Abstract
Objective:
The management of early hypotension in extremely low gestational age neonates (ELGANs) varies greatly between centers. The objective of this study was to provide updated data on the use of vasoactive medications in ELGANs during the first postnatal week.
Study design:
We identified ELGANs (22-27 weeks gestational age) cared for at Pediatrix neonatal intensive care units from 2009-2018. We evaluated the frequency of exposure to vasoactive medications by gestational age, and compared use of vasoactive medications between 2 epochs (2009-2013 and 2014-2018).
Results:
A total of 10 070/34 234 (29%) ELGANs received ≥1 vasoactive medication. Dopamine was the most frequently used vasoactive medication. The majority (83%) of treated ELGANs initiated therapy on postnatal day 0-1. Overall use of vasoactive medications was slightly lower in 2014-2018 than 2009-2013 (28% vs 31%, p<0.001).
Conclusion:
A substantial proportion of ELGANs were exposed to vasoactive medications during the first postnatal week.
INTRODUCTION
There is no clear consensus regarding the diagnosis or management of early hypotension in extremely low gestational age neonates (ELGANS). Low blood pressure (BP) is a common concern in ELGANs during the first postnatal week,1, 2 and it can be challenging for clinicians to differentiate normal, transitional hemodynamic changes from pathophysiologic hypotension that requires treatment. Several studies have shown that early hypotension in ELGANs is associated with both short and long-term adverse outcomes, including intraventricular hemorrhage (IVH), neurodevelopmental impairment, and death.1, 3, 4 These findings have led many clinicians to use medications in order to raise BP to “normal” values. Treatment strategies include volume expansion, vasoactive medications, corticosteroids, or a combination of therapies. Unfortunately, the majority of studies have not demonstrated decreased morbidity and mortality in neonates treated for hypotension.5, 6, 7, 8, 9 Some studies even suggest that treatment of hypotension is independently associated with harm.2, 5, 6, 7, 8, 10, 11, 12, 13, 14, 15
Vasoactive medications (vasopressors and inotropes) are frequently used in the management of early hypotension in ELGANs, despite a paucity of efficacy and safety data.5, 10 These medications have varying developmentally-regulated mechanisms of action and potential adverse effects.16, 17 They have not been extensively studied in preterm neonates, and none are currently labeled by the United States (US) Food and Drug Administration for use in this population. Several randomized controlled trials (RCTs) have compared vasoactive medications in neonates with hypotension and have not demonstrated clear superiority of any one drug.18, 19, 20, 21, 22 In the absence of data to guide use of antihypotensive therapies such as vasoactive medications, it is not surprising that there is significant practice variation between centers in both intervention thresholds and treatment of early hypotension in preterm neonates.10, 11, 23, 24, 25 Evidence suggests that while the incidence of hypotension in ELGANs has remained relatively stable, treatment strategies have changed, including an overall decline in the use of vasoactive medications.8, 23, 24, 25 Whether this observation is related to a higher BP threshold for intervention, increased comfort with cautious observation in some neonates, or some other practice variation is unknown. The objective of this study is to provide updated data on the use of vasoactive medications during the first postnatal week among ELGANs cared for at US neonatal intensive care units (NICUs) from 2009-2018.
METHODS
Study design
We identified neonates born at 22-27 weeks gestational age (GA) and cared for at US Pediatrix Medical Group NICUs from 2009-2018. We excluded neonates if they had a major congenital anomaly, congenital heart disease (excluding isolated patent ductus arteriosus, atrial septal defect, or patent foramen ovale), were outborn, or received no medications (as these neonates were likely to have either missing data or have received comfort care only). Neonatal demographic and clinical data were obtained from the Pediatrix BabySteps Clinical Data Warehouse (CDW).26 The CDW contains de-identified data extracted from clinical care documentation including patient demographics, medical history, diagnoses, medications, and laboratory results. This study was approved by the Duke University Health System Institutional Review Board with a waiver of informed consent.
Definitions
We included data on receipt of vasoactive medications (dopamine, dobutamine, epinephrine, norepinephrine, vasopressin, and milrinone) during the first postnatal week for any clinical indication and any duration of use. We defined bacteremia as any blood culture positive with an organism not typically considered a contaminant prior to postnatal day 7. For blood cultures positive for coagulase-negative Staphylococcus (CoNS), we defined definite CoNS bacteremia as 2 positive cultures on the same day27 and probable CoNS bacteremia as 2 positive cultures for CoNS within a 4-day period, 3 positive cultures within a 7-day period, or 4 positive cultures within a 10-day period.28 We included definite and probable CoNS bacteremia in the analysis.
Statistical methods
We used descriptive statistics such as frequencies (with percentages) and medians (with 25th and 75th percentiles) to describe discrete variables and continuous variables, respectively. We compared the distribution of demographic and clinical characteristics between neonates with and without any vasoactive medication exposure during the first postnatal week using the Chi-squared test. We used the Kruskal-Wallis or Chi-squared test to compare the distribution of vasoactive medication use, the concurrent use of 2 or 3 vasoactive medications, the distribution of the postnatal start day of treatment, and the total duration of treatment with vasoactive medication(s) by GA group. We used the Chi-squared test to compare the proportions of neonates exposed to the total number of vasoactive medications, each individual vasoactive medication, and combinations of vasoactive medications during the first postnatal week between 2 epochs (2009-2013 and 2014-2018). These 5-year epochs were chosen to compare exposure to vasoactive medications among similar numbers of ELGANs. P-values <0.05 were considered statistically significant. All analyses were conducted using Stata version 16.0 (College Station, TX).
RESULTS
Between 2009-2018, a total of 34 234 ELGANs cared for at 345 Pediatrix NICUs met inclusion criteria. This cohort of neonates had a median GA of 26 weeks (25th, 75th percentiles: 24, 27) and median birth weight of 779 g (635, 930). A total of 10 070 (29%) neonates were exposed to ≥1 vasoactive medication in the first postnatal week (Table 1). Among neonates who received a vasoactive medication, 8331/10 070 (83%) initiated therapy on postnatal day 0 or 1 and the median number of days of exposure was 3 (25th, 75th percentiles: 1, 4) (Table 2). Earlier GA was associated with exposure to a greater number of vasoactive medications for a greater number of days. There were significant differences in all demographics for ELGANs with and without exposure to vasoactive medications (Table 1). Exposure was more common with lower GA; 328/548 (60%) of neonates born at 22 weeks were exposed compared with 1388/9493 (15%) at 27 weeks. Exposure was highest among ELGANs in the lowest birth weight category of <500 g. ELGANs with vasoactive medication exposure were more likely to be male, SGA, non-white race, and born by cesarean section; they were significantly less likely to have received antenatal corticosteroids. They were also more likely to have a 5 minute APGAR score <5, require mechanical ventilation, receive surfactant and/or systemic corticosteroids, and have bacteremia.
Table 1:
Demographics and clinical characteristics by vasoactive medication exposure during the first postnatal week, 2009-2018
| Vasoactive med. exposure | p-value† | ||
|---|---|---|---|
| Yes N=10 070 |
No N=24 164 |
||
| Gestational age (weeks), N (%) | <0.001 | ||
| 22 | 328 (3) | 220 (0.9) | |
| 23 | 1868 (19) | 1612 (7) | |
| 24 | 2549 (25) | 3457 (14) | |
| 25 | 2201 (22) | 4649 (19) | |
| 26 | 1736 (17) | 6121 (25) | |
| 27 | 1388 (14) | 8105 (34) | |
| Birth weight (g), N (%) | <0.001 | ||
| <500 | 1176 (12) | 902 (4) | |
| 500-749 | 5243 (52) | 7933 (33) | |
| ≥750 | 3651 (36) | 15 329 (63) | |
| Male, N (%) | 5650 (56) | 12 403 (51) | <0.001 |
| SGA, N (%) | 2057 (21) | 2907 (12) | <0.001 |
| White race, N (%) | 3734 (39) | 9441 (41) | <0.001 |
| Cesarean section, N (%) | 7329 (74) | 16 721 (70) | <0.001 |
| 5 min. APGAR score <5, N (%) | 3502 (36) | 4607 (19) | <0.001 |
| Antenatal steroids, N (%) | 7922 (79) | 20 406 (84) | <0.001 |
| Mechanical ventilation, N (%)* | 9765 (97) | 19 125 (79) | <0.001 |
| Surfactant, N (%) | 9168 (91) | 19 535 (81) | <0.001 |
| Systemic corticosteroid, N (%)* | 2585 (26) | 601 (2) | <0.001 |
| Bacteremia, N (%)* | 528 (5) | 637 (3) | <0.001 |
Prior to postnatal day 7
p-value for Chi-squared test
Table 2:
Trends in vasoactive medication use during the first postnatal week by gestational age, 2009-2018
| Gestational age at birth (weeks) | p-value† | |||||||
|---|---|---|---|---|---|---|---|---|
| 22 N=548 |
23 N=3480 |
24 N=6006 |
25 N=6850 |
26 N=7857 |
27 N=9493 |
Total N=34 234 |
||
| Vasoactive med. exposure, N (%) | 328 (60) | 1868 (54) | 2549 (42) | 2201 (32) | 1736 (22) | 1388 (15) | 10 070 (29) | <0.001 |
| Number of vasoactive meds., N (%) | <0.001 | |||||||
| 1 | 196 (36) | 1225 (35) | 1874 (31) | 1651 (24) | 1370 (17) | 1105 (12) | 7421 (22) | |
| 2 | 110 (20) | 500 (14) | 523 (9) | 431 (6) | 290 (4) | 224 (2) | 2078 (6) | |
| 3 | 22 (4) | 136 (4) | 145 (2) | 111 (2) | 72 (0.9) | 52 (0.6) | 538 (2) | |
| ≥4 | 0 (0) | 7 (0.2) | 7 (0.12) | 8 (0.1) | 4 (0.1) | 7 (0.1) | 33 (0.1) | |
| Dopamine, N (%) | 283 (52) | 1675 (48) | 2301 (38) | 2004 (29) | 1574 (20) | 1258 (13) | 9095 (27) | <0.001 |
| Epinephrine, N (%) | 116 (21) | 541 (16) | 591 (10) | 458 (7) | 307 (4) | 246 (3) | 2259 (7) | <0.001 |
| Dobutamine, N (%) | 79 (14) | 410 (12) | 449 (7) | 381 (6) | 273 (3) | 200 (2) | 1792 (5) | <0.001 |
| Milrinone, N (%) | 1 (0.2) | 17 (0.5) | 27 (0.5) | 26 (0.4) | 20 (0.3) | 21 (0.2) | 112 (0.3) | 0.05 |
| Vasopressin, N (%) | 3 (0.6) | 18 (0.5) | 14 (0.2) | 9 (0.1) | 7 (0.1) | 13 (0.1) | 64 (0.2) | <0.001 |
| Norepinephrine, N (%) | 0 (0) | 0 (0) | 1 (<0.1) | 0 (0) | 1 (<0.1) | 0 (0) | 2 (<0.1) | 0.70 |
| 2 med. combinations, N (%) | ||||||||
| Dopa. + Dobuta. | 72 (13) | 365 (10) | 395 (7) | 328 (5) | 227 (3) | 161 (2) | 1548 (5) | <0.001 |
| Dopa. + Epi. | 75 (14) | 387 (11) | 392 (7) | 307 (4) | 185 (2) | 152 (2) | 1498 (4) | <0.001 |
| Dopa. + Norepi. | 0 (0) | 0 (0) | 1 (<0.1) | 0 (0) | 1 (<0.1) | 0 (0) | 2 (<0.1) | 0.70 |
| Dopa. + Vaso. | 1 (0.2) | 14 (0.4) | 11 (0.2) | 8 (0.1) | 6 (0.1) | 11 (0.1) | 51 (0.2) | 0.002 |
| Dopa. + Milrinone | 1 (0.2) | 15 (0.4) | 24 (0.4) | 20 (0.3) | 17 (0.2) | 19 (0.2) | 96 (0.3) | 0.09 |
| Other | 4 (1) | 11 (0.3) | 11 (0.2) | 13 (0.2) | 10 (0.1) | 7 (0.1) | 56 (0.2) | 0.001 |
| 3 med. combinations, N (%) | ||||||||
| Dopa. + Epi. + Vaso. | 0 (0) | 6 (0.2) | 8 (0.1) | 5 (0.1) | 1 (<0.1) | 6 (0.1) | 26 (0.1) | 0.04 |
| Dobuta. + Epi. + Vaso. | 1 (0.2) | 4 (0.1) | 3 (0.1) | 3 (<0.1) | 0 (0) | 3 (<0.1) | 14 (<0.1) | 0.05 |
| Other | 21 (4) | 136 (4) | 144 (2) | 113 (2) | 75 (1) | 53 (0.6) | 542 (2) | <0.001 |
| Postnatal start day of first vasoactive med., N (%) | <0.01 | |||||||
| 0 | 213 (65) | 1099 (59) | 1441 (57) | 1263 (57) | 970 (56) | 777 (56) | 5763 (57) | |
| 1 | 87 (27) | 471 (25) | 653 (26) | 522 (24) | 472 (27) | 363 (26) | 2568 (26) | |
| 2 | 14 (4) | 91 (5) | 139 (5) | 158 (7) | 115 (7) | 112 (8) | 629 (6) | |
| 3 | 2 (0.6) | 53 (3) | 109 (4) | 83 (4) | 55 (3) | 59 (4) | 361 (4) | |
| 4 | 3 (0.9) | 42 (2) | 71 (3) | 61 (3) | 39 (2) | 32 (2) | 248 (2) | |
| 5 | 5 (2) | 55 (3) | 69 (3) | 51 (2) | 52 (3) | 29 (2) | 261 (3) | |
| 6 | 4 (1) | 57 (3) | 67 (3) | 63 (3) | 33 (2) | 16 (1) | 240 (2) | |
| Days of exposure, median (25th, 75th%) | 2 (1,4) | 2 (1,5) | 3 (1,5) | 3 (1,5) | 3 (1,4) | 3 (1,4) | 3 (1,4) | <0.001 |
p-value for Kruskal-Wallis or Chi-squared test
The most frequently used vasoactive medication was dopamine, followed by epinephrine, dobutamine, milrinone, vasopressin, and norepinephrine (Table 2). Use of milrinone, vasopressin, and norepinephrine was uncommon (<1% of ELGANs for each). A total of 2649/34 234 (7.7%) ELGANs received ≥2 vasoactive medications and 571/34 234 (1.7%) received ≥3 vasoactive medications. The most common medication combinations were dopamine plus dobutamine and dopamine plus epinephrine (Table 2). From 2009-2013 to 2014-2018, there was a statistically significant decrease in the percentage of ELGANs exposed to vasoactive medications (31% vs 28%, p<0.001). There was a statistically significant decrease in the use of dopamine and dobutamine, whereas use of vasopressin increased (Table 3, Figure 1). There was no significant change in the use of epinephrine, norepinephrine, or milrinone. Initial exposure to vasoactive medications occurred later and was for shorter duration in 2014-2018 compared with 2009-2013 (Table 3).
Table 3:
Trends in vasoactive medication use during the first postnatal week by time period, 2009-2018
| 2009-2013 N=16 908 |
2014-2018 N=17 326 |
p-value† |
|
|---|---|---|---|
| Vasoactive med. exposure, N (%) | 5236 (31) | 4834 (28) | <0.001 |
| Total number of vasoactive meds., N (%) | <0.001 | ||
| 1 | 3815 (23) | 3606 (21) | |
| 2 | 1106 (7) | 972 (6) | |
| 3 | 305 (2) | 233 (1) | |
| ≥4 | 10 (0.1) | 23 (0.1) | |
| Dopamine, N (%) | 4791 (28) | 4304 (25) | <0.001 |
| Epinephrine, N (%) | 1076 (6) | 1183 (7) | 0.08 |
| Dobutamine, N (%) | 1043 (6) | 749 (4) | <0.001 |
| Milrinone, N (%) | 59 (0.4) | 53 (0.3) | 0.49 |
| Vasopressin, N (%) | 13 (0.1) | 51 (0.3) | <0.001 |
| Norepinephrine, N (%) | 0 (0) | 2 (<0.1) | 0.16 |
| Postnatal start day of vasoactive med., N (%) | <0.001 | ||
| 0 | 3109 (59) | 2654 (55) | |
| 1 | 1356 (26) | 1212 (25) | |
| 2 | 288 (6) | 341 (7) | |
| 3 | 170 (3) | 191 (4) | |
| 4 | 113 (2) | 135 (3) | |
| 5 | 111 (2) | 150 (3) | |
| 6 | 89 (2) | 151 (3) | |
| Days of exposure, median (25th, 75th%) | 3 (2,5) | 2 (1,4) | <0.001 |
p-value for Chi-squared test or Wilcoxon rank sum test
Figure 1.
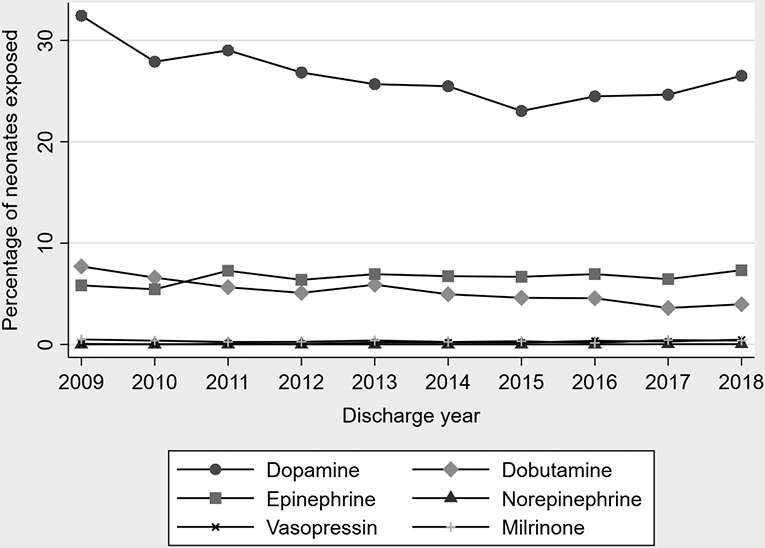
Vasoactive medications in ELGANs during the first postnatal week, 2009-2018.
DISCUSSION
The diagnosis of early hypotension in preterm neonates is controversial. Early neonatal BP is influenced by a number of factors, including GA, postnatal age, and birth weight,2, 4, 10, 11, 24, 29, 30, 31, 32, 33, 34 In ELGANs, these relationships are less clear and early BP measurements can vary widely.2, 31 Receipt of antenatal corticosteroids and delayed cord clamping are associated with higher early BP, whereas conditions such as perinatal asphyxia and sepsis can result in low BP.2, 24, 29, 31, 35 In ELGANs, additional factors that cause or exacerbate hypotension include immature myocardium, relative adrenal insufficiency, patent ductus arteriosus, and decreased venous return related to the use of positive pressure ventilation.36, 37, 38
This study evaluated the use of vasoactive medications during the first postnatal week among ELGANs cared for at Pediatrix NICUs from 2009-2018. Similar to prior database studies,8, 24, 25 we found that a substantial proportion (29%) of ELGANs were exposed to ≥1 vasoactive medication. The majority (83%) of these neonates began treatment within 2 days of birth. Treatment was associated with lower GA, lower birth weight, male sex, SGA status, non-white race, and a lack of antenatal corticosteroids. Not unexpectedly, markers of illness severity such as 5 minute APGAR score <5, bacteremia, receipt of surfactant, and need for mechanical ventilation, were also associated with treatment.
Prior observational studies and practice surveys have identified dopamine as a first-line agent for hypotension in neonates.23, 24, 25, 39 We found that in our cohort of ELGANs, dopamine remained the most frequently prescribed vasoactive medication, followed by epinephrine and dobutamine. This finding aligns with another US database study showing that use of epinephrine surpassed that of dobutamine in the mid-2000s as a result of an overall decline in the use of dobutamine.23 Use of norepinephrine, vasopressin, and milrinone was infrequent in our study cohort (<1% of neonates each), though there was a small increase in the proportion of neonates exposed to vasopressin in 2014-2018 compared with 2009-2013. Unfortunately, we did not have data on the indication for use of specific vasoactive medications. For example, milrinone is typically used in full term neonates to lower pulmonary vascular resistance in the setting of pulmonary hypertension. This issue is less common in ELGANs, which may explain the low rates of milrinone use in our study cohort. Also, at some centers, dopamine is often used in preterm neonates to increase renal perfusion for oliguria (in the presence or absence of hypotension). As we did not have access to BP values or urine output, some of these ELGANs may have received dopamine for the primary indication of oliguria rather than hypotension. Finally, we may have included use of epinephrine boluses for cardiac arrest or bradyarrhythmias in either the delivery room or NICU, in accordance with Neonatal Resuscitation Program guidelines. Despite the potential inclusion of ELGANs exposed to vasoactive medications for indications other than hypotension, the distribution of exposure to individual vasoactive medications did not differ significantly from those reported in other US database studies.23, 25
Prior studies have shown declining rates in the treatment of early neonatal hypotension.23 Between 2009-2018, the overall proportion of ELGANs exposed to vasoactive medications at Pediatrix NICUs also decreased slightly from 31 to 28% (p<0.001). This was driven by the decreased use of dopamine and dobutamine, a trend also observed from 2001-2012 among US Pediatric Health Information System (PHIS) database centers.23 Vasoactive medications were started later, and used for a shorter duration in 2014-2018 compared with 2009-2013. As we did not have access to vital signs (including BP), we cannot relate this finding to the incidence of hypotension or the threshold(s) used for intervention. It is also possible that another practice change, such as increased use of antenatal or postnatal corticosteroids, contributed to the decreased use of vasoactive medications.
This study contributes to the limited evidence on the use of vasoactive medications in ELGANs, and has several strengths. First, it provides updated data on the use of vasoactive medications over a recent 10-year period. Second, the study cohort encompasses a generalizable population of ELGANs cared for by a large number of US NICUs. The Pediatrix Medical Group includes NICUs in 35 states, as well as Puerto Rico, which care for nearly 25% of the US’s NICU patients. Third, we focused specifically on extremely preterm neonates, a group for which data on medication safety and efficacy is lacking. By demonstrating the frequent “off-label” use of vasoactive medications in ELGANs, this study provides justification for prospective safety and efficacy research.
There are limitations to this study. First, we did not have access to vital signs, including BP. As a result, we could not evaluate differences in BP treatment thresholds between individual neonates or between centers. We also could not evaluate the severity or duration of hypotension, how quickly BP increased, or if BP was “overcorrected.” Second, we did not have data on the indications for use of vasoactive medications, which may have been for reasons other than systemic hypotension (for example, use of epinephrine for delivery room resuscitation). Third, we did not have data on receipt of intravenous fluid boluses, total fluids per day, or medication doses, so we could not evaluate the impact of these factors on the use of vasoactive medications. Finally, we did not evaluate the association between vasoactive medication exposure and clinical outcomes such as death. There are inherent challenges to studying the relationship between treatment for hypotension, hypotension itself, and negative outcomes, as it is difficult to control for illness severity. In this retrospective study, we did not have data on BP or other important clinical indicators of illness severity such as receipt of cardiopulmonary resuscitation or blood gas values. This challenge highlights the need for prospective research on the management of hypotension and clinical outcomes in this population.
In conclusion, ELGANs are at high risk for hypotension and treatment with vasoactive medications during the first postnatal week. There is controversy regarding the definition and management of hypotension in this population, with limited evidence to support best practices. We found that a substantial proportion of ELGANs cared for at Pediatrix NICUs from 2009-2018 were exposed to vasoactive medications, though overall use of vasoactive medications decreased slightly over the study period. Whether this observation reflects a higher threshold for treatment or another practice change, such as increased use of antenatal or postnatal corticosteroids, delayed cord clamping, or avoidance of mechanical ventilation, is not clear. Early hypotension in ELGANs is a complex and often multifactorial problem that requires an individualized approach. Newer technologies such as near-infrared spectroscopy and functional echocardiography may augment traditional hemodynamic monitoring and help tailor treatment.40, 41 Data from clinical trials is needed to better delineate which ELGANs benefit from treatment of hypotension and which can be cautiously observed, though issues relating to eligibility and enrollment can pose major challenges for investigators.42 Finally, given the frequent “off-label” use of vasoactive medications in preterm neonates, particularly in the first 1-2 postnatal days, prospective efficacy and safety studies are greatly needed.
FUNDING INFORMATION
The authors did not receive funding for this study.
Footnotes
CONFLICT OF INTEREST
The authors declare that they have no conflict of interest.
REFERENCES
- 1.Al-Aweel I, Pursley DM, Rubin LP, Shah B, Weisberger S, Richardson DK. Variations in prevalence of hypotension, hypertension, and vasopressor use in NICUs. J Perinatol 2001, 21(5): 272–278. [DOI] [PubMed] [Google Scholar]
- 2.Batton B, Batton D, Riggs T. Blood pressure during the first 7 days in premature infants born at postmenstrual age 23 to 25 weeks. Am J Perinatol 2007, 24(2): 107–115. [DOI] [PubMed] [Google Scholar]
- 3.Fanaroff AA, Fanaroff JM. Short- and long-term consequences of hypotension in ELBW infants. Semin Perinatol 2006, 30(3): 151–155. [DOI] [PubMed] [Google Scholar]
- 4.Faust K, Hartel C, Preuss M, Rabe H, Roll C, Emeis M, et al. Short-term outcome of very-low-birthweight infants with arterial hypotension in the first 24 h of life. Arch Dis Child Fetal Neonatal Ed 2015, 100(5): F388–392. [DOI] [PubMed] [Google Scholar]
- 5.Batton B, Li L, Newman NS, Das A, Watterberg KL, Yoder BA, et al. Use of antihypotensive therapies in extremely preterm infants. Pediatrics 2013, 131(6): e1865–1873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dempsey EM, Al Hazzani F, Barrington KJ. Permissive hypotension in the extremely low birthweight infant with signs of good perfusion. Arch Dis Child Fetal Neonatal Ed 2009, 94(4): F241–244. [DOI] [PubMed] [Google Scholar]
- 7.Fanaroff JM, Wilson-Costello DE, Newman NS, Montpetite MM, Fanaroff AA. Treated hypotension is associated with neonatal morbidity and hearing loss in extremely low birth weight infants. Pediatrics 2006, 117(4): 1131–1135. [DOI] [PubMed] [Google Scholar]
- 8.Wong J, Shah PS, Yoon EW, Yee W, Lee S, Dow K. Inotrope use among extremely preterm infants in Canadian neonatal intensive care units: variation and outcomes. Am J Perinatol 2015, 32(1): 9–14. [DOI] [PubMed] [Google Scholar]
- 9.Durrmeyer X, Marchand-Martin L, Porcher R, Gascoin G, Roze JC, Storme L, et al. Abstention or intervention for isolated hypotension in the first 3 days of life in extremely preterm infants: association with short-term outcomes in the EPIPAGE 2 cohort study. Arch Dis Child Fetal Neonatal Ed 2017, 102(6): 490–496. [DOI] [PubMed] [Google Scholar]
- 10.Batton B, Li L, Newman NS, Das A, Watterberg KL, Yoder BA, et al. Early blood pressure, antihypotensive therapy and outcomes at 18-22 months' corrected age in extremely preterm infants. Arch Dis Child Fetal Neonatal Ed 2016, 101(3): F201–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Laughon M, Bose C, Allred E, O'Shea TM, Van Marter LJ, Bednarek F, et al. Factors associated with treatment for hypotension in extremely low gestational age newborns during the first postnatal week. Pediatrics 2007, 119(2): 273–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ewer AK, Tyler W, Francis A, Drinkall D, Gardosi JO. Excessive volume expansion and neonatal death in preterm infants born at 27-28 weeks gestation. Paediatr Perinat Epidemiol 2003, 17(2): 180–186. [DOI] [PubMed] [Google Scholar]
- 13.St Peter D, Gandy C, Hoffman SB. Hypotension and Adverse Outcomes in Prematurity: Comparing Definitions. Neonatology 2017, 111(3): 228–233. [DOI] [PubMed] [Google Scholar]
- 14.Verma RP, Dasnadi S, Zhao Y, Chen HH. Complications associated with the current sequential pharmacological management of early postnatal hypotension in extremely premature infants. Proc (Bayl Univ Med Cent) 2019, 32(3): 355–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kuint J, Barak M, Morag I, Maayan-Metzger A. Early treated hypotension and outcome in very low birth weight infants. Neonatology 2009, 95(4): 311–316. [DOI] [PubMed] [Google Scholar]
- 16.Dempsey E, Rabe H. The Use of Cardiotonic Drugs in Neonates. Clin Perinatol 2019, 46(2): 273–290. [DOI] [PubMed] [Google Scholar]
- 17.Noori S, Seri I. Neonatal blood pressure support: the use of inotropes, lusitropes, and other vasopressor agents. Clin Perinatol 2012, 39(1): 221–238. [DOI] [PubMed] [Google Scholar]
- 18.Rios DR, Kaiser JR. Vasopressin versus dopamine for treatment of hypotension in extremely low birth weight infants: a randomized, blinded pilot study. J Pediatr 2015, 166(4): 850–855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Baske K, Saini SS, Dutta S, Sundaram V. Epinephrine versus dopamine in neonatal septic shock: a double-blind randomized controlled trial. Eur J Pediatr 2018, 177(9): 1335–1342. [DOI] [PubMed] [Google Scholar]
- 20.Subhedar NV, Shaw NJ. Dopamine versus dobutamine for hypotensive preterm infants. Cochrane Database Syst Rev 2003(3): CD001242. [DOI] [PubMed] [Google Scholar]
- 21.Valverde E, Pellicer A, Madero R, Elorza D, Quero J, Cabanas F. Dopamine versus epinephrine for cardiovascular support in low birth weight infants: analysis of systemic effects and neonatal clinical outcomes. Pediatrics 2006, 117(6): e1213–1222. [DOI] [PubMed] [Google Scholar]
- 22.Osborn D, Evans N, Kluckow M. Randomized trial of dobutamine versus dopamine in preterm infants with low systemic blood flow. J Pediatr 2002, 140(2): 183–191. [DOI] [PubMed] [Google Scholar]
- 23.Rios DR, Moffett BS, Kaiser JR. Trends in pharmacotherapy for neonatal hypotension. J Pediatr 2014, 165(4): 697–701 e691. [DOI] [PubMed] [Google Scholar]
- 24.Burns ML, Stensvold HJ, Risnes K, Guthe HJ, Astrup H, Nordhov SM, et al. Inotropic Therapy in Newborns, A Population-Based National Registry Study. Pediatr Crit Care Med 2016, 17(10): 948–956. [DOI] [PubMed] [Google Scholar]
- 25.Lasky T, Greenspan J, Ernst FR, Gonzalez L. Dopamine and dobutamine use in preterm or low birth weight neonates in the premier 2008 database. Clin Ther 2011, 33(12): 2082–2088. [DOI] [PubMed] [Google Scholar]
- 26.Spitzer AR, Ellsbury DL, Handler D, Clark RH. The Pediatrix BabySteps Data Warehouse and the Pediatrix QualitySteps improvement project system--tools for "meaningful use" in continuous quality improvement. Clin Perinatol 2010, 37(1): 49–70. [DOI] [PubMed] [Google Scholar]
- 27.Zaidi AK, Harrell LJ, Rost JR, Reller LB. Assessment of similarity among coagulase-negative staphylococci from sequential blood cultures of neonates and children by pulsed-field gel electrophoresis. J Infect Dis 1996, 174(5): 1010–1014. [DOI] [PubMed] [Google Scholar]
- 28.Horan TC, Andrus M, Dudeck MA. CDC/NHSN surveillance definition of health care-associated infection and criteria for specific types of infections in the acute care setting. Am J Infect Control 2008, 36(5): 309–332. [DOI] [PubMed] [Google Scholar]
- 29.Fanaroff JM, Fanaroff AA. Blood pressure disorders in the neonate: hypotension and hypertension. Semin Fetal Neonatal Med 2006, 11(3): 174–181. [DOI] [PubMed] [Google Scholar]
- 30.Nuntnarumit P, Yang W, Bada-Ellzey HS. Blood pressure measurements in the newborn. Clin Perinatol 1999, 26(4): 981–996, x. [PubMed] [Google Scholar]
- 31.Batton B, Li L, Newman NS, Das A, Watterberg KL, Yoder BA, et al. Evolving blood pressure dynamics for extremely preterm infants. J Perinatol 2014, 34(4): 301–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Logan JW, O'Shea TM, Allred EN, Laughon MM, Bose CL, Dammann O, et al. Early postnatal hypotension and developmental delay at 24 months of age among extremely low gestational age newborns. Arch Dis Child Fetal Neonatal Ed 2011, 96(5): F321–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Spinazzola RM, Harper RG, de Soler M, Lesser M. Blood pressure values in 500- to 750-gram birthweight infants in the first week of life. J Perinatol 1991, 11(2): 147–151. [PubMed] [Google Scholar]
- 34.Cordero L, Timan CJ, Waters HH, Sachs LA. Mean arterial pressures during the first 24 hours of life in < or = 600-gram birth weight infants. J Perinatol 2002, 22(5): 348–353. [DOI] [PubMed] [Google Scholar]
- 35.Demarini S, Dollberg S, Hoath SB, Ho M, Donovan EF. Effects of antenatal corticosteroids on blood pressure in very low birth weight infants during the first 24 hours of life. J Perinatol 1999, 19(6 Pt 1): 419–425. [DOI] [PubMed] [Google Scholar]
- 36.Sahni M, Jain S. Hypotension in Neonates. NeoReviews 2016, 17(10): e579–e587. [Google Scholar]
- 37.El-Khuffash A, McNamara PJ. Hemodynamic Assessment and Monitoring of Premature Infants. Clin Perinatol 2017, 44(2): 377–393. [DOI] [PubMed] [Google Scholar]
- 38.Wu TW, Azhibekov T, Seri I. Transitional Hemodynamics in Preterm Neonates: Clinical Relevance. Pediatr Neonatol 2016, 57(1): 7–18. [DOI] [PubMed] [Google Scholar]
- 39.Stranak Z, Semberova J, Barrington K, O'Donnell C, Marlow N, Naulaers G, et al. International survey on diagnosis and management of hypotension in extremely preterm babies. Eur J Pediatr 2014, 173(6): 793–798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pellicer A, Greisen G, Benders M, Claris O, Dempsey E, Fumagalli M, et al. The SafeBoosC phase II randomised clinical trial: a treatment guideline for targeted near-infrared-derived cerebral tissue oxygenation versus standard treatment in extremely preterm infants. Neonatology 2013, 104(3): 171–178. [DOI] [PubMed] [Google Scholar]
- 41.Jain A, Sahni M, El-Khuffash A, Khadawardi E, Sehgal A, McNamara PJ. Use of targeted neonatal echocardiography to prevent postoperative cardiorespiratory instability after patent ductus arteriosus ligation. J Pediatr 2012, 160(4): 584–589 e581. [DOI] [PubMed] [Google Scholar]
- 42.Batton BJ, Li L, Newman NS, Das A, Watterberg KL, Yoder BA, et al. Feasibility study of early blood pressure management in extremely preterm infants. J Pediatr 2012, 161(1): 65–69 e61. [DOI] [PMC free article] [PubMed] [Google Scholar]


