This cohort study investigates changes in optic nerve head and retinal morphology during spaceflight and brief in-flight exposure to lower-body negative pressure.
Key Points
Question
How do optic nerve head and retinal morphology change during spaceflight and during brief in-flight exposure to lower-body negative pressure (LBNP)?
Findings
In this cohort study of 14 spaceflight crew members, neuroretinal rim and peripapillary retinal thickness increased, optic cup volume and macular thickness decreased, and Bruch membrane opening moved posteriorly in association with spaceflight. A 10- to 20-minute exposure to LBNP during spaceflight was not associated with changes in ocular structure.
Meaning
These findings suggest that peripapillary and macular retinal thickness may be affected differently by spaceflight and do not respond rapidly to acute fluid shift reversal with LBNP; longer-duration exposure to countermeasures may be necessary to mitigate the effects of spaceflight-associated neuro-ocular syndrome.
Abstract
Importance
Countermeasures that reverse the headward fluid shift experienced in weightlessness have the potential to mitigate spaceflight-associated neuro-ocular syndrome. This study investigated whether use of the countermeasure lower-body negative pressure during spaceflight was associated with changes in ocular structure.
Objective
To determine whether changes to the optic nerve head and retina during spaceflight can be mitigated by brief in-flight application of 25-mm Hg lower-body negative pressure.
Design, Setting, and Participants
In the National Aeronautics and Space Administration’s “Fluid Shifts Study,” a prospective cohort study, optical coherence tomography scans of the optic nerve head and macula were obtained from US and international crew members before flight, in-flight, and up to 180 days after return to Earth. In-flight scans were obtained both under normal weightless conditions and 10 to 20 minutes into lower-body negative pressure exposure. Preflight and postflight data were collected in the seated, supine, and head-down tilt postures. Crew members completed 6- to 12-month missions that took place on the International Space Station. Data were analyzed from 2016 to 2021.
Interventions or Exposures
Spaceflight and lower-body negative pressure.
Main Outcomes and Measures
Changes in minimum rim width, optic cup volume, Bruch membrane opening height, peripapillary total retinal thickness, and macular thickness.
Results
Mean (SD) flight duration for the 14 crew members (mean [SD] age, 45 [6] years; 11 male crew members [79%]) was 214 (72) days. Ocular changes on flight day 150, as compared with preflight seated, included an increase in minimum rim width (33.8 μm; 95% CI, 27.9-39.7 μm; P < .001), decrease in cup volume (0.038 mm3; 95% CI, 0.030-0.046 mm3; P < .001), posterior displacement of Bruch membrane opening (−9.0 μm; 95% CI, −15.7 to −2.2 μm; P = .009), and decrease in macular thickness (fovea to 500 μm, 5.1 μm; 95% CI, 3.5-6.8 μm; P < .001). Brief exposure to lower-body negative pressure did not affect these parameters.
Conclusions and Relevance
Results of this cohort study suggest that peripapillary tissue thickening, decreased cup volume, and mild central macular thinning were associated with long-duration spaceflight. Acute exposure to 25-mm Hg lower-body negative pressure did not alter optic nerve head or retinal morphology, suggesting that longer durations of a fluid shift reversal may be needed to mitigate spaceflight-induced changes and/or other factors are involved.
Introduction
Approximately two-thirds of crew members on long-duration missions to the International Space Station develop spaceflight-associated neuro-ocular syndrome (SANS).1,2 This condition has important implications for future space travel, as mission durations are expected to increase and chronic optic disc edema (ODE), a hallmark SANS finding, could potentially lead to irreversible vision loss.
Although the etiology of SANS is not well established, the chronic cephalad fluid shift that occurs during weightlessness is considered the primary initiating factor. Despite overlapping signs with idiopathic intracranial hypertension (IIH), recent studies suggest that intracranial pressure (ICP) is not pathologically elevated in weightlessness but may instead be slightly less than that experienced in a supine posture on Earth.3 On Earth, humans spend approximately two-thirds of the day in an upright posture, in which the hydrostatic pressure gradient shifts fluid footward. The inability to “stand up” during spaceflight and shift fluid away from the head has been proposed to result in a mean 24-hour ICP value greater than that habitually experienced on Earth, which may contribute to ODE development.4
Lower-body negative pressure (LBNP) is a potential SANS countermeasure that aims to reverse the headward fluid shift by redistributing fluid from the upper body to the legs and abdomen. Studies on Earth that used head-down tilt (HDT) or supine postures to simulate spaceflight found that LBNP reduced the headward fluid shift, as evidenced by significant reductions in invasive and noninvasive surrogate measures of ICP.5,6,7,8,9 Invasive ICP measures have not been reported during spaceflight; however, investigating changes in optic nerve head (ONH) and retinal morphology could provide insight regarding the pathophysiology of ODE in SANS, as well as relative changes in fluid shifts under normal weightless conditions and during LBNP application.
The goal of this study was to determine how ocular morphology changes during spaceflight and during acute fluid-shift reversal with in-flight LBNP. Although changes in tissue thickness at the ONH have been described during long-duration spaceflight,1,10,11,12 in-flight changes in optic cup size, ONH anterior-posterior position, and macular thickness have not been established. We hypothesized that the spaceflight-induced chronic headward fluid shift results in structural changes at the ONH but not at the macula and that acute application of LBNP partially reverses these structural changes.
Methods
Participants
Crew members, including astronauts and cosmonauts, completed long-duration International Space Station missions in the National Aeronautics and Space Administration’s (NASA’s) “Fluid Shifts Study.” Six individuals had prior spaceflight experience. Additional details and outcome variables for this cohort have been reported.13,14,15 This study was approved by the NASA Johnson Space Center institutional review board and the human research multilateral review board, and it adhered to the tenets of the Declaration of Helsinki. Informed written consent was obtained from all crew members, and crew members were not offered compensation or incentives to participate. Participant enrollment was open to all crew members from NASA, the European Space Agency, the Japan Aerospace Exploration Agency, the Canadian Space Agency, and Roscosmos. We were not able to report race and ethnicity of the participants owing to confidentiality concerns.
This study followed Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guidelines (eAppendix in the Supplement).
Study Design
Preflight and postflight optical coherence tomography (OCT) were performed in the Cardiovascular and Vision Laboratory at NASA’s Johnson Space Center. During spaceflight, scans were acquired both under normal weightless conditions and approximately 10 to 20 minutes into a session of 25-mm Hg LBNP application with the Russian Chibis-M system16 on approximately flight day (FD) 50 and FD150. The magnitude and duration of LBNP were chosen owing to logistical constraints and to maximize the potential stimulus, while limiting the possibility of syncope. Four crew members were also imaged on approximately FD250 (range, 223-293 days). Postflight scans were acquired approximately 10 days (range, 8-13 days; return to Earth [R] + 10), approximately 30 days (range, 27-50 days; R + 30), and approximately 180 days (range, 167-216 days; R + 180) after return to Earth. For preflight and R + 10 time points, participants were imaged in seated, supine, and 15°-HDT postures after resting in each position for approximately 10 to 15 minutes.
Optical Coherence Tomography
The Spectralis OCT1 and OCT2 systems (Heidelberg Engineering) were used to acquire images from the left eye of each participant, including a 24-line (20°) radial scan centered on the ONH and a 12-line (20°) radial scan centered on the macula (Figure 1). The AutoRescan feature aligned all images to the preflight seated baseline scan. All scans were inspected by 2 readers of a pool of 3 readers (including C.R.F. and L.P.P.) who corrected segmentation errors and manually selected Bruch membrane opening (BMO). Data were exported, and calculations were performed using MATLAB (MathWorks). Each measurement represents the mean of the 2 readers’ results.
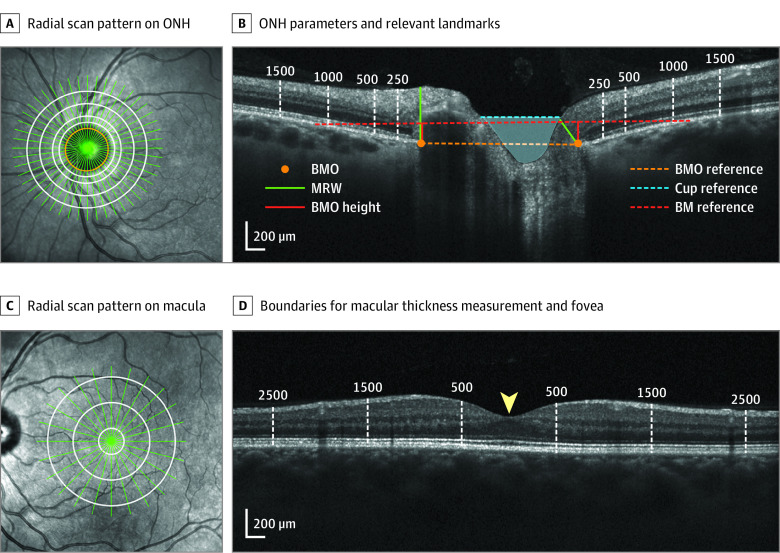
Figure 1. Optic Nerve Head (ONH) and Retinal Optical Coherence Tomography Parameters.
A, 30° Infrared image showing a radial scan pattern (green) centered on the ONH, best-fit Bruch membrane opening (BMO) ellipse (orange), and concentric ellipses forming the boundaries for total retinal thickness (TRT) measurement (white). B, Radial B-scan illustrating ONH parameters and relevant landmarks, including BMO, minimum rim width (MRW), BM reference, BMO height, BMO reference, cup reference, optic cup (blue shaded region), and the boundaries for TRT measurement (labeled white dashed lines). C, 30° Infrared image illustrating a radial scan pattern (green) centered on the macula and boundaries for macular thickness measurements (white). D, Radial B-scan showing the boundaries for macular thickness measurement (labeled white dashed lines) and fovea (yellow arrowhead).
The neuroretinal rim minimum rim width (MRW), the minimum distance from each BMO point to the internal limiting membrane (ILM), was constrained to the region between BMO points. The anterior-posterior BMO position (BMO height) was quantified as the minimum distance from each BMO point to a 4-mm BM reference line centered on the ONH.10 A line parallel and 200-μm anterior to a reference line connecting the BMO points defined the anterior boundary of the optic cup; cup volume was quantified as the region contained within this boundary and the ILM, linearly interpolated between B-scans in 3-dimensional space. Peripapillary total retinal thickness (TRT) was calculated in 4 annular regions: BMO to 250 μm (TRT250), 250 to 500 μm (TRT500), 500 to 1000 μm (TRT1000), and 1000 to 1500 μm (TRT1500) (Figure 1A-B).1,10 Macular thickness (MT) was quantified within a 500 μm radius of the fovea (MT500) and in annuli extending from 500 to 1500 μm (MT1500) and 1500 to 2500 μm (MT2500) (Figure 1C-D).
Statistical Analysis
Statistical analyses were performed using Stata software, version 17.0 (StataCorp), with an emphasis on characterizing the observed effects with modeled means and 95% CIs. Statistically significant differences were determined against a 2-tailed null hypothesis of no differences with α = .05. All model assumptions were evaluated before reporting effects, resulting in the elimination of a few overly influential observations where necessary to meet model requirements. Although there were some missing data, full information maximum likelihood (FIML) mixed modeling maximized the number of observations informing each model and reduced bias associated with listwise elimination.
Each continuously scaled outcome was submitted to separate statistical mixed models with a priori fixed-effects parameters. All models included random y-intercepts to accommodate the nesting of observations within participants and FIML estimations using analysis of variance–based degrees of freedom with small-n adjustments. Longitudinal changes in outcomes collected in-flight and postflight were evaluated relative to preflight (seated position). Another set of models addressed the potential effects of LBNP on ocular structure by comparing observations collected with vs without LBNP within time points; comparisons were also made between in-flight time points without LBNP. Finally, models evaluating the effects of posture and spaceflight included fully factorialized coefficients for posture (supine, HDT, relative to seated), day (preflight vs postflight), and the posture-by-day simple interaction effects (relative to preflight seated). Data were analyzed from 2016 to 2021.
Results
Spaceflight and Ocular Morphology
A total of 14 US and international crew members (mean [SD] age, 45 [6] years; 11 male participants [79%]; 3 female participants [21%]) were included in the study. Mean (SD) flight duration was 214 (72) days. ODE, defined as an increase in TRT250 exceeding 19.4 μm,17 occurred in 4 of 14 participants (29%) by FD50 and 9 of 13 participants (69%) by FD150. However, only 1 participant developed Frisén grade 1 edema,18 as determined by NASA’s Flight Medicine Clinic. Mean values for all parameters before, during, and after spaceflight are listed in the Table (exact P values in eTable 1 in the Supplement).
Table. Estimated Marginal Mean (95% CI) for Each Parameter Before, During, and After Spaceflight.
| Variable | Preflight seated | FD50 | FD150 | R + 10a | R + 30 | R + 180 |
|---|---|---|---|---|---|---|
| MRW, μm | 361.1 (330.4 to 391.8) | 381.7 (351.0 to 412.4) | 394.9 (364.1 to 425.4) | 378.3 (347.4 to 409.2) | 369.0 (338.3 to 399.7) | 358.9 (328.2 to 389.6) |
| Cup volume, mm3 | 0.193 (0.103 to 0.283) | 0.167 (0.077 to 0.258) | 0.158 (0.068 to 0.248) | 0.163 (0.072 to 0.253) | 0.177 (0.087 to 0.268) | 0.193 (0.103 to 0.284) |
| BMO height, μm | −118.3 (−147.1 to −89.4) | −124.7 (−153.5 to −95.8) | −127.2 (−156.1 to −98.3) | −117.2 (−146.4 to −88.0) | −118.4 (−147.3 to −89.5) | −122.3 (−151.2 to −93.4) |
| TRT250,b μm | 395.8 (376.4 to 415.1) | 407.8 (388.4 to 427.1) | 418.9 (399.5 to 438.2) | 411.7 (392.1 to 431.2) | 403.0 (383.7 to 422.4) | 393.7 (374.4 to 413.1) |
| TRT500,c μm | 371.8 (359.1 to 384.5) | 378.0 (365.3 to 390.7) | 384.0 (371.3 to 396.8) | 381.3 (368.5 to 394.1) | 376.2 (363.5 to 388.9) | 370.1 (357.4 to 382.8) |
| TRT1000,d μm | 338.0 (328.1 to 347.9) | 338.3 (328.4 to 348.2) | 340.0 (330.1 to 349.9) | 339.1 (329.1 to 349.1) | 339.3 (329.4 to 349.2) | 336.0 (326.1 to 346.0) |
| TRT1500,e μm | 302.5 (294.2 to 310.9) | 303.2 (294.8 to 311.5) | 302.7 (294.4 to 311.1) | 301.9 (293.5 to 310.3) | 302.6 (294.2 to 310.9) | 300.6 (292.3 to 309.0) |
| MT500,f μm | 272.4 (262.6 to 282.1) | 268.7 (258.9 to 278.4) | 267.2 (257.5 to 277.0) | 267.2 (257.4 to 277.0) | 269.9 (260.1 to 279.6) | 270.9 (260.1 to 279.6) |
| MT1500,g μm | 348.1 (339.4 to 356.8) | 345.5 (336.8 to 354.2) | 344.5 (335.8 to 353.2) | 344.7 (336.0 to 353.5) | 347.4 (338.7 to 356.1) | 347.0 (338.3 to 355.7) |
| MT2500,h μm | 315.7 (308.1 to 323.4) | 314.8 (307.1 to 322.4) | 314.7 (307.0 to 322.4) | 314.3 (306.6 to 322.0) | 316.1 (308.5 to 323.8) | 315.2 (307.6 to 322.9) |
Abbreviations: BMO, Bruch membrane opening; FD, flight day; MRW, minimum rim width; MT, macular thickness; R+, days after return to Earth; TRT, total retinal thickness.
The R + 10 time point includes only 8 participants, as several international crewmembers did not return directly to Houston.
TRT250, TRT from BMO to 250 μm.
TRT500, TRT from 250 to 500 μm.
TRT1000, TRT from 500 to 1000 μm.
TRT1500, TRT from 1000 to 1500 μm.
MT500, MT from the fovea to 500 μm.
MT1500, MT from 500 to 1500 μm.
MT2500, MT from 1500 to 2500 μm.
The greatest effects of spaceflight occurred at the ONH. MRW increased by 20.6 μm (95% CI, 15.0-26.3 μm; P < .001) on FD50 and 33.8 μm (95% CI, 27.9-39.7 μm; P < .001) on FD150 (Figure 2A). These changes were accompanied by corresponding decreases in cup volume of 0.028 mm3 (95% CI, 0.020-0.036 mm3; P < .001) on FD50 and 0.038 mm3 (95% CI, 0.030-0.046 mm3; P < .001) on FD150 (Figure 2B). The individual who developed grade 1 edema did not have a quantifiable optic cup at any time point and was excluded from cup volume analyses. After return to Earth, changes in MRW and cup volume persisted through R + 30 (MRW: 7.9 um; 95% CI, 2.2-13.7 μm; P = .007; cup volume: −0.017 mm3; 95% CI, −0.025 to −0.009 mm3; P < .001); however, by R + 180, neither measure differed from baseline. BMO height tended to be lower than baseline (ie, shifted posteriorly) on FD50, but the value was not statistically significant. However, BMO height was significantly lower on FD150 (−9.0 μm; 95% CI, −15.7 to −2.2 μm; P = .009) (Figure 2C). The participant with grade 1 edema exhibited the greatest change in BMO height (−53.3 μm).
Figure 2. Changes in Optic Nerve Head Morphology During Spaceflight.
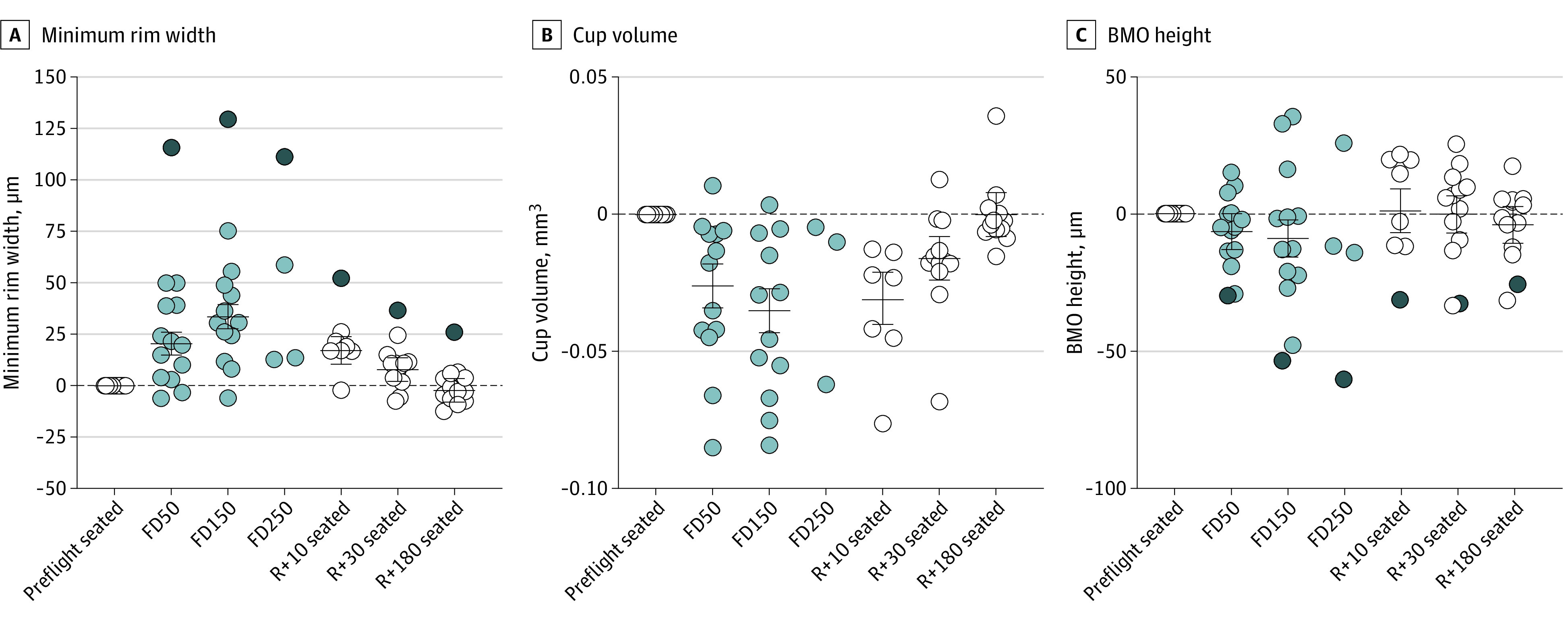
A, Minimum rim width increased on flight day (FD)50 and FD150 and gradually recovered after return to Earth, with no difference from the preflight baseline by return to Earth (R) + 180. B, Cup volume followed a similar timeline as minimum rim width, initially decreasing on FD50 and returning to baseline by R + 180. C, Bruch membrane opening (BMO) height was significantly reduced (ie, posteriorly displaced) on FD150 but did not differ from baseline at any other time point. Circles show all individual subject data representing data obtained on Earth (white), data obtained during spaceflight (light gray), and the participant with Frisén grade 1 disc edema (dark gray); this individual did not have a detectable optic cup and was therefore excluded from the cup volume analysis. Horizontal bars represent the estimated marginal mean values across participants, and error bars represent the 95% CIs. P values for the change relative to the preflight seated baseline value are provided in eTable 1 in the Supplement. Statistics were not performed on FD250 data owing to the small sample size (n = 4).
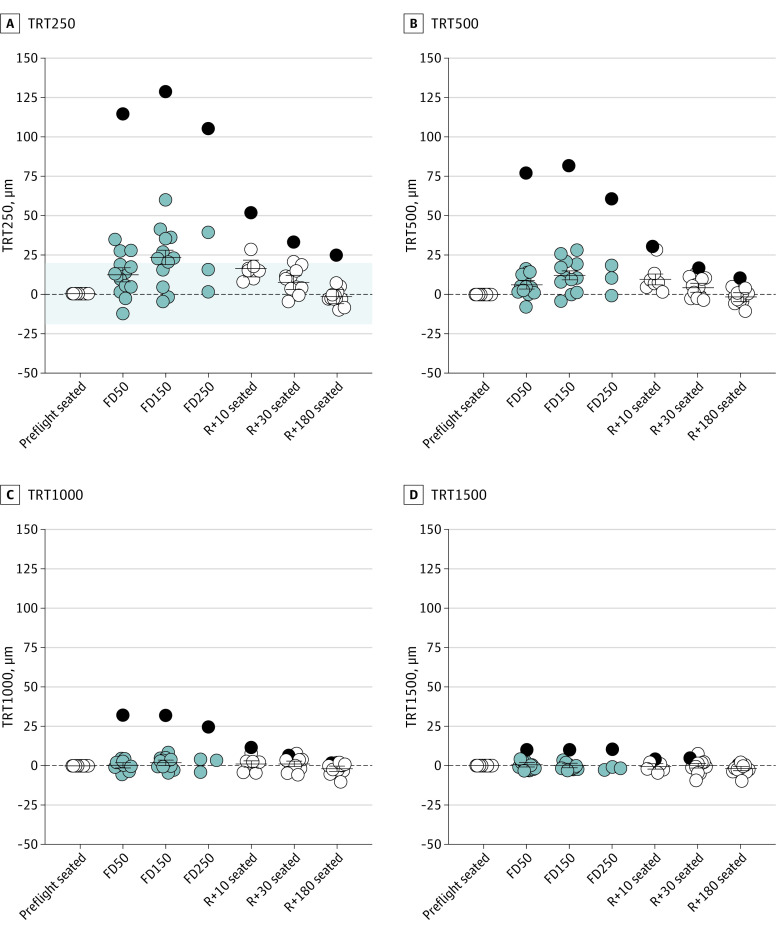
Peripapillary TRT followed a similar pattern as MRW, although the magnitude of change was smaller and decreased with distance from the ONH (Figure 3). On FD50, TRT250 and TRT500 increased by 12.0 μm (95% CI, 7.6-16.4 μm; P < .001) and 6.2 μm (95% CI, 3.5-9.0 μm; P < .001), respectively. By FD150, TRT1000 also increased; changes were as follows: TRT250, 23.1 μm (95% CI, 18.6-27.6 μm; P < .001); TRT500, 12.3 μm (95% CI, 9.4-15.1 μm; P < .001); and TRT1000, 2.0 μm (95% CI, 0.3-3.7 μm; P = .02). TRT1000 recovered by R + 10, whereas changes in TRT250 and TRT500 persisted through R + 30 (TRT250: 7.3 μm; 95% CI, 2.8-11.7 μm; P = .002; TRT500: 4.4 μm; 95% CI, 1.6-7.2 μm; P = .002) but recovered by R + 180. Despite no changes in TRT1500 during spaceflight, both TRT1000 and TRT1500 values were slightly less than preflight values on R + 180, decreasing 2.0 μm (95% CI, 0.3-3.6 μm; P = .02) and 1.9 μm (95% CI, 0.5-3.3 μm; P = .007), respectively.
Figure 3. Changes in Peripapillary Total Retinal Thickness (TRT) During Spaceflight .
TRT250 (A) and TRT500 (B) increased on flight day (FD) 50 and FD150 and did not return to preflight baseline values until return to Earth (R) + 180. C, TRT1000 was only significantly increased on FD150 and was slightly reduced relative to the preflight seated baseline on R + 180. D, TRT1500 did not increase during spaceflight and was also slightly reduced relative to baseline on R + 180. Circles show all individual subject data representing data obtained on Earth (white), data obtained during spaceflight (light gray), and the participant with Frisén grade 1 disc edema (dark gray). Horizontal bars represent the estimated marginal mean values across participants, and error bars represent the 95% CIs. The shaded area in panel A represents the predefined range of normal day-to-day variation in TRT250 (±19.4) μm.17 P values for the change relative to the preflight seated baseline value are provided in eTable 1 in the Supplement. Statistics were not performed on FD250 data owing to the small sample size (n = 4). TRT250 indicates TRT from BMO to 250 μm; TRT500, TRT from 250 to 500 μm; TRT1000, TRT from 500 to 1000 μm; TRT1500, TRT from 1000 to 1500 μm.
In contrast to the increase in TRT adjacent to the ONH, central MT decreased during spaceflight. MT500 decreased by 3.7 μm (95% CI, 2.1-5.3 μm; P < .001) on FD50 and 5.1 μm (95% CI, 3.5-6.8 μm; P < .001) on FD150 (Figure 4A). The magnitude of thinning decreased with distance from the fovea; MT1500 decreased by 2.6 μm (95% CI, 1.2-3.0 μm; P < .001) on FD50 and by 3.6 μm (95% CI, 2.2-5.0 μm; P < .001) on FD150 (Figure 4B), and there were no changes in MT2500 during or after spaceflight (Figure 4C). MT1500 recovered by R + 30, whereas MT500 did not recover until R + 180.
Figure 4. Changes in Macular Thickness During Spaceflight.
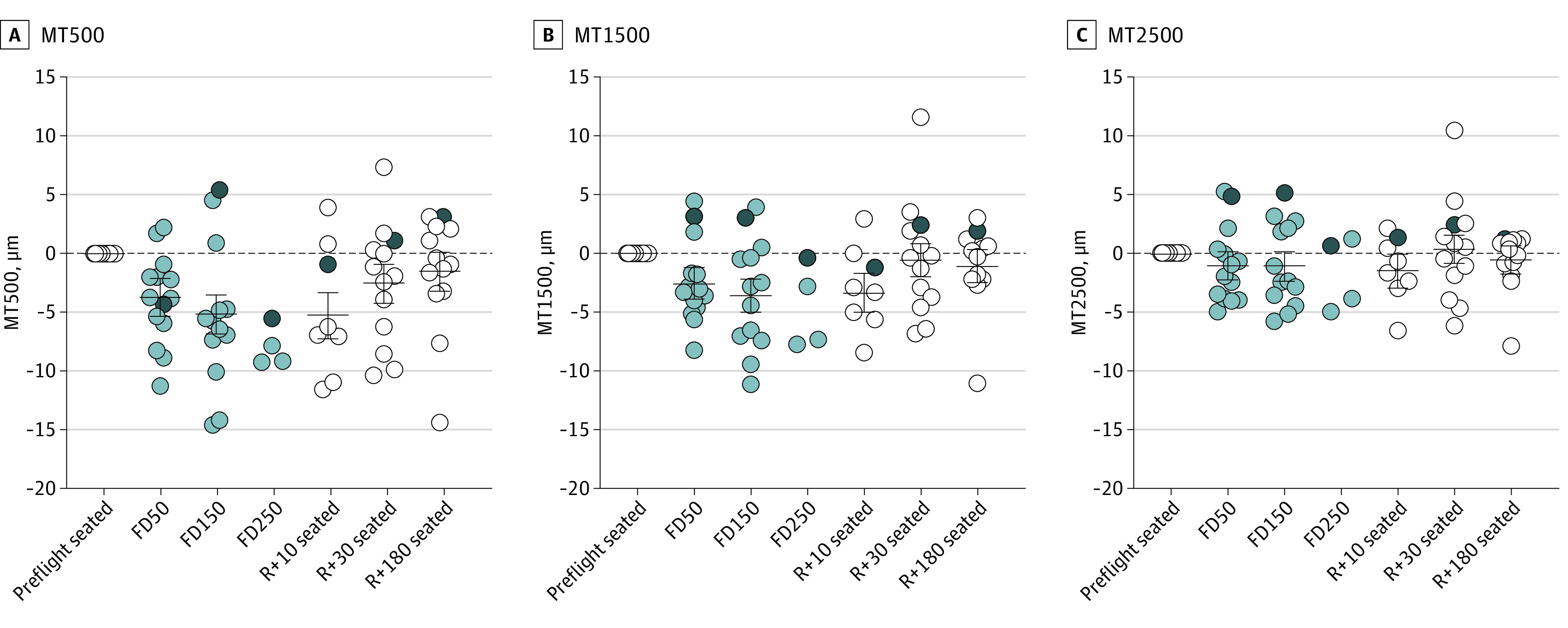
A, Macular thickness (MT) 500 μm decreased on flight day (FD) 50 and FD150, and it did not return to preflight baseline values until return to Earth (R) + 180. B, MT1500 also decreased on FD50 and FD150 but returned to baseline by R + 30. C, There were no significant changes in MT2500 during or after spaceflight. Circles show all individual subject data representing data obtained on Earth (white), data obtained during spaceflight (light gray), and the participant with Frisén grade 1 disc edema (dark gray). Horizontal bars represent the estimated marginal mean values across participants, and error bars represent the 95% CI. P values for the change relative to the preflight seated baseline value are provided in eTable 1 in the Supplement. Statistics were not performed on FD250 data owing to the small sample size (n = 4). MT500 indicates MT from the fovea to 500 μm; MT1500, MT from 500 to 1500 μm; MT2500, MT from 1500 to 2500 μm.
Fluid Shifts and Ocular Morphology
Changes in ocular structure were not identified during LBNP (eTables 2 and 3 in the Supplement). Values under normal weightless conditions differed between FD50 and FD150 for MRW, cup volume, TRT250, TRT500, TRT1000, and MT500 (MRW: 12.2 μm; 95% CI, 7.2-17.2 μm; P < .001; cup volume: −0.010 mm3; 95% CI, −0.006 to −0.014 mm3; P < .001; TRT250: 11.3 μm; 95% CI, 7.9-14.8 μm; P < .001; TRT500: 5.9 μm; 95% CI, 3.0-8.7 μm, P < .001; TRT1000: 1.5 μm; 95% CI, 0.1-2.9 μm; P = .04; MT500: −1.5 μm; 95% CI, 0.0 to −3.0 μm; P = .046), suggesting that the magnitudes of ODE and central macular thinning were associated with an increase in flight duration. Although posterior BMO displacement only became significant on FD150, there was no difference in BMO height between FD50 and FD150.
The effects of acute posture changes on Earth were investigated to provide context for measures obtained during LBNP. Supine and 15°-HDT postures cause headward fluid shifts that are similar to and exceed that of spaceflight, respectively.14 There was no significant posture-by-day interaction for any parameter. MRW and TRT250 were the only parameters to demonstrate a simple main effect of posture, with MRW being 4.1 μm greater in HDT than when seated (95% CI, 1.1-7.1 μm; P = .007) and TRT250 being 2.5 μm thinner supine than when seated (95% CI, 0.1-4.9 μm; P = .04) (eTable 4 in the Supplement).
Discussion
Although the increase in peripapillary tissue thickness and decrease in optic cup size that occur during long-duration spaceflight were consistent with ODE, the posterior BMO displacement and reversible macular thinning observed in this cohort study suggest differences between SANS and IIH. In-flight fluid shift reversal via 10 to 20 minutes of 25-mm Hg LBNP does not reverse ocular structural changes associated with spaceflight, possibly because a longer duration of exposure is required and/or other mechanisms are involved.
The increases in MRW (33.8 μm) and TRT (23.1 μm) observed on FD150 in the present study were similar in magnitude to those previously described in 11 astronauts completing 6-month missions,1 and both studies demonstrated that peripapillary retinal thickening increases in magnitude and expands radially with flight duration. Furthermore, MRW and TRT measured closest to the ONH recovered between R + 30 and R + 180, similar to previous reports of recovery at R + 90.1 The comparable findings between studies highlights the mild nature of the edema during approximately 6 months of spaceflight. The individual who developed Frisén grade 1 ODE was the only participant with a nonexistent preflight optic cup. Future investigations will determine whether crowded ONH morphology is related to the development of ODE during spaceflight.
ODE in SANS is hypothesized to result from the chronic headward fluid shift that occurs in weightlessness. Current evidence from parabolic flight suggests that ICP in acute weightlessness is not elevated to pathological levels (ie, >25 cm H2O)19,20 but is slightly less than that in a supine posture on Earth.3 However, it is unknown if ICP measured during brief periods of weightlessness in individuals who previously received central nervous system chemotherapy to treat hematologic malignancy3 reflects ICP levels during long-duration spaceflight. The influence that an absence of diurnal change in ICP has on the development of ODE is also unknown. It is possible that a chronic, unremitting ICP elevation, regardless of magnitude, may be sufficient to persistently decrease the translaminar pressure difference (TLPD, intraocular pressure [IOP] − ICP) and disrupt axoplasmic flow, leading to axonal swelling and ODE which manifests as an increase in ONH tissue thickness and a decrease in cup volume.
The observed posterior BMO displacement is inconsistent with a decreased TLPD. Patients with IIH often exhibit anterior BMO displacement, thought to be a mechanical deformation caused by elevated ICP exerting a force at the ONH21,22,23; therefore, we hypothesized that any sustained ICP stimulus at the posterior pole during spaceflight would also result in anterior BMO displacement. Instead, BMO height was posteriorly displaced by −9.0 μm, similar to a previous study that measured BMO height within approximately 1-week postflight (−9.9 μm),10 and the individual with grade 1 ODE demonstrated the greatest posterior displacement. Either a relative reduction in ICP or increase in IOP would be consistent with posterior BMO displacement. IOP only increased by approximately 1 mm Hg during spaceflight in the present participants,13 suggesting that ICP would need to remain low or decrease during spaceflight if these were the only factors contributing to the displacement. A notable difference between IIH and SANS is that the latter often presents with choroidal thickening.1,13 An increase in choroid thickness could anteriorly displace the BM reference used to calculate BMO height, causing an apparent relative posterior displacement of the BMO. Although use of a choroid/sclera reference could circumvent this issue, the sclera is also not a stationary landmark, as globe flattening at the ONH is common in SANS.1,2,24 Further work is needed to better understand factors that influence BMO position during spaceflight and to characterize differences in peripapillary shape deformations between SANS and IIH. Although changes in the position of the lamina cribrosa could provide insight regarding changes in the TLPD, laminar position was not assessed in this study owing to unreliable visibility.
We hypothesized that spaceflight-induced changes in retinal thickness would be isolated to the peripapillary region. Although no retinal thickening was detectable past TRT1000, retinal thinning occurred in the central macula. The mild decrease in MT (approximately 5 μm) was consistent across time points, exceeded the mean posture-induced changes, and returned to preflight levels after spaceflight. This thinning presumably cannot be attributed to a loss of retinal ganglion cells or their axons, as thinning recovered after return to Earth and was greatest at the fovea where inner retinal structures are laterally displaced.25 Anatomical differences at the fovea may make it particularly susceptible to mechanical deformations. For example, the foveal avascular zone (approximately 200-1100 μm diameter)26 is hypothesized to be more deformable than vascularized retinal regions and therefore more vulnerable to the effects of IOP.27,28 Additionally, the foveola does not contain rods, astrocytes, or microglia,25 which may influence tissue properties. Muller cells in the central fovea also express increased glial fibrillary acidic protein, which may indicate increased susceptibility to mechanical stress.25 It is possible that the chronic, albeit mild,13 increase in IOP that occurs during spaceflight exerts a compressive force at the macula, as has been observed after cataract surgery.29 The spaceflight-induced choroidal thickening reported for this cohort13 may also contribute an opposing compressive force at the macula.
Brief application of 25-mm Hg LBNP during spaceflight reduced both internal jugular vein pressure and IOP in the present participants,13,14 suggesting that this magnitude of negative pressure is sufficient to reverse the fluid shift at the head and eye. However, this LBNP exposure did not affect choroid thickness13 and did not significantly alter ONH or retinal morphology presented here. Similarly, a previous study on Earth determined that brief LBNP application did not reverse mild choroidal thickening in a supine posture.5 To our knowledge, the association of LBNP with ONH morphology has not previously been studied. Given that approximately 10 to 15 minutes of exposure to 15° HDT had no effect, the lack of a response to a similar duration of LBNP during spaceflight may be a result of insufficient exposure duration rather than the magnitude of the fluid shift. Evidence that changes in MRW and TRT occur over hours in response to pressure and posture changes30,31 suggests that it may be possible for longer-duration LBNP application to reverse spaceflight-induced ocular changes.
Limitations
There were a few study limitations. Given crew-scheduling constraints, obtaining OCT scans without and with LBNP on the same day and testing participants at the exact same time of day were not possible. However, efforts were made to minimize the days between the in-flight conditions, and all testing occurred during the first half of the day. The study also had a limited sample size and did not have a control group that was not exposed to spaceflight, as is common in spaceflight research. Therefore, cause-and-effect relationships for spaceflight could not be confirmed.
Conclusions
The increased peripapillary TRT and decreased optic cup size observed in this cohort study were consistent with mild ODE. However, in-flight posterior ONH displacement and thinning of the central macula suggest that ICP alone cannot explain the ocular findings associated with long-duration spaceflight. Brief in-flight application of 25-mm Hg LBNP did not influence ONH or retinal morphology, suggesting that longer-duration exposure to headward fluid shift countermeasures may be required to prevent or reverse ocular changes associated with spaceflight, or that other factors contribute to SANS.
eAppendix. STROBE Reporting Guidelines: Additional Details and Exceptions
eTable 1. P Values for Change in Each Parameter During and After Spaceflight Relative to the Preflight Seated Value
eTable 2. Estimated Marginal Mean (95% CI) for Each Parameter Without and With Lower-Body Negative Pressure During Spaceflight
eTable 3. P Values for Change in Each Parameter With LBNP and Between-Flight Days Without LBNP
eTable 4. Estimated Marginal Mean (95% CI) for Each Parameter With Posture Changes Before and After Spaceflight
References
- 1.Macias BR, Patel NB, Gibson CR, et al. Association of long-duration spaceflight with anterior and posterior ocular structure changes in astronauts and their recovery. JAMA Ophthalmol. 2020;138(5):553-559. doi: 10.1001/jamaophthalmol.2020.0673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mader TH, Gibson CR, Pass AF, et al. Optic disc edema, globe flattening, choroidal folds, and hyperopic shifts observed in astronauts after long-duration space flight. Ophthalmology. 2011;118(10):2058-2069. doi: 10.1016/j.ophtha.2011.06.021 [DOI] [PubMed] [Google Scholar]
- 3.Lawley JS, Petersen LG, Howden EJ, et al. Effect of gravity and microgravity on intracranial pressure. J Physiol. 2017;595(6):2115-2127. doi: 10.1113/JP273557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang LF, Hargens AR. Spaceflight-induced intracranial hypertension and visual impairment: pathophysiology and countermeasures. Physiol Rev. 2018;98(1):59-87. doi: 10.1152/physrev.00017.2016 [DOI] [PubMed] [Google Scholar]
- 5.Marshall-Goebel K, Macias BR, Laurie SS, et al. Mechanical countermeasures to headward fluid shifts. J Appl Physiol (1985). 2021;130(6):1766-1777.doi: 10.1152/japplphysiol.00863.2020 [DOI] [PubMed] [Google Scholar]
- 6.Macias BR, Liu JHK, Grande-Gutierrez N, Hargens AR. Intraocular and intracranial pressures during head-down tilt with lower body negative pressure. Aerosp Med Hum Perform. 2015;86(1):3-7. doi: 10.3357/AMHP.4044.2015 [DOI] [PubMed] [Google Scholar]
- 7.Marshall-Goebel K, Terlević R, Gerlach DA, Kuehn S, Mulder E, Rittweger J. Lower-body negative pressure reduces optic nerve sheath diameter during head-down tilt. J Appl Physiol (1985). 2017;123(5):1139-1144.doi: 10.1152/japplphysiol.00256.2017 [DOI] [PubMed] [Google Scholar]
- 8.Watkins W, Hargens AR, Seidl S, Clary EM, Macias BR. Lower-body negative pressure decreases noninvasively measured intracranial pressure and internal jugular vein cross-sectional area during head-down tilt. J Appl Physiol (1985). 2017;123(1):260-266.doi: 10.1152/japplphysiol.00091.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Petersen LG, Lawley JS, Lilja-Cyron A, et al. Lower-body negative pressure to safely reduce intracranial pressure. J Physiol. 2019;597(1):237-248. doi: 10.1113/JP276557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Patel N, Pass A, Mason S, Gibson CR, Otto C. Optical coherence tomography analysis of the optic nerve head and surrounding structures in long-duration International Space Station astronauts. JAMA Ophthalmol. 2018;136(2):193-200. doi: 10.1001/jamaophthalmol.2017.6226 [DOI] [PubMed] [Google Scholar]
- 11.Macias BR, Ferguson CR, Patel N, et al. Changes in the optic nerve head and choroid over 1 year of spaceflight. JAMA Ophthalmol. 2021;139(6):663-667. doi: 10.1001/jamaophthalmol.2021.0931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stenger MB, Laurie SS, Sadda SR, Sadun AA, Macias BR, Huang AS. Focus on the optic nerve head in spaceflight-associated neuro-ocular syndrome. Ophthalmology. 2019;126(12):1604-1606. doi: 10.1016/j.ophtha.2019.09.009 [DOI] [PubMed] [Google Scholar]
- 13.Greenwald SH, Macias BR, Lee SMC, et al. Intraocular pressure and choroidal thickness respond differently to lower body negative pressure during spaceflight. J Appl Physiol (1985). 2021;131(2):613-620.doi: 10.1152/japplphysiol.01040.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Marshall-Goebel K, Laurie SS, Alferova IV, et al. Assessment of jugular venous blood flow stasis and thrombosis during spaceflight. JAMA Netw Open. 2019;2(11):e1915011. doi: 10.1001/jamanetworkopen.2019.15011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Arbeille P, Zuj KA, Macias BR, et al. Lower-body negative pressure reduces jugular and portal vein volumes and counteracts the elevation of middle cerebral vein velocity during long-duration spaceflight. J Appl Physiol (1985). 2021;131(3):1080-1087.doi: 10.1152/japplphysiol.00231.2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yarmanova EN, Kozlovskaya IB, Khimoroda NN, Fomina EV. Evolution of Russian microgravity countermeasures. Aerosp Med Hum Perform. 2015;86(12)(suppl):A32-A37. doi: 10.3357/AMHP.EC05.2015 [DOI] [PubMed] [Google Scholar]
- 17.Laurie SS, Lee SMC, Macias BR, et al. Optic disc edema and choroidal engorgement in astronauts during spaceflight and individuals exposed to bed rest. JAMA Ophthalmol. 2020;138(2):165-172. doi: 10.1001/jamaophthalmol.2019.5261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Frisén L. Swelling of the optic nerve head: a staging scheme. J Neurol Neurosurg Psychiatry. 1982;45(1):13-18. doi: 10.1136/jnnp.45.1.13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dandy WE. Intracranial pressure without brain tumor: diagnosis and treatment. Ann Surg. 1937;106(4):492-513. doi: 10.1097/00000658-193710000-00002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Smith JL. Whence pseudotumor cerebri? J Clin Neuroophthalmol. 1985;5(1):55-56. [PubMed] [Google Scholar]
- 21.Kupersmith MJ, Sibony P, Mandel G, Durbin M, Kardon RH. Optical coherence tomography of the swollen optic nerve head: deformation of the peripapillary retinal pigment epithelium layer in papilledema. Invest Ophthalmol Vis Sci. 2011;52(9):6558-6564. doi: 10.1167/iovs.10-6782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sibony P, Kupersmith MJ, Rohlf FJ. Shape analysis of the peripapillary RPE layer in papilledema and ischemic optic neuropathy. Invest Ophthalmol Vis Sci. 2011;52(11):7987-7995. doi: 10.1167/iovs.11-7918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pardon LP, Cheng H, Tang RA, Saenz R, Frishman LJ, Patel NB. Custom optical coherence tomography parameters for distinguishing papilledema from pseudopapilledema. Optom Vis Sci. 2019;96(8):599-608. doi: 10.1097/OPX.0000000000001408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sater SH, Sass AM, Rohr JJ, et al. Automated MRI-based quantification of posterior ocular globe flattening and recovery after long-duration spaceflight. Eye (Lond). 2021;35(7):1869-1878.doi: 10.1038/s41433-021-01408-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bringmann A, Syrbe S, Görner K, et al. The primate fovea: structure, function and development. Prog Retin Eye Res. 2018;66:49-84. doi: 10.1016/j.preteyeres.2018.03.006 [DOI] [PubMed] [Google Scholar]
- 26.Dubis AM, Hansen BR, Cooper RF, Beringer J, Dubra A, Carroll J. Relationship between the foveal avascular zone and foveal pit morphology. Invest Ophthalmol Vis Sci. 2012;53(3):1628-1636. doi: 10.1167/iovs.11-8488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Springer AD, Hendrickson AE. Development of the primate area of high acuity. 1. use of finite element analysis models to identify mechanical variables affecting pit formation. Vis Neurosci. 2004;21(1):53-62. doi: 10.1017/S0952523804041057 [DOI] [PubMed] [Google Scholar]
- 28.Chen K, Weiland JD. Anisotropic and inhomogeneous mechanical characteristics of the retina. J Biomech. 2010;43(7):1417-1421. doi: 10.1016/j.jbiomech.2009.09.056 [DOI] [PubMed] [Google Scholar]
- 29.Lee YC, Chung FL, Chen CC. Intraocular pressure and foveal thickness after phacoemulsification. Am J Ophthalmol. 2007;144(2):203-208. doi: 10.1016/j.ajo.2007.04.020 [DOI] [PubMed] [Google Scholar]
- 30.Pardon LP, Harwerth RS, Patel NB. Neuroretinal rim response to transient changes in intraocular pressure in healthy non-human primate eyes. Exp Eye Res. 2020;193:107978. doi: 10.1016/j.exer.2020.107978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pardon LP, Cheng H, Chettry P, Patel NB. Optic nerve head morphological changes over 12 hours in seated and head-down tilt postures. Invest Ophthalmol Vis Sci. 2020;61(13):21. doi: 10.1167/iovs.61.13.21 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eAppendix. STROBE Reporting Guidelines: Additional Details and Exceptions
eTable 1. P Values for Change in Each Parameter During and After Spaceflight Relative to the Preflight Seated Value
eTable 2. Estimated Marginal Mean (95% CI) for Each Parameter Without and With Lower-Body Negative Pressure During Spaceflight
eTable 3. P Values for Change in Each Parameter With LBNP and Between-Flight Days Without LBNP
eTable 4. Estimated Marginal Mean (95% CI) for Each Parameter With Posture Changes Before and After Spaceflight