Abstract
The economic potential of the cactus species Cereus peruvianus Mill. (syn. C. hildmannianus K. Schum.) has already been demonstrated through the generation of products and patents. However, the phenolic compounds and antioxidant activity have not yet been evaluated. The aim of our study was to determine the total phenolic compounds, evaluate the antioxidant activity and characterize the phenolic compounds of cladode extracts from C. peruvianus grown in the southern region of Brazil, in two collection periods. Higher total content of phenolic compounds and antioxidant activity were detected in the cladode extract collected in 2016 than in the cladode extract collected in 2015. The profile of phenolic compounds identified five flavonoids that had not previously been reported in species of the genus Cereus. The phenolic compounds linked to antioxidant activities identified in the cladode extract from C. peruvianus support the use of this species in human food as a source of natural antioxidants.
Keywords: Cactus, UHPLC-ESI-QTOF-MS, Flavonoids, Polyphenols, Therapeutic potential
Graphical abstract
Highlights
-
•
Total phenolic compounds were determined in cladode extracts from Cereus peruvianus.
-
•
The antioxidant activity and the phenolic compounds were evaluated and characterized.
-
•
Different total content of phenolic compounds was detected in two collection periods.
-
•
The production of phenolic compounds by the same plant underwent annual variation.
-
•
Five flavonoids that had not previously been reported in Cereus genus were identified.
1. Introduction
The identification of novel antioxidants from natural sources is currently one of the main research focuses on the development of human diets and the preventive role of antioxidants in various oxidative stress-related conditions. Lin et al. (2016) highlighted the importance of phenolic compounds in human nutrition and management of type 2 diabetes. The health benefits and toxicity of phenolic compounds were widely reported by Bhuyan and Basu (2017). Phenolic compounds derived from various natural sources are linked to antioxidant, anti-inflammatory, anti-allergic, anti-carcinogenic, antihypertensive, cardioprotective, anti-arthritic and antimicrobial activities (review by Bhuyan and Basu, 2017). The phenolic compounds which participate in plant defense against ultraviolet rays, pathogens, and several predators also have a preventive role in cardiovascular and neurodegenerative diseases, including antiviral, anticancer, and anti-inflammatory activities (Cory et al., 2018; Forni et al., 2019; Potì et al., 2019). Alara et al. (2021) reported a summary of some recent studies and their results, regarding different sources of polyphenols. Several species of fruit and vegetables have been reported as important sources of phenolic compounds (Alara et al., 2021). However, species of Cactaceae are not yet on this list. Although medicinal properties and health benefits of several cactus species have been reported (review by Chandra et al., 2019), few studies have been done to characterize phenolic compounds derived from cacti species. Cacti have been singled out as the food and fuel of the future (FAO, 2017), due to morphological and physiological adaptations that allow these plants to resist global warming and the marked climate change that are already evident across the entire globe.
Phenolic compounds, chemical variability and antioxidant activity have been mainly reported in species and cultivars of Opuntia and Nopalea genera (Santos-Zea et al., 2011; Alves et al., 2017; Bargougui et al., 2019; Chbani et al., 2020; Santiago et al., 2021). Phenolic compounds in cacti of the genus Stenocereus and Hylocereus have been mainly evaluated in fruits (Esquivel et al., 2007; Beltrán-Orozco et al., 2009; Choo and Yong, 2011; Som et al., 2019; Attar et al., 2022). Assessments of phenolic compounds in cacti of the genus Cereus are scarce, and they have been restricted to only one species. The characterization and antioxidant activity of phenolic compounds in cladodes and fruits of C. jamacaru, a cactus used as food and in the traditional medicine in the northeastern region of Brazil, have supported its use in human food and also revealed its therapeutic potential (Dutra et al., 2018, 2019; Santos et al., 2018; Soares et al., 2021). The genus Cereus Miller is described in the subfamily Cactoideae, tribe Cereeae, and it is widely distributed from Mexico to Argentina, with a center of diversity in Brazil (Zappi et al., 2015). The origin of Cereus genus was dated during the Pliocene-Pleistocene transition and the Cerrado was identified as the probable ancestral area (Franco et al., 2017). Different species of Cereus have been described in the northern, northeastern, midwestern, southeastern and southern regions of Brazil (Taylor and Zappi, 2004; Hunt et al., 2006).
Plants of the Cereus genus cultivated in the south of Brazil identified as C. peruvianus Mill., also known as C. hildmannianus K. Schumann, have also been used in popular and herbal medicine. An extract from cladodes from C. peruvianus has shown beneficial effects on human health as well as for the treatment of different disorders. According to Assis et al. (2011), C. peruvianus is a synonym of C. hildmannianus in the southern region of Brazil. The formulations with C. peruvianus cladodes showed inhibition of the gastric lesions (Tanaka et al., 2010) and effective results in reducing body weight in Wistar rats submitted to a high-fat diet (Bernardi et al., 2013). Beneficial health effects of cactus cladodes have been attributed to dietary fiber (Osuna-Martínez et al., 2014), and an extract of Cereus is marketed as a herbal medicine with the purpose of losing weight (ViaFarma, 2017). C. hildmannianus cladodes have been indicated for the treatment of kidney disorders and in the topical treatment of wounds (Carvalho et al., 2018) and as a diuretic (Sousa et al., 2019).
The economic potential of C. hildmannianus has already been demonstrated through the generation of products and patents. Nutraceutical products include powdered (≤0.2 mm) of C. hildmannianus (syn. C. peruvianus) associated with vitamins, mineral nutrients, and fiber, which may be consumed in the form of tablets, capsules, chewing gum and sweets, and may also be added to juices or teas (BR patent number, 2009002296) (Luna, 2012). Despite the medicinal properties and several utilities found, and the chemical characterizations that have already been carried out for C. peruvianus or C. hildmannianus (review by Santos et al., 2020), the phenolic compounds and antioxidant activity have not yet been evaluated. The aim of the present study was to determine the total phenolic compounds, evaluate the antioxidant activity and characterize the phenolic compounds of cladode extracts from C. peruvianus grown in the southern region of Brazil and collected during two different periods to verify whether the production and characterization of phenolic compounds by a plant is conserved in both periods.
2. Material and methods
2.1. Collection and preparation of cladodes from C. peruvianus
Cladodes of C. peruvianus were collected in 2015 (September) and 2016 (August) from a cultivated plant in an experimental area in southern Brazil (Maringá, state of Paraná; 23°25′38″S; 51°56′15″W). Cladodes from only one plant were collected in the two periods since the aim of our study was to investigate whether the production and characterization of phenolic compounds by a plant is conserved or whether it can undergo annual variation. According to data from the Main Climatic Station of the State University of Maringá (UEM), mean indicators of climatic conditions in the month in which the samples were collected comprised 273.0 h of sunshine, 24.0 °C with 53.7 mm of rain in 2015, and 267.0 h of sunshine, 20.8 °C with 64.8 mm of rain in 2016.
The cladodes of C. peruvianus were cut (20 cm in length), washed in running water and the spines and vascular bundles were removed. After cleaning, the cladodes were cut into smaller pieces. The cladode pieces (150 g of each sample) were lyophilized at −60 °C, until completely dry. The weight of materials after freeze-drying was 10.0 g. The cladode collected in 2015 was analyzed in 2015 and cladode collected in 2016 was analyzed in 2016.
2.2. Extraction of phenolic compounds
Phenolic compounds were extracted by a procedure described by Wiczkowski et al. (2013). The cladodes of each lyophilized sample (10 g) were homogenized with 200 mL of a solution prepared with methanol/water/trifluoroacetic acid (0.58:0.38:0.04, v/v/v) at room temperature under mechanical stirring (blender) for 5 min at 5 °C. Then, the extracts were filtered and centrifuged at 5,000 rpm for 30 min at 5 °C. After centrifugation, the supernatants were concentrated in a rotary evaporator at 35 °C, and then lyophilized at −60 °C until completely dry. After the lyophilization process, 3 g of crude extract were obtained. The extracts were stored at −20 °C until used.
2.3. Determination of total phenolic compounds
The total phenolic compounds in the cladode extracts of C. peruvianus were determined using 100 mg of the crude extracts suspended in 10 mL of methanol (10 mg/mL) employing the Folin-Ciocalteu method described by Astello-Garcia et al. (2015). An aliquot (20 μL) of the methanol solution was added to 1.58 mL of distilled water. Then, 100 μL of Folin-Ciocalteu phenol reagent was added, and after 3 min 300 μL of 20% Na2CO3 was added. The mixtures were left to stand for 1 h at room temperature in the dark. Afterwards, the reading was taken in a Libra S60PC spectrophotometer (Biochrom, Cambourne, CBE, United Kingdom) at 765 nm. Gallic acid (100–800 mg mL−1) was used as a standard in the calibration curve to determine the content of total phenolic compounds. Results were expressed in milligrams of gallic acid equivalents per gram of extract (mg EAG·g−1 of extract). The determinations for each extract were performed in triplicates.
2.4. Evaluation of antioxidant action by the DPPH• method
The scavenging ability of the DPPH• (2,2-diphenyl-1-picrylhydrazyl) radical was measured using the method described by Ma et al. (2011). C. peruvianus crude extracts were diluted in three concentrations of methanol (10, 25 and 50 mg mL−1), and 25 μL of each dilution was transferred to test tubes with 2 mL of 0.625 μM DPPH• methanol solution. The solutions were mixed and left to stand at room temperature in the dark. After 30 min, the reading was carried out in a Libra S60PC spectrophotometer (Biochrom, Cambourne, CBE, United Kingdom) at 517 nm, and the same volume of methanol was used as control. Trolox methanol solutions at concentrations 100–2000 μM L−1 were used to construct the calibration curve; antioxidant capacity was expressed as μM equivalent trolox·g−1 extract (μM ET·g−1 extract). Assays were performed in triplicates.
2.5. Evaluation of antioxidant action by the ABTS• method
The ABTS [2, 2′-azino-bis(3-ethylbenzothiazolin)-6-sulfonic] assay was performed as described by Rufino et al. (2007). The ABTS• radical was prepared with 5 mL of an ABTS• stock solution (7 mM) and 88 μL of a potassium persulfate stock solution (140 mM). The solution was left to stand for 16 h at room temperature (25 °C) in the dark. After that, the ABTS• solution was diluted in ethanol to the initial absorbance value of 0.700 (±0.05) at 734 nm. The assay was performed using 30 μL of the crude extract resuspended in three concentrations of ethanol (10, 25 and 50 mg mL−1), and 30 μL of each extract was transferred to test tubes with 3.0 mL of ABTS• radical and homogenized in a shaker. After 6 min, the reading was carried out in a Libra S60PC spectrophotometer (Biochrom, Cambourne, CBE, United Kingdom) at 734 nm, and the same volume of ethanol was used as control. Solutions of trolox ET (100–1500 μM L−1) were used to construct the calibration curve, with the antioxidant capacity was expressed as μM equivalent trolox·g−1 extract (μM ET·g−1 extract). Assays were performed in triplicates.
2.6. Identification of phenolic compounds by UHPLC-ESI-QTOF-MS
The identification of phenolic compounds by UHPLC-ESI-QTOF-MS was performed using crude extracts of C. peruvianus samples collected in 2015 and 2016, resuspended in methanol. Aliquots of the extracts were analyzed by UHPLC- ESI-QTOF-MS using a Nexera X2 high performance liquid chromatography system. The system was equipped with two LC30AD pumps, Shimadzu XR-ODSIII column (2.0 mm i.d. x 75 mm, 1.6 μm), injection volume of 1 μL and kept at 40 °C. Water (0.1% formic acid; A) and acetonitrile (0.1% formic acid; B), both with LC-MS purity grade, at a flow rate of 0.2 mL min−1, with a linear gradient of elution were used as solvents. The gradient used was: 95% solvent A and 5% solvent B (1 min), 50% solvent A and 50% solvent B (10 min), 5% solvent A and 95% solvent B (12 min), 5% solvent A and 95% solvent B (13 min), 95% solvent A and 5% solvent B (17 min), and 95% solvent A and 5% solvent B (20 min).
The chromatographic system was coupled to a Q-TOF Impact II model mass spectrometer (Bruker, Germany) equipped with an ESI source operating with AutoMS/MS acquisition mode. The three most intense ions from each chromatographic peak were selected. The acquisition rate was 5 Hz (MS and MS/MS) and the adjustment of the equipment in the range of m/z 70–1300. Analyses were performed in positive ionization mode, with capillary voltage set at 4.50 kV, source temperature of 200 °C and desolvation gas flow of 8 L min−1. The positive mode was employed since most natural compounds are preferentially ionized in the positive mode in untargeted studies. Negative mode could be used. However, the richness of substances in the C. peruvianus cladodes would not be evidenced and only a portion of the compounds would be visible, being attributed to the compounds that tend to ionize and become negative. It is easier and more common to use the negative mode for analysis of this class. However, this same class is also perfectly possible to be analyzed in a positive way. The advantage of using the positive mode of analysis has been reported by Liigand et al. (2017) and Grabsk et al. (2017).
Precursor scans were performed using collision-induced dissociation (CID). The results were obtained using a collision energy ramp in the range of 15–40 eV and a collision gas pressure of 3.06·10-3 mBar in the collision chamber. The ion chromatograms and MS and MS/MS spectra were visualized using the Data Analysis 4.3 software, compared to the literature and identified through a mass spectrometry database such as MassBank (http://www.massbank.jp/Index), ReSpect for phytochimicals (http://spectra.psc.riken.jp/) and from the error calculation (Brenton and Godfrey, 2010), using the following equation: Mass error (ppm) = (TEM-OEM)/TEM) x 1000000, where TM is the theoretical mass, OEM is the observed exact mass, and TEM is the theoretical exact mass.
2.7. Data analysis
Measurements were taken in triplicate for each assay and the results were analyzed as the mean for each year of collection (2015 and 2016) with Sisvar statistical program (Ferreira, 2019). ANOVA and Tukey's test (p < 0.05) were used to compare means.
3. Results and discussion
3.1. Determination of total phenolic compounds
Higher total content of phenolic compounds (14.91 ± 0.015 mg GAE·g−1) was detected in cladode extract of C. peruvianus collected in 2016 than in cladode extract collected in 2015 (11.03 ± 0.008 mg GAE·g−1) [sum of squares = 0.0032; p < 0.05; coefficient of variation: CV = 8.14%) (Table 1). Variations in climate and time conditions may exert an important influence on the secondary metabolism, inducing changes in chemical composition (Alves et al., 2017). These changes were observed in the amount of total phenolic compounds in cladodes of C. peruvianus plants cultivated in the south of Brazil. The fruiting phenological stage also seems to exert an influence on the secondary metabolism, inducing changes in the total flavonoid content of C. jamacaru from northeastern Brazil. The extract of fruiting cladodes showed a higher flavonoid content than that of vegetative cladodes (Dutra et al., 2019). Seasonal variations in the chemical composition of secondary metabolites in cladodes from other cactus species were also related to edaphic factors in the place of cultivation, seasons and age of the plants (Stintzing and Carle, 2005). The different processes that an extract goes through before component analysis can also lead to a differential evaluation of the total phenolic compounds (Avila-Nava et al., 2014). The highest total content of phenolic compounds was detected in cladode extract collected in 2016 showing that the production of phenolic compounds by the same plant is not conserved and it can undergo annual variation. Thus, the expectation is that a higher amount of total phenolic compounds may be observed in cladodes from other plants of C. peruvianus, or in cladodes from the same plant as used in the present study, if collected in different periods of the year. Other factors involved with changes in the amount of total phenolic compounds of the same plant not resulting from seasonal variations should be investigated in the future. Li et al. (2019) has showed that mechanical stresses such as tissues cutting have induced the increase of total phenolic amount and antioxidant activity in pitaya fruits of the Hylocereus undatus cactus.
Table 1.
Phenolic compounds content and antioxidant capacity of cladodes extract from C. peruvianus collected in 2015 and 2016.a
| Collection period | Total phenolic contentb | DPPH•c | ABTS•d |
|---|---|---|---|
| 2015 | 11.03 ± 0.008b | 17.26 ± 0.11b | 8.63 ± 0.06b |
| 2016 | 14.91 ± 0.015a | 24.04 ± 0.13a | 17.08 ± 0.11a |
c/d μM of equivalent Trolox (ET)·g−1 of extract.
a Values are expressed as mean of triplicates ± SD. Different letters in the same column are statistically different (Tukey test p ≤ 0.05, n = 3).
b mg of gallic acid equivalent (GAE)·g−1 of extract.
3.2. Profile of the phenolic compounds by UHPLC-ESI-QTOF-MS analysis
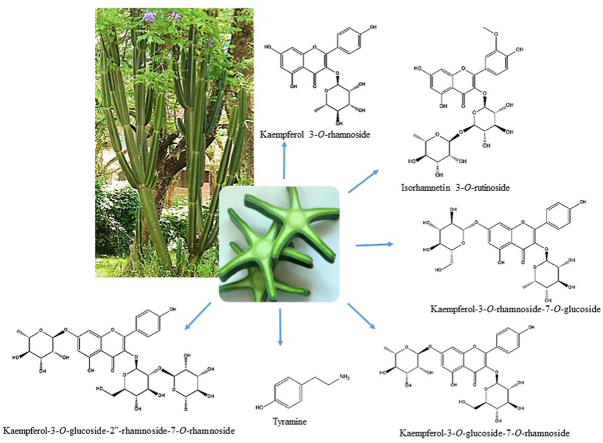
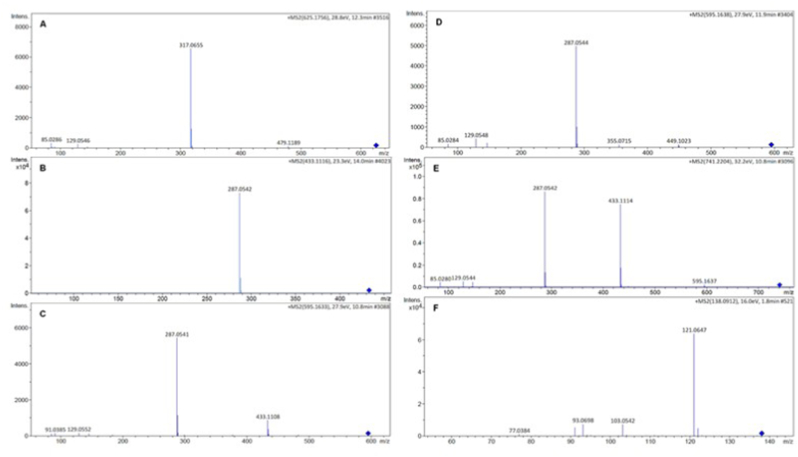
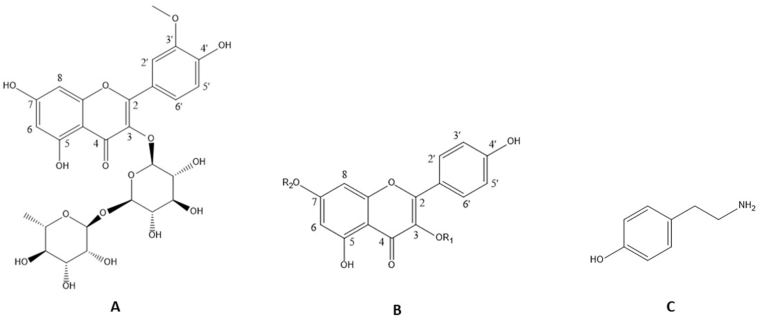
Phenolic compounds were identified by comparison with the open access database, m/z values and MS2 fragmentation patterns. The structures and fragmentation spectra of the six compounds identified are shown in Fig. 1 and Fig. 2. The six compounds shown in Table 2 belong to the class of flavonoids and alkaloids. The UHPLC-ESI-QTOF-MS profiles of each collection period were sent in a supplementary file.
Fig. 1.
Fragmentation spectra of compounds detected in C. peruvianus cladodes. Compound 1: isorhamnetin 3-O-rutinoside (A); Compound 2: kaempferol 3-O-rhamnoside (B); Compound 3: kaempferol-3-O-glucoside-7-O-rhamnoside (C); Compound 4: kaempferol-3-O-rhamnoside-7-O-glucoside (D); Compound 5: kaempferol-3-glucoside-2″-rhamnoside-7-O-rhamnoside (E); Compound 6: tyramine (F).
Fig. 2.
Phenolic compounds detected by UHPLC-ESI-QTOF-MS from extracts rich in phenolic compounds of C. peruvianus cladodes. A: Isorhamnetina 3-O-rutinoside (1); B: Kaempferol 3-O-rhamnoside (2) – R1 = Rha e R2 = H, Kaempferol-3-O-glucoside-7-O-rhamnoside (3) – R1 = Glc e R2 = Rha, Kaempferol-3-O-rhamnoside-7-O-glucoside (4) – R1 = Rha e R2 = Glc, Kaempferol-3-O-glucoside-2″-rhamnoside-7-O-rhamnoside (5) – R1 = Rha-Glc e R2 = Rha; C: Tyramine (6).
Table 2.
Secondary metabolites detected in cladode extract from C. peruvianus. The identification was performed based on information obtained from the MS2 spectra in positive mode [M+H]+ and retention time (RT).
| Compound | RT (min) | [M+H]+m/z | Fragments [M+H]+ | Identification | Reference | Error (ppm) |
|---|---|---|---|---|---|---|
| 1 | 12.3 | 625.1756 | 317.0655 | Isorhamnetin 3-O-rutinoside | ReSpect for Phytochemicals (riken.jp) | 1.96 |
| 2 | 14.0 | 433.1116 | 287.0542 | Kaempferol 3- O -rhamnoside | ReSpect for Phytochemicals (riken.jp) | 4.33 |
| 3 | 10.8 | 595.1633 | 287.0541/433.1108 | Kaempferol-3-O-glucoside-7-O-rhamnoside | Kerhoas et al. (2006) | 4.15 |
| 4 | 11.9 | 595.1638 | 287.0544/449.1023 | Kaempferol-3-O-rhamnoside-7-O-glucoside | Kerhoas et al. (2006) | 4.99 |
| 5 | 10.8 | 741.2204 | 287.0542/433.1114/595.1637 | Kaempferol-3-O-glucoside-2″-rhamnoside-7-O-rhamnoside | Kerhoas et al. (2006) | 5.61 |
| 6 | 1.8 | 138.0912 | 93.0698/103.0542/121.0647 | Tyramine | MassBank of North America (ucdavis.edu) | 4.96 |
Error (ppm) = (TEM-OEM)/TEM) x 1000000, where TM is the theoretical mass, OEM is the observed exact mass, and TEM is the theoretical exact mass.
According to the database (ReSpect: accession PS042004) the compound 1 (12.3 min), a di-glycoside, was identified as isorhamnetin 3-O-rutinoside (narcissin) and was detected only in cladode extract of C. peruvianus collected in 2015. The precursor ion m/z 625.1756 [M+H]+ gave rise to the fragment ion at m/z 317.0655 [M+H]+, characteristic of the aglycone isorhamnetin, revealing the loss of 308 Da, which corresponds to the elimination of a residue of rutinose (rhamnose-glucose) (Fig. 1A). The flavonol isorhamnetin 3-O-rutinoside is well known and reported by several authors in cladodes (Qiu et al., 2002; Guevara-Figueroa et al., 2010; Astello-Garcia et al., 2015) and fruits (Lee et al., 2003; Moussa-Ayoub et al., 2014; Son et al., 2014) of species of the genus Opuntia.
According to the database (ReSpect: accession PM008005) the compound 2 (14.0 min) was identified as kaempferol 3-O-rhamnoside (kaempferin) (Fig. 1B). The precursor ion m/z 433.1116 [M + H]+ gave rise to the fragment ion at m/z 287.0542 [M + H]+ due to the loss of 146 Da, indicating the presence of a deoxyhexose (rhamnose). Previous reports indicated the presence of kaempferol 3-O-rhamnoside in fruits of Opuntia ficus indica (Amaya-Cruz et al., 2019).
Compounds 3 (10.8 min) and 4 (11.9 min) both with the same precursor ion, but different retention times (RT), produced distinct fragments, although resulting in the same main fragment ion at m/z 287 [M + H]+ (Table 2). In the MS2 spectrum of the precursor ion m/z 595.1633 [M + H]+ of compound 3 (Fig. 1C), the fragment ion was observed at m/z 433.1108 [M + H]+ generated by the loss of 162 Da, which corresponds to the elimination of a hexose residue, followed by the loss of 146 Da, which corresponds to the elimination of the deoxyhexose residue (rhamnose). The inverse was observed in the fragmentation spectrum of compound 4 of the precursor ion m/z 595.1638 [M + H]+ (Fig. 1D), in which the first observed loss was 146 Da, producing the fragment ion in m/z 449.1023 [M + H]+, which corresponded to the elimination of the rhamnose residue. After that, the loss of 162 Da was observed, corresponding to the elimination of a hexose residue. Typical low-mass ions were also found, confirming the assignment of rhamnosyl (m/z 147, 129) and hexosyl (m/z 145, 127) (Kerhoas et al., 2006). Compounds 3 and 4 were identified as kaempferol-3-O-glucoside-7-O-rhamnoside and kaempferol-3-O-rhamnoside-7-O-glucoside, respectively, when compared with the MS2 fragments reported by Kerhoas et al. (2006). The flavonol kaempferol-glucosyl-rhamnoside was also detected in Opuntia cladodes (Santos-Zea et al., 2011).
In the MS2 spectrum of the ion m/z 741,2204 [M + H]+ of compound 5 (10.8 min) (Fig. 1E), the fragment ion was observed at m/z 595.1637 [M + H]+ generated by the loss of 146 Da (deoxyhexose). After that, losses of 162 Da (hexose) and 146 Da (deoxyhexose) were observed, producing the fragment ions at m/z 433.1114 [M + H]+ and m/z 287.0542 [M + H]+, respectively. According to the fragmentation pattern reported by Kerhoas et al. (2006), compound 5 was identified as kaempferol-3-O-glucoside-2″-rhamnoside-7-O-rhamoside (Fig. 1E). The flavonol kaempferol-3-O-glucoside-2″-rhamnoside-7-O-rhamoside was also reported in Opuntia cladodes (Astello-Garcia et al., 2015).
The five flavonoids (isorhamnetina 3-O-rutinoside, kaempferol 3-O-rhamnoside, kaempferol-3-O-glucopyranoside-7-O-rhamnoside, kaempferol-3-O-rhamnoside-7-O-glucopyranoside and kaempferol-3-O-glucoside-2″-rhamnoside-7-O-rhamnoside) have already been identified in Cactaceae, in cladodes and fruits of species of the genus Opuntia. However, they have not yet been reported in species of the genus Cereus. Thus, our study is the first report of isorhamnetin 3-O-rutinoside, kaempferol 3-O-rhamnoside, kaempferol-3-O-glucopyranoside-7-O-rhamnoside, kaempferol-3-O-rhamnoside-7-O-glucopyranoside and kaempferol-3-O-glucoside-2″-rhamnoside-7-O-rhamnoside in cladodes of C. peruvianus. Furthermore, our results are in line with the kaempferol biosynthesis scheme proposed by Jones et al. (2003) and Kerhoas et al. (2006).
A spectrum of therapeutic activities has been attributed to isorhamnetin and kaempferol. Isorhamnetin, quercetin and kaempferol are flavonoids detected in a wide variety of fruits and are described as having anticancer properties (Birt et al., 2001). Isorhamnetine, kaempferol, and quercetin have been associated with a decrease in advanced recurrent adenomas (Bobe et al., 2008). Saud et al. (2013) showed a protective action of isorhamnetine in male mice treated with mutagens. Mice treated with isorhamnetin showed an increase in survival rate of 80%, suggesting chemoprotective effects in colon cancer. Fang et al. (2005) reported that the four glycosylated forms of kaempferol inhibited the secretion of cytokines, which are glycoproteins produced during allergic and inflammatory reactions. The anti-inflammatory potential of kaempferol was also reported by Kempuraj et al. (2005), suggesting its use in the treatment of inflammatory and allergic diseases. In addition to anti-inflammatory activity, kaempferol and its derivatives showed significant contraceptive activity, without inducing toxicity and gastric damage (Toker et al., 2004).
In the MS2 spectrum of the ion m/z 138.0912 [M+H]+ (1.8 min) of compound 6 (Fig. 1F), the fragments m/z 93.0698 [M+H]+, m/z 103.0542 [M+H]+, and m/z 121.0647 [M+H]+, which corresponds to tyramine, according to the database (MassBanK: accession PR311153) were observed (Fig. 2). Tyramine is an alkaloid derived from tyrosine produced via the shikimic acid pathway and an important precursor of several groups of alkaloids and flavonoids (Robinson, 1981; Dewick, 2002). The tyramine alkaloid was previously obtained from cladodes and callus cultures of C. peruvianus (Oliveira and Machado, 2003) and from cladodes of C. jamacaru (Davet et al., 2009; Dutra et al., 2018; Medeiros et al., 2019). Tyramine is described as a neurotransmitter/neuromodulator in insects. Nagayaa et al. (2002) investigated the neuromodulatory effect of tyramine on Drosophila melanogaster, and they concluded that tyramine is a neuromodulatory agent in a wide range of invertebrate phyla. Due to the neurological effects promoted by tyramine on insects, some researchers have focused their attention on its corresponding receptor, which is believed to be a potentially promising target for the development of new and specific insecticides (Roeder, 2005).
In plants, a review by Santos et al. (2020) reported that cladode extracts from C. hildmannianus (syn. C. peruvianus) have shown great potential in folk medicine, and exhibited numerous pharmacological activities, in vitro and in vivo, such as gastroprotective, antioxidant, antifungal, ovicidal, hemagglutinating and anticancer activity. The substances in these extracts are different classes of chemical compounds, such as fatty acids, polysaccharides, terpenes, alkaloids, phenolic acids, and flavonoids. Thus, C. peruvianus plants appear to show vast potential for commercial exploitation with pharmaceutical and nutraceutical properties. The results of the present study support the use of cladodes from C. hildmannianus (syn. C. peruvianus) as a source of natural antioxidants.
3.3. Antioxidant activity of the C. peruvianus cladode extract
Higher antioxidant activity (24.04 ± 0.13 μM ET·g−1) was detected in the cladode extract of C. peruvianus collected in 2016 than in the cladode extract collected in 2015 (17.26 ± 0.11 μM ET·g−1), employing the DPPH• method. The antioxidant activity that employed the ABTS• method was also higher (17.08 ± 0.11 μM ET·g−1) in cladodes collected in 2016 than in the cladode extract collected in 2015 (8.63 ± 0.06 μM ET·g−1). Thus, the highest antioxidant activity in the cladode extract of C. peruvianus, employing the DPPH• and ABTS• methods, coincided with the higher total content of phenolic compounds (14.91 ± 0.015 mg GAE·g−1) in cladodes collected in 2016 than in cladode extract collected in 2015 (11.03 ± 0.008 mg GAE·g−1).
Different values for the antioxidant capacity of extracts from different tissues have been described in cactus species. Lower antioxidant activity than that observed in C. peruvianus cladode extracts was reported in fresh fruit extracts of the species Opuntia ficus-indica (5.22 ± 0.89 μM ET·g−1), O. stricta (4.72 ± 0.25 μM ET·g−1), and O. undulata (1.08 ± 0.44 μM ET·g−1) (Fernández-López et al., 2010), while higher antioxidant activity was reported in dry pulp of Stenocereus pruinosus fruits (48.8 ± 1.29 μM ET·g−1) (Rodríguez-Sánchez et al., 2017) and in pulp and skin of Hylocereus polyhizus fruits (28.03 ± 0.83 μM ET·g−1) (Wu et al., 2006). As well as the total content of phenolic compounds, seasonal variations in the chemical composition of secondary metabolites in cladodes of other cactus species (related to edaphic factors of the place of cultivation, seasons and age of the plants) may also lead to a differential evaluation of the antioxidant activity. In this way, the perspective is that a different antioxidant activity may be observed in cladodes from other plants of C. peruvianus, or in cladodes from the same plant used in the present study if collected in different periods of the year. Different light intensity, for example, is a factor that may determine different antioxidant activity. The higher incidence of UV light can stimulate the production of phenolic compounds since phenylalanine lyase (PAL; EC 4.3.1.24), a fundamental enzyme in the production of phenolics, is activated by light.
The different processes that the extract goes through before component analysis can also lead to a differential evaluation of the antioxidant activity. The antioxidant activity of C. jamacaru cladode extract was expressed in half-maximal effective concentration (EC50). Higher oxidant activity (427.74 ± 5.80 μg mL−1) was observed when employing the DPPH• method than when ABTS• was used (270.57 ± 4.99 μg mL−1) (Dutra et al., 2018). The extract of seed, fruit pulp and cladode of C. jamacaru showed different antioxidant activity levels when employing the DPPH• and ABTS• methods (Dutra et al., 2019). The antioxidant activity of C. jamacaru fruit peel extract has been also expressed in μM ET·g−1 sample. The extract of fruit peel showed different antioxidant activity employing the ABTS•: 6.88 μM ET·g−1 sample, 8.31 μM ET·g−1 sample and 10.14 μM ET·g−1 sample (Melo et al., 2017), and 7.23 μM ET·g−1 sample (Santos et al., 2018).
Despite the divergent ways of expressing the antioxidant activity in extracts from different tissues and cactus species, the relevant data in the present study showed the antioxidant activity of cladode extracts from C. peruvianus. Phenolic and alkaloid compounds in the extract of C. jamacaru were related to antioxidant activity, increasing its ability in metal chelating activity and promoting anti-cytotoxic activity against cisplatin (Dutra et al., 2018). Dutra et al. (2018) also showed that the antioxidant activity may act on the cell cycle of the tumor cells in vitro and in vivo, leading to anticancer effects and tumor reduction. Evidence of antioxidant activity in the cladode extract from C. peruvianus paves the way for the use of C. peruvianus cladodes as a natural antioxidant and anticancer agent. Flavonoids were not separately quantified in the present study. The primary objective of our study was to trace a metabolic profile of the detected flavonoids since the phenolic compounds had not been identified until then. The future perspective is to quantify the flavonoids. Quantification is essential to know whether flavonoids are in sufficient concentrations to be used by the industrial sector to explore the previously reported therapeutic and nutraceutical potential for cladode extract from C. peruvianus.
4. Conclusions
The phenolic compounds linked to antioxidant activities identified in cladode extracts from C. peruvianus support the use of this species in human food as a source of natural antioxidants and suggest further study of the additional therapeutic potential of the extract as an anticancer agent. The highest or lowest content of total phenolic compounds, different phenolic compounds, as well as the antioxidant activity, may vary in cladode extracts from C. peruvianus, depending on variations in climate and weather conditions. Despite the variations depending on climate and weather conditions, our study was notable showing that in addition to the fruits mainly reported in other cactus species, the cladode extracts from C. peruvianus may also be a source of natural antioxidants. Thus, C. peruvianus cladodes can be an alternative for the food industry as a source of natural antioxidants.
Funding
Current study was funded by the Coordination for the Upgrading of Higher Education Personnel (Coordenação de Aperfeiçoamento de Pessoal de Nível Superior – CAPES) [Finance Code 001].
CRediT author statement
CAM, ARCN, AJBO and MFPSM formulated the hypothesis for present study and selected and collected the plant material; ARCN, AJBO and RACG designed the experiments extraction of phenolic compounds, determination of total phenolic compounds, evaluation of antioxidant action by the DPPH• method, evaluation of antioxidant action by the ABTS• method; ARCN, ASA, RTRA and EJP carried out the experiments to identify the phenolic compounds by UHPLC-ESI-QTOF-MS and data analysis. All authors discussed results and contributed towards the drafting of the manuscript. All authors have read and approved the final manuscript.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The authors would like to thank CAPES (Coordenação de Aperfeiçoamento de Pessoal de Nível Superior, Brasília DF Brazil) for financial support [Finance Code 001], Conselho Nacional de Desenvolvimento Científico e Tecnológico (Brasília DF Brazil) [grant number 304447/2016-1] and Fundação Araucária (Curitiba, PR Brazil) [grant number 002/2017].
Handling editor: Alejandro G. Marangoni
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.crfs.2022.05.014.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- Alara O.R., Abdurahman N.H., Ukaegbu C.I. Extraction of phenolic compounds: a review. Curr. Res. Food Sci. 2021;4:200–214. doi: 10.1016/j.crfs.2021.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alves F.A.L., Andrade A.P., Bruno R.L.A., Silva M.G.V., Souza M.F.V., Santos D.C. Seasonal variability of phenolic compounds and antioxidant activity in prickly pear cladodes of Opuntia and Nopalea generes. Food Sci. Technol. 2017;37:536–543. doi: 10.1590/1678-457X.19316. [DOI] [Google Scholar]
- Amaya-Cruz D.M., Pérez-Ramírez I.F., Delgado-García J., Mondragón-Jacobo C., Dector-Espinoza J., Reynoso-Camacho R. An integral profile of bioactive compounds and functional properties of prickly pear (Opuntia ficus indica L.) peel with different tonalities. Food Chem. 2019;278:568–578. doi: 10.1016/j.foodchem.2018.11.031. [DOI] [PubMed] [Google Scholar]
- Assis J.G.A., Resende S.V., Bellintani M.C., Coelho P.J.A., Correia D., Marchi M.N.G., Cruz B.M., Nahoum P.I.V., Menezes M.O.T., Meiado M.V. In: Plano de ação nacional para a conservação das Cactáceas: série espécies ameaçadas. Icmbio, Brasília, Brazil. Silva S.R., Zappi D., Taylor N., Machado M., Org, editors. Instituto Chico Mendes de Conservação da Biodiversidade; 2011. Conservação ex situ; pp. 44–54. [Google Scholar]
- Astello-Garcia M.G., Cervantes I., Nair V., Santos-Diaz M.S., Reyes-Agueero A., Gueraud F., Negre-Salvayre A., Rossignol M., Cisneros-Zevallos L., La Rosa A.P.B. Chemical composition and phenolic compounds profile of cladodes from Opuntia spp. cultivars with different domestication gradient. J. Food Compos. Anal. 2015;43:119–130. doi: 10.1016/j.jfca.2015.04.016. [DOI] [Google Scholar]
- Attar S.H., Gündesli M.A., Urün I., Kafkas S., Kafkas N.E., Ercisli S., Ge C., Mlcek J., Adamkova A. Nutritional analysis of red-purple and white-fleshed pitaya (Hylocereus) species. Molecules. 2022;27:808. doi: 10.3390/molecules27030808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avila-Nava A., Calderón-Olivera M., Medina-Campos O.N., Zou T., Gu L., Torres N., Pedraza-Chaverri J. Extract of cactus (Opuntia ficus indica) cladodes scavenges reactive oxygen species in vitro and enhances plasma antioxidant capacity in humans. J. Funct.Foods. 2014;10:13–24. doi: 10.1016/j.jff.2014.05.009. [DOI] [Google Scholar]
- Bargougui A., Tag M.H., Bouaziz M., Triki S. Antimicrobial, antioxidant, total phenols and flavonoids content of four cactus (Opuntia ficus-indica) Cult. Biomed. Pharmacol. J. 2019;12:1353–1368. doi: 10.13005/bpj/1764. [DOI] [Google Scholar]
- Beltrán-Orozco M.C., Oliva-Coba T.G., Gallardo-Velázquez T., Osorio-Revilla G. Ascorbic acid, phenolic content, and antioxidant capacity of red, cherry, yellow and white types of pitaya cactus fruit (Stenocereus stellatus Riccobono) Agrociencia. 2009;43:153–162. [Google Scholar]
- Bernardi M.M., Spinosa H.S., Ricci E.L., Silva T.M.R. Preclinical study of an antiobesity phytotherapic compound obtained from cactus Cereus plants in male and female rats fed with high-fat diet: comparison with sibutramine. J. Health Sci. Inst. 2013;31:320–323. [Google Scholar]
- Bhuyan D.J., Basu A. In: Utilisation of Bioactive Compounds from Agricultural and Food Production Waste. Vuong Q.V., editor. CRC Press, Taylor & Francis Group; 2017. Phenolic compounds: potential health benefits and toxicity; pp. 27–59. Chapter: 2 Publisher. [Google Scholar]
- Birt D.F., Hendrich S., Wanfg W. Dietary agents in cancer prevention: flavonoids and isoflavonoids. Pharmacol. Therapeut. 2001;90:157–177. doi: 10.1016/S0163-7258(01)00137-1. [DOI] [PubMed] [Google Scholar]
- Bobe G., Sansbury L.B., Albert P.S., Cross A.J., Kahle L., Ashby J., Kikendall J.W. Dietary flavonoids and colorectal adenoma recurrence in the polyp prevention trial. Cancer Epidemiol. Biomark. Prev. 2008;17:1344–1353. doi: 10.1158/1055-9965.EPI-07-0747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenton A.G., Godfrey A.R. Accurate mass measurement: terminology and treatment of data. J. Am. Soc. Mass Spectrom. 2010;21:1821–1835. doi: 10.1016/j.jasms.2010.06.006. [DOI] [PubMed] [Google Scholar]
- Carvalho A.C.B., Lana T.N., Perfeito J.P.S., Silveira D. The Brazilian market of herbal medicinal products and the impacts of the new legislation on traditional medicines. J. Ethnopharmacol. 2018;212:29–35. doi: 10.1016/j.jep.2017.09.040. [DOI] [PubMed] [Google Scholar]
- Chandra R., Bhandari P., Sharma S.C., Emmanuel I., Alam A. Health benefits of cactus. Ann. Phytomed. 2019;8:179–185. doi: 10.21276/ap.2019.8.2.23. [DOI] [Google Scholar]
- Chbani M., Matthäus B., Charrouf Z., El Monfalouti H., Kartah B., Gharby S., Willenberg I. Characterization of phenolic compounds extracted from cold pressed cactus (Opuntia ficus-indica L.) seed oil and the effect of roasting on their composition. Foods. 2020;9:1098. doi: 10.3390/foods9081098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choo W.S., Yong W.K. Antioxidant properties of two species of Hylocereus fruits. Adv. Appl. Sci. Res. 2011;2:418–425. www.pelagiaresearchlibrary.com [Google Scholar]
- Cory H., Passarelli S., Szeto J., Tamez M., Mattei J. The role of polyphenols in human health and food systems: a mini review. Front. Nutr. 2018;5:87. doi: 10.3389/fnut.2018.00087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davet A., Carvalho J.L.S., Dadalt R.C., Vituoso S., Dias J.F., Miguel M.D., Miguel O.G. Cereus jamacaru: a non-buffered LC quantification method to nitrogen compounds. Chromatographia. 2009;69:245–247. doi: 10.1365/s10337-009-1130-z. [DOI] [Google Scholar]
- Dewick P.M. Medicinal Natural Products. A Biosynthetic Approach. second ed. John Wiley & Sons Ltd; 2002. Secondary metabolism: the building blocks and construction mechanisms; pp. 7–33. [Google Scholar]
- Dutra J.C.V., Ferreira J.M., Pereira P.R.C., Oliveira J.B.-H., Gervásio S.V., Xavier M.B., Mota M.M., Luz A.C., Pretti I.R., França H.S., Jamal C.M., Batitucci M.C.P. Cereus jamacaru D.C. hydroalcoholic extract promotes anti-cytotoxic and antitumor activity. Pharmaceuticals. 2018;11:130–148. doi: 10.3390/ph11040130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutra J.C.V., Oliveira J.B.H., Santos V.S., Pereira P.R.C., Ferreira J.M., Batitucci M.D.C.P. Fruiting increases total content of flavonoids and antiproliferative effects of Cereus jamacaru DC cladodes in sarcoma 180 cells in vitro. Asian Pac. J. Trop. Biomed. 2019;9:66. doi: 10.4103/2221-1691.250857. [DOI] [Google Scholar]
- Esquivel P., Stintzinga F.C., Carle R. Phenolic compound profiles and their corresponding antioxidant capacity of purple pitaya (Hylocereus sp.) genotypes. Z. Naturforsch. 2007;62c:636–644. doi: 10.1515/znc-2007-9-1003. https://www.researchgate.net/publication/5779581 [DOI] [PubMed] [Google Scholar]
- Fang S.H., Rao Y.K., Tzeng Y.M. Inhibitory effects of flavonol glycosides from Cinnamomum osmophilia on inflammatory mediators in LPS/IFN-γ-activated murine macrophages. Bioorg. Med. Chem. 2005;13:2381–2388. doi: 10.1016/j.bmc.2005.01.050. [DOI] [PubMed] [Google Scholar]
- FAO (Food and Agriculture Organization) 2017. The Future of Food and Agriculture – Trends and Challenges; p. 163. Rome. [Google Scholar]
- Fernández-López J.A., Almela L., Obón J.M., Castellar R. Determination of antioxidant constituents in cactus pear fruits. Plant Foods Hum. Nutrition. 2010;65:253–259. doi: 10.1007/s11130-010-0189-x. [DOI] [PubMed] [Google Scholar]
- Ferreira D.F. SISVAR: a Computer analysis system to fixed effects split plot type designs. Rev. Brasil. Biometria. 2019;37:529–535. doi: 10.28951/rbb.v37i4.450. [DOI] [Google Scholar]
- Forni C.F., Bartoli M., Pieretti S., Facchino A., D'Arcangelo D., Norelli S., Valle G., Nisini R., Beninati S., Tabolacci C., Jadeja R.N. Beneficial role of phytochemicals on oxidative stress and age-related diseases. BioMed Res. Int. 2019 doi: 10.1155/2019/8748253. Article ID 8748253, 16 pages. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franco F.F., Silva G.A.R., Moraes E.M., Taylor N., Zappi D.C., Jojima C.L., Machado M.C. Plio-Pleistocene diversification of Cereus (Cactaceae, Cereeae) and closely allied genera. Bot. J. Linn. Soc. 2017;183:199–210. doi: 10.1093/BOTLINNEAN/BOW010. [DOI] [Google Scholar]
- Grabsk A.H.A., Avincola A.S., Claus T., Porto C., Visentainer J.V., Pilau E.J. Direct incorporation of ginger and oregano antioxidants in canola oil. J. Braz. Chem. Soc. 2017;28:995–1002. doi: 10.21577/0103-5053.20160252. [DOI] [Google Scholar]
- Guevara-Figueroa T., Jiménez-Islas H., Reyes-Escogido M.L., Mortensen A.G., Laursen B.B., Lin L.W., De La Rosa A.P.B. Proximate composition, phenolic acids, and flavonoids characterization of commercial and wild nopal (Opuntia spp.) J. Food Compos. Anal. 2010;23:525–532. doi: 10.1016/j.jfca.2009.12.003. [DOI] [Google Scholar]
- Hunt D.R., Taylor N.P., Charles G. The New Cactus Lexicon. Milborne Port; 2006. (England) [Google Scholar]
- Jones P., Messner B., Nakajima J.-I., Schäffner A.R., Saito K. UGT73C6 and UGT78D1, glycosyltransferases involved in flavonol glycoside biosynthesis in Arabidopsis thaliana. J. Biol. Chem. 2003;278:43910–43918. doi: 10.1074/jbc.M303523200. [DOI] [PubMed] [Google Scholar]
- Kempuraj D., Madhappan B., Christodoulou S., Boucher W., Cao J., Papadopoulou N., Theoharides T.C. Flavonols inhibit proinflammatory mediator release, intracellular calcium ion levels and protein kinase C theta phosphorylation in human mast cells. Br. J. Pharmacol. 2005;145:934–944. doi: 10.1038/sj.bjp.0706246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerhoas L., Aouak D., Cingöz A., Routaboul J.M., Lepiniec L., Einhorn J., Birlirakis N. Structural characterization of the major flavonoid glycosides from Arabidopsis thaliana seeds. J. Agric. Food Chem. 2006;54:6603–6612. doi: 10.1021/jf061043n. [DOI] [PubMed] [Google Scholar]
- Lee E.H., Kim H.J., Song Y.S., Jin C., Lee K.T., Cho J., Lee Y.S. Constituents of the stems and fruits of Opuntia ficus-indica var. saboten. Arch Pharm. Res. (Seoul) 2003;26:1018–1023. doi: 10.1007/BF02994752. [DOI] [PubMed] [Google Scholar]
- Li X., Li M., Ji N., Ji P., Zhang J., Zheng Y., Zhang X., Li F. Cold plasma treatment induces phenolic accumulation and enhances antioxidant activity in fresh-cut pitaya (Hylocereus undatus) fruit. LWT - Food Sci. Technol. (Lebensmittel-Wissenschaft -Technol.) 2019;115 doi: 10.1016/j.lwt.2019.108447. [DOI] [Google Scholar]
- Liigand P., Kaupmees K., Haav K., Liigand J., Leito I., Girod M., Antoine R., Kruve A. Think negative: finding the best electrospray ionization/MS mode for your analysis. Anal. Chem. 2017;89:5665–5668. doi: 10.1021/acs.analchem.7b00096. [DOI] [PubMed] [Google Scholar]
- Lin D., Xiao M., Zhao J., Li Z., Xing B., Li X., Kong M., Li L., Zhang Q., Liu Y., Chen H., Qin W., Wu H., Chen S. An overview of plant phenolic compounds and their importance in human nutrition and management of type 2 diabetes. Molecules. 2016;21:1374–1393. doi: 10.3390/molecules21101374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luna F.P. CAplus; Brasil: 2012. Cactus-containing Nutraceutical Compositions and Their Uses; p. 31. BR 2009002296, A2. [Google Scholar]
- Ma X., Wu H., Liu L., Yao Q., Wang S., Zhan R., Zhou Y. Polyphenolic compounds and antioxidant properties in mango fruits. Sci. Hortic. (Canterb.) 2011;129:102–107. doi: 10.1016/j.scienta.2011.03.015. [DOI] [Google Scholar]
- Medeiros I.U., Medeiros R.A., Bortolin R.H., Queiroz F.M., Silbiger V.N., Pflugmacher S., Schwarz A. Genotoxicity and pharmacokinetic characterization of Cereus jamacaru ethanolic extract in rats. Biosci. Rep. 2019;39 doi: 10.1042/BSR20180672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melo R.S., Silva S.M., Sousa A.S.B., Lima R.P., Dantas A.L., Dantas R.L., Figueiredo V.M. Maturação e qualidade de frutos de mandacaru (Cereus jamacaru P.D.C.) de diferentes bioclimas do estado da Paraíba. Rev. Agropecu. Téc. 2017;38:160–168. doi: 10.25066/agrotec.v38i3.33818. [DOI] [Google Scholar]
- Moussa-Ayoub T., El-Hady E.S.A.A., Omran H., El-Samahy S.K., Kroh L.W., Rohn S. Influence of cultivar and origin on the flavonol profile of fruits and cladodes from cactus Opuntia ficus-indica. Food Res. Int. 2014;64:864–872. doi: 10.1016/j.foodres.2014.08.021. [DOI] [PubMed] [Google Scholar]
- Nagayaa Y., Kutsukakeb M., Chigusab S.I., Komatsu A. A trace amine, tyramine, functions as a neuromodulator in Drosophila melanogaster. Neurosci. Lett. 2002;329:324–328. doi: 10.1016/s0304-3940(02)00596-7. [DOI] [PubMed] [Google Scholar]
- Oliveira A.J.B., Machado M.F.P.S. Alkaloid production by callous tissue cultures of Cereus peruvianus (Cactaceae) Appl. Biochem. Biotechnol. 2003;104:149–155. doi: 10.1385/abab:104:2:149. [DOI] [PubMed] [Google Scholar]
- Osuna-Martínez U., Reyes-Esparza J., Rodríguez-Fragoso L. Cactus (Opuntia ficus-indica): a review on its antioxidant properties and potential pharmacological use in chronic diseases. Nat. Prod. Chem. Res. 2014;2:2–8. doi: 10.4172/2329-6836.1000153. [DOI] [Google Scholar]
- Potì F., Santi D., Spaggiari G., Zimetti F., Zanotti I. Polyphenol health effects on cardiovascular and neurodegenerative disorders: a review and meta-analysis. Int. J. Mol. Sci. 2019;20:351–377. doi: 10.3390/ijms20020351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu Y., Chen Y., Pei Y., Matsuda H., Yoshikawa M. Constituents with radical scavenging effect from Opuntia dillenii: structures of new R-pyrones and flavonol glycoside. Chem. Pharmaceut. Bull. 2002;50:1507–1510. doi: 10.1248/cpb.50.1507. [DOI] [PubMed] [Google Scholar]
- Robinson T. The Biochemistry of Alkaloids. Molecular Biology Biochemistry and Biophysics. second ed. Springer-Verlag; 1981. Simple amino acid derivatives and protoalkaloids; pp. 15–23. [Google Scholar]
- Rodríguez-Sánchez J.A., Victoria M.T.C., Barragán-Huerta B.E. Betaxanthins and antioxidant capacity in Stenocereus pruinosus: stability and use in food. Food Res. Int. 2017;91:63–71. doi: 10.1016/j.foodres.2016.11.023. [DOI] [PubMed] [Google Scholar]
- Roeder T. Tyramine and octopamine: ruling behavior and metabolism. Annu. Rev. Entomol. 2005;50:447–477. doi: 10.1146/annurev.ento.50.071803.130404. [DOI] [PubMed] [Google Scholar]
- Rufino M.S.M., Alves R.E., Brito E.S., Morais S.M., Sampaio C.G., Perez-Jimenez J., Saura-Calixto F.D. Comunicado Técnico; 2007. Metodologia científica: Determinação da atividade antioxidante total em frutas pela captura do radical livre ABTS•. Fortaleza: Embrapa Angroindústria Tropical; pp. 36–44. [Google Scholar]
- Santiago E., Juániz I., Cid C., Peña M.-P. Extraction of (poly)phenolic compounds of cactus (Opuntia ficus-indica (L.) Mill.) cladodes. Food Anal. Methods. 2021;14:1167–1175. doi: 10.1007/s12161-020-01946-6. [DOI] [Google Scholar]
- Santos É.S., Oliveira A.J.B., Machado M.F.P.S., Mangolin C.A., Gonçalves R.A.C. Cereus hildmannianus (K.) Schum. (Cactaceae): ethnomedical uses, phytochemistry and biological activities. J. Ethnopharmacol. 2020;264 doi: 10.1016/j.jep.2020.113339. [DOI] [PubMed] [Google Scholar]
- Santos I.A., Pereira T.S., Almeida R.L.J., Muniz C.E.S., Silva J.N. 2018. Bioactive Compounds (Phenolic Compounds and Antioxidant Activity) and Physico-Chemical Characteristics of Mandacaru Fruit Shell (Cereus jamacaru) [DOI] [Google Scholar]
- Santos-Zea L., Gutiérrez-Uribe J.A., Serna-Saldivar S.O. Comparative analyses of total phenols, antioxidant activity, and flavonol glycoside profile of cladode flours from different varieties of Opuntia spp. J. Agric. Food Chem. 2011;59:7054–7061. doi: 10.1021/jf200944y. [DOI] [PubMed] [Google Scholar]
- Saud S.M., Young M.R., Jones-Hall Y.L., Ileva L., Evbuomwan M.O., Wise J., Bobe G. Chemopreventive activity of plant flavonoid isorhamnetin in colorectal cancer is mediated by oncogenic Src and β-catenin. Cancer Res. 2013;73:5473–5484. doi: 10.1158/0008-5472.CAN-13-0525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soares L.M.N., Silva G.M., Alonso-Buriti F.C., Alves H.S. Cereus jamacaru D.C. (Mandacaru): a promising native Brazilian fruit as a source of nutrients and bioactives derived from its pulp and skin. Plant Foods Hum. Nutr. 2021;76:170–178. doi: 10.1007/s11130-021-00885-9. [DOI] [PubMed] [Google Scholar]
- Som A.M., Ahmat N., Hamid H.A.A., Azizuddin N.M. A comparative study on foliage and peels of Hylocereus undatus (white dragon fruit) regarding their antioxidant activity and phenolic content. Heliyon. 2019;5 doi: 10.1016/j.heliyon.2019.e01244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Son J.-E., Lee B.H., Nam T.G., Im S., Chung D.K., Lee J.M., Chun O.K., Kim D.-O. Flavonols from the ripe fruits of Opuntia fícus-indica var. saboten protect neuronal PC-12 cells against oxidative stress. J. Food Biochem. 2014;38:518–526. doi: 10.1111/jfbc.12088. [DOI] [Google Scholar]
- Sousa D.M.D., Sousa M.D., Macedo J.L., Silva S.S., Silva R.R.C., Nascimento L.L.B., Miranda Junior R.N.C. Fitoterápicos utilizados para perda de peso comercializados em farmácias. Res. Soc. Dev. 2019;8:1–15. doi: 10.33448/rsd-v8i4.930. [DOI] [Google Scholar]
- Stintzing F.C., Carle R. Cactus stems (Opuntia spp.): a review on their chemistry, technology, and uses. Mol. Nutr. Food Res. 2005;49:175–194. doi: 10.1002/mnfr.200400071. [DOI] [PubMed] [Google Scholar]
- Tanaka L.Y.A., Oliveira A.J.B., Gonçalves J.E., Cipriani T.R., Souza L.M., Marques M.C.A., Iacomini M. An arabinogalactan with anti-ulcer protective effects isolated from Cereus peruvianus. Carbohydr. Polym. 2010;82:714–721. doi: 10.1016/j.carbpol.2010.05.043. [DOI] [Google Scholar]
- Taylor N.P., Zappi D.C. Royal Botanic Gardens; Kew: 2004. The Cacti of Eastern Brazil; p. 499. [Google Scholar]
- Toker G., Küpeli E., Memisoğlu M., Yesilada E. Flavonoids with antinociceptive and anti-inflammatory activities from the leaves of Tilia argentea (silver linden) J. Ethnopharmacol. 2004;95:393–397. doi: 10.1016/j.jep.2004.08.008. [DOI] [PubMed] [Google Scholar]
- ViaFarma . 2017. Cactus Cereus Extrato Seco.https://www.dermomanipulacoes.com.br/assets/uploads/Koubo.pdf [Google Scholar]
- Wiczkowski W., Nowak D.S., Topolska J. Red cabbage anthocyanins: profile, isolation, identification, and antioxidant activity. Food Res. Int. 2013;51:303–309. doi: 10.1016/j.foodres.2012.12.015. [DOI] [Google Scholar]
- Wu L.C., Hsu H.W., Chen Y.C., Chiu C.C., Lin Y.I., Ho J.A.A. Antioxidant and antiproliferative activities of red pitaya. Food Chem. 2006;95:319–327. doi: 10.1016/j.foodchem.2005.01.002. [DOI] [Google Scholar]
- Zappi D., Taylor N.P., Santos M.R., Larocca J. 2015. Cactaceae in Lista de Espécies da Flora do Brasil. Jardim Botânico do Rio de Janeiro.http://floradobrasil.jbrj.gov.br/jabot/floradobrasil/FB1434 Available at: [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.