Abstract
Background:
Non-alcoholic fatty liver disease (NAFLD) has become a major contributor to the rising incidence of hepatocellular carcinoma (HCC) in the US and other developed countries. Iron, an essential metal primarily stored in hepatocytes, may play a role in the development of NAFLD-related HCC. Epidemiological data on iron overload without hemochromatosis in relation to HCC are sparse. This study aimed to examine the associations between serum biomarkers of iron and the risk of HCC in NAFLD patients.
Methods:
We identified 18,569 patients with NAFLD using the University of Pittsburgh Medical Center electronic health records from 2004 through 2018. After an average 4.34 years of follow-up, 244 patients developed HCC. Cox proportional hazard regression was used to calculate hazard ratios (HRs) and 95% confidence intervals (CIs) of HCC incidence associated with elevated levels of iron biomarkers with adjustment for age, sex, race, body mass index, history of diabetes and tobacco smoking.
Results:
The HRs (95% CIs) of HCC for clinically defined elevation of serum iron and transferrin saturation were 2.91 (1.34–6.30) and 2.02 (1.22–3.32), respectively, compared with their respective normal range. No statistically significant association was observed for total iron-binding capacity or serum ferritin with HCC risk.
Conclusions:
Elevated levels of serum iron and transferrin saturation were significantly associated with increased risk of HCC among patients with NAFLD without hemochromatosis or other major underlying causes of chronic liver diseases.
Impact:
Clinical surveillance of serum iron level may be a potential strategy to identify patients with NAFLD who are at high risk for HCC.
Keywords: hepatocellular carcinoma, iron overload, non-alcoholic fatty liver disease, electronic health records
INTRODUCTION
The mortality rate of primary liver cancer in the US has doubled since the mid-1980s1. Worldwide the death toll of primary liver cancer is projected to reach one million in 2030 with the current trend2,3. Hepatocellular carcinoma (HCC) is the major subtype of primary liver cancer4, accounting for approximately 75–90% of total primary liver cancer cases5. Incidence rates of HCC vary dramatically across different geographic regions – the highest rate in sub-Saharan Africa and Eastern Asia (>20.0 per 100,000 persons) and the lowest in North America and Northern Europe (<5.0 per 100,000 persons)6. Chronic infection with hepatitis B virus (HBV) and/or hepatitis C virus (HCV) accounts for approximately 50% or more of all HCC cases in the US7. Heavy alcohol use and cigarette smoking are also recognized as risk factors for HCC8. In high-risk regions, exposure to dietary aflatoxin plays a significant role in the development of HCC9.
Non-alcoholic fatty liver disease (NAFLD) and its associated conditions including obesity and diabetes has been emerged as major risk factors for HCC10. NAFLD is considered as one of the most common chronic liver diseases in developed countries11. Globally, it is estimated that the prevalence of NAFLD is 30–40% in men and 15–20% in women12. NAFLD encompasses a wide spectrum of liver conditions from simple steatosis and nonalcoholic steatohepatitis (NASH) to fibrosis and cirrhosis, which may eventually progress to HCC13,14.
The mechanism for the disease progression from NAFLD to HCC remains unclear. One of the potential contributing factors may be iron overload. On average, healthy well-nourished adults have 1–3 grams of iron in their bodies15. Dietary iron is the major source for humans. Under normal condition, approximately 10% of dietary iron is absorbed through intestine16. Iron is not secreted through urine but naturally through the loss of blood16. Most of the iron in the body is contained in the hemoglobin of matured red blood cells17. The rest of body iron is present in ferritin complexes stored in bone marrow, liver and spleen15. The liver ferritin is the primary iron reservoir for physiologic supply when needed18,19. Two clinical studies in Iran and Egypt found that high ferritin level may accelerate the liver injury or liver fibrosis in people with metabolic disease such as NAFLD20,21. However, the role of serum iron biomarkers on the progression of NAFLD to HCC are unknown.
Iron overload occurs when there are excess stores of iron in the body, which is defined as the total amount of the body iron surpasses 5 grams22. The primary iron overload or hereditary hemochromatosis is caused by genetic variants in the HFE gene, resulting in excess absorption of iron from the intestine16,23 Up to 90% of individuals with hemochromatosis diagnosis carry the HFE mutations24,25. The secondary iron overload would result from excess absorption of iron from the food, repeated blood transfusions or excess intake of iron-containing substances27. In the US, one in 200 Caucasians is in the iron overload status26. Regardless of the underlying cause, iron overload may cause hepatotoxicity28 and lead to liver cirrhosis29. Numerous studies examined and found a statistically significant association for hereditary hemochromatosis with risk of cirrhosis and HCC30–34. However, epidemiological data on elevated iron levels without hemochromatosis in relation to HCC risk is sparse.
In the present study, we examined the association between systemic biomarkers of iron status and risk of HCC among a large cohort of NAFLD patients without diagnosis of hemochromatosis, alcoholic and viral liver disease and other liver conditions in a large healthcare network system in the Commonwealth of Pennsylvania (PA).
MATERIALS AND METHODS
Study Population
We conducted a retrospective cohort study of participants with non-alcoholic fatty liver disease (NAFLD) of the University of Pittsburgh Medical Center (UPMC) Health Insurance Plan (referred as the UPMC NAFLD Cohort Study). UPMC is a major medical service provider with 40 hospitals and 700 doctors’ offices and outpatient sites that serves more than 3 million patients annually throughout western PA in the US. Data was requested through the Health Record Research Requestion (R3) provided by the University of Pittsburgh Biomedical Informatics Services. For the present analysis we requested deidentified electronic health records (EHRs) of all UPMC Health Plan participants, 40–89 years of age when they had any medical service at the UPMC Healthcare Network System during January 1, 2004 through December 31, 2018. The study was approved by the University of Pittsburgh Internal Review Board, and conducted in accordance with the recognized ethical guidance.
Inclusion and Exclusion Criteria for the NAFLD Cohort
Eligibility criteria for the UPMC NAFLD Cohort Study were patients with any of the following diagnoses according to the International Classification of Diseases 9th revision, clinical modification (ICD-9-CM) or 10th revision, clinical modification (ICD-10-CM) codes: non-alcoholic fatty liver (NAFL), non-alcoholic steatohepatitis (NASH), cirrhosis of liver without mention of alcohol, hepatic failure, or hepatic encephalopathy (see ICD codes in Supplementary Table 1). Exclusion criteria for the UPMC NAFLD Cohort were patients with any of the following diagnoses based on their ICD-9-CM or ICD-10-CM codes on the EHRs: alcoholic liver disease, alcohol use disorder, somatic consequences of alcohol, autoimmune liver disease, alpha-1-antitrypsin deficiency, secondary or unspecified biliary cirrhosis, drug use disorder except nicotine/caffeine, hemochromatosis, Budd-Chiari syndrome, viral hepatitis, unspecified chronic hepatitis, or Wilson’s disease (see ICD codes in Supplementary Table 2). These inclusion and exclusion criteria were based on the recent consensus statement by an expert panel for the definition of NAFLD using administrative coding in electronic health care records35. The UPMC NAFLD Cohort Study consisted of 47,165 unique patients.
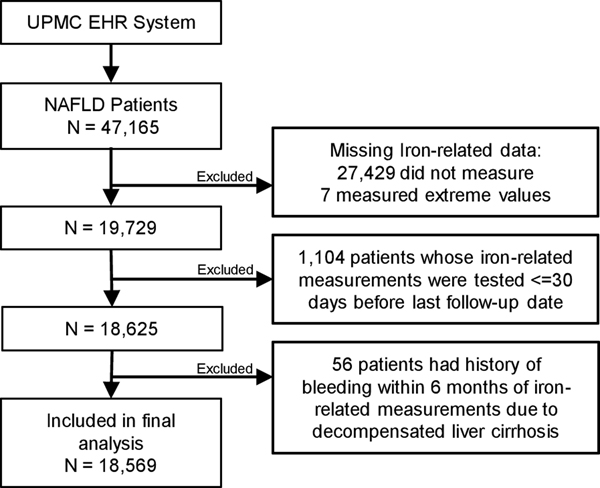
The primary exposure variables for the present analysis were serum biomarkers of iron status – iron, transferrin saturation, total iron binding capacity (TIBC) and ferritin. Patients who did not have any one of the 4 iron biomarker measurement were excluded from the present analysis (n= 27,429 excluded). We further excluded 7 patients with extreme values of iron-related measurements, which deemed as physiologically impossible values (Figure 1). The covariates extracted from the EHRs included age, sex, race, body mass index, smoking status, and histories of type 2 diabetes, dyslipidemia and hypertension.
Figure 1: Diagram for the construction of the final analytic dataset for the UPMC NAFLD Cohort Study, 2004 – 2018.
The primary outcome of the present analysis was incident HCC with the initial diagnosis date from January 1, 2004 through December 31, 2018. All HCC cases were identified based on ICD-9-CM code 155.0 or ICD-10-CM codes C22.0 and C22.8.
Any subject whose earliest measurement of any iron biomarker was performed within 30 days prior to or after the latest date of follow-up were excluded from the present analysis to minimize the potential impact of diagnostic and treatment procedures for HCC on the levels of serum iron biomarkers (n = 1,104 excluded). In addition, we excluded patients whose earliest measurement of any iron biomarker was done less than 180 days after the occurrence of gastrointestinal bleeding (n = 56 excluded), which might have resulted in reduced serum iron level (Figure 1). One hundred seventeen NAFLD patients (4 HCC cases and 113 non-HCC cases) who had a history of gastrointestinal bleeding more than 180 days before serum iron biomarker measurement. Their average time interval from the gastrointestinal bleeding to the serum irone test was 32.2 months, which would have sufficient time to produce mature red blood cells. The final analysis included 18,569 NAFLD patients including 244 incident HCC cases (Figure 1). As expected, a small percentage of NALFD patients were NASH (n = 2,517, 13.6%) because its diagnosis required an invasive liver biopsy procedure.
Statistical Analysis
The distributions of serum iron, transferrin saturation, and ferritin were skewed. Thus, we used the non-parametric Wilcox test to examine the differences in median values of these iron-related biomarkers between patients with incident HCC and those without HCC. Chi-square and pooled two-sample t test were used to compare the differences in the distributions of nominal or categorical variables and continuous variables, respectively, between HCC cases and non-HCC subjects. The Cox proportional hazard regression method was used to assess the associations between serum levels of iron biomarkers and HCC risk. Person-years at risk for each study subject was counted from the date of iron measurements to the date of HCC diagnosis, death, last encounter with any UPMC Healthcare Network facility, whichever occurred first. All study subjects were grouped into low (below lower limit of normal), normal, and high levels (above upper limit of normal) according to the recently recommended ranges for clinical use: the normal range was 75–175 μg/dl for serum iron36,37, 25%−35% for transferrin saturation38,39, and 240–450 μg/dl for TIBC39,40 for both men and women. The normal range of serum ferritin was 3–30 μg/dl for men and 1–20 μg/dl for women41,42.
Statistical analyses were carried out using SAS software version 9.4 (SAS Institute, Cary, NC). All p values reported are two-sided. The p values of less than 0.05 were considered being statistically significant.
RESULTS
The present analysis included 18,569 patients with NAFLD. After an average 4.34 years of follow-up, 244 developed HCC. Men were more likely to develop HCC than women. Patients who developed HCC were older, ever cigarette smokers, or having a history of type 2 diabetes, dyslipidemia or hypertension (All P-values<0.05) (Table 1). A stepwise Cox regression model identified the following independent variables that were statistically significantly associated with HCC risk: age (years), sex, BMI (kg/m2), race (white vs. non-white), history of type 2 diabetes (yes vs. no), and smoking status (never vs. ever smoker). These variables were included as covariates in the final multivariate Cox regression models examining the associations between serum iron biomarkers and HCC risk.
Table 1.
Distribution of selected risk factors in subjects by incident HCC status The UPMC NAFLD Cohort Study, 2004 – 2018
| Characteristics | Incident HCC | Free of HCC | P* |
|---|---|---|---|
|
| |||
| Number of subjects | 244 | 18,325 | |
| Age (years), mean ± SD | 66.1 ± 10.8 | 59.9 ± 12.0 | <0.001 |
| BMI* (Kg/m2), mean ± SD | 32.5 ± 7.7 | 33.8 ± 7.8 | 0.014 |
| Sex, n (%) | |||
| Women | 125 (51.2) | 11,454 (62.5) | 0.003 |
| Men | 119 (48.8) | 6,871 (37.5) | |
| Race, n (%) | |||
| White | 226 (92.6) | 16,714 (91.2) | 0.438 |
| Non-white | 18 (7.4) | 1,611 (8.8) | |
| Cigarette smoking status, n (%) | |||
| Never Smoker | 94 (38.5) | 9,178 (50.1) | <0.001 |
| Ever Smokers | 115 (47.1) | 7,814 (42.6) | |
| Missing | 35 (14.4) | 1,333 (7.3) | |
| History of type 2 diabetes, n (%) | |||
| No | 150 (61.5) | 9,724 (53.1) | < 0.001 |
| Yes | 94 (38.5) | 8,601 (46.9) | |
| History of dyslipidemia, n (%) | |||
| No | 85 (34.8) | 4,952 (27.0) | < 0.006 |
| Yes | 159 (65.2) | 13,373 (73.0) | |
| History of hypertension, n (%) | |||
| No | 30 (12.3) | 3,671 (20.0) | |
| Yes | 214 (87.7) | 14,654 (80.0) | 0.003 |
BMI: body mass index; HCC: hepatocellular carcinoma; NAFLD: non-alcoholic fatty liver disease.
Derived from the t-test (for continuous variables) or Chi-square test (categorical or nominal variables).
The median values of the four iron biomarkers for patients with and without HCC are shown in Table 2. Serum iron was slightly higher whereas serum ferritin and TIBC were lower in patients with HCC than those without HCC. However, their differences were not statistically significant (P>0.05).
Table 2.
Median values of serum iron biomarkers in subjects by incident HCC status The UPMC NAFLD Cohort Study, 2004 – 2018
| Serum iron biomarkers | Incident HCC | Free of HCC | |||
|---|---|---|---|---|---|
|
|
|
||||
| N | Median (5%, 95%) | N | Median (5%, 95%) | P* | |
|
| |||||
| Iron (μg/dl) | 188 | 70.5 (19.0, 171.0) | 15,586 | 69.0 (19.0, 141.0) | 0.897 |
| Transferrin Saturation ( %) | 175 | 19.4 (5.0, 49.0) | 13,697 | 20.0 (5.4, 44.0) | 0.704 |
| TIBC (μg/dl) | 224 | 335.5 (174.0, 503.0) | 16,304 | 348.0 (191.0, 485.0) | 0.180 |
| Ferritin (μg /dl) | 219 | 8.3 (0.8, 98.1) | 16,111 | 10.0 (0.9, 70.0) | 0.445 |
HCC: hepatocellular carcinoma; NAFLD: non-alcoholic fatty liver disease; TIBC: total iron binding capacity.
Derived from the Wilcoxon rank sum test.
Higher level of serum iron was associated with significantly elevated risk of developing HCC (Table 3). Compared with the normal range, the risk of HCC for NAFLD patients with iron level above the upper limit of normal was increased by a statistically significant 191% (HR=2.91; 95% CI: 1.34–6.30) after adjusting for age, sex, race, BMI, history of type 2 diabetes, and smoking status. Similarly, HCC risk was doubled (HR = 2.02, 95% CI: 1.22–3.32) for NAFLD patients with above normal range of transferrin saturation (>35%) as compared with the normal range. No statistically significant association was observed for TIBC or serum ferritin with HCC risk.
Table 3.
The association between serum iron biomarkers and risk of developing HCC The UPMC NAFLD Cohort Study, 2004 – 2018
| Serum iron biomarkers | Total No. of subjects | Total No. of person-years | Incident HCC cases | HR (95% CI)* | P |
|---|---|---|---|---|---|
|
| |||||
| Serum iron (μg/dl) | |||||
| Low (<75) | 8,830 | 36329.4 | 102 | 0.90 (0.67, 1.22) | 0.505 |
| Normal (75–175) | 6,707 | 28144.4 | 79 | 1.00 (Reference) | |
| High (>175) | 237 | 796.5 | 7 | 2.91 (1.34, 6.30) | 0.007 |
| P for trend | 0.066 | ||||
| Continuous (Log2) | 1.09 (0.92, 1.29) | 0.336 | |||
| Transferrin saturation (%) | |||||
| Low (<25) | 9,109 | 38996.9 | 113 | 1.15 (0.78, 1.71) | 0.482 |
| Normal (25–35) | 3,156 | 13095.1 | 32 | 1.00 (Reference) | |
| High (>35) | 1,607 | 5466.5 | 30 | 2.02 (1.22, 3.32) | 0.006 |
| P for trend | 0.053 | ||||
| Continuous (Log2) | 1.10 (0.93,1.30) | 0.288 | |||
| TIBC (μg/dl) | |||||
| Low (<240) | 1,806 | 6438.6 | 31 | 1.14 (0.77, 1.68) | 0.524 |
| Normal (240–450) | 13,045 | 60808.3 | 166 | 1.00 (Reference) | |
| High (>450) | 1,677 | 8466.1 | 27 | 1.33 (0.88, 2.00) | 0.174 |
| P for trend | 0.632 | ||||
| Continuous (Log2) | 0.90 (0.66, 1.23) | 0.503 | |||
| Serum ferritin (μg/dl) | |||||
| Low (<3/<1)† | 1,428 | 6948.3 | 27 | 1.38 (0.91, 2.09) | 0.127 |
| Normal† | 11,150 | 51386.4 | 141 | 1.00 (Reference) | |
| High (>30/>20)† | 3,752 | 15653.9 | 51 | 1.03 (0.75, 1.42) | 0.868 |
| P for trend | 0.368 | ||||
| Continuous (Log2) | 0.96 (0.90, 1.04) | 0.317 | |||
BMI: body mass index; HCC: hepatocellular carcinoma; NAFLD: non-alcoholic fatty liver disease; TIBC: total iron binding capacity.
Adjusted for age (years), race, body mass index (kg/m2), history of type 2 diabetes, cigarette smoking status.
The normal range of serum ferritin was 3–30 μg/dl for men and 1–20 μg/dl for women,
Serum iron levels were highly correlated with transferrin saturation (the correlation coefficient (r) = 0.83, P < 0.00), but not correlated with TIBC (r = 0.04) or serum ferritin (r = 0.11). The correlation of serum ferritin with other iron biomarker measurements was low or moderate (Supplementary Table 3).
Besides the NAFLD, iron deficiency anemia was the most common indication for serum iron test. As expected, a higher proportion of NAFLD patients tested as having low serum iron level had a history of anemia (17.8%) than those with high serum iron (8.7%) (Supplementary Table 4). Low serum iron was also associated with higher BMI, female gender, non-white race/ethnicity, and history of type 2 diabetes and dyslipidemia. To eliminate the potential confounding effect of the iron test indication, we performed a sensitivity analysis after excluding all subjects with a history of iron deficiency anemia, the results remained the same. The adjusted HR of HCC for the high serum iron was 3.19 (95% CI = 1.47–6.94, P = 0.004) compared with the normal range of serum iron.
DISCUSSION
We investigated the associations between four serum biomarkers of iron status and HCC risk in a retrospective cohort study of 18,569 patients derived from the UPMC NAFLD Cohort Study with clinical diagnosis of NAFLD without hemochromatosis or any other major underlying causes of chronic liver disease. We found that elevated levels of both serum iron and transferrin saturation were significantly associated with 2- to 3-fold increased risks of HCC than normal range of these iron biomarker measurements. There were no statistically significant association for serum ferritin and TIBC with the risk of HCC.
NAFLD has become the major cause of chronic liver disease in the U.S.43. However, only minority of the patients with fatty liver progress to cirrhosis, at 1.2% after 20 years of follow-up44. Among NAFLD patients with cirrhosis, the HCC incidence rate was around 1% per year45. The additional risk factors such as iron overload, may accelerate the progression of NAFLD to cirrhosis and HCC. Liver iron deposits have been frequently observed in patients with NAFLD46. A hospital-based case-control study involving 51 HCC cases and 102 matched controls free of HCC demonstrated that iron deposits (corrected total iron score>0) were significantly more frequent in patients with NASH-related cirrhosis with HCC than in HCC-free controls46. A recent meta-analysis summarized 9 previous studies published before 201947. These studies used the design of a prospective cohort or nested case-control study within a prospective cohort in various populations in Asia, Europe and USA47. Of the 9 studies, three investigated the serum iron and risk of liver cancer and found a pooled HR of 2.47 (95% CI: 1.31, 4.63) for the highest level of serum iron compared with the lowest iron, although different cut-offs for high or low serum iron were used. Our findings with an HR of 2.91 (95% CI: 1.34–6.30) for HCC with clinically defined high level of serum iron were consistent with the pooled HR of this meta-analysis. In addition, a statistically significant increased risk of HCC with elevated transferrin saturation in our study was also consistent with similar findings from a previous study48. Given the high correlation between serum iron and transferrin saturation, we were not able to separate their effect on HCC risk.
Serum ferritin, a biomarker for body iron reservoir, has been associated with increased risk of HCC. In a hospital-based case-control study of 24 HCC cases and 48 age- race-, and sex-matched controls of blacks in South Africa, the iron-loaded subjects (defined as a raised serum ferritin concentration in combination with a transferrin saturation ≥60% due to high intake of iron from home-made alcoholic beverage) were at significantly increased risk of HCC (odds ratio = 10.6, 95% CI: 1.5–76.8) compared with normal ferritin level49. The meta-analysis described above also found a statistically significant, 49% increased risk of HCC associated with higher levels of serum ferritin, although there was a significant heterogeneity among the 6 studies included47. These studies used different cut-off values for serum ferritin, different study populations, the patients with different underlying causes of HCC or chronic liver disease. These differences may explain the different results of the meta-analysis from ours that used a clinically defined sex-specific cut-off values for serum ferritin and NAFLD patients only.
Our current study has several strengths. First, we retrospectively constructed a cohort of more than 18,000 patients with clinical diagnosis of NAFLD and iron measurements who were free of other chronic liver diseases including hemochromatosis and alcoholic and viral hepatitis. The exposure to iron was defined by four commonly used clinical measurements of serum iron-related biomarkers and quantified on average 4.34 years prior to the occurrence of HCC, which minimized the potential impact of diagnostic and treatment procedures for HCC on serum biomarker levels. All cut-off values of the iron biomarkers included in the present study were derived from most recently defined values with clinical relevance.
Out study also has several limitations. First, random errors of the four iron measurements due to incorrect data entry or at single time point may not be reflective of their change over time. However, measurement errors due to random variations usually attenuate the observed iron biomarker-HCC risk association towards the null. Second, although the present study included more than 18,000 NAFLD patients with more than 4 years of follow-up, the number of HCC cases was relatively small given its low incidence rate, which did not allow for subgroup analysis stratified by gender, obesity status or history of type 2 diabetes. Third, we cannot completely rule out potential residual confounding by relatively low levels of alcohol consumption on the observed iron-HCC risk association due to incomplete recording of moderate or lower alcohol intake history in patients’ EHRs, although patients with heavy alcohol consumptions or related alcohol use disorders were excluded from the study population50. Finally as with any observational studies, confounding and/or selection bias might play a role on the observed association between serum iron and HCC risk.
In conclusion, in a large retrospective cohort study of 18,569 NAFLD patients, we found that the elevations of pre-diagnostic serum iron and transferrin saturation were significantly associated with increased risk of HCC. Our findings provide supporting evidence for the detrimental role of iron overload on the progression of NAFLD to the development of HCC. Further prospective studies with large samples in diverse study populations are warranted to confirm our findings.
Supplementary Material
ACKNOWLEDGEMENTS
This research project was partially supported by an US NIH grant (No. R01CA255809 to J. Behari and J.-M. Yuan), the University of Pittsburgh Medical Center Hillman Cancer Center Start-up fundings (to H.N. Luu and J.-M. Yuan). C.E. Thomas was supported by an US NIH training grant No. T32CA186873 (to J.-M. Yuan). We acknowledge the University of Pittsburgh Biomedical Informatics Services for providing de-identified electronic health records.
Financial support statement:
This study was partially supported by the University of Pittsburgh Medical Center Hillman Cancer Center start-up fund.
Abbreviations:
- BMI
body mass index
- EHR
electric health record
- HBV
hepatitis B virus
- HCC
hepatocellular carcinoma
- HCV
hepatitis C virus
- HR
hazard ratio
- ICD
International classification of disease
- NAFL
Non-alcoholic fatty liver
- NAFLD
non-alcoholic fatty liver disease
- NASH
non-alcoholic steatohepatitis
- PA
Commonwealth of Pennsylvania
- SD
standard deviation
- UPMC
University of Pittsburgh Medical Center
Footnotes
Conflict of interest statement:
No conflict of interest was declared by any other authors
REFERENCES
- 1.Endeshaw M, Hallowell BD, Razzaghi H, Senkomago V, McKenna MT, Saraiya M. Trends in liver cancer mortality in the United States: Dual burden among foreign- and US-born persons. Cancer. 2019;125(5):726–734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anstee QM, Reeves HL, Kotsiliti E, Govaere O, Heikenwalder M. From NASH to HCC: current concepts and future challenges. Nature Reviews Gastroenterology & Hepatology. 2019;16(7):411–428. [DOI] [PubMed] [Google Scholar]
- 3.Akinyemiju T, Abera S, Ahmed M, et al. The Burden of Primary Liver Cancer and Underlying Etiologies From 1990 to 2015 at the Global, Regional, and National Level: Results From the Global Burden of Disease Study 2015. JAMA Oncol. 2017;3(12):1683–1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Balogh J, Victor D 3rd, Asham EH, et al. Hepatocellular carcinoma: a review. J Hepatocell Carcinoma. 2016;3:41–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Petrick JL, McGlynn KA. The changing epidemiology of primary liver cancer. Curr Epidemiol Rep. 2019;6(2):104–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.El-Serag HB. Epidemiology of Viral Hepatitis and Hepatocellular Carcinoma. Gastroenterology. 2012;142(6):1264–1273.e1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.White DL, Thrift AP, Kanwal F, Davila J, El-Serag HB. Incidence of Hepatocellular Carcinoma in All 50 United States, From 2000 Through 2012. Gastroenterology. 2017;152(4):812–820.e815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Petrick JL, Campbell PT, Koshiol J, et al. Tobacco, alcohol use and risk of hepatocellular carcinoma and intrahepatic cholangiocarcinoma: The Liver Cancer Pooling Project. Br J Cancer. 2018;118(7):1005–1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Magnussen A, Parsi MA. Aflatoxins, hepatocellular carcinoma and public health. World J Gastroenterol. 2013;19(10):1508–1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Younes R, Bugianesi E. Should we undertake surveillance for HCC in patients with NAFLD? J Hepatol. 2018;68(2):326–334. [DOI] [PubMed] [Google Scholar]
- 11.Byrne CD, Targher G. NAFLD: a multisystem disease. J Hepatol. 2015;62(1 Suppl):S47–64. [DOI] [PubMed] [Google Scholar]
- 12.Browning JD, Szczepaniak LS, Dobbins R, et al. Prevalence of hepatic steatosis in an urban population in the United States: Impact of ethnicity. Hepatology. 2004;40(6):1387–1395. [DOI] [PubMed] [Google Scholar]
- 13.Li B, Zhang C, Zhan Y-T. Nonalcoholic Fatty Liver Disease Cirrhosis: A Review of Its Epidemiology, Risk Factors, Clinical Presentation, Diagnosis, Management, and Prognosis. Can J Gastroenterol Hepatol. 2018;2018:2784537–2784537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Siddiqui MS, Fuchs M, Idowu MO, et al. Severity of nonalcoholic fatty liver disease and progression to cirrhosis are associated with atherogenic lipoprotein profile. Clin Gastroenterol Hepatol. 2015;13(5):1000–1008.e1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Abbaspour N, Hurrell R, Kelishadi R. Review on iron and its importance for human health. J Res Med Sci. 2014;19(2):164–174. [PMC free article] [PubMed] [Google Scholar]
- 16.Pietrangelo A.Hereditary hemochromatosis. Biochimica et Biophysica Acta (BBA) - Molecular Cell Research. 2006;1763(7):700–710. [DOI] [PubMed] [Google Scholar]
- 17.Andrews NC. Disorders of iron metabolism. N Engl J Med. 1999;341(26):1986–1995. [DOI] [PubMed] [Google Scholar]
- 18.Toyokuni S.Role of iron in carcinogenesis: cancer as a ferrotoxic disease. Cancer Sci. 2009;100(1):9–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chung JW, Shin E, Kim H, et al. Hepatic iron overload in the portal tract predicts poor survival in hepatocellular carcinoma after curative resection. Liver International. 2018;38(5):903–914. [DOI] [PubMed] [Google Scholar]
- 20.Ghamarchehreh ME, Jonaidi-Jafari N, Bigdeli M, Khedmat H, Saburi A. Iron Status and Metabolic Syndrome in Patients with Non-Alcoholic Fatty Liver Disease. Middle East J Dig Dis. 2016;8(1):31–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.El Nakeeb N, Saleh SA, Massoud YM, Hussein A, Hamed R. Serum ferritin as a non-invasive marker in the prediction of hepatic fibrosis among Egyptian patients with non-alcoholic fatty liver disease. JGH Open. 2017;1(3):112–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zacharski LR, Ornstein DL, Woloshin S, Schwartz LM. Association of age, sex, and race with body iron stores in adults: analysis of NHANES III data. Am Heart J. 2000;140(1):98–104. [DOI] [PubMed] [Google Scholar]
- 23.Rochette J, Pointon JJ, Fisher CA, et al. Multicentric Origin of Hemochromatosis Gene (HFE) Mutations. The American Journal of Human Genetics. 1999;64(4):1056–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rochette J, Pointon JJ, Fisher CA, et al. Multicentric origin of hemochromatosis gene (HFE) mutations. Am J Hum Genet. 1999;64(4):1056–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Acton RT, Barton JC, Snively BM, et al. Geographic and racial/ethnic differences in HFE mutation frequencies in the Hemochromatosis and Iron Overload Screening (HEIRS) Study. Ethn Dis. 2006;16(4):815–821. [PubMed] [Google Scholar]
- 26.McDowell LA, Kudaravalli P, Sticco KL. Iron Overload. StatPearls. Treasure Island (FL)2020. [PubMed] [Google Scholar]
- 27.Wallace DF. The Regulation of Iron Absorption and Homeostasis. Clin Biochem Rev. 2016;37(2):51–62. [PMC free article] [PubMed] [Google Scholar]
- 28.Bloomer SA, Brown KE. Iron-Induced Liver Injury: A Critical Reappraisal. Int J Mol Sci. 2019;20(9):2132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kowdley KV. Iron Overload in Patients With Chronic Liver Disease. Gastroenterol Hepatol (N Y). 2016;12(11):695–698. [PMC free article] [PubMed] [Google Scholar]
- 30.Fargion S, Valenti L, Fracanzani AL. Beyond hereditary hemochromatosis: new insights into the relationship between iron overload and chronic liver diseases. Dig Liver Dis. 2011;43(2):89–95. [DOI] [PubMed] [Google Scholar]
- 31.Nahon P, Sutton A, Rufat P, et al. Liver iron, HFE gene mutations, and hepatocellular carcinoma occurrence in patients with cirrhosis. Gastroenterology. 2008;134(1):102–110. [DOI] [PubMed] [Google Scholar]
- 32.Kowdley KV. Iron, hemochromatosis, and hepatocellular carcinoma. Gastroenterology. 2004;127(5):S79–S86. [DOI] [PubMed] [Google Scholar]
- 33.Kew MC. Hepatic iron overload and hepatocellular carcinoma. Liver Cancer. 2014;3(1):31–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shiani A, Narayanan S, Pena L, Friedman M. The Role of Diagnosis and Treatment of Underlying Liver Disease for the Prognosis of Primary Liver Cancer. Cancer Control. 2017;24(3):1073274817729240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hagström H, Adams LA, Allen AM, et al. Administrative Coding in Electronic Health Care Record-Based Research of NAFLD: An Expert Panel Consensus Statement. Hepatology. 2021;74(1):474–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ji X, Cui N, Liu J. Neurocognitive Function Is Associated With Serum Iron Status in Early Adolescents. Biol Res Nurs. 2017;19(3):269–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Balan V, Baldus W, Fairbanks V, Michels V, Burritt M, Klee G. Screening for hemochromatosis: a cost-effectiveness study based on 12,258 patients. Gastroenterology. 1994;107(2):453–459. [DOI] [PubMed] [Google Scholar]
- 38.Koerper MA, Dallman PR. Serum iron concentration and transferrin saturation in the diagnosis of iron deficiency in children: normal developmental changes. J Pediatr. 1977;91(6):870–874. [DOI] [PubMed] [Google Scholar]
- 39.Faruqi A, Mukkamalla SKR. Iron Binding Capacity. StatPearls Publishing, Treasure Island (FL); 2020. [PubMed] [Google Scholar]
- 40.Åsberg A, Thorstensen K, Mikkelsen G, Åsberg AE. The diagnostic accuracy of unbound iron binding capacity (UIBC) as a test for empty iron stores. Scand J Clin Lab Invest. 2013;73(3):208–213. [DOI] [PubMed] [Google Scholar]
- 41.Kratz A, Ferraro M, Sluss PM, Lewandrowski KB. Case records of the Massachusetts General Hospital. Weekly clinicopathological exercises. Laboratory reference values. N Engl J Med. 2004;351(15):1548–1563. [DOI] [PubMed] [Google Scholar]
- 42.Kratz A, Ferraro M, Sluss PM, Lewandrowski KB. Normal Reference Laboratory Values. The New England journal of medicine. 2004;351(15):1548–1563. [DOI] [PubMed] [Google Scholar]
- 43.Sanyal AJ, Friedman SL, McCullough AJ, et al. Challenges and opportunities in drug and biomarker development for nonalcoholic steatohepatitis: findings and recommendations from an American Association for the Study of Liver Diseases-U.S. Food and Drug Administration Joint Workshop. Hepatology (Baltimore, Md). 2015;61(4):1392–1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dam-Larsen S, Becker U, Franzmann MB, Larsen K, Christoffersen P, Bendtsen F. Final results of a long-term, clinical follow-up in fatty liver patients. Scand J Gastroenterol. 2009;44(10):1236–1243. [DOI] [PubMed] [Google Scholar]
- 45.Ioannou GN, Green P, Lowy E, Mun EJ, Berry K. Differences in hepatocellular carcinoma risk, predictors and trends over time according to etiology of cirrhosis. PLoS One. 2018;13(9):e0204412-e0204412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sorrentino P, D’Angelo S, Ferbo U, Micheli P, Bracigliano A, Vecchione R. Liver iron excess in patients with hepatocellular carcinoma developed on non-alcoholic steato-hepatitis. J Hepatol. 2009;50(2):351–357. [DOI] [PubMed] [Google Scholar]
- 47.Tran KT, Coleman HG, McCain RS, Cardwell CR. Serum Biomarkers of Iron Status and Risk of Primary Liver Cancer: A Systematic Review and Meta-Analysis. Nutr Cancer. 2019;71(8):1365–1373. [DOI] [PubMed] [Google Scholar]
- 48.Ellervik C, Tybjærg-Hansen A, Nordestgaard BG. Risk of cancer by transferrin saturation levels and haemochromatosis genotype: population-based study and meta-analysis. Journal of Internal Medicine. 2012;271(1):51–63. [DOI] [PubMed] [Google Scholar]
- 49.Mandishona E, MacPhail AP, Gordeuk VR, et al. Dietary iron overload as a risk factor for hepatocellular carcinoma in Black Africans. Hepatology. 1998;27(6):1563–1566. [DOI] [PubMed] [Google Scholar]
- 50.Tai B, McLellan AT. Integrating information on substance use disorders into electronic health record systems. J Subst Abuse Treat. 2012;43(1):12–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.