Treatment group differences were assessed using a Cox regression model assuming proportional hazards, with treatment group, stratification factors, age, race, and time since prostate cancer diagnosis as fixed effects, and study site and patient as random effects. Treatment group differences in incidence of prostate cancer progression, negative biopsy results, and a secondary rise in serum PSA levels were assessed using logistic regression, with treatment group, stratification factors, age, race, and time since prostate cancer diagnosis as fixed effects and study site and patient as random effects. Treatment group differences in the percentage of cancer-positive cores were analyzed using a mixed-model–repeated-measures model, with treatment group, stratification factors, visit, visit-by-treatment, and baseline score as fixed effects, study site and patient as random effects, and a Bonferroni-Holm test to adjust for multiplicity. Additional analyses of primary and secondary end points were analyzed using the same methods. The PROs were analyzed using descriptive statistics. Adverse events were graded based on the National Cancer Institute Common Terminology Criteria for Adverse Event, version 4.03, and coded using the Medical Dictionary for Regulatory Activities, version 23.0.
Results
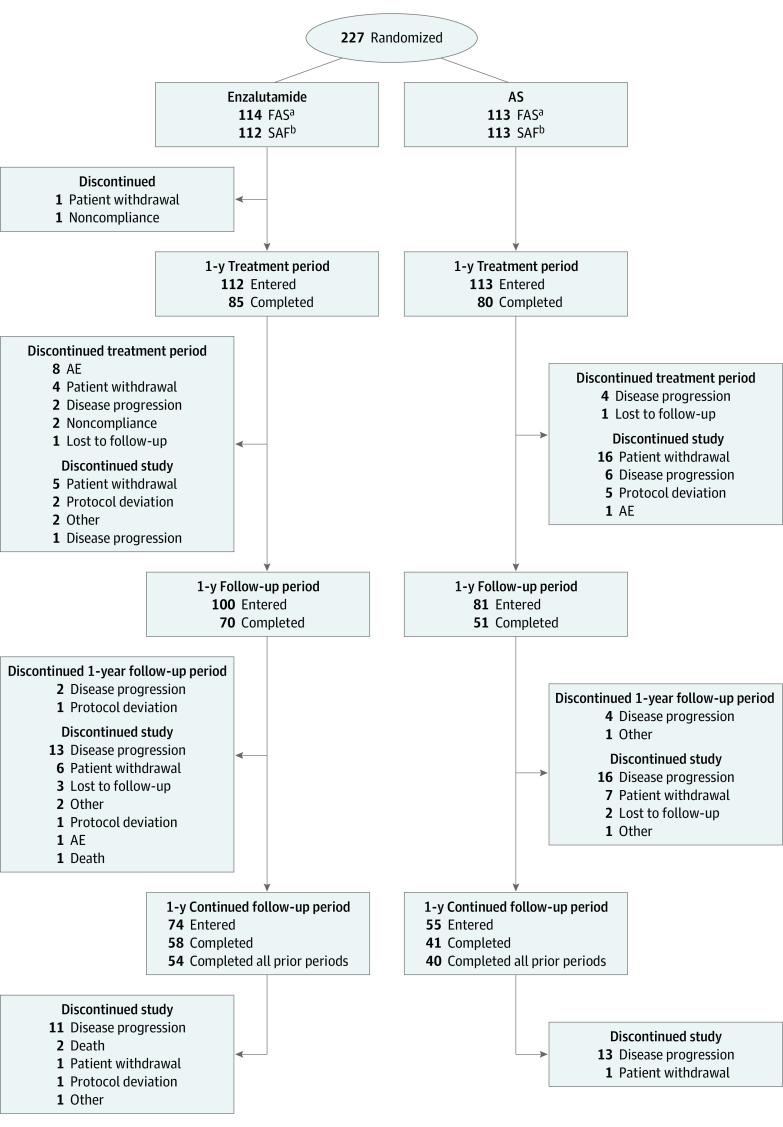
Patient disposition is presented in Figure 1. Overall, 227 men were randomized across 66 sites. Of the 114 men randomized to receive treatment with enzalutamide, 85 (74.6%) completed 1 year of AS, 70 (61.4%) completed 1 year of follow-up, and 58 (50.9%) completed 1 year of continued follow-up. Of the 113 men randomized to undergo AS, 80 (70.8%) completed 1 year of treatment, 51 (45.1%) completed 1 year of follow-up, and 41 (36.3%) completed 1 year of continued follow-up. Fifty-four patients (47.4%) receiving enzalutamide and 40 patients (35.4%) undergoing AS completed all study periods. Disease progression and patient withdrawal were generally the most commonly reported reasons for discontinuation in both treatment arms throughout the study. Median follow-up was 492.5 days (range, 1.0-1078.0 days) for patients receiving enzalutamide and 270.5 days (range, 1.0-806.0 days) for patients undergoing AS.
Figure 1. Patient Disposition.
Patients could discontinue a study period yet remain in the study. AE indicates adverse event; AS, active surveillance.
aThe full analysis set (FAS) comprised all randomized patients.
bThe safety analysis set (SAF) comprised all randomized patients who received study treatment. Two patients randomized to the enzalutamide treatment arm were excluded from the SAF; 1 patient did not receive the study drug but remained in the study and 1 patient withdrew from the study after randomization and before taking the study drug.
Baseline demographic characteristics and disease characteristics were similar between treatment arms (Table 1). Overall, 121 men (53.3%) had low-risk prostate cancer and 172 (75.8%) underwent non–mpMRI-targeted follow-up biopsies. The median enzalutamide treatment duration was 352 days (range, 1-393 days). During the 1-year treatment period, enzalutamide treatment was interrupted in 15 men (13.2%) and the enzalutamide dose was reduced in 14 men (12.3%), primarily owing to AEs.
Table 1. Baseline Demographic and Disease Characteristics.
| Parameter | No. (%) | ||
|---|---|---|---|
| Enzalutamide (n = 114) | AS (n = 113) | Total (N = 227) | |
| Age, mean (SD), y | 65.2 (8.2) | 66.9 (7.3) | 66.1 (7.8) |
| Median (range) | 65.0 (41-87) | 66.0 (52-87) | 65.0 (41-87) |
| Racea | |||
| Asian | 0 | 1 (0.9) | 1 (0.4) |
| Black or African American | 8 (7.0) | 13 (11.5) | 21 (9.3) |
| Hispanic | 1 (0.9) | 0 | 1 (0.4) |
| White | 105 (92.1) | 99 (87.6) | 204 (89.9) |
| BMI, mean (SD) | 29.7 (4.4) | 28.7 (4.4) | 29.2 (4.4) |
| Prostate cancer risk | |||
| Lowb | 61 (53.5) | 60 (53.1) | 121 (53.3) |
| Intermediatec | 53 (46.5) | 53 (46.9) | 106 (46.7) |
| Type of follow-up biopsy | |||
| mpMRI-targeted | 27 (23.7) | 28 (24.8) | 55 (24.2) |
| Non–mpMRI-targeted | 87 (76.3) | 85 (75.2) | 172 (75.8) |
| Time since prostate cancer diagnosis, mean (SD), y | 0.3 (0.51) | 0.2 (0.24) | 0.3 (0.40) |
| Median (range) | 0.2 (0-5) | 0.2 (0-2) | 0.2 (0-5) |
| Clinical tumor stage at prostate cancer diagnosis | |||
| T1-T1b | 0 | 1 (0.9) | 1 (0.4) |
| T1c-T2a | 107 (93.9) | 106 (93.8) | 213 (93.8) |
| T2b-T2c | 7 (6.1) | 6 (5.3) | 13 (5.7) |
| Clinical lymph nodes at prostate cancer diagnosis | |||
| NX | 50 (43.9) | 42 (37.2) | 92 (40.5) |
| N0 | 64 (56.1) | 71 (62.8) | 135 (59.5) |
| Distant metastases at prostate cancer diagnosis | |||
| MX | 53 (46.5) | 48 (42.5) | 101 (44.5) |
| M0 | 61 (53.5) | 65 (57.5) | 126 (55.5) |
| Total Gleason score at prostate cancer diagnosis | |||
| 6 | 67 (58.8) | 66 (58.4) | 133 (58.6) |
| 7 (3 + 4 pattern only) | 46 (40.4) | 47 (41.6) | 93 (41.0) |
| Unknown | 1 (0.9) | 0 | 1 (0.4) |
| LS cancer-positive cores, mean (SE), %d | 25.43 (1.61) | 23.12 (1.58) | NA |
| Serum PSA levels, median (range), ng/mL | 5.8 (1-17) | 5.9 (1-23) | 5.9 (1-23) |
Abbreviations: AS, active surveillance; BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); LS, least squares; mpMRI, multiparametric magnetic resonance imaging; NA, not available; PSA, prostate-specific antigen.
SI conversion factor: To convert PSA to μg/L, multiply by 1.
Self-reported.
Low-risk prostate cancer was defined per National Comprehensive Cancer Network guidelines as T1c-T2a, PSA levels of less than 10 ng/mL, N0, M0, and Gleason score of 6 or lower.
Intermediate-risk prostate cancer was defined per National Comprehensive Cancer Network guidelines as T2b-T2c, PSA level of less than 20 ng/mL, N0, M0, and Gleason score of 7 or lower (3 + 4 pattern only).
Based on the most recent biopsy taken during the 6 months before screening.
Primary End Point
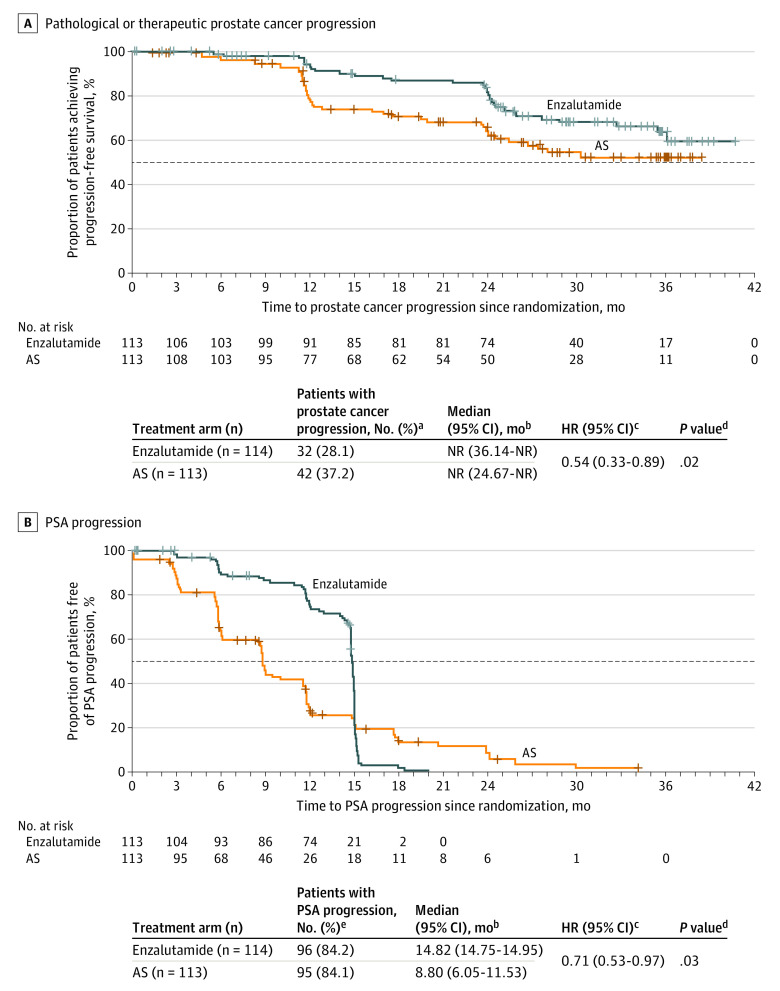
Disease progression was observed in 32 patients (28.1%) receiving treatment with enzalutamide and 42 (37.2%) of those undergoing AS. The median time to pathological or therapeutic prostate cancer progression was not reached in either treatment arm (Figure 2A). However, treatment with enzalutamide significantly reduced the risk of prostate cancer progression by 46% vs AS (HR, 0.54; 95% CI, 0.33-0.89; P = .02).
Figure 2. Time to Pathological or Therapeutic Prostate Cancer Progression and Prostate-Specific Antigen (PSA) Progression.
Pathological progression was defined as an increase in primary or secondary Gleason pattern by 1 or more or a higher proportion of cancer-positive cores (≥15% increase). Therapeutic progression was defined as the earliest occurrence of primary therapy for prostate cancer (prostatectomy, radiation, focal therapy, or systemic therapy). Prostate-specific antigen progression was defined as a secondary rise in serum PSA levels of 25% or more of the baseline, an increase of 25% or more than the nadir, or an absolute increase of 2 or more ng/mL (to convert to μg/L multiply by 1). AS indicates active surveillance; NR, not reached.
aPatients with no prostate cancer progression at the time of study completion, discontinuation, or death were censored at the last assessment date. Patients who switched therapy during the study were censored at the time of the initial therapy switch, and patients who discontinued receiving therapy were censored at the time of study discontinuation.
bCalculated using a 2-sided, log-rank test.
cCalculated using a Cox regression model assuming proportional hazards, with treatment group, stratification factors, age, race, and time since prostate cancer diagnosis as fixed effects, and study site and patient as random effects. A hazard ratio (HR) of less than 1 favored enzalutamide.
dCalculated using a 2-sided, stratified, log-rank test.
ePatients with no PSA progression at the time of study completion, discontinuation, or death were censored at the last assessment date. Patients who switched therapy during the study were censored at the time of the initial therapy switch, and patients who discontinued receiving therapy were censored at the time of study discontinuation.
The incidence of pathological or therapeutic prostate cancer progression was lower with enzalutamide (7.9%) vs AS (23.0%) at 1 year (odds ratio [OR], 0.3; 95% CI, 0.11-0.60; P < .01). At 2 years, incidence was similar between treatment arms (16.0% with enzalutamide vs 16.4% with AS; OR, 0.9; 95% CI, 0.36-2.24; P = .81).
Secondary End Points
The odds of a negative biopsy result at 1 year were significantly increased with treatment with enzalutamide vs AS (OR, 3.5; 95% CI, 1.76-6.92; P < .001). At 2 years, although there were more patients with negative biopsy results in the enzalutamide arm than the AS arm, the difference was not statistically significant (Table 2). The mean percentage of cancer-positive cores at 1 year was significantly lower with enzalutamide than AS (difference in least squares mean [SE], −10.07 [2.40]; 95% CI, −14.79 to −5.34; P < .001; Table 2). Although there was no statistically significant difference in cancer-positive cores between the treatment arms at 2 years, there was a statistically significant reduction of 6.7% between baseline and year 2 with enzalutamide (95% CI, −11.36 to −2.00).
Table 2. Secondary Efficacy End Points.
| End point | No. (%) | |
|---|---|---|
| Enzalutamide | AS | |
| Incidence of negative biopsy result at 1 y, No. | 114 | 113 |
| Biopsy result | ||
| Negative | 40 (35.1) | 16 (14.2) |
| Positive | 53 (46.5) | 74 (65.5) |
| Unknown | 21 (18.4) | 23 (20.4) |
| Missing | 11 (9.6) | 2 (1.8) |
| Discontinued | 10 (8.8) | 21 (18.6) |
| OR (95% CI)a,b | 3.5 (1.76-6.92) | |
| P valuec | <.001 | |
| Incidence of negative biopsy result at 2 y, No. | 100 | 83 |
| Biopsy result | ||
| Negative | 19 (19.0) | 10 (12.0) |
| Positive | 47 (47.0) | 40 (48.2) |
| Unknown | 34 (34.0) | 33 (39.8) |
| Missing | 11 (11.0) | 5 (6.0) |
| Discontinued | 23 (23.0) | 26 (31.3) |
| OR (95% CI)a,b | 1.6 (0.66-4.00) | |
| P valuec | .29 | |
| Percentage of cancer-positive cores at 1 y, No.d | 90 | 81 |
| LS change from baseline, mean (SE) [95% CI]e | −12.97 (1.99) [−16.9 to −9.03] | −2.90 (2.03) [−6.93 to 1.12] |
| Difference in LS, mean (SE) [95% CI] | −10.07 (2.40) [−14.79 to −5.34] | |
| P valuef | <.001 | |
| Percentage of cancer-positive cores at 2 y, No.d | 53 | 42 |
| LS change from baseline, mean (SE) [95% CI]e | −6.68 (2.37) [−11.36 to −2.00] | −1.53 (2.57) [−6.61 to 3.55] |
| Difference in LS, mean (SE) [95% CI] | −5.15 (3.17) [−11.40 to 1.11] | |
| P valuef | .11 | |
| Incidence of a secondary rise in serum PSA at 1 y, No. | 114 | 113 |
| PSA responseg | 28 (24.6) | 78 (69.0) |
| OR (95% CI)a,b | 0.1 (0.08-0.26) | |
| P valuec | <.001 | |
| Incidence of a secondary rise in serum PSA levels at 2 y, No. | 100 | 83 |
| PSA responseg | 92 (92.0) | 77 (92.8) |
| OR (95% CI)a,b | 1.1 (0.37-3.53) | |
| P valuec | 0.81 | |
Abbreviations: AS, active surveillance; LS, least squares; OR, odds ratio; PSA, prostate-specific antigen.
Calculated using an exact logistic regression model with treatment group, stratification factors, age, race, and time since prostate cancer diagnosis as fixed effects and study site and patient as random effects.
Calculated based on exact binomial distribution.
Calculated based on exact binomial distribution from the logistic regression model.
Analyzed using a mixed-mode–repeated-measures model, with treatment group, stratification factors, visit, visit-by-treatment, and baseline score as fixed effects and study site and patient as random effects.
Most recent biopsy taken during the 6 months before screening.
A Bonferroni-Holm test was used to adjust for multiplicity.
PSA response was defined as a secondary rise in serum PSA levels of 25% or more of the baseline, an increase of 25% or more than the nadir, or an absolute increase of 2 or more ng/mL (to convert to μg/L, multiply by 1).
Time to PSA progression was significantly delayed by 6 months with treatment with enzalutamide vs AS (HR, 0.71; 95% CI, 0.53-0.97; P = .03; Figure 2B). The odds of a secondary rise in serum PSA levels at 1 year were significantly reduced with treatment with enzalutamide vs AS (OR, 0.1; 95% CI, 0.08-0.26; P < .001), but not at 2 years (Table 2). Treatment with enzalutamide was not associated with clinically significant worsening of PROs, with the exception of sexual and physical function, which resolved by month 24 after treatment cessation.
Additional Analyses
Results of additional analyses of time to prostate cancer progression by pathological vs therapeutic progression were consistent with the FAS results, with a median time to prostate cancer progression not reached in either treatment arm or group (eFigure 2 in Supplement 3). The HRs were not statistically significant because of the small sample size.
For time to pathological or therapeutic prostate cancer progression by low (6) vs high (7) GS (eFigure 3 in Supplement 3), data were generally consistent with the FAS. The median time to prostate cancer progression was not reached in either treatment arm in patients with a low GS. However, among patients with a high GS, the median time to prostate cancer progression was 30 months with AS, for whom the number of progression events nearly doubled compared with enzalutamide (40.4% vs 21.7%; median not reached with treatment with enzalutamide). Analyses of the incidence of prostate cancer by risk indicated that, regardless of whether receiving enzalutamide or undergoing AS, pathological progression was higher in patients with low-risk disease, whereas therapeutic progression was higher in patients with intermediate-risk disease (eTable 1 in Supplement 3).
Results of the analyses in patients with a consistent biopsy type (eTable 2 in Supplement 3) were consistent with the FAS, further strengthening the findings in the overall study population. The risk of prostate cancer progression was reduced by 55% with treatment with enzalutamide vs AS, and the odds of disease progression were 80% lower at 1 year. In patients treated with enzalutamide, the odds of having a negative biopsy result were 3.5 times higher at 1 year, the mean percentage of cancer positive cores was 8.4% lower at 1 year, time to PSA progression was 6 months longer, and the odds of having a secondary rise in serum PSA levels at 1 year were 80% lower vs AS.
Safety
During the 1-year treatment period, the incidence of AEs was higher in patients receiving enzalutamide (92.0%) compared with AS (54.9%). During the 1-year follow-up period, the corresponding numbers were 39.3% and 23.0%, respectively, further decreasing to 14.3% and 10.6%, respectively, during the 1-year continued follow-up period (eTable 3 in Supplement 3). The incidence of serious AEs was low across treatment arms and periods (eTable 3 in Supplement 3).
The most commonly reported AEs during treatment with enzalutamide were fatigue (62 [55.4%]), gynecomastia (41 [36.6%]), nipple pain (34 [30.4%]), breast tenderness (29 [25.9%]), and erectile dysfunction (20 [17.9%]); the only AE that occurred in 5% or more of patients in the AS arm was hypertension (8 [7.1%]; Table 3). Drug-related AEs were reported in 99 men (88.4%) during treatment with enzalutamide, of which only 2.7% were considered serious and 7.1% led to study drug discontinuation (eTable 3 in Supplement 3).
Table 3. AEs Reported During the 1-Year Treatment Period by Gradea.
| Characteristic | No. (%) | |||||||
|---|---|---|---|---|---|---|---|---|
| Enzalutamide, 160 mg (n = 112) | AS (n = 113) | |||||||
| Gradeb | Total | Gradeb | Total | |||||
| 1 | 2 | ≥3 | 1 | 2 | ≥3 | |||
| Any AE | 34 (30.4) | 58 (51.8) | 11 (9.8)c | 103 (92.0) | 27 (23.9) | 25 (22.1) | 10 (8.8)d | 62 (54.9) |
| Drug-related AEs | 47 (42.0) | 46 (41.1) | 6 (5.4) | 99 (88.4) | NA | NA | NA | NA |
| AEs reported in ≥5% of patients in any treatment arme | ||||||||
| Fatigue | 49 (43.8) | 11 (9.8) | 2 (1.8) | 62 (55.4) | 3 (2.7) | 1 (0.9) | 0 | 4 (3.5) |
| Gynecomastia | 26 (23.2) | 14 (12.5) | 1 (0.9) | 41 (36.6) | 2 (1.8) | 0 | 0 | 2 (1.8) |
| Nipple pain | 30 (26.8) | 4 (3.6) | 0 | 34 (30.4) | 0 | 0 | 0 | 0 |
| Breast tenderness | 25 (22.3) | 4 (3.6) | 0 | 29 (25.9) | 1 (0.9) | 0 | 0 | 1 (0.9) |
| Erectile dysfunction | 9 (8.0) | 11 (9.8) | 0 | 20 (17.9) | 2 (1.8) | 0 | 0 | 2 (1.8) |
| Alopecia | 10 (8.9) | 1 (0.9) | 0 | 11 (9.8) | 0 | 0 | 0 | 0 |
| Decreased libido | 8 (7.1) | 1 (0.9) | 0 | 9 (8.0) | 1 (0.9) | 0 | 0 | 1 (0.9) |
| Hypertension | 3 (2.7) | 5 (4.5) | 0 | 8 (7.1) | 3 (2.7) | 2 (1.8) | 3 (2.7) | 8 (7.1) |
| Breast enlargement | 5 (4.5) | 2 (1.8) | 0 | 7 (6.3) | 0 | 0 | 0 | 0 |
| Diarrhea | 3 (2.7) | 3 (2.7) | 0 | 6 (5.4) | 0 | 0 | 0 | 0 |
| Hot flush | 4 (3.6) | 2 (1.8) | 0 | 6 (5.4) | 0 | 0 | 0 | 0 |
| Nausea | 6 (5.4) | 0 | 0 | 6 (5.4) | 0 | 0 | 0 | 0 |
| Pollakiuria | 4 (3.6) | 2 (1.8) | 0 | 6 (5.4) | 3 (2.7) | 1 (0.9) | 0 | 4 (3.5) |
| Upper respiratory tract infection | 4 (3.6) | 2 (1.8) | 0 | 6 (5.4) | 2 (1.8) | 2 (1.8) | 0 | 4 (3.5) |
| Weight decreased | 4 (3.6) | 2 (1.8) | 0 | 6 (5.4) | 0 | 0 | 0 | 0 |
Abbreviations: AE, adverse event; AS, active surveillance; NA, not applicable.
The 1-year treatment period was defined as from the date of the first dose (enzalutamide) or randomization (AS) until the date discontinued or completed 12 months on study +30 days.
Adverse events were graded based on the National Cancer Institute Common Terminology Criteria for Adverse Events, version 4.03.
Including 10 grade 3 AEs and 1 grade 5 AE (death).
Including 9 grade 3 AEs and 1 grade 4 AE.
Adverse events were coded using the Medical Dictionary for Regulatory Activities, version 23.0.
Most AEs experienced during the 1-year treatment period were grade 1 or 2 (Table 3). Of the 9.8% of AEs experienced by men in the enzalutamide treatment arm that were grade 3 or higher, only grade 3 fatigue was reported by more than 1 patient (reported by 2 patients). No grade 4 AEs were experienced during treatment with enzalutamide, and only 1 grade 5 AE (homicide) was reported. Six grade 3 AEs (5.4%) in the enzalutamide arm were considered to be drug related (1 occurrence each of gait disturbance, gynecomastia, myocardial infarction, and syncope and 2 occurrences of fatigue). In the AS arm, 9 grade 3 AEs and 1 grade 4 AE were reported, with only grade 3 hypertension reported by more than 1 patient (reported by 3 patients).
During the study, 3 men (2.7%) in the enzalutamide treatment arm died (eTable 3 in Supplement 3); none were receiving enzalutamide at the time of death. One death was because of homicide during the 1-year treatment period after the patient had discontinued enzalutamide treatment because of noncompliance with the study drug, and 2 deaths occurred during the continued follow-up period (intracranial hemorrhage and metastatic cholangiocarcinoma). No deaths were considered by the investigators to be related to treatment or disease progression.
Discussion
In the phase 2 ENACT randomized clinical trial, treatment with enzalutamide monotherapy significantly reduced the risk of prostate cancer and PSA progression vs AS in patients with low-risk or intermediate-risk localized prostate cancer. Patients receiving enzalutamide also had a significant improvement in the odds of a negative biopsy result, as well as significant reductions in the percentage of cancer-positive cores and odds of a secondary rise in serum PSA levels at 1 year compared with AS. Enzalutamide monotherapy was well-tolerated, with the observed AE data consistent with the known safety profile of enzalutamide.
The REDEEM trial was the first to assess the efficacy and safety of any pharmacological treatment in men with low-risk prostate cancer who were undergoing AS.5 Dutasteride was found to significantly reduce the risk of pathological or therapeutic prostate cancer progression by 38% during the 3-year treatment period vs AS (P = .01). The observed 46% risk reduction (P = .02) in ENACT is consistent with this finding. Similar to REDEEM, AEs related to sexual function and breast disorders were among the most commonly reported treatment-related AEs in ENACT. In contrast to REDEEM, ENACT used a treatment with proven clinical benefit in more advanced prostate cancer and included men with intermediate-risk prostate cancer.
Limitations
The ENACT PSA progression analysis results were potentially confounded by the effect of enzalutamide on PSA levels and the definition of PSA progression, which resulted in a potential bias toward AS. The PSA rebound was observed following cessation of enzalutamide treatment, although the mean PSA levels in this arm remained less than baseline throughout the study. In addition, the findings may not be generalizable to all racial and ethnic minority groups. While the additional analyses by pathological vs therapeutic progression, low vs high GS, low-risk vs intermediate-risk disease, and in patients with consistent biopsy types provided results that support those from the overall ENACT study population, these findings should be interpreted with caution, given that the study was not designed or powered for such analyses.
Conclusions
This randomized clinical trial found that enzalutamide monotherapy was well tolerated and demonstrated a significant treatment response in patients with low-risk or intermediate-risk localized prostate cancer. To our knowledge, ENACT represents the first trial to compare the effects of a novel androgen receptor antagonist as monotherapy vs AS in patients with low-risk or intermediate-risk localized prostate cancer. Results suggest that enzalutamide may offer an alternative short-term treatment option for this patient population, potentially reducing the need for more aggressive treatment approaches.
Trial protocol
Statistical analysis plan
eFigure 1. Study Design
eFigure 2. Time to Prostate Cancer Progression by (A) Pathological and (B) Therapeutic Progression
eFigure 3. Time to Pathological or Therapeutic Prostate Cancer Progression by (A) Low and (B) High GS
eTable 1. Incidence of Prostate Cancer Progression (Pathological vs. Therapeutic) by Risk (Low vs. Intermediate)
eTable 2. Additional Analyses of Primary and Secondary Efficacy Endpoints in Patients With Consistent Biopsy
eTable 3. Overview of AEs
Data sharing statement
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70(1):7-30. doi: 10.3322/caac.21590 [DOI] [PubMed] [Google Scholar]
- 2.National Cancer Institute . Stage distribution of SEER incidence cases, 2009-2018. Accessed June 1, 2021. https://seer.cancer.gov/explorer/application.html?site=66&data_type=1&graph_type=4&compareBy=race&chk_race_1=1&chk_race_5=5&chk_race_4=4&chk_race_3=3&chk_race_6=6&chk_race_8=8&chk_race_2=2&hdn_sex=2&age_range=1&advopt_precision=1&advopt_display=2#tableWrap.
- 3.National Comprehensive Cancer Network . NCCN clinical practice guidelines in oncology: prostate cancer version 2.2021. Accessed April 1, 2021. https://www.nccn.org/professionals/physician_gls/pdf/prostate_blocks.pdf.
- 4.Hu JC, Chang E, Natarajan S, et al. Targeted prostate biopsy in select men for active surveillance: do the Epstein criteria still apply? J Urol. 2014;192(2):385-390. doi: 10.1016/j.juro.2014.02.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fleshner NE, Lucia MS, Egerdie B, et al. Dutasteride in localised prostate cancer management: the REDEEM randomised, double-blind, placebo-controlled trial. Lancet. 2012;379(9821):1103-1111. doi: 10.1016/S0140-6736(11)61619-X [DOI] [PubMed] [Google Scholar]
- 6.Montgomery B, Tretiakova MS, Joshua AM, et al. Neoadjuvant enzalutamide prior to prostatectomy. Clin Cancer Res. 2017;23(9):2169-2176. doi: 10.1158/1078-0432.CCR-16-1357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tombal B, Borre M, Rathenborg P, et al. Long-term efficacy and safety of enzalutamide monotherapy in hormone-naive prostate cancer: 1- and 2-year open-label follow-up results. Eur Urol. 2015;68(5):787-794. doi: 10.1016/j.eururo.2015.01.027 [DOI] [PubMed] [Google Scholar]
- 8.Scher HI, Fizazi K, Saad F, et al. ; AFFIRM Investigators . Increased survival with enzalutamide in prostate cancer after chemotherapy. N Engl J Med. 2012;367(13):1187-1197. doi: 10.1056/NEJMoa1207506 [DOI] [PubMed] [Google Scholar]
- 9.Beer TM, Armstrong AJ, Rathkopf DE, et al. ; PREVAIL Investigators . Enzalutamide in metastatic prostate cancer before chemotherapy. N Engl J Med. 2014;371(5):424-433. doi: 10.1056/NEJMoa1405095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hussain M, Fizazi K, Saad F, et al. Enzalutamide in men with nonmetastatic, castration-resistant prostate cancer. N Engl J Med. 2018;378(26):2465-2474. doi: 10.1056/NEJMoa1800536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Armstrong AJ, Szmulewitz RZ, Petrylak DP, et al. ARCHES: A randomized, phase III study of androgen deprivation therapy with enzalutamide or placebo in men with metastatic hormone-sensitive prostate cancer. J Clin Oncol. 2019;37(32):2974-2986. doi: 10.1200/JCO.19.00799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Davis ID, Martin AJ, Stockler MR, et al. ; ENZAMET Trial Investigators and the Australian and New Zealand Urogenital and Prostate Cancer Trials Group . Enzalutamide with standard first-line therapy in metastatic prostate cancer. N Engl J Med. 2019;381(2):121-131. doi: 10.1056/NEJMoa1903835 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial protocol
Statistical analysis plan
eFigure 1. Study Design
eFigure 2. Time to Prostate Cancer Progression by (A) Pathological and (B) Therapeutic Progression
eFigure 3. Time to Pathological or Therapeutic Prostate Cancer Progression by (A) Low and (B) High GS
eTable 1. Incidence of Prostate Cancer Progression (Pathological vs. Therapeutic) by Risk (Low vs. Intermediate)
eTable 2. Additional Analyses of Primary and Secondary Efficacy Endpoints in Patients With Consistent Biopsy
eTable 3. Overview of AEs
Data sharing statement