Key Points
Question
Do patients with neovascular age-related macular degeneration (nAMD) treated with a surgically implanted port delivery system (PDS) with ranibizumab prefer the PDS over intravitreal injections of anti–vascular endothelial growth factor agents?
Findings
This phase 3 randomized clinical trial found that treatment satisfaction was high with both PDS and intravitreal treatment, but almost all patients in the PDS arm preferred treatment delivered via the PDS at week 40 vs previous intravitreal injections.
Meaning
Although PDS treatment was preferred by most patients assigned to PDS over previous intravitreal injections, both delivery methods have high treatment satisfaction.
This randomized clinical trial evaluates patient treatment satisfaction with a port delivery system for ranibizumab vs intravitreal injections in patients with neovascular age-related macular degeneration.
Abstract
Importance
The port delivery system (PDS) with ranibizumab has demonstrated noninferior and equivalent efficacy compared with monthly intravitreal injections of ranibizumab, an anti–vascular endothelial growth factor (VEGF) agent, in patients with neovascular age-related macular degeneration (nAMD), but evaluating patient preference is important to help inform clinical decision-making.
Objective
Evaluate treatment satisfaction for ranibizumab delivered via PDS vs intravitreal injections as well as patient preference among those assigned to PDS.
Design, Setting, and Participants
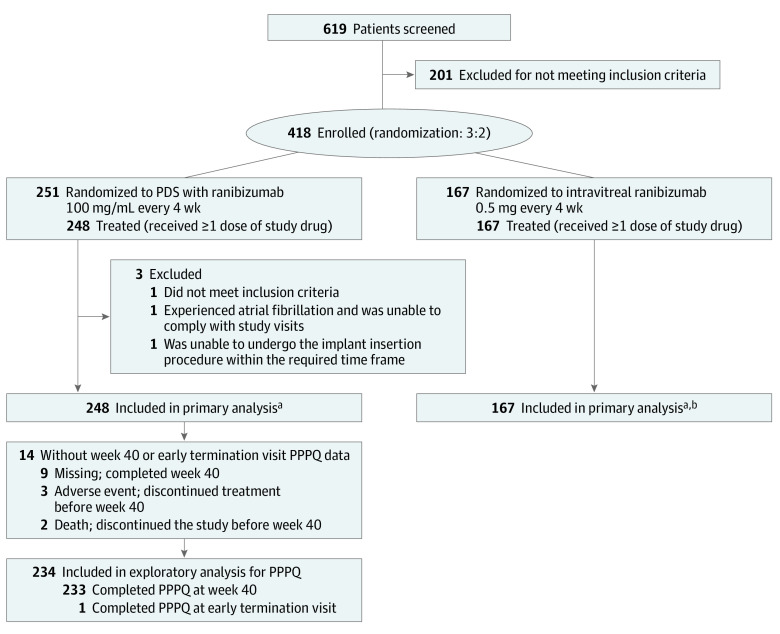
Archway was a phase 3 randomized active-comparator open-label clinical trial conducted at 78 sites in the US. Patients 50 years and older with nAMD diagnosed within 9 months of screening with a documented response to anti-VEGF therapy were included. Of 619 patients screened, 418 were enrolled; 415 were included in the primary analysis and 234 were included in the secondary exploratory analysis. The Archway study ran from September 12, 2019, through primary readout on May 22, 2020.
Interventions
Patients were randomized 3:2 to PDS with ranibizumab, 100 mg/mL, with fixed refill exchanges every 24 weeks or intravitreal ranibizumab injections, 0.5 mg, every 4 weeks.
Main Outcomes and Measures
Treatment satisfaction was measured using the Macular Disease Treatment Satisfaction Questionnaire in the PDS and intravitreal injection arms at week 40. Patient preference was assessed using the content-validated PDS Patient Preference Questionnaire (PPPQ), which measured the proportion of patients in the PDS arm with monthly monitoring who preferred treatment with the PDS at week 40 over previous intravitreal injections or concurrent fellow-eye injections. Both outcomes were exploratory end points.
Results
The mean (SD) age of participants at baseline was 75.0 (7.9) years; 234 participants (59%) were women and 162 (41%) were men. At week 40, differences in overall treatment satisfaction scores were minimal for the PDS and intravitreal injection arms (mean, 68.0; 95% CI, 67.4-68.6; n = 237 and mean, 66.1; 95% CI, 64.9-67.3; n = 159, respectively; difference, 1.9; 95% CI, 0.7-3.1). A total of 234 of 248 patients (94.4%) in the PDS arm were included in the PPPQ analysis. At week 40, almost all patients in the PDS arm preferred treatment via PDS (218 of 234 [93.2%]) vs previous intravitreal injections (3 of 234 [1.3%]), including 172 of 234 (73.5%) with a very strong preference for the PDS. In patients who received concurrent fellow-eye injections (n = 78), 72 (92.3%) preferred the PDS.
Conclusions and Relevance
Although PDS treatment was preferred by almost all patients assigned to PDS over previous intravitreal injections, both delivery methods have high treatment satisfaction. These findings provide further evidence for the PDS as a meaningful alternative treatment option for patients with nAMD.
Trial Registration
ClinicalTrials.gov Identifier: NCT03677934
Introduction
Since the introduction of anti–vascular endothelial growth factor (VEGF) agents, treatment outcomes for patients with neovascular age-related macular degeneration (nAMD) have transformed, shifting from managing vision loss to patients achieving vision gain or stabilization.1 However, optimal long-term anti-VEGF therapy involves frequent injections and monitoring, which contribute to a high management burden and associated noncompliance,2,3,4 and ultimately result in lower visual acuity gains than observed in controlled clinical trials.5,6,7
The port delivery system with ranibizumab (PDS) is an innovative drug delivery system that has been approved by the US Food and Drug Administration for the treatment of nAMD in adults who have previously responded to at least 2 anti-VEGF injections. The PDS includes a surgically placed permanent indwelling and refillable ocular implant that provides continuous release of a customized formulation of ranibizumab.8,9,10 The PDS is refilled with ranibizumab in an in-clinic setting approximately every 6 months and, compared with the requirement for frequent intravitreal injections, has the potential to lead to a reduction in treatment frequency and monitoring visits.8,9,10 The phase 3 Archway trial9 evaluating PDS with ranibizumab, 100 mg/mL with fixed refill exchanges every 24 weeks, vs intravitreal ranibizumab, 0.5 mg injections every 4 weeks, demonstrated that continuous ranibizumab delivery via PDS had equivalent visual acuity efficacy to monthly ranibizumab injections and a generally well-tolerated safety profile.
As in other medical fields, the patient voice is becoming increasingly valuable in clinical decision-making in ophthalmology because traditional efficacy measures do not fully assess all aspects of treatment that are important to patients (eg, quality of life, symptoms, costs, ease of treatment, and treatment satisfaction).11,12,13 Although the PDS is a new treatment for nAMD, a disease for which many other treatment options exist, the drug-device combination and surgical nature of the PDS, with its unique surgical and device-related risks, distinguish it from other options involving intravitreal injections.
When new treatments become available, clinicians may take account of patient preferences. To quantify this, it is necessary to prospectively evaluate patient preference, strength of preference, and reasons for preference using standardized and validated instruments. Patient-reported outcome measures offer clinicians the ability to capture patient views in aspects of treatment beyond efficacy measures. The Macular Disease Treatment Satisfaction Questionnaire (MacTSQ) is a validated patient-reported outcome measures that has been used to measure treatment satisfaction for injections of anti-VEGF agents in patients with nAMD in both clinical trial14 and clinical practice settings.13 However, other specific and validated patient-reported outcome measures in patients with nAMD are lacking, and opportunities to inform clinical decision-making incorporating the views of patients have been limited.
Patients who receive their preferred treatment (ie, through shared decision-making, choice, or assessed preferences) may have higher treatment satisfaction rates, increased adherence and compliance, and superior clinical outcomes.15 The potential for fewer visits and reduced treatment burden with the PDS vs intravitreal injections may influence patient choice. To evaluate patient preference between PDS and intravitreal injections, a PDS Patient Preference Questionnaire (PPPQ) was developed based on the previously reported Patient Preference Questionnaire.16,17 We report treatment satisfaction with the PDS vs intravitreal injections as measured by the MacTSQ in the phase 3 Archway trial9 and the phase 2 Ladder trial.8,10 In addition, among the patients in the PDS arms, we report the PPPQ whereby we evaluated patient preference in the for the PDS vs intravitreal anti-VEGF injections. To further assess the patient voice in our PDS trials, we also report development and content validity of the PPPQ in a subpopulation of patients in Ladder.8,10
Methods
Study Design and Participants
The Archway study ran from September 12, 2019, through primary readout on May 22, 2020. Details of the primary analysis of Archway9 have been published. Briefly, Archway was a phase 3 randomized visual assessor–masked active-comparator open-label trial of the PDS for patients nAMD conducted at 78 sites in the US. The trial adhered to the tenets of the Declaration of Helsinki18 and was conducted in accordance with International Conference on Harmonisation E6 Guidelines for Good Clinical Practice19 and applicable local, state, and federal laws. All trial sites received institutional review board approval before trial initiation and all patients provided written informed consent before enrollment. Participants were compensated for their travel expenses.
Patients were 50 years and older with nAMD-related neovascular lesions involving the macula diagnosed in the study eye within 9 months of screening. Patients had to have received at least 3 previous anti-VEGF intravitreal injections (ranibizumab, bevacizumab, or aflibercept) within 6 months of screening and demonstrated response to anti-VEGF therapy. Study eye exclusion criteria have been previously reported9 and included history of surgical or therapeutic treatment for nAMD other than ranibizumab or prior participation in a clinical trial involving anti-VEGF agents.
Patients were assigned randomly 3:2 to receive ranibizumab either every 24 weeks via the PDS or monthly via intravitreal injection. Implant insertion at day 1 and refill-exchange procedures at 24 weeks were performed as detailed previously.8 Patient monitoring in both treatment arms occurred monthly, and criteria for supplemental treatment in the PDS arm has been described previously.9 Details of Ladder8,10 have been reported previously and are summarized in the eMethods in Supplement 3.
Patient-Reported Outcomes, End Points, and Statistical Analyses
The MacTSQ14 was used to evaluate patient-reported treatment satisfaction with ranibizumab delivered via the PDS for 40 weeks compared with monthly ranibizumab as an exploratory end point in Archway.9 The MacTSQ was assessed at baseline and week 40 before any other study procedures were conducted. The MacTSQ provides a total score and 2 subscale scores. MacTSQ total score ranges from 0 to 72, where a score of at least 60 indicates high satisfaction; the 2 subscales are the Information Provision and Convenience and the Impact of Treatment. Both subscale scores range from 0 to 36, where a score of at least 30 indicates high satisfaction. MacTSQ scores were summarized using appropriate descriptive statistics based on observed data. For patients who discontinued the study early and were administered the MacTSQ at the early termination visit, the last assessment available before week 40 was used in the analysis. The MacTSQ was also used to assess treatment satisfaction in the PDS and intravitreal injection groups in the phase 2 Ladder trial.8 Details of assessment of treatment satisfaction in Ladder are provided in the eMethods in Supplement 3. To assess impact of the experience of ocular serious adverse events (SAEs) on treatment satisfaction, subgroup analyses were performed for patients with and without ocular SAEs in both Archway9 and Ladder.8,10
Patient preference was assessed using the PPPQ in Archway9 to evaluate patient preference for treatment delivered via the PDS vs previous intravitreal injections as an exploratory end point. The 3-item PPPQ captures a patient’s preference for treatment, the strength of their preference, and reasons for their preference (eFigure 1 in Supplement 3). Development and content validation of the PPPQ in a subpopulation in Ladder8,10 are described in the eMethods in Supplement 3. The PPPQ content validation study20 included 11 patients (eTable 1 in Supplement 3), 10 of whom (90.9%) preferred the PDS vs 1 (9.1%) who preferred intravitreal injections (eFigure 2A in Supplement 3). Trained study site personnel administered the PPPQ at week 40 (before any other study procedures) to patients in the PDS arm, all of whom had received at least 3 anti-VEGF injections before day 1. Assessment of the PPPQ at week 40 was to coincide with assessment of the primary end point in Archway.9 Patients were asked to compare the experience of PDS treatment with intravitreal anti-VEGF treatment. For patients who discontinued the study early and were administered the PPPQ at the early termination visit, the last assessment available before week 40 was used in the analysis. A subgroup analysis was carried out in patients with bilateral nAMD in the PDS arm who received at least 1 anti-VEGF intravitreal injection in the fellow eye within the study through week 40. Additionally, to assess impact of the experience of ocular SAEs on patient preference, a subgroup analysis was performed for patients with and without ocular SAEs. Strength of preference and reasons for preference with the PPPQ were summarized descriptively.
Results
MacTSQ
The mean (SD) age of participants at baseline was 75.0 (7.9) years; 234 participants (59%) were women and 162 (41%) were men. All patients in Archway9 were included in the analysis of MacTSQ at baseline (248 in the PDS arm; 167 in the monthly injection arm). In both the PDS and monthly injection arms, patients had high overall treatment satisfaction, with MacTSQ total scores of at least 60 and subscale scores of at least 30 in both treatment arms at baseline (Figure 1). At week 40, high total treatment satisfaction scores were observed in the PDS (mean, 68.0; 95% CI, 67.4-68.6; n = 237 and mean, 66.1; 95% CI, 64.9-67.3; n = 159, respectively; difference, 1.9; 95% CI, 0.7-3.1) (Figure 1 and Table). Subscale scores also showed high treatment satisfaction in both arms. Treatment satisfaction was high for both treatment methods in patients with and without ocular SAEs (eTable 2 in Supplement 3).
Figure 1. Macular Disease Treatment Satisfaction Questionnaire (MacTSQ) Overall Treatment Satisfaction and Subscale Scores for Ranibizumab Via the Port Delivery System (PDS) vs Intravitreal Delivery.
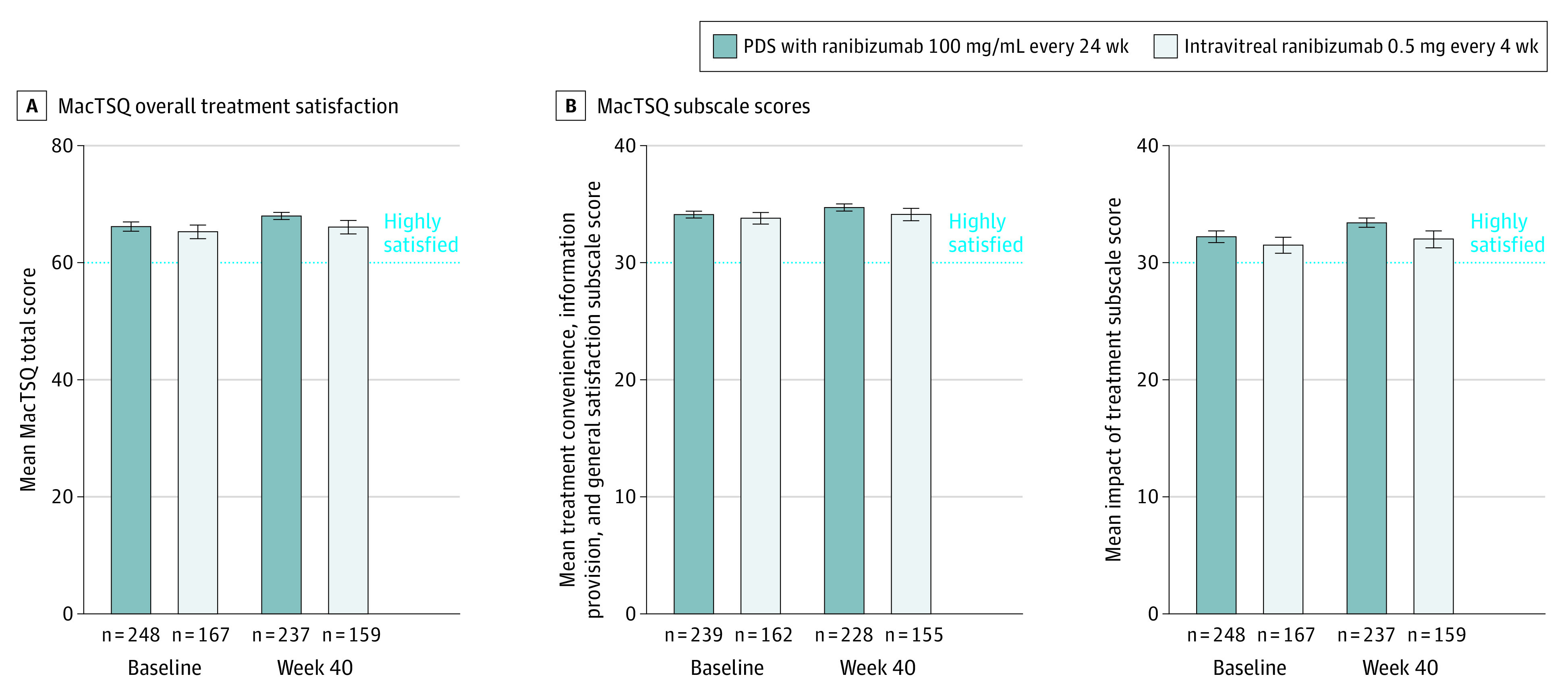
The overall MacTSQ score is on a scale of 1 to 72, with scores of at least 60 indicating high satisfaction. Each MacTSQ subscale is on a scale of 1 to 36, with scores of at least 30 indicating high satisfaction. Error bars represent 95% CIs.
Table. Macular Disease Treatment Satisfaction Questionnaire (MacTSQ) Overall Treatment Satisfaction and Subscale Scores at Week 40a.
| Scale | PDS with ranibizumab, 100 mg/mL every 24 wk | Intravitreal ranibizumab, 0.5 mg every 4 wk | Difference (95% CI) | ||
|---|---|---|---|---|---|
| No. | Mean (95% CI) | No. | Mean (95% CI) | ||
| Overall treatment satisfaction scoreb | 237 | 68.0 (67.4-68.6) | 159 | 66.1 (64.9-67.3) | 1.9 (0.7 to 3.1) |
| Treatment convenience, information provision, and general satisfaction subscale scorec | 228 | 34.7 (34.4-34.9) | 155 | 34.1 (33.6-34.7) | 0.6 (–0.1 to 1.2) |
| Impact of treatment subscale scorec | 237 | 33.4 (32.9-33.8) | 159 | 32.0 (31.2-32.7) | 1.4 (0.6 to 2.2) |
Abbreviation: PDS, port delivery system with ranibizumab.
Treatment group means, differences, and associated 95% CIs were estimated with an analysis of variance model with randomized treatment as the independent variable.
The overall MacTSQ score is on a scale of 1 to 72, with scores of at least 60 indicating high satisfaction.
Each MacTSQ subscale is on a scale of 1 to 36, with scores of at least 30 indicating high satisfaction.
High treatment satisfaction was also observed with PDS and monthly intravitreal injections throughout Ladder8,10 and in patients with and without ocular SAEs in the PDS arm (eResults, eTables 3 and 4, and eFigure 3 in Supplement 3).
PPPQ
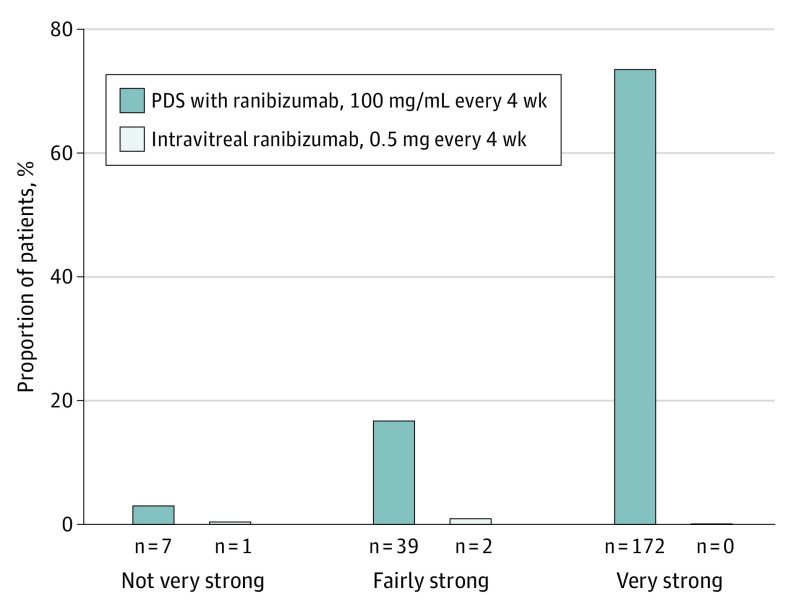
PPPQ was evaluated only in patients assigned to the PDS arm. A total of 234 of 248 patients (94.4%) completed the PPPQ and were included in the analysis (Figure 2). As reported in our primary analysis of Archway,9 almost all patients in the PDS group (218 of 234 [93.2%]) preferred treatment via PDS at week 40 vs intravitreal injections (3 of 234 [1.3%]); 13 patients (5.6%) had no preference for either regimen. Most patients in the PDS group (172 of 234 [73.5%]) had a very strong preference for PDS (Figure 3). The most common reasons cited for preference for PDS were fewer treatments (175 of 218 [80.3%]), less discomfort (164 of 218 [75.2%]), and less worry or nervousness (117 of 218 [53.7%]) (Figure 4).
Figure 2. Patient Flow Diagram for the Exploratory Analysis of the Port Delivery System (PDS) Patient Preference Questionnaire (PPPQ).
aDetails of the primary analysis of Archway have been published elsewhere.9
bPatients in the intravitreal ranibizumab group were not included in the exploratory analysis for PPPQ.
Figure 3. Treatment Strength of Preference for the Port Delivery System (PDS) Patient Preference Questionnaire.
For patients with missing week-40 values, the last postbaseline observation was used. Percentages are based on the total number of patients who completed the measure (n = 234).
Figure 4. Reason for Treatment Preference in Patients in the Port Delivery System (PDS) Arm as Assessed by the PDS Patient Preference Questionnaire.
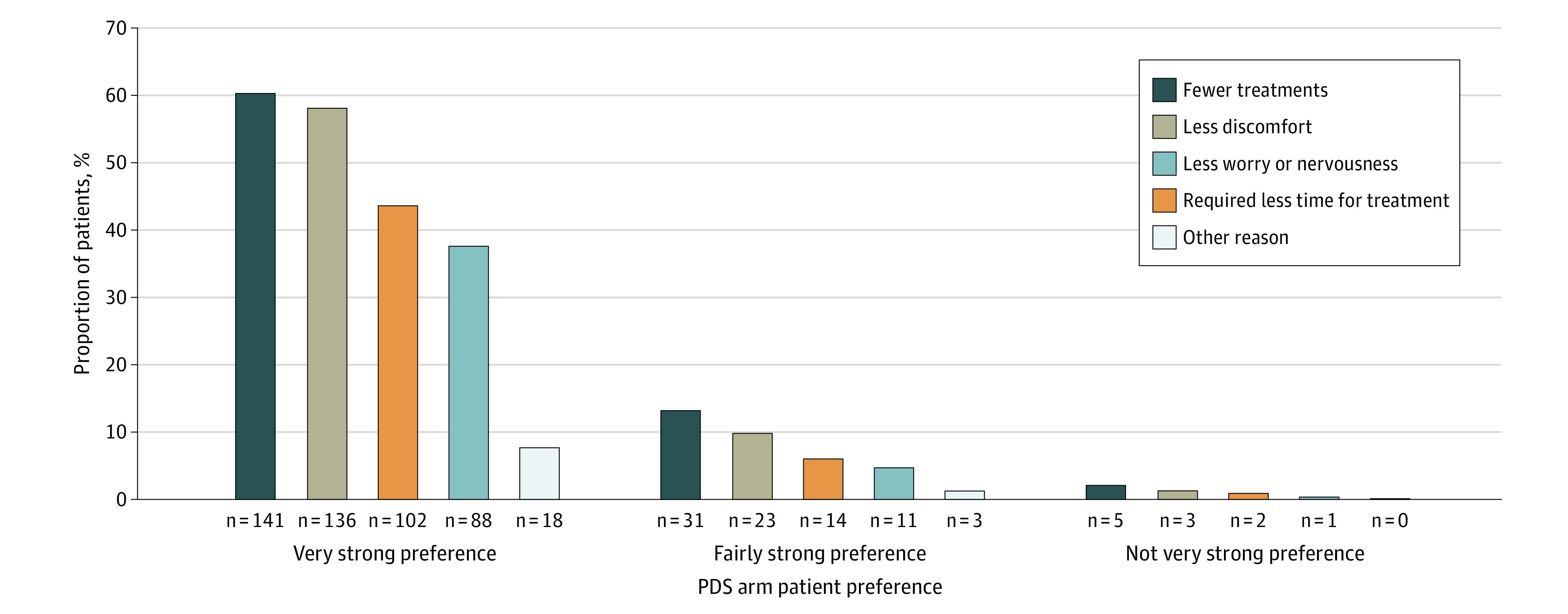
Patients could select multiple reasons for their preference. For patients with missing week-40 values, the last postbaseline observation was used. Percentages are based on the total number of patients who completed the measure (n = 234).
Results from the subgroup analysis in patients with bilateral nAMD in the PDS group (n = 78 [33.3%]) who received at least 1 anti-VEGF intravitreal injection in the fellow eye through week 40 were consistent with the results in the entire PDS population. Of 78 patients in this subgroup, 72 (92.3%) preferred the PDS, whereas 2 (2.6%) preferred intravitreal injections.
Among PDS patients who were administered the PPPQ and did not have an ocular SAE (n = 222), 209 (94.1%) expressed preference for the PDS. Of the 12 patients who were administered the PPPQ and had an ocular SAE, 9 (75.0%) expressed preference for the PDS.
Discussion
Systematic evaluation of patient preference is emerging as an important factor in determining the most appropriate treatments, especially when different treatments show equivalent efficacy. Objective cohort preference data are increasingly helpful to physicians, patients, and health care systems in determining treatment choices. Archway9 was the first phase 3 trial of the PDS; it demonstrated that longer-acting anti-VEGF treatment via the PDS provided equivalent visual acuity efficacy, with some safety concerns unique to the PDS, compared with monthly intravitreal anti-VEGF injections. To better understand the patient experience in Archway9 and Ladder,8,10 we used the MacTSQ to measure treatment satisfaction for patients treated with the PDS vs patients treated with intravitreal injections. Patients in both Archway and Ladder reported not only a high overall treatment satisfaction for both the PDS and intravitreal injections, but post hoc analyses did not demonstrate a difference in treatment satisfaction between treatment methods. Another clinical trial14 in patients with nAMD using the MacTSQ to assess treatment satisfaction after injection of anti-VEGF agents showed that visual acuity gains were an important determinant of patient satisfaction. In Archway, visual acuity was equivalent between the PDS and intravitreal injections arms and may have led to similar outcomes for treatment satisfaction. However, when using the MacTSQ to assess treatment satisfaction in patients with nAMD in a clinical practice setting, visual acuity gains were found to be less important, and patients’ perception of satisfaction was more related to aspects of their care and disease process, including in-clinic service, quality of life, and duration of disease.13 These findings indicate that the patient’s experience of treatments is likely to differ depending on setting and may encompass other aspects besides efficacy.
Here, using the content-validated PPPQ with patients assigned to the PDS arm, we found that 93.2% of patients preferred treatment with ranibizumab delivered via the PDS vs only 1.3% of patients who preferred intravitreal injections.9 In addition, we found that most patients (73.5%) in the PDS group had a very strong preference for the PDS, with the most common reasons for PDS preference being fewer treatments, less discomfort, and less worry or nervousness. This is perhaps unsurprising because many patients receiving repeated intravitreal injections report discomfort and anxiety arising from fear of discomfort,21 and fear and anxiety are commonly given reasons for nonadherence among patients receiving intravitreal anti-VEGF injections.22 We found that a similarly high proportion of patients preferred the PDS vs intravitreal injections in the subpopulation of patients with bilateral disease who received concurrent intravitreal injections in the fellow eye. These findings in Archway9 were consistent with the results from our earlier PPPQ content validation study.20
Given the concerns associated with the safety profile of the PDS, we performed a subgroup analysis for patients with and without ocular SAEs to assess whether preference for the PDS was driven by those without serious complications. Although patient preference for the PDS was lower in patients with ocular SAEs vs those without, most patients (75.0%) with ocular SAEs preferred PDS treatment. These data further contextualize the overall strong patient preference for the PDS observed in Archway.9
Patient-reported outcome measures provide a unique opportunity to aid clinical development because they capture aspects of treatment that are important to patients and generate standardized outcomes that can be used to inform treatment decision-making. However, there is a need for specific and validated instruments in patients with nAMD to accurately capture the patient’s voice. The PPPQ was developed, and its content was validated using a subpopulation of patients with nAMD who completed Ladder.8,10 The aim of developing the PPPQ was to provide clinicians with a robust and standardized method of assessing patient preference for the PDS. After minor modifications to the PPPQ based on feedback in the PPPQ content validation study,20 we successfully demonstrated the content validity of the PPPQ.
Long-term and frequent use of intravitreal anti-VEGF injections is associated with substantial burden to patients, caregivers, and health care professionals.2,3,4 Other studies evaluating patient preference for different anti-VEGF treatments in clinical practice settings showed that the main factor in patient treatment preference was impact on visual acuity.23,24,25 However, treatment burden (eg, frequency and length of visits and costs to patients and health care professionals) was another important consideration.23,24,25 One study comparing pro re nata vs monthly injections with ranibizumab in patients with nAMD reported that a higher proportion of patients favored a less burdensome pro re nata regimen over monthly injections (53% vs 38%, respectively).26 Another analysis of anti-VEGF regimens in patients with nAMD reported a preference for treat-and-extend (46%), followed by pro re nata (44%), and fixed (4%) anti-VEGF regimens, thereby reducing the burden for patients and caregivers and use of health care resources.27 These studies highlight the unmet need for improvements in the treatment approach for patients with nAMD. In addition, these findings suggest that patients may prefer less burdensome treatment regimens. Evidence from other treatment settings suggest that saving time and less frequent injection regimen are key reasons underlying patient preference.28,29
Limitations
In addition to the open-label study design, there are several limitations of this investigation that could affect interpretation of results. In Archway,9 the PPPQ was assessed at week 40 in patients who were diagnosed with nAMD within 9 months of screening and who were responsive to anti-VEGF therapy. Further studies are required to evaluate patient preference with longer-term disease, with longer-term follow-up, and in a clinical practice setting. This is particularly important in patients with nAMD because nAMD is a life-long condition. Patients’ preference for treatment may vary during the course of the disease and be influenced by factors outside those that can be controlled in a clinical trial. Another potential limitation of the current study is the risk of bias. Patients who were open to receiving a surgical procedure agreed to participate in Archway.9 Further, because patients with the PDS were asked to recall their previous experience with intravitreal injections (ie, before PDS implantation at baseline), results at week 40 may suffer from recall bias. However, because results from the 33.3% of patients with bilateral disease who received intravitreal injections in the fellow eye through week 40 showed that a similarly high proportion of patients (more than 90%) preferred the PDS vs intravitreal injections, the potential impact of this bias on patient preference is assumed to be minimal. Neither Archway9 nor Ladder8,10 was designed to evaluate the number of visits required for monitoring patients treated via PDS. The potential role of decreased monitoring cannot be assessed in this study because patients in the PDS arm came in for monthly visits. Future studies may determine the optimal visit schedule for patients treated with the PDS and investigate the potential of the PDS to reduce the treatment and monitoring burden among patients with nAMD. Another important consideration is assessment of real-world economics of any treatment; hence, appropriate evaluations of preference for the PDS accounting for economics are warranted, and will be feasible in the future given recent approval of the PDS.
Conclusions
Although PDS treatment was preferred over previous intravitreal injections by almost all patients assigned to the PDS, both delivery methods had high treatment satisfaction. Using the content-validated PPPQ in patients with nAMD only assigned to the PDS in Archway,9 we found that continuous delivery of ranibizumab via the PDS was preferred by almost all patients in the PDS group vs intravitreal injections. Preference for the PDS was supported by findings in patients with bilateral nAMD who received concurrent intravitreal injections in the fellow eye. Most patients assigned to the PDS who experienced ocular SAEs preferred treatment with PDS. The overall results appear to be driven by the perceived reductions in treatment burden reported as the need for fewer treatments, requiring less time for treatment, and less discomfort. We previously reported that the PDS had equivalent efficacy and was noninferior to intravitreal anti-VEGF injections in Archway and had a well-understood safety profile that needs to be kept in mind. Together with the results of the current analysis, these findings suggest that the PDS could be a meaningful alternative treatment option for patients with nAMD.
Trial protocol
Statistical analysis plan
eMethods
eResults
eReferences
eTable 1. Baseline Characteristics and Demographics for the PPPQ Content Validation Population
eTable 2. MacTSQ Overall Treatment Satisfaction and Subscale Scores From Archway for the PDS 100 mg/mL q24w (n = 248) and Intravitreal Ranibizumab 0.5 mg q4w Injection (n = 167) Arms for Patients With and Without Ocular SAEs
eTable 3. Difference in MacTSQ Overall Treatment Satisfaction Subscale Scores From Ladder for the PDS 100 mg/mL PRN (n = 59) and Monthly Intravitreal Ranibizumab 0.5 mg Injection (n = 41) Arms
eTable 4. MacTSQ Overall Treatment Satisfaction and Subscale Scores From Ladder for the PDS 100 mg/mL PRN (n = 179) and Monthly Intravitreal Ranibizumab 0.5 mg Injection (n = 41) Arms for Patients With and Without Ocular SAEs
eFigure 1. Baseline Characteristics and Demographics for the PPPQ Content Validation Population
eFigure 2. Treatment Preference (A) and Strength of Preference (B) for the PPPQ (PPPQ Content Validation Population; n = 11)
eFigure 3. MacTSQ Overall Treatment Satisfaction (A) and Subscale Scores (B) From Ladder for the PDS 100 mg/mL PRN (n = 59) and Monthly Intravitreal Ranibizumab 0.5 mg Injection (n = 41) Arms
Data sharing statement
References
- 1.Flaxel CJ, Adelman RA, Bailey ST, et al. Age-related macular degeneration preferred practice pattern. Ophthalmology. 2020;127(1):1-P65. doi: 10.1016/j.ophtha.2019.09.024 [DOI] [PubMed] [Google Scholar]
- 2.Gohil R, Crosby-Nwaobi R, Forbes A, Burton B, Hykin P, Sivaprasad S. Caregiver burden in patients receiving ranibizumab therapy for neovascular age related macular degeneration. PLoS One. 2015;10(6):e0129361. doi: 10.1371/journal.pone.0129361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Prenner JL, Halperin LS, Rycroft C, Hogue S, Williams Liu Z, Seibert R. Disease burden in the treatment of age-related macular degeneration: findings from a time-and-motion study. Am J Ophthalmol. 2015;160(4):725-31.e1. doi: 10.1016/j.ajo.2015.06.023 [DOI] [PubMed] [Google Scholar]
- 4.Varano M, Eter N, Winyard S, Wittrup-Jensen KU, Navarro R, Heraghty J. Current barriers to treatment for wet age-related macular degeneration (WAMD): findings from the WAMD patient and caregiver survey. Clin Ophthalmol. 2015;9:2243-2250. doi: 10.2147/OPTH.S92548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cohen SY, Mimoun G, Oubraham H, et al. ; LUMIERE Study Group . Changes in visual acuity in patients with wet age-related macular degeneration treated with intravitreal ranibizumab in daily clinical practice: the LUMIERE study. Retina. 2013;33(3):474-481. doi: 10.1097/IAE.0b013e31827b6324 [DOI] [PubMed] [Google Scholar]
- 6.Holekamp NM, Liu Y, Yeh WS, et al. Clinical utilization of anti-VEGF agents and disease monitoring in neovascular age-related macular degeneration. Am J Ophthalmol. 2014;157(4):825-833.e1. doi: 10.1016/j.ajo.2013.12.018 [DOI] [PubMed] [Google Scholar]
- 7.Holz FG, Tadayoni R, Beatty S, et al. Multi-country real-life experience of anti-vascular endothelial growth factor therapy for wet age-related macular degeneration. Br J Ophthalmol. 2015;99(2):220-226. doi: 10.1136/bjophthalmol-2014-305327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Campochiaro PA, Marcus DM, Awh CC, et al. The port delivery system with ranibizumab for neovascular age-related macular degeneration: results from the randomized phase 2 Ladder clinical trial. Ophthalmology. 2019;126(8):1141-1154. doi: 10.1016/j.ophtha.2019.03.036 [DOI] [PubMed] [Google Scholar]
- 9.Holekamp NM, Campochiaro PA, Chang M, et al. Archway randomized phase 3 trial of the port delivery system with ranibizumab for neovascular age-related macular degeneration. Ophthalmology. 2022;129(3):295-307. doi: 10.1016/j.ophtha.2021.09.016 [DOI] [PubMed] [Google Scholar]
- 10.Khanani AM, Callanan D, Dreyer R, et al. ; of the Ladder Investigators . End-of-study results for the Ladder phase 2 trial of the port delivery system with ranibizumab for neovascular age-related macular degeneration. Ophthalmol Retina. 2021;5(8):775-787. doi: 10.1016/j.oret.2020.11.004 [DOI] [PubMed] [Google Scholar]
- 11.Braithwaite T, Calvert M, Gray A, Pesudovs K, Denniston AK. The use of patient-reported outcome research in modern ophthalmology: impact on clinical trials and routine clinical practice. Patient Relat Outcome Meas. 2019;10:9-24. doi: 10.2147/PROM.S162802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dean S, Mathers JM, Calvert M, et al. “The patient is speaking”: discovering the patient voice in ophthalmology. Br J Ophthalmol. 2017;101(6):700-708. doi: 10.1136/bjophthalmol-2016-309955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gohil R, Crosby-Nwaobi R, Forbes A, Burton BJ, Hykin P, Sivaprasad S. Treatment satisfaction of patients undergoing ranibizumab therapy for neovascular age-related macular degeneration in a real-life setting. Patient Prefer Adherence. 2016;10:949-955. doi: 10.2147/PPA.S105536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mitchell J, Bradley C. Design and development of the MacTSQ measure of satisfaction with treatment for macular conditions used within the IVAN trial. J Patient Rep Outcomes. 2017;2(1):5. doi: 10.1186/s41687-018-0031-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lindhiem O, Bennett CB, Trentacosta CJ, McLear C. Client preferences affect treatment satisfaction, completion, and clinical outcome: a meta-analysis. Clin Psychol Rev. 2014;34(6):506-517. doi: 10.1016/j.cpr.2014.06.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pivot X, Gligorov J, Müller V, et al. ; PrefHer Study Group . Preference for subcutaneous or intravenous administration of trastuzumab in patients with HER2-positive early breast cancer (PrefHer): an open-label randomised study. Lancet Oncol. 2013;14(10):962-970. doi: 10.1016/S1470-2045(13)70383-8 [DOI] [PubMed] [Google Scholar]
- 17.Rummel M, Kim TM, Aversa F, et al. Preference for subcutaneous or intravenous administration of rituximab among patients with untreated CD20+ diffuse large B-cell lymphoma or follicular lymphoma: results from a prospective, randomized, open-label, crossover study (PrefMab). Ann Oncol. 2017;28(4):836-842. doi: 10.1093/annonc/mdw685 [DOI] [PubMed] [Google Scholar]
- 18.World Medical Association . WMA Declaration of Helsinki—ethical principles for medical research involving human subjects. Accessed September 3, 2021. https://www.wma.net/policies-post/wma-declaration-of-helsinki-ethical-principles-for-medical-research-involving-human-subjects/
- 19.International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use . ICH harmonised guideline integrated addendum to ICH E6(R1): guideline for Good Clinical Practice ICH E6(R2) ICH consensus guideline. Accessed September 3, 2021. https://ichgcp.net/
- 20.Tschosik E, Kapre A, Ferrara D, Chang M. Content validity of the port delivery System with ranibizumab Patient Preference Questionnaire. Presented at: Association for Research in Vision and Ophthalmology; April 28-May 2, 2019; Vancouver, BC, Canada. [Google Scholar]
- 21.McClard CK, Wang R, Windham V, et al. Questionnaire to Assess Life Impact of Treatment by Intravitreal Injections (QUALITII): development of a patient-reported measure to assess treatment burden of repeat intravitreal injections. BMJ Open Ophthalmol. 2021;6(1):e000669. doi: 10.1136/bmjophth-2020-000669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ehlken C, Ziemssen F, Eter N, et al. Systematic review: non-adherence and non-persistence in intravitreal treatment. Graefes Arch Clin Exp Ophthalmol. 2020;258(10):2077-2090. doi: 10.1007/s00417-020-04798-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Baxter JM, Fotheringham AJ, Foss AJ. Determining patient preferences in the management of neovascular age-related macular degeneration: a conjoint analysis. Eye (Lond). 2016;30(5):698-704. doi: 10.1038/eye.2016.18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bhagat D, Kirby B, Bhatt H, Jager R, George M, Sheth V. Patient preferences associated with anti-vascular endothelial growth factor therapies for neovascular age-related macular degeneration and diabetic macular edema. Clin Ophthalmol. 2020;14:2975-2982. doi: 10.2147/OPTH.S273564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mueller S, Agostini H, Ehlken C, Bauer-Steinhusen U, Hasanbasic Z, Wilke T. Patient preferences in the treatment of neovascular age-related macular degeneration: a discrete choice experiment. Ophthalmology. 2016;123(4):876-883. doi: 10.1016/j.ophtha.2015.12.001 [DOI] [PubMed] [Google Scholar]
- 26.Droege KM, Caramoy A, Kersten A, et al. Patient preference of ranibizumab treatment regimen for neovascular age-related macular degeneration—monthly injections versus pro re nata. Graefes Arch Clin Exp Ophthalmol. 2014;252(1):31-34. doi: 10.1007/s00417-013-2412-6 [DOI] [PubMed] [Google Scholar]
- 27.Pina Marín B, Gajate Paniagua NM, Gómez-Baldó L, Gallego-Pinazo R. Burden of disease assessment in patients with neovascular age-related macular degeneration in Spain: results of the AMD-MANAGE study. Eur J Ophthalmol. 2022;32(1):385-394. Published online March 15, 2021. doi: 10.1177/11206721211001716 [DOI] [PubMed] [Google Scholar]
- 28.McNamara M, Turner-Bowker DM, Westhead H, et al. Factors driving patient preferences for growth hormone deficiency (GHD) injection regimen and injection device features: a discrete choice experiment. Patient Prefer Adherence. 2020;14:781-793. doi: 10.2147/PPA.S239196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Metz M, Semsek D, Rogmans G, et al. Patient, nurse, and physician preferences: final results of the CONVENIENCE study evaluating pegfilgrastim prophylaxis via pre-filled syringe or on-body injector in cancer patients. Support Care Cancer. 2021;29(11):6633-6643. doi: 10.1007/s00520-021-06230-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial protocol
Statistical analysis plan
eMethods
eResults
eReferences
eTable 1. Baseline Characteristics and Demographics for the PPPQ Content Validation Population
eTable 2. MacTSQ Overall Treatment Satisfaction and Subscale Scores From Archway for the PDS 100 mg/mL q24w (n = 248) and Intravitreal Ranibizumab 0.5 mg q4w Injection (n = 167) Arms for Patients With and Without Ocular SAEs
eTable 3. Difference in MacTSQ Overall Treatment Satisfaction Subscale Scores From Ladder for the PDS 100 mg/mL PRN (n = 59) and Monthly Intravitreal Ranibizumab 0.5 mg Injection (n = 41) Arms
eTable 4. MacTSQ Overall Treatment Satisfaction and Subscale Scores From Ladder for the PDS 100 mg/mL PRN (n = 179) and Monthly Intravitreal Ranibizumab 0.5 mg Injection (n = 41) Arms for Patients With and Without Ocular SAEs
eFigure 1. Baseline Characteristics and Demographics for the PPPQ Content Validation Population
eFigure 2. Treatment Preference (A) and Strength of Preference (B) for the PPPQ (PPPQ Content Validation Population; n = 11)
eFigure 3. MacTSQ Overall Treatment Satisfaction (A) and Subscale Scores (B) From Ladder for the PDS 100 mg/mL PRN (n = 59) and Monthly Intravitreal Ranibizumab 0.5 mg Injection (n = 41) Arms
Data sharing statement