Abstract
Background
In sub‐Saharan Africa, adult outpatients with symptoms of acute infectious illness are not routinely tested for prevalent or acute HIV infection (AHI) when seeking healthcare.
Methods
Adult symptomatic outpatients aged 18–39 years were evaluated by a consensus AHI risk score. Patients with a risk score ≥ 2 and no previous HIV diagnosis were enrolled in a stepped‐wedge trial of opt‐out delivery of point‐of‐care (POC) HIV‐1 nucleic acid testing (NAAT), compared with standard provider‐initiated HIV testing using rapid tests in the observation period. The primary outcome was the number of new diagnoses in each study period. Generalized estimating equations with a log‐binomial link and robust variance estimates were used to account for clustering by health facility. The trial is registered with ClinicalTrials.gov NCT03508908.
Results
Between 2017 and 2020, 13 (0.9%) out of 1374 participants in the observation period and 37 (2.5%) out of 1500 participants in the intervention period were diagnosed with HIV infection. Of the 37 newly diagnosed cases in the intervention period, two (5.4%) had AHI. Participants in the opt‐out intervention had a two‐fold greater odds of being diagnosed with HIV (odds ratio = 2.2, 95% confidence interval: 1.39–3.51) after adjustment for factors imbalanced across study periods.
Conclusions
Among symptomatic adults aged 18–39 years targeted by our POC NAAT intervention, we identified one chronic HIV infection for every 40 patients and one AHI patient for every 750 patients tested. Although AHI yield was low in this population, routinely offered opt‐out testing could diagnose twice as many patients as an approach relying on provider discretion.
Keywords: acute HIV infection, diagnostic tests, HIV infection, partner notification, point of care, serology, viral load
INTRODUCTION
Kenya has the fifth largest HIV epidemic in the world, with an estimated adult population prevalence of 4.9% and 1.3 million adults living with HIV in 2018 [1]. Although 80% of people living with HIV (PLWH) know their status, one in five PLWH remain undiagnosed [1]. HIV testing is the essential first step for the UNAIDS 95‐95‐95 goals to be reached, and strategies to improve HIV case finding should be optimized [2].
Finding undiagnosed PLWH in a declining HIV epidemic is challenging [1, 3]. While conventional facility‐based HIV testing and counselling (HTC) has not achieved high testing coverage in sub‐Saharan Africa [4], facility‐based HIV testing presents several unique opportunities to identify PLWH, offer HIV care and treatment to people newly diagnosed, and ensure that prevention options are extended to partners of PLWH. First, symptomatic people seeking healthcare are concerned about their health and entitled to a diagnosis or the exclusion thereof. Second, providers need to know the HIV status of their patients in order to make appropriate diagnosis, treatment, and management plans. Third, communities in the catchment areas of health facilities are entitled that all is done to mitigate ongoing transmission of a preventable illness, and lastly, healthcare policy‐makers should expect returns on HIV testing policies targeting the population at large.
In sub‐Saharan Africa, provider‐initiated testing and counselling (PITC) is offered to only approximately one in five adult patients seeking healthcare [4]. Challenges impacting uptake of PITC include logistics, data systems and human resources and management – reflecting the weaknesses of health systems in the region [5]. Most patients are willing to be tested [6, 7], but the yield of PITC even in highly endemic areas has recently been estimated at around 1% [3]. Therefore, HIV testing of patients seeking healthcare should be strategic and targeted to increase both the yield and number of newly diagnosed patients.
One weakness, however, of routine rapid tests for HIV diagnosis is that they do not identify patients with acute HIV infection (AHI) [8, 9], which is typically defined as the first weeks after HIV acquisition, during which HIV antibodies are undetectable [10]. Although some AHI patients remain asymptomatic, most experience an acute illness approximately 2 weeks following infection, and the majority of these patients seek healthcare [11, 12, 13, 14, 15]. Common symptoms of AHI include fever, joint and muscle pains, headache and fatigue [16]. Diagnosing AHI is important, as patients with AHI have very high viral loads in the few weeks following acquisition, and their sexual behaviour is unlikely to change until they are diagnosed, linked to HIV care and initiated on antiretroviral therapy (ART) [17, 18]. Acute HIV infection is not easily diagnosed using point‐of‐care (POC) antigen‐antibody assays such as the Determine HIV 1/2 Ab/Ag Combo Rapid Test (Alere, Freehold, NJ, USA), which unfortunately has had inconsistent results and low sensitivity in sub‐Saharan Africa [19, 20], but can be diagnosed using nucleic acid amplification testing (NAAT) or p24‐antigen testing [21].
Previously, we showed that among adults at high risk for HIV‐1 acquisition followed in a research cohort, 69% of those who seroconverted sought healthcare at the research clinic or elsewhere due to AHI symptoms [15]. Although not all patients with AHI develop symptoms compatible with an acute retroviral syndrome [22], AHI symptoms are more common among patients infected with subtype A than subtype C or D, and most HIV infections in Kenya are subtype A [23]. Undiagnosed AHI is associated with substantial risk of onward HIV transmission and a common occurrence overlooked [24]. In two pilot studies conducted by our group in coastal Kenya in 2013 and 2016, we showed that AHI could be detected in around 1% of symptomatic adults who had negative or discordant HIV rapid test results [25, 26]. For the present study, we used a consensus AHI risk score derived from pooled data from Kenya, Malawi and South Africa [16]. With the recent availability of POC HIV‐1 NAAT platforms in sub‐Saharan Africa, real‐time AHI testing before the patient leaves the clinic has become feasible [27], and could be used to supplement opt‐out testing approaches in health facilities.
In the present proof‐of‐concept study, we evaluated the yield of a targeted HIV‐1 testing intervention offered to adults aged 18–39 years who were seeking healthcare at four public and two private health facilities in coastal Kenya, before and after intervention delivery, using a modified stepped‐wedge trial design. The HIV‐1 testing intervention consisted of opt‐out POC HIV‐1 NAAT followed by standard rapid antibody tests to distinguish AHI from chronic HIV among all adult outpatients presenting for healthcare who met our AHI risk score and agreed to enrol [16]. HIV‐1 testing in the observation period consisted of PITC using standard rapid tests without additional support to facility staff for implementation. The primary outcome was new HIV diagnoses in each study period. Secondary outcomes included linkage to HIV care and ART initiation among newly diagnosed index participants, and the yield of HIV partner notification (HPN) using the HIV‐1 testing intervention, when offered to their partners [28]. Our objective with the present analysis was to compare primary and secondary outcomes across study periods (pre‐ and post‐intervention).
METHODS
Study setting
We conducted our study at four public and two private health facilities in a peri‐urban area (population > 100 000) in Kilifi and Mombasa County, coastal Kenya (Figure 1). HIV prevalence estimates in Kilifi and Mombasa counties in 2018 were 2.3% and 5.6%, respectively [1]. This area has been the site of a KEMRI HIV/Sexually Transmitted Infection (STI) Research Clinic since 2005. We identified the participating health facilities (three in Kilifi county; three in Mombasa county) due to their size, location (within 20 km of our KEMRI Research Clinic), patient volume (> 500 patients aged 18–39 years seen over 3 months) and willingness to collaborate with the research team. All clinics served general population patients; the four public facilities included offered limited programming for key populations.
FIGURE 1.
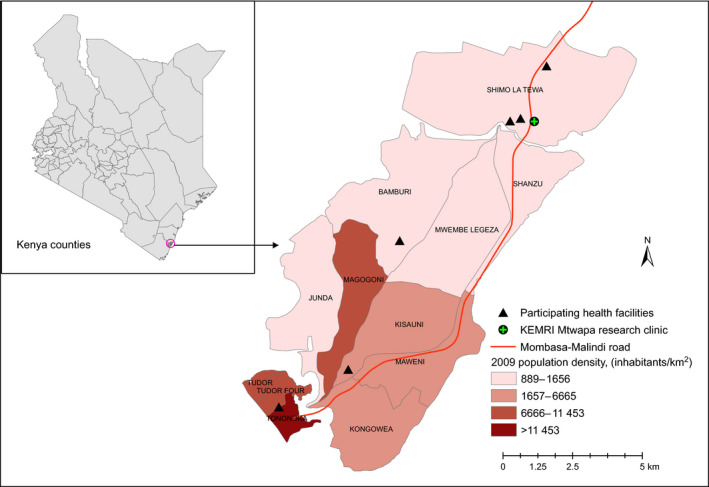
Map of Tambua Mapema Plus study sites [Colour figure can be viewed at wileyonlinelibrary.com]
Study design
We used a modified stepped‐wedge design to evaluate the yield of the HIV‐1 NAAT intervention, before (1375 patients) and after (1500 patients) intervention delivery [28]. We chose a modified stepped‐wedge trial design as the intervention would do more good than harm and it was not practical to deliver the intervention simultaneously to all participants, due to resource constraints. Each phase lasted 6 months per site, with the exception of the first site having only 3 months of observation [28]. The primary outcome was a new HIV diagnosis, combining chronic HIV and AHI diagnoses.
Study population
Eligibility criteria for participation included: age 18–39 years; not previously diagnosed with HIV infection; and a score ≥ 2 on our published AHI risk score algorithm [16], with scoring as follows: age 18–29 years (1), fever (1), fatigue (1), body pains (1), diarrhoea (1), sore throat (1) and genital ulcer disease (GUD) (3). Patients who agreed to screening and were eligible were offered enrolment after consenting by the research team.
Observation period
In the observation period, HIV testing was only done if ordered by the primary care clinician and was carried out using HIV rapid testing per Kenyan Ministry of Health guidelines [29]. Up to 20 participants were targeted for enrolment each week (maximum of four per day). Following the clinical consultation and prior to HIV testing (if ordered), research procedures in the observation period consisted of a computer‐assisted self‐interview (CASI)/computer‐assisted personal interview (CAPI) to assess sociodemographic characteristics, HIV testing history and sexual risk behaviour. Participants found to be HIV‐negative and those not tested ended their participation at the baseline visit. Those newly diagnosed with HIV had a follow‐up home visit at 6 weeks, which included a second CASI/CAPI assessing linkage to HIV care and treatment, as well as partner outcomes. Those who had not yet notified partners at this time point were offered standard HPN services [30].
Intervention period
Screening and enrolment procedures were similar as in the observation period. However, all enrolled participants were routinely offered the POC HIV‐1 NAAT, followed by two rapid HIV tests when the X‐pert was positive (details below). Test results were provided to participants in real time, with post‐test counselling by research staff. Newly diagnosed intervention period study participants were offered enrolment in an HIV care cohort at the nearby KEMRI Research Clinic and encouraged to start government‐provided ART upon linkage (the same day as diagnosis or as soon as possible). These index patients were asked about sexual partners in the past year (chronic HIV infection) or the past 3 months (AHI) and offered enhanced HPN, in which the HIV‐1 NAAT intervention was used to test all partners successfully reached. Index participants judged to be at moderate or high risk of intimate partner violence were excluded from enhanced HPN. Partners who tested positive were offered enrolment in the KEMRI ART cohort, while those who tested negative and were in an ongoing relationship with the index participant were offered enrolment into a pre‐exposure prophylaxis (PrEP) cohort. Follow‐up in the ART and PrEP cohorts was for 12 months.
Laboratory testing
A 4 mL blood sample was obtained and tested using the Xpert® HIV Qual assay (GeneXpert® HIV‐1 Qual Cepheid, Sunnyvale, CA, USA) on‐site at the health facility where the participant was recruited and enrolled. This assay has been found to be easy to use and feasible in a community‐based facility with limited or no laboratory infrastructure [24]. For participants in whom HIV‐1 nucleic acid was detected, a laboratory technician conducted rapid antibody testing (currently Determine®, Abbott Laboratories; and First Response® (Mumbai, India), Premier Medical Corporation, Princeton, NJ, USA) in accordance with Kenyan HIV testing guidelines [29], to distinguish acute (nucleic acid‐positive, antibody‐negative or indeterminate) from chronic (antibody‐positive) HIV infection. This algorithm obviated the need to conduct both rapid antibody tests and Xpert HIV‐1 Qual test on the vast majority of samples, reducing overall test costs.
Statistical analysis
We compared the number and reasons of individuals who accepted vs. refused screening in the observation vs. intervention phase, the number and reasons for screening out participants in each phase, and, among those eligible, the number and reasons for refusing study participation in each phase, using χ2 or Fisher’s exact tests for categorical variables as appropriate. We compared the proportion of patients with the following outcomes in the observation and intervention periods: (1) tested for HIV infection; (2) newly diagnosed with chronic HIV infection (i.e. HIV seropositive); and (3) newly diagnosed with AHI.
We conducted analyses on the individual‐level data, using generalized estimating equation (GEE) models to account for clustering by health facility, with a log link, binomial distribution, and robust variance estimates [29]. Using GEE, we first compared the primary outcome (i.e. new HIV diagnoses) across arms for an unadjusted estimate. We next compared baseline characteristics between individuals in the observation and intervention groups to identify imbalances across study periods. Where imbalances were identified (i.e. differences significant at P < 0.20), we controlled for these potential confounders in secondary analyses using GEE models of the primary outcome as described earlier. We compared the age of newly diagnosed cases by Wilcoxson rank‐sum test in the intervention and observation periods.
Ethical considerations
The study received ethical approval by the KEMRI Scientific and Ethical Review Unit (KEMRI/SERU/CGMRC‐C/051/3280), the Human Subjects Division at the University of Washington (STUDY00001808), and the Oxford Tropical Research Ethics Committee (OxTREC) at the University of Oxford (reference: 46‐16). The protocol was approved by the Division of AIDS (DAIDS), National Institute of Allergy and Infectious Diseases (NIAID), andn the National Institutes of Health (NIH) (DAIDS‐ES 38181).
RESULTS
A total of 19 464 patients aged 18–39 years sought healthcare at one of the six study health facilities during the period December 2017 to March 2020: 9062 patients in the observation period and 10 402 in the intervention period (Figure 2). Slightly more patients refused study screening in the observation period (0.4%) compared with the intervention period (0.1%, P = 0.008). Most patients (59.9%) who were ineligible had a risk score < 2, while being outside the age range, prior HIV diagnosis, and previous participation in the study were less common reasons for ineligibility. More eligible patients refused enrolment in the intervention period (318/1818 or 17.5%), than in the observation period (118/1495 or 7.9%, P < 0.001), with notable increases in refusal due to HIV testing (29.3% of eligible intervention period patients vs. no eligible observation period patients).
FIGURE 2.
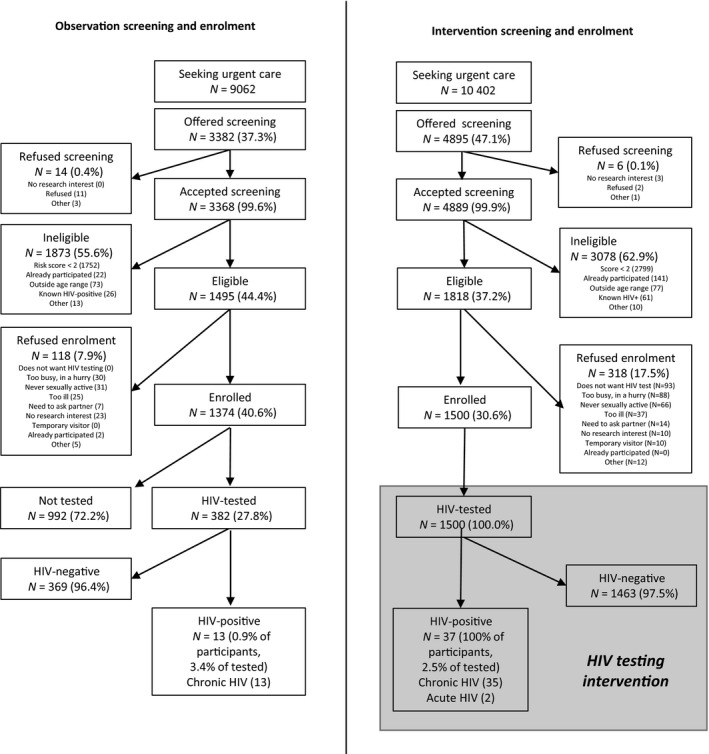
Consort diagram: Tambua Mapema Plus Trial profile. Five (4.2%) and twelve (3.7%) participants in the observation and intervention phases, respectively, provided more than one reason when they refused research participation
In total, 3374 participants enrolled: 1374 in the observation period and 1500 in the intervention period (Table 1). Overall, 61.7% of participants were female, 40.6% were employed, and the median (interquartile range, IQR) age was 25 (23–30) years. The frequency of reported symptoms was as follows: fever (48.1%), diarrhoea (15.2%), fatigue (72.1%), body pains (66.1%), sore throat (28.7%) and genital ulcers (7.1%). Among those who were sexually active in the last 6 weeks (69.9%), 27.5% of participants reported unprotected sex with a partner of unknown HIV status and 7.8% reported multiple partners. Among men, 0.2% reported same‐sex behaviour.
TABLE 1.
Characteristics of enrolled participants, Tambua Mapema Plus Trial, coastal Kenya, 2017–2020
|
Total (N = 2874) |
Observation phase (N = 1374a) | Intervention phase, (N = 1500) | P‐value | |
|---|---|---|---|---|
| Sex | ||||
| Male | 1101 (38.3) | 488 (35.5) | 613 (40.9) | 0.088 |
| Female | 1773 (61.7) | 886 (64.5) | 887 (59.1) | |
| Age (median, IQR) | 25 (23, 30) | 25 (23, 29) | 26 (23, 30) | 0.143 |
| Education levela | ||||
| Primary or below | 1102 (38.4) | 513 (37.4) | 589 (39.3) | 0.611 |
| Secondary | 1068 (37.2) | 542 (39.6) | 526 (35.1) | |
| Tertiary | 699 (24.4) | 315 (23.0) | 384 (25.6) | |
| Marital statusa | ||||
| Single | 1302 (45.4) | 649 (47.4) | 653 (43.6) | 0.402 |
| Married | 1394 (48.6) | 635 (46.3) | 759 (50.6) | |
| Separated/divorced/widowed | 173 (6.0) | 86 (6.3) | 87 (5.8) | |
| Religiona | ||||
| Christian | 2216 (77.6) | 1053 (76.9) | 1163 (77.6) | 0.484 |
| Muslim | 608 (21.2) | 295 (21.5) | 313 (20.9) | |
| None | 45 (1.6) | 22 (1.6) | 23 (1.5) | |
| Source of incomea | ||||
| Employed | 1164 (40.6) | 529 (38.6) | 635 (42.4) | 0.046 |
| Self‐employed (casual) | 768 (26.8) | 364 (26.6) | 404 (26.9) | |
| Unemployed | 937 (32.7) | 477 (34.8) | 460 (30.7) | |
| Symptoms reported | ||||
| Fever | 1383 (48.1) | 663 (48.3) | 720 (48.0) | 0.955 |
| Diarrhoea | 437 (15.2) | 195 (14.2) | 242 (16.1) | 0.261 |
| Fatigue | 2072 (72.1) | 904 (65.8) | 1168 (77.9) | < 0.001 |
| Body aches | 1899 (66.1) | 886 (64.5) | 1013 (67.5) | 0.185 |
| Sore throat | 824 (28.7) | 376 (27.4) | 448 (29.9) | 0.401 |
| Genital ulcer | 204 (7.1) | 91 (6.6) | 113 (7.5) | 0.653 |
| Sexual risk behaviour | ||||
| Risk groupa | ||||
| Heterosexuals | 1948 (67.9) | 897 (65.5) | 1051 (70.1) | 0.842 |
| Key populations | 61 (2.1) | 19 (1.4) | 42 (2.8) | |
| Populations not sexually active in past 6 weeks | 860 (30.0) | 454 (33.1) | 406 (27.1) | |
| Unprotected sex with partner of unknown HIV statusb | 788 (27.5) | 277 (20.2) | 511 (34.1) | < 0.001 |
| Unprotected sex with partner of known HIV‐positive statusb | 26 (0.9) | 5 (0.4) | 21 (1.4) | 0.001 |
| Multiple sex partnersb | 223 (7.8) | 76 (5.5) | 147 (9.8) | < 0.001 |
| Transactional sexb | 52 (1.8) | 15 (1.1) | 37 (2.5) | < 0.001 |
| Male same‐sex behaviourb | 7 (0.2) | 3 (0.2) | 4 (0.3) | 0.833 |
| People who inject drugsb | 3 (0.1) | 1 (0.1) | 2 (0.1) | 0.543 |
| HIV testing history | ||||
| Never tested for HIV | 300 (10.4) | 147 (10.7) | 153 (10.2) | 0.820 |
| Tested for HIV in last year | 1062 (37.0) | 499 (36.3) | 563 (37.5) | 0.649 |
| Tested for HIV in last 3 months | 404 (14.1) | 234 (17.0) | 170 (11.3) | 0.018 |
Abbreviation: IQR, interquartile range.
One participant who enrolled twice excluded after closure of the observation phase.
Missing in five participants (four observation, one intervention).
In the observation phase, 352 (25.6%) participants were tested for HIV based on orders from a primary care clinician, of whom 13 (3.7%, or 0.9% of all observation study participants) were newly diagnosed with chronic HIV infection. In the intervention phase, all participants were tested for HIV, of whom 37 (2.5%) participants were newly diagnosed with HIV, including 35 (2.3%) with chronic HIV infection and two (0.1%) with AHI (Figure 2). The yield of observational testing (3.7%) was not statistically significantly different from the yield of intervention testing (2.5%, P = 0.2). The unadjusted odds of an HIV diagnosis in the intervention period were 2.71 [95% confidence interval (CI): 1.66–4.33, P < 0.001).
Characteristics that differed in the observation and intervention periods at P < 0.10 included sex, source of income, reported fatigue, sexual risk behaviour and history of HIV testing. Compared with the observational period, fewer participants in the HIV‐1 testing intervention period were female (59.1% vs. 64.5%); more were employed (42.4% vs. 38.6%) and more reported fatigue (77.9% vs. 65.8%). In addition, more intervention participants reported sex with a partner of unknown status (34.1% vs. 20.2%), sex with a known‐positive partner (1.4% vs. 0.4%), multiple partners (9.8% vs. 5.5%) and transactional sex (2.5% vs. 1.1%).
After adjustment for factors that were imbalanced across study periods, the odds of an HIV diagnosis in the intervention period were 2.21 (95% CI: 1.39–3.51, P = 0.001). Of note, the female to male ratios of newly diagnosed index participants in the observation and intervention periods were similar (6/7 vs. 20/17, P = 0.98). The median ages of newly diagnosed women in the observation vs. the intervention period was statistically significantly different (33.5 vs. 26.2, P = 0.03); the median ages for newly diagnosed men across the two periods were not different (32.1 vs. 33.6, P = 0.39).
At the visit 6 weeks after diagnosis, 33 (89.2%) of 37 intervention index participants vs. nine (69.2%, P = 0.05, one‐sided) out of 13 observation index participants were successfully linked to HIV care and had initiated ART. Of the 30 intervention index participants who enrolled for HIV care at the KEMRI HIV/STI Research Clinic (81.1% of the total), 16 (53.3%) agreed to enhanced HPN.
Ten partners (27.0%) of 37 index participants were tested in the intervention period, compared with three partners (23.1%, P = 0.78) of 13 index participants in the observation period. Of these, two intervention period partners (2/10, or 20.0%) and two observation period partners (2/3, or 66.7%, P = 0.12) were newly diagnosed with chronic HIV infection. No intervention period partners were diagnosed with AHI. Details of the newly diagnosed cases in the intervention period, their viral load at diagnosis, and their linkage and partner notification outcomes are presented in Table 2; detailed data were not available for observation period cases, as they were referred to the clinic at which they were diagnosed. Four regular male partners of index participants started PrEP in the intervention period research cohort. No partner started PrEP in the observational period, when clinics delivered standard care.
TABLE 2.
Characteristics of intervention period index participants and partner testing outcomes, Tambua Mapema Plus Trial, coastal Kenya, 2017–2020
| No. | Sex | Age (years) | Chronic or acute HIV infection | Linkage outcome | Log10viral load | Regular partners reported | Casual partners reported | Consented to HPN | Positive tests/no. tested | Partner HIV status and linkage outcome |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Female | 19 | Acute | Enrolled | NDa | 1 | 0 | Yes | 0/1 | Spouse confirmed negative and initiated on PrEP |
| 2 | Female | 23 | Chronic | Enrolled | 4.74 | 2 | 1 | Yes | 0/1 | Spouse confirmed negative and initiated on PrEP; other regular partner status unknown; casual partner status unknown |
| 3 | Female | 23 | Chronic | Otherb | N/A | 1 | 0 | N/A | Status unknown | |
| 4 | Female | 24 | Chronic | Enrolled | 2.96 | 1 | 0 | Yes | 0/1 | Spouse confirmed negative and initiated on PrEP |
| 5 | Female | 24 | Chronic | Enrolled | NDc | 1 | 0 | No | Status unknown | |
| 6 | Female | 25 | Chronic | Not enrolled | Not done | Not assessed | Not assessed | N/A | N/A | |
| 7 | Female | 26 | Chronic | Enrolled | 5.13 | 1 | 0 | Yes | Status unknown | |
| 8 | Female | 26 | Chronic | Enrolled | 5.01 | 2 | 0 | Yes | 0/1 | Regular partner confirmed negative initiated on PrEP; spouse status unknown |
| 9 | Female | 26 | Chronic | Enrolled | 6.06 | 1 | 0 | Yes | Status unknown | |
| 10 | Female | 26 | Acute | Enrolled | 6.91 | 1 | 0 | No | Regular partner tested positive first, linked at testing facility. Index case referred to study team with AHI symptoms | |
| 11 | Female | 26 | Chronic | Enrolled | 6.17 | 1 | 5 | Yes | 0/2 | Spouse status unknown; two casual partners confirmed negative (not in an ongoing relationship); three other casual partners status unknown |
| 12 | Female | 27 | Chronic | Enrolled | 4.22 | 1 | 0 | Yes | 0/1 | Regular partner confirmed negative, not in an ongoing relationship |
| 13 | Female | 27 | Chronic | Enrolled | 4.81 | 1 | 0 | No | Status unknown | |
| 14 | Female | 28 | Chronic | Enrolled | 6.42 | 1 | 0 | No | Spouse reported to be known positive and on treatment | |
| 15 | Female | 28 | Chronic | Enrolled | 4.69 | 0 | 0 | No | Regular partner deceased | |
| 16 | Female | 31 | Chronic | Enrolled | 4.73 | 1 | 1 | Yes | Regular partner and casual partner status unknown | |
| 17 | Female | 31 | Chronic | Enrolled | 3.60 | 1 | 5 | Passive referral | 0/1 | Spouse confirmed negative initiated on PEPd; casual partners' status unknown |
| 18 | Female | 32 | Chronic | Enrolled | 4.23 | 1 | 0 | Passive referral | 1/1 | Regular partner newly diagnosed with chronic HIV |
| 19 | Female | 34 | Chronic | Enrolled | 4.71 | 1 | 0 | No | Status unknown | |
| 20 | Female | 39 | Chronic | Enrolled | 5.05 | 1 | 0 | Yes | Spouse reported to have tested elsewhere (self‐reported negative) | |
| 21 | Male | 20 | Chronic | Enrolled | 4.85 | 1 | 0 | No | Status unknown | |
| 22 | Male | 24 | Chronic | Enrolled | 4.76 | 1 | 0 | Yes | Spouse reported to be known positive and on treatment | |
| 23 | Male | 24 | Chronic | Enrolled | 4.50 | 2 | 2 | Yes | Spouse tested elsewhere (self‐reported negative); other regular partner status unknown; casual partners' status unknown | |
| 24 | Male | 25 | Chronic | LTFU | 6.16 | 1 | 0 | No | Status unknown | |
| 25 | Male | 29 | Chronic | Enrolled | 3.72 | 1 | 8 | Yes | Regular partner status unknown; casual partners' status unknown | |
| 26 | Male | 30 | Chronic | Enrolled | 4.90 | 1 | 0 | No | Regular partner reported to be known positive and on treatment | |
| 27 | Male | 31 | Chronic | Not enrolled | Not done | 2 | 0 | N/A | Spouse reported to be known positive and on treatment; other regular partner status unknown | |
| 28 | Male | 31 | Chronic | Enrolled | 6.58 | 1 | 1 | No | Spouse and casual partner status unknown | |
| 29 | Male | 34 | Chronic | Not enrolled | Not done | Not assessed | Not assessed | N/A | N/A | |
| 30 | Male | 36 | Chronic | Enrolled | 3.20 | 2 | 2 | Yes | Spouse tested elsewhere (self‐reported negative); other regular partners' status unknown; casual partners' status unknown | |
| 31 | Male | 36 | Chronic | Enrolled | 5.73 | 0 | 0 | No | N/A | |
| 32 | Male | 36 | Chronic | Enrolled | 4.21 | 2 | 0 | Yes | Spouse tested elsewhere (self‐reported negative); other regular partner known positive and on treatment | |
| 33 | Male | 36 | Chronic | Enrolled | 4.01 | 1 | 0 | No | N/A | |
| 34 | Male | 38 | Chronic | Othere | N/A | 2 | 0 | Passive referral | One spouse reported to be a known positive. Second spouse newly diagnosed with chronic HIV and linked at facility of index enrolment | |
| 35 | Male | 39 | Chronic | Other e , f | N/A | Not assessed | Not assessed | N/A | N/A | |
| 36 | Male | 39 | Chronic | Enrolled | 5.47 | 0 | 1 | No | Casual partner status unknown | |
| 37 | Male | 39 | Chronic | Enrolled | 4.14 | 2 | 0 | Yes | 1/1 | Regular partner newly diagnosed with chronic HIV and initiated on ART; spouse status unknown |
Abbreviations: HPN, HIV partner notification; LTFU, lost to follow‐up; ND, not detected; PrEP, pre‐exposure prophylaxis.
Elite controller.
Relocated outside of study area.
Participant denied prior HIV testing or use of ART (CD4 count at diagnosis was 168 cells/mL).
Participant initiated on post‐exposure prophylaxis (PEP) with HIV exposure < 72 h; no PrEP continuation because of COVID‐19 lockdown.
Linked to care at testing facility.
Participant linked to care at testing facility but died before week 6 visit.
DISCUSSION
This study evaluated a targeted HIV‐1 NAAT intervention to detect acute and chronic HIV infection that was systematically offered to adult outpatients aged 18–39 years seeking healthcare for symptoms compatible with AHI. Implementation of this intervention resulted in a 2.2‐fold higher odds of new HIV diagnosis among participants in the intervention period compared with the observation period, in which standard PITC was conducted, with testing ordered by the primary care clinician. In addition, intensive efforts to link patients to HIV care and expedite ART initiation, test partners and link partners to treatment or prevention as indicated were successful and may have improved clinical outcomes by decreasing time to viral suppression among participants living with HIV and protecting their HIV‐negative partners in ongoing relationships via PrEP initiation.
Our study aimed to increase the number of new HIV diagnoses by consistently implementing a targeted opt‐out HIV testing approach using the GeneXpert® HIV‐1 Qual assay as a POC test. All patients willing to enrol in the intervention period were tested by a team whose procedures were incorporated into the patient flow. This intervention took the burden off the provider to recommend testing and ensured that all participants who were willing to be tested would leave the facility knowing their status. Provider‐initiated testing and counselling are infrequently offered in African health facilities for various logistical reasons, including high patient load, time constraints, staff shortages and fluctuations in test kit supplies [31]. While our intervention required additional resources, our study provides proof‐of‐concept that most Kenyan patients are willing to be tested and can be linked to HIV care and partner notification within facilities, provided a system is set up to ensure HIV testing. Offering oral HIV self‐testing to patients presenting with symptoms of acute infectious illness would be one approach to increase feasibility; this strategy has proven effective in settings similar to ours [6, 32]. While oral HIV self‐testing would not identify AHI, if applied in our study, it would have identified one chronic HIV infection for every 40 participants tested in the intervention period.
In the present study, we found only two AHI cases, suggesting that AHI is uncommon in the population studied. In an earlier study conducted in a different town in coastal Kenya in 2016, targeted opt‐out HIV‐1 NAAT supported by rapid antibody testing led to detection of 24 (3.4%) chronic HIV‐1 infections and six (0.9%) acute HIV infections among 706 care‐seeking participants aged 18–35 years who were identified using the same AHI risk score algorithm as in the present study, for a 25% increase in new HIV diagnoses [26]. The 0.9% AHI prevalence in our pilot study was close to the 1% AHI prevalence among sexually transmitted diseases patients reported by Rutstein et al. [33] in Malawi, using a risk score algorithm that combined sexual risk behaviour (i.e. more than one partner in the previous 2 months), symptoms including GUD, and discordant rapid test results [34]. However, in the present study, AHI prevalence was much lower, at only 0.1%, and HIV‐1 NAAT increased the number of new HIV diagnoses by only 6% (from 35 to 37 cases). It is of interest that our engagement with providers in the community led to referral of a symptomatic woman whose male partner had just been diagnosed with chronic HIV for AHI screening, demonstrating that knowledge of AHI and available HIV‐1 NAAT could be helpful in the right clinical situation. As the availability of Xpert machines increases for other clinical uses (e.g. HIV viral load monitoring, TB diagnosis), use of the Xpert HIV‐1 Qual or Quant assays for the purpose of AHI diagnosis may become more common [35].
Although the yield of PITC among those tested in the observation phase (3.7%) was not significantly different from the yield of HIV testing (2.5%) among all participants in the intervention phase, it suggests that healthcare providers target patients who are more likely to have HIV. In an analysis of who was offered PITC in the observation period, healthcare providers more often offered PITC to older participants, men, those who had tested > 1 year ago or never tested, and participants with certain symptoms (i.e. sore throat or ulcer disease) [7]. Because providers tested only about one in four patients seeking healthcare in the observation period, they probably missed a substantial number of cases, especially among younger women, who made up a greater proportion of the cases diagnosed in the intervention period.
While screening acceptability was similar among both arms, more eligible patients (17.5% vs. 7.9%, P < 0.001) refused enrolment in the intervention period, and 29.2% of intervention period refusals were due to the HIV testing offered. Refusals in the observation period more often occurred because patients were not interested in research participation or felt too ill. Of interest, the intervention period population included more participants who reported risky sexual behaviour (e.g. unprotected sex with a partner of unknown status, or multiple sex partners) than participants in the observation period, suggesting that patients with a higher risk perception may have been more likely to enrol when testing was presented as a standard component of the study.
In this trial of an HIV‐1 NAAT intervention, we identified one AHI case for 750 participants meeting our age and symptom criteria algorithm. Planned modelling and cost‐effectiveness work will help to elucidate the potential impact of opt‐out AHI detection as done in this study, as compared with opt‐out HIV testing using standard rapid tests, on the Kenyan HIV epidemic and the situations in which such testing would be cost‐effective. We note that our testing intervention and intensive linkage to HIV care with HPN were conducted by a team of researchers fully committed to this work, representing a substantial investment of personnel and other resources. In reality, HIV is only one of the many problems that patients may present with, patient numbers at many public and private health facilities are large, and healthcare providers may prefer testing options that take less time or are conducted by patients themselves using approaches such as HIV oral self‐testing [32].
Our study had several limitations. First, our offer of HIV testing to all intervention participants as part of study procedures led to differences in the study population enrolled in each period. Second, we did not obtain a blood sample from all observation period participants, and were therefore unable to assess the number of acute and chronic HIV infections missed by standard care. Third, we did not collect data on the reasons providers offered PITC to specific patients. Fourth, our study sample had only a small proportion (< 3%) of individuals from key populations at higher risk for HIV infection, such as men who have sex with men, male and female sex workers, and people who inject drugs. Fifth, our study will have missed patients with no or few symptoms who actually had AHI, as they would not have met our eligibility criteria. Lastly, we did not exclude participants who were not sexually active in the 6 weeks prior to study screening (one‐third of the study population), who would therefore not have been at risk for AHI.
Despite these limitations, our study is the first study in Africa to have tested the effect of an opt‐out HIV‐1 NAAT intervention to diagnose both acute and chronic HIV infections among care‐seeking outpatients. We have shown that while AHI was very uncommon, this intervention was very acceptable to patients and increased the odds of a new HIV diagnosis by over two‐fold. Linkage to immediate treatment and HPN, with PrEP provision to uninfected partners was also acceptable and efficient when done by the same team. Planned modelling and cost‐effectiveness evaluations will help in evaluating the potential impact of such an approach. Regardless, health facilities are a promising and important site for identification patients with undiagnosed HIV infection.
ACKNOWLEDGEMENTS
We thank staff at the six health facilities in Kilifi and Mombasa counties for their support, study participants for their commitment to the study, and staff at the KEMRI‐Wellcome Trust Programme facilitating the conduct of the study. The KEMRI HIV/Sexually Transmitted Infection (STI) Research Clinic in Mtwapa is financially supported by the International AIDS vaccine Initiative (IAVI) which receives generous support of the American people through the United States Agency for International Development (USAID).
The views expressed in this publication are those of the authors and not necessarily those of AAS, NEPAD Agency, Wellcome Trust, or the UK government. This manuscript was submitted for publication with the permission from the Director of KEMRI.
Sanders EJ, Agutu C, van der Elst E, et al. Effect of an opt‐out point‐of‐care HIV‐1 nucleic acid testing intervention to detect acute and prevalent HIV infection in symptomatic adult outpatients and reduce HIV transmission in Kenya: a randomized controlled trial. HIV Med.2022;23:16–28. 10.1111/hiv.13157
Funding information
Funding for the trial was provided by the National Institute of Allergy and Infectious Diseases (NIAID), grant R01 AI124968. SMG was also supported by the Robert W. Anderson Endowed Chair in Medicine. We acknowledge Usha Sharma of the NIAID Division of AIDS for the scientific support for the Tambua Mapema Plus study. This work was also supported through the Sub‐Saharan African Network for TB/HIV Research Excellence, a Developing Excellence in Leadership, Training and Science (DELTAS) Africa Initiative (grant no. DEL‐15‐006). The DELTAS Africa Initiative is an independent funding scheme of the African Academy of Sciences (AAS)'s Alliance for Accelerating Excellence in Science in Africa and supported by the New Partnership for Africa's Development (NEPAD) Planning and Coordinating Agency with funding from the Wellcome Trust (grant no. 107752/Z/15/Z) and the UK government. Truvada was supplied by Gilead Sciences Inc.
[The copyright line for this article was changed on 14 July 2022 after original online publication.]
AUTHOR CONTRIBUTIONS
SMG and EJS designed the trial, wrote the protocol, provided study oversight, conducted data analysis, and prepared the draft manuscript. CA led the trial implementation. All authors reviewed and contributed to the final manuscript.
REFERENCES
- 1. NASCOP Preliminary KENPHIA 2018 Report. 2020; NASCOP. Accessed August 14, 2021. http://www.nascop.or.ke/KENPHIA
- 2. UNAIDS . Fast‐Track: accelerating action to end the AIDS epidemic by 2030. JC 2743. 2015; Geneva, Switzerland.
- 3. De Cock KM, Barker JL, Baggaley R, El Sadr WM. Where are the positives? HIV testing in sub‐Saharan Africa in the era of test and treat. AIDS. 2019;33(2):349‐352. [DOI] [PubMed] [Google Scholar]
- 4. Sharma M, Ying R, Tarr G, Barnabas R. Systematic review and meta‐analysis of community and facility‐based HIV testing to address linkage to care gaps in sub‐Saharan Africa. Nature. 2015;528(7580):S77‐S85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Roura M, Watson‐Jones D, Kahawita TM, Ferguson L, Ross DA. Provider‐initiated testing and counselling programmes in sub‐Saharan Africa: a systematic review of their operational implementation. AIDS. 2013;27(4):617‐626. [DOI] [PubMed] [Google Scholar]
- 6. Johnson C, Neuman M, MacPherson P, et al. Use and awareness of and willingness to self‐test for HIV: an analysis of cross‐sectional population‐based surveys in Malawi and Zimbabwe. BMC Public Health. 2020;20(1):779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Agutu CA, Oduor TH & Kombo B High patient acceptability but low coverage of provider‐initiated HIV testing among adult outpatients with symptoms of acute infectious illness in coastal Kenya. PlOS One 2021: e0246444. 10.1371/journal.pone.0246444 [DOI] [PMC free article] [PubMed]
- 8. Fiscus SA, Pilcher CD, Miller WC, et al. Rapid, real‐time detection of acute HIV infection in patients in Africa. J Infect Dis. 2007;195(3):416‐424. [DOI] [PubMed] [Google Scholar]
- 9. Elliott T, Sanders EJ, Doherty M, et al. Challenges of HIV diagnosis and management in the context of pre‐exposure prophylaxis (PrEP), post‐exposure prophylaxis (PEP), test and start and acute HIV infection: a scoping review. J Int AIDS Soc. 2019;22(12):e25419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Rutstein SE, Ananworanich J, Fidler S, et al. Clinical and public health implications of acute and early HIV detection and treatment: a scoping review. J Int AIDS Soc. 2017;20(1):21579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Tindall B, Barker S, Donovan B, et al. Characterization of the acute clinical illness associated with human immunodeficiency virus infection. Arch Intern Med. 1988;148(4):945‐949. [PubMed] [Google Scholar]
- 12. Schacker T, Collier AC, Hughes J, Shea T, Corey L. Clinical and epidemiologic features of primary HIV infection. Ann Intern Med. 1996;125(4):257‐264. [DOI] [PubMed] [Google Scholar]
- 13. Lindback S, Thorstensson R, Karlsson AC, et al. Diagnosis of primary HIV‐1 infection and duration of follow‐up after HIV exposure. Karolinska Institute Primary HIV Infection Study Group. AIDS. 2000;14 (15):2333‐2339. [DOI] [PubMed] [Google Scholar]
- 14. Cooper DA, Gold J, Maclean P, et al. Acute AIDS retrovirus infection. Definition of a clinical illness associated with seroconversion. Lancet. 1985;1(8428):537‐540. [DOI] [PubMed] [Google Scholar]
- 15. Sanders EJ, Wahome E, Mwangome M, et al. Most adults seek urgent healthcare when acquiring HIV‐1 and are frequently treated for malaria in coastal Kenya. AIDS. 2011;25(9):1219‐1224. [DOI] [PubMed] [Google Scholar]
- 16. Sanders EJ, Wahome E, Powers KA, et al. Targeted screening of at‐risk adults for acute HIV‐1 infection in sub‐Saharan Africa. AIDS. 2015;29(Suppl 3):S221‐S230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Cohen MS, Shaw GM, McMichael AJ, Haynes BF. Acute HIV‐1 Infection. N Engl J Med. 2011;364(20):1943‐1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Brenner BG, Roger M, Routy JP, et al. High rates of forward transmission events after acute/early HIV‐1 infection. J Infect Dis. 2007;195(7):951‐959. [DOI] [PubMed] [Google Scholar]
- 19. Adetunji AA, Adewumi MO, Michael OS, Fayemiwo SA, Ogunniyi A, Taiwo BO. Rapid HIV antigen‐antibody assays and detection of acute HIV infection in Sub‐Saharan Africa. Am J Trop Med Hyg. 2019;101(2):285‐286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Eshleman SH, Piwowar‐Manning E, Sivay MV, et al. Performance of the BioPlex 2200 HIV Ag‐Ab assay for identifying acute HIV infection. J Clin Virol. 2018;99–100:67‐70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Peters PJ, Westheimer E, Cohen S, et al. Screening yield of HIV antigen/antibody combination and pooled HIV RNA testing for acute HIV infection in a high‐prevalence population. JAMA. 2016;315(7):682‐690. [DOI] [PubMed] [Google Scholar]
- 22. Robb ML, Eller LA, Kibuuka H, et al. Prospective study of acute HIV‐1 infection in adults in East Africa and Thailand. N Engl J Med. 2016;374(22):2120‐2130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sanders EJ, Price MA, Karita E, et al. Differences in acute retroviral syndrome by HIV‐1 subtype in a multicentre cohort study in Africa. AIDS. 2017;31(18):2541‐2546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Powers KA, Cohen MS. Acute HIV‐1 infection in sub‐Saharan Africa: a common occurrence overlooked. AIDS. 2014;28(9):1365‐1367. [DOI] [PubMed] [Google Scholar]
- 25. Sanders EJ, Mugo P, Prins HA, et al. Acute HIV‐1 infection is as common as malaria in young febrile adults seeking care in coastal Kenya. AIDS. 2014;28(9):1357‐1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Sanders EJ, Chirro O, Oduor C, et al. Point‐of‐care HIV RNA testing and immediate antiretroviral therapy initiation in young adults seeking out‐patient care in Kenya. AIDS. 2019;33(5):923‐926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Agutu CA, Ngetsa CJ, Price MA, et al. Systematic review of the performance and clinical utility of point of care HIV‐1 RNA testing for diagnosis and care. PLoS One. 2019;14(6):e0218369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Graham SM, Agutu C, van der Elst E, et al. A novel HIV‐1 RNA testing intervention to detect acute and prevalent HIV infection in young adults and reduce HIV transmission in Kenya: protocol for a randomized controlled trial. JMIR Res Protoc. 2020;9(8):e16198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. MoH . Guidelines for HIV testing services in Kenya. Nairobi: Minstry of Health, Government of Kenya; 2015. [Google Scholar]
- 30. WHO Guidelines on HIV self‐testing and partner notification: supplement to consolidated guidelines on HIV testing services. Geneva, Switzerland: World Health Organization; 2016. Accessed August 14, 2021. https://apps.who.int/iris/bitstream/handle/10665/251655/9789241549868‐eng.pdf [PubMed]
- 31. Prins HA, Mugo P, Wahome E, et al. Diagnosing acute and prevalent HIV‐1 infection in young African adults seeking care for fever: a systematic review and audit of current practice. Int Health. 2014;6(2):82‐92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Dovel K, Shaba F, Offorjebe OA, et al. Effect of facility‐based HIV self‐testing on uptake of testing among outpatients in Malawi: a cluster‐randomised trial. Lancet Glob Health. 2020;8(2):e276‐e287. [DOI] [PubMed] [Google Scholar]
- 33. Rutstein SE, Pettifor AE, Phiri S, et al. Incorporating acute HIV screening into routine HIV testing at sexually transmitted infection clinics, and HIV testing and counseling centers in Lilongwe, Malawi. J Acquir Immune Defic Syndr. 2016;71(3):272‐280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Powers KA, Miller WC, Pilcher CD, et al. Improved detection of acute HIV‐1 infection in sub‐Saharan Africa: development of a risk score algorithm. AIDS. 2007;21(16):2237‐2242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Bulterys MA, Oyaro P, Brown E, et al. Costs of point‐of‐care viral load testing for adults and children living with HIV in Kenya. Diagnostics. 2021;11(1):140. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
SMG and EJS designed the trial, wrote the protocol, provided study oversight, conducted data analysis, and prepared the draft manuscript. CA led the trial implementation. All authors reviewed and contributed to the final manuscript.


