Abstract
Introduction
In the phase 3 study involving the use of durvalumab with or without tremelimumab in combination with platinum-based chemotherapy in untreated extensive-stage SCLC (CASPIAN study), first-line durvalumab plus platinum-etoposide (EP) significantly improved overall survival (OS) versus EP alone (p = 0.0047). We report exploratory subgroup analyses of treatment patterns and outcomes according to the presence of baseline brain or central nervous system metastases.
Methods
Patients (WHO performance status 0 or 1), including those with asymptomatic or treated-and-stable brain metastases, were randomized to four cycles of durvalumab plus EP followed by maintenance durvalumab until progression or up to six cycles of EP and optional prophylactic cranial irradiation. Prespecified analyses of OS and progression-free survival (PFS) in subgroups with or without brain metastases used unstratified-Cox proportional hazards models. The data cutoff was on January 27, 2020.
Results
At baseline, 28 out of 268 patients (10.4%) in the durvalumab plus EP arm and 27 out of 269 patients (10.0%) in the EP arm had known brain metastases, of whom 3 of 28 (10.7%) and 4 of 27 (14.8%) had previous brain radiotherapy, respectively. Durvalumab plus EP (versus EP alone) prolonged OS (hazard ratio, 95% confidence interval) in patients with (0.79, 0.44–1.41) or without (0.76, 0.62–0.92) brain metastases, with similar PFS results (0.73, 0.42–1.29 and 0.80, 0.66–0.97, respectively). Among patients without brain metastases, similar proportions in each arm developed new brain lesions as part of their first progression (8.8% and 9.5%), although 8.3% in the EP arm received prophylactic cranial irradiation. Similar proportions in each arm received subsequent brain radiotherapy (20.5% and 21.2%), although more common in patients with than without baseline brain metastases (45.5% and 18.0%).
Conclusions
The OS and PFS benefit with first-line durvalumab plus EP were maintained irrespective of the presence of brain metastases, further supporting its standard-of-care use.
Keywords: CASPIAN, Brain metastases, Extensive-stage SCLC, Immunotherapy, Central nervous system
Introduction
SCLC, characterized by aggressive tumor growth and early widespread disease, comprises approximately 15% of lung cancers.1, 2, 3 Approximately two-thirds of patients are diagnosed with extensive-stage (ES) disease,1 which has a particularly poor prognosis.3 However, after decades of limited progress, immunotherapy targeting programmed cell death ligand-1 (PD-L1) combined with platinum-based chemotherapy has exhibited prolonged overall survival (OS) in patients with ES-SCLC.4,5
Durvalumab is a selective, high-affinity human immunoglobulin G1 monoclonal antibody that blocks PD-L1 binding to programmed cell death protein-1 (PD-1) and CD80.6 The phase 3 CASPIAN trial (NCT03043872) evaluated the first-line durvalumab, with or without the anticytotoxic T lymphocyte-associated antigen-4 antibody tremelimumab, combined with etoposide plus cisplatin or carboplatin (EP), versus EP alone, in patients with ES-SCLC. At the planned interim analysis (data cutoff: March 11, 2019), first-line durvalumab plus EP, versus EP, significantly improved OS (primary end point) with hazard ratio (HR) of 0.73 (95% confidence interval [CI]: 0.59−0.91, p = 0.0047).4 Accordingly, first-line durvalumab plus EP has been approved globally for ES-SCLC.7,8 These findings were reinforced by updated analyses (at 2- and 3-y follow-up), exhibiting sustained OS benefit (2-y follow-up: HR = 0.75, 95% CI: 0.62−0.91; median OS 12.9 versus 10.5 mo), observed across all subgroups,9,10 including patients with liver metastases (posthoc analysis) or any extent of baseline disease (i.e., thoracic-only or any extrathoracic).9,11 Durvalumab plus tremelimumab plus EP, versus EP, was not associated with statistically significant improvement in OS (HR = 0.82, 95% CI: 0.68–1.00, p = 0.0451).9
At diagnosis, approximately 20% of patients with SCLC have brain metastases and up to 80% have central nervous system (CNS) involvement during the next 2 years.12,13 Whole-brain radiotherapy has historically been the mainstay of treatment for brain metastases in SCLC and other solid tumors but is associated with neurocognitive toxicity.14 Understanding the outcomes for patients with brain metastases receiving immunotherapy plus EP is of significant interest.
Here, we report the subgroup analyses of treatment patterns and outcomes in the CASPIAN study according to the presence or absence of baseline brain or CNS metastases. An exploratory analysis of time to progression in the brain or CNS is also reported. The analyses focus on patients treated with first-line durvalumab plus EP, versus EP, omitting the tremelimumab arm because it was not associated with significantly improved outcomes.
Materials and Methods
Patients and Study Design
CASPIAN is an open-label phase 3 study. The design (Supplementary Fig. 1) has been reported.4,9 Treatment-naive patients with ES-SCLC having WHO performance status (PS) 0 or 1 (eligibility criteria in Supplementary Methods) were randomized 1-to-1-to-1 to durvalumab plus EP, durvalumab plus tremelimumab plus EP, or EP. Patients received less than or equal to four cycles of durvalumab at a 1500-mg dose (with or without tremelimumab 75 mg) plus EP every 3 weeks, followed by maintenance durvalumab every 4 weeks until progression, or less than or equal to six cycles of EP every 3 weeks. EP-arm patients could receive prophylactic cranial irradiation (PCI) after chemotherapy per the investigator's discretion (PCI was not permitted in the immunotherapy groups before discontinuation of all study treatments). Local treatment of isolated lesions, excluding target lesions, with palliative intent (e.g., by local surgery or radiotherapy) was permitted during study treatment. Tumor imaging by computed tomography (preferred) or magnetic resonance imaging was done every 6 weeks for the first 12 weeks from randomization and every 8 weeks thereafter until confirmed objective disease progression. Patients with untreated-asymptomatic or treated-and-stable brain metastases were eligible. Brain metastases were deemed stable if patients were off steroids and anticonvulsants at least 1 month before entry. Patients with leptomeningeal disease were excluded. Brain imaging, per local practice, was suggested for suspected brain metastases but not mandated at screening or during treatment.
Statistical Analysis
Efficacy was analyzed in the intention-to-treat (ITT) population using the 2-year analysis (data cutoff, January 27, 2020). Safety analyses included all patients who received at least one study dose. OS and investigator-assessed progression-free survival (PFS) (Response Evaluation Criteria in Solid Tumors version 1.1) were estimated by the Kaplan-Meier method. Prespecified OS and PFS analyses in patient subgroups with or without baseline brain or CNS metastases were conducted using unstratified-Cox proportional hazards models (only covariate, study treatment) to calculate HRs and 95% CIs for between-arm differences. In the exploratory analysis of all randomized patients, we assessed the time to disease progression (defined by Response Evaluation Criteria in Solid Tumors) in the brain or brain radiotherapy, whichever occurred first (described in Supplementary Methods).
Results
Patients and Treatment
Overall, 805 patients were randomized, including 268 to durvalumab plus EP and 269 to EP, of whom 28 (10.4%) and 27 (10.0%) had known baseline brain or CNS metastases, respectively. Among these patients, three out of 28 (10.7%) and four out of 27 (14.8%) had previous brain radiotherapy, respectively (Table 1), and 2 of 28 (7.1%) and 1 of 27 (3.7%) had previous brain tumor resection, respectively (untreated patients were presumed to be asymptomatic). Among patients with brain metastases, there were some differences in baseline characteristics between arms, as expected with small patient numbers (Supplementary Table 1). In the durvalumab plus EP arm, there was a higher percentage of female patients and a lower percentage of patients who were Asian or had PS of 1. In the EP arm, 8.3% had PCI or brain radiotherapy after chemotherapy (2 of 27 [7.4%] with and 20 of 239 [8.4%] without baseline brain metastases). Two patients in the durvalumab plus EP arm received concomitant PCI after completing EP therapy (protocol deviations).
Table 1.
Radiotherapy to the Brain Before, During, and After Study Treatment in Patients With or Without Brain Metastases at Baseline
| n (%) | Brain Metastases at Baseline |
No Brain Metastases at Baseline |
||
|---|---|---|---|---|
| Durvalumab + EP (n = 28) | EP (n = 27) | Durvalumab + EP (n = 240) | EP (n = 242) | |
| Radiotherapy to brain before study | 3 (10.7) | 4 (14.8) | 0 | 1 (0.4)a |
| Radiotherapy to brain concurrent with study treatment | 2 (7.1) | 0 | 3 (1.3)b | 0 |
| Radiotherapy to brain subsequent to study treatmentc | 13 (46.4) | 12 (44.4) | 42 (17.5) | 45 (18.6) |
EP, etoposide and either cisplatin or carboplatin; PCI, prophylactic cranial irradiation.
In one patient who received previous irradiation to the brain, his or her lesions were no longer present at baseline.
Excludes two patients who received concomitant PCI (given over approximately a 2-week period in between durvalumab cycles) after completing EP therapy (protocol deviations).
Excludes patients who received optional PCI after chemotherapy in the EP arm (two patients with and 20 patients without brain metastases at baseline); no patients in the durvalumab plus EP arm received subsequent PCI.
Efficacy
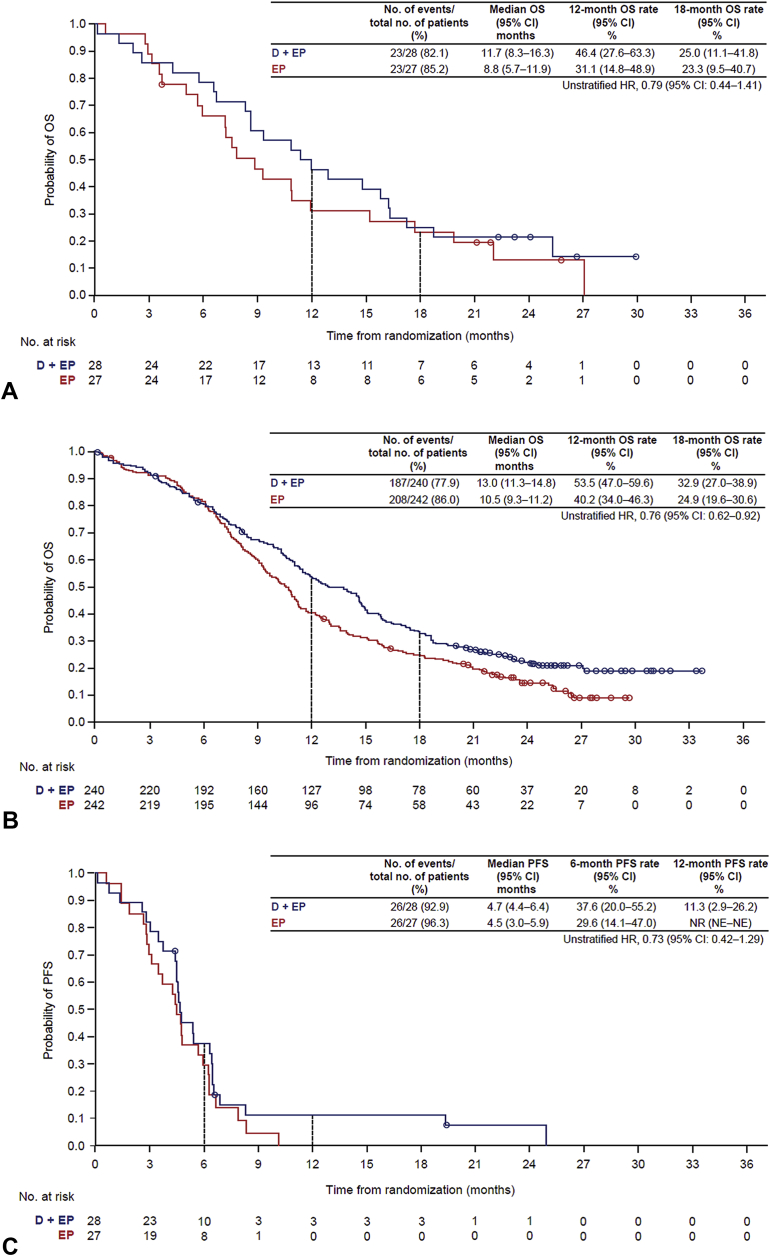
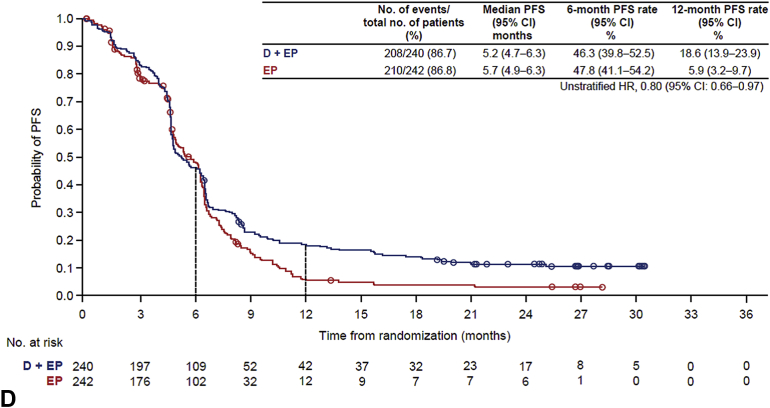
Durvalumab plus EP, versus EP, prolonged OS in patients with (HR = 0.79, 95% CI: 0.44−1.41) or without (HR = 0.76, 95% CI: 0.62−0.92) baseline brain metastases (Fig. 1 and Supplementary Fig. 2). PFS was also prolonged with durvalumab plus EP, versus EP, in patients with (HR = 0.73, 95% CI: 0.42−1.29) or without (HR = 0.80, 95% CI: 0.66−0.97) brain metastases (Fig. 1 and Supplementary Fig. 2).
Figure 1.
OS in patients (A) with and (B) without brain metastases at baseline and overall PFS in patients (C) with and (D) without brain metastases at baseline. CI, confidence interval; D, durvalumab; EP, etoposide and either cisplatin or carboplatin; HR, hazard ratio; NE, not evaluable; NR, not reached; OS, overall survival; PFS, progression-free survival.
Among patients without baseline brain metastases, similar proportions in each arm developed new brain or CNS metastases as part of their first progression (durvalumab plus EP: 21 of 240 [8.8%]; EP: 23 of 242 [9.5%]) (Supplementary Table 2), although 8.3% of patients in the EP arm received PCI after chemotherapy. In the EP arm (all patients, N = 269), the proportion who developed new brain metastases as part of their first progression was similar, regardless of whether they had received PCI (2 of 22 [9.1%]) or not (29 of 247 [11.7%]), albeit on the basis of a small number who had received PCI.
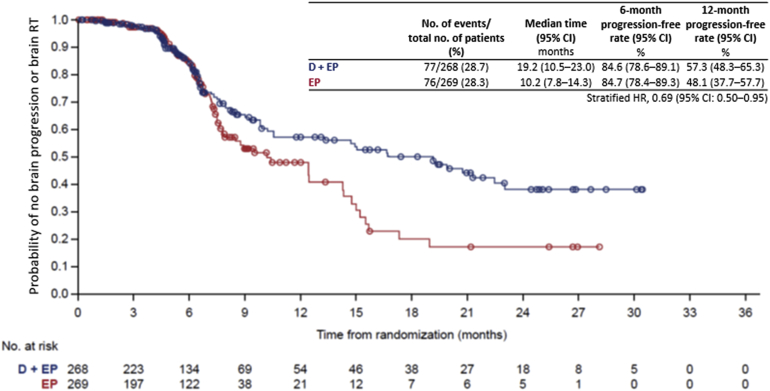
In the exploratory analysis of all randomized patients, durvalumab plus EP, versus EP, prolonged the time to disease progression in the brain or brain radiotherapy, whichever occurred first (HR = 0.69, 95% CI: 0.50−0.95) (Fig. 2).
Figure 2.
Time to RECIST-defined disease progression in the brain or brain radiotherapy (excluding PCI), whichever occurred first. CI, confidence interval; D, durvalumab; EP, etoposide, and either cisplatin or carboplatin; HR, hazard ratio; PCI, prophylactic cranial irradiation; RECIST, Response Evaluation Criteria in Solid Tumors; RT, radiotherapy.
Radiotherapy and Subsequent Systemic Therapy Use
Before study entry, overall, three patients in the durvalumab plus EP arm and five in the EP arm received brain radiotherapy (Table 1). No patients in the EP arm received concurrent brain radiotherapy, whereas five (1.9%) in the durvalumab plus EP arm received brain radiotherapy during durvalumab maintenance (Table 1). Overall, similar proportions in the durvalumab plus EP and EP arms received brain radiotherapy after study treatment (55 of 268 [20.5%] and 57 of 269 [21.2%]), although more common among patients with baseline brain metastases versus those without (25 of 55 [45.5%] and 87 of 482 [18.0%]) (Table 1).
Among patients with baseline brain metastases, 57.1% (16 of 28) in the durvalumab plus EP arm and 48.1% (13 of 27) in the EP arm received subsequent systemic anticancer therapy. Among patients without brain metastases, 44.6% (107 of 240) and 46.3% (112 of 242) received subsequent therapy.
Safety
Among patients with baseline brain metastases, fewer in the durvalumab plus EP arm, versus EP arm, experienced all-cause grade 3 or 4 adverse events (AEs) (67.9% versus 85.2%) and serious AEs (28.6% versus 59.3%), whereas the safety profiles among patients without brain metastases were generally similar across arms (Supplementary Table 3). Overall, more patients in the durvalumab plus EP arm reported immune-mediated AEs irrespective of the presence of brain metastases; however, most such events were low-grade and endocrine-related.
Discussion
In the CASPIAN study, first-line durvalumab plus EP, versus EP, significantly improved OS at the planned interim analysis, which was sustained at updated analyses after 2- and 3-years follow-up.4,9,10 In the subgroup analyses reported here, the OS and PFS benefit were maintained with first-line durvalumab plus EP irrespective of the presence of baseline brain or CNS metastases, consistent with the ITT analyses. It should be noted that the 95% CI for the OS HR and PFS HR crossed one for the subgroup with brain metastases, as may be expected on the basis of the small sample size (n = 55) and resultant wide CI. Safety findings in each subgroup were also consistent with the ITT results and the known profiles of each agent. These results suggest that, whereas for symptomatic brain metastases whole-brain radiotherapy or stereotactic radiosurgery remain to be standard,14 systemic treatments should be immediately prioritized for patients with asymptomatic brain metastases and that they need not be excluded from immuno-oncology SCLC trials.
These findings, although based on small subgroups, seem promising compared with separately reported analyses for atezolizumab or pembrolizumab plus chemotherapy in this setting.5,15 In addition, they are consistent with prospective, albeit limited, data from recent studies of immune checkpoint inhibitors in patients with NSCLC having brain metastases, and supported by preclinical data challenging the notion that the brain is an immune-privileged site.16, 17, 18, 19 Furthermore, the 3-year OS data from the CASPIAN study continue to exhibit similar benefits with durvalumab plus EP in patients with or without baseline brain metastases.10
There are, however, potential limitations of these analyses, including the small subgroups, which preclude, for example, an assessment of the effects of durvalumab in the presence or absence of previous or concurrent brain radiotherapy as there are too few patients who received either. The proportion of patients with brain metastases seems lower than that reported in a real-world ES-SCLC setting,12,13 likely owing to the inclusion criteria (e.g., enrollment of patients with WHO PS 0 or 1). Baseline computed tomography or magnetic resonance imaging was not mandated (consistent with current clinical practice guidelines20), so the number of patients with asymptomatic brain metastases may have been underestimated. PCI exclusion in the durvalumab plus EP arm prevents assessment of its potential role with immunotherapy. In addition, although PFS and OS analyses were prespecified, the study was not powered for subgroup comparisons.
Finally, an exploratory analysis of all patients revealed that durvalumab plus EP prolonged the time to brain progression or radiotherapy, suggesting that this combination may delay intracranial progression—a major challenge in ES-SCLC treatment. However, this analysis must be interpreted carefully. Collection of brain imaging was not mandated in the protocol and scan collection was capped at progression (+1 follow-up); therefore, brain progression events may have been missed, particularly any preceded by non-brain disease progression.
Whereas exploratory subgroup analyses such as this provide important insights, the value of immune checkpoint inhibition remains to be formally documented in patients with brain metastases from SCLC. Future prospective trials evaluating the combination of immunotherapy and brain radiotherapy, or directly comparing immunotherapy plus chemotherapy with radiotherapeutic approaches, are needed to better understand the potential role of immunotherapy and how it fits alongside radiotherapy in the management of brain metastases in ES-SCLC.
In conclusion, this exploratory analysis further supports the use of first-line durvalumab plus EP as the standard of care for patients with ES-SCLC, irrespective of whether patients have baseline brain metastases.
CRediT Authorship Contribution Statement
Yuanbin Chen: Conceptualization, Investigation, Supervision, Writing - review & editing.
Luis Paz-Ares: Conceptualization, Investigation, Writing - review & editing.
Niels Reinmuth, Marina Chiara Garassino, Galina Statsenko, Maximilian J. Hochmair, Mustafa Özgüroğlu, Francesco Verderame, Libor Havel, György Losonczy, Nikolay V. Conev, Katsuyuki Hotta, Jun Ho Ji, Tapashi Dalvi, Jonathan W. Goldman: Investigation, Writing - review & editing.
Stuart Spencer: Formal Analysis, Writing - review & editing.
Haiyi Jiang: Conceptualization, Visualization, Investigation, Writing - review & editing.
Data Sharing Statement
Data underlying the findings described in this manuscript may be obtained in accordance with AstraZeneca’s data sharing policy described at: https://astrazenecagrouptrials.pharmacm.com/ST/Submission/Disclosure.
Acknowledgments
This study (NCT03043872) was sponsored by AstraZeneca. The authors thank the patients, their families, and caregivers. Medical writing support, under the direction of the authors, was provided by Andrew Gannon and Craig Turner of Ashfield MedComms (New York, NY), an Ashfield Health company, and was funded by AstraZeneca.
Footnotes
Disclosure: Dr. Chen reports receiving honoraria from Amgen, United States, AstraZeneca, United Kingdom, Bristol-Myers Squibb, Guardant Health, Jazz Pharmaceutical, Merck, United States, Pfizer, and Takeda and a research contract and support for meeting attendance/travel from Ipsen, France, all outside the submitted work. Dr. Paz-Ares reports receiving grants from AstraZeneca, Bristol-Myers Squibb, Merck Sharp & Dohme, United Kingdom, and Pfizer; consulting fees from Amgen, AstraZeneca, Bayer, Blueprint Medicines, Bristol-Myers Squibb, Ipsen, Eli Lilly, Merck, Mirati, Merck Sharp & Dohme, Novartis, Pfizer, PharmaMar, Roche, Sanofi, and Servier, France; honoraria from AstraZeneca, Janssen, Merck, Mirati, and Sanofi; and reports having a leadership role with Genomica and Altum Sequencing, all outside the submitted work. Dr. Reinmuth reports receiving honoraria from Amgen, AstraZeneca, Boehringer Ingelheim, Germany, Bristol-Myers Squibb, Daiichi Sankyo, Eli Lilly, Hoffmann-La Roche, Merck, Merck Sharp & Dohme, Pfizer, and Takeda and consulting fees from Amgen, AstraZeneca, Boehringer Ingelheim, Bristol-Myers Squibb, Daiichi Sankyo, Hoffmann-La Roche, Merck, Merck Sharp & Dohme, Pfizer, and Takeda, Japan, all outside the submitted work. Dr. Garassino reports receiving grants from AstraZeneca and Merck and personal fees from AstraZeneca, Boehringer Ingelheim, Bristol-Myers Squibb, Celgene, Eli Lilly, GlaxoSmithKline, United Kingdom, Merck, Novartis, Pfizer, Roche, and Takeda, all outside the submitted work. Dr. Hochmair reports receiving speakers’ honoraria from AstraZeneca, Boehringer Ingelheim, Merck Sharp & Dohme, Pfizer, Roche, and Takeda outside the submitted work. Dr. Verderame reports receiving grants from Roche SpA, Boehringer Ingelheim, Servier, AstraZeneca, GlaxoSmithKline, Takeda, and Merck Sharp & Dohme, all outside the submitted work. Dr. Hotta reports receiving grants and personal fees from AstraZeneca during the conduct of the study; grants from Bristol-Myers Squibb, Chugai, Japan, Eli Lilly, and Merck Sharp & Dohme outside the submitted work; and honoraria from AstraZeneca, Boehringer Ingelheim, Bristol-Myers Squibb, Chugai, Eli Lilly, Merck Sharp & Dohme, Nippon Kayaku, Japan, Ono, Pfizer, United States, Taiho, and Takeda outside the submitted work. Mr. Spencer and Dr. Dalvi report full-time employment and stock ownership with AstraZeneca. Dr. Jiang reports full-time employment and stock ownership with AstraZeneca and a patent pending for the CASPIAN study trial design. Dr. Goldman reports receiving research grants from AbbVie, United States, AstraZeneca, Bristol-Myers Squibb, United States, Genentech, United States, and Merck; consulting fees from AbbVie, AstraZeneca, Bristol-Myers Squibb, and Genentech; and support for travel from AstraZeneca, all outside the submitted work. The remaining authors declare no conflict of interest.
Cite this article as: Chen Y, Paz-Ares L, Reinmuth N, et al. Impact of brain metastases on treatment patterns and outcomes with first-line durvalumab plus platinum-etoposide in extensive-stage SCLC (CASPIAN): a brief report. JTO Clin Res Rep. 2022;3:100330.
Note: To access the supplementary material accompanying this article, visit the online version of the JTO Clinical and Research Reports at www.jtocrr.org/ and at https://doi.org/10.1016/j.jtocrr.2022.100330.
Supplementary Data
References
- 1.Oronsky B., Reid T.R., Oronsky A., Carter C.A. What’s new in SCLC? A review. Neoplasia. 2017;19:842–847. doi: 10.1016/j.neo.2017.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang S., Tang J., Sun T., et al. Survival changes in patients with small cell lung cancer and disparities between different sexes, socioeconomic statuses and ages. Sci Rep. 2017;7:1339. doi: 10.1038/s41598-017-01571-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Byers L.A., Rudin C.M. Small cell lung cancer: where do we go from here? Cancer. 2015;121:664–672. doi: 10.1002/cncr.29098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Paz-Ares L., Dvorkin M., Chen Y., et al. Durvalumab plus platinum-etoposide versus platinum-etoposide in first-line treatment of extensive-stage small-cell lung cancer (CASPIAN): a randomised, controlled, open-label, phase 3 trial. Lancet. 2019;394:1929–1939. doi: 10.1016/S0140-6736(19)32222-6. [DOI] [PubMed] [Google Scholar]
- 5.Horn L., Mansfield A.S., Szczesna A., et al. First-line Atezolizumab plus chemotherapy in extensive-stage small-cell lung cancer. N Engl J Med. 2018;379:2220–2229. doi: 10.1056/NEJMoa1809064. [DOI] [PubMed] [Google Scholar]
- 6.Stewart R., Morrow M., Hammond S.A., et al. Identification and characterization of MEDI4736, an antagonistic anti-PD-L1 monoclonal antibody. Cancer Immunol Res. 2015;3:1052–1062. doi: 10.1158/2326-6066.CIR-14-0191. [DOI] [PubMed] [Google Scholar]
- 7.AstraZeneca. Imfinzi (durvalumab) prescribing information. https://www.azpicentral.com/imfinzi/imfinzi.pdf#page=1
- 8.European Medicines Agency Durvalumab summary of product characteristics. https://www.ema.europa.eu/en/documents/product-information/imfinzi-epar-product-information_en.pdf
- 9.Goldman J.W., Dvorkin M., Chen Y., et al. Durvalumab, with or without tremelimumab, plus platinum-etoposide versus platinum-etoposide alone in first-line treatment of extensive-stage small-cell lung cancer (CASPIAN): updated results from a randomised, controlled, open-label, phase 3 trial. Lancet Oncol. 2021;22:51–65. doi: 10.1016/S1470-2045(20)30539-8. [DOI] [PubMed] [Google Scholar]
- 10.Paz-Ares L., Chen Y., Reinmuth N., et al. Durvalumab, with or without tremelimumab, plus platinum-etoposide in first-line treatment of extensive-stage small-cell lung cancer: 3-year overall survival update from CASPIAN. ESMO Open. 2022;7:100408. doi: 10.1016/j.esmoop.2022.100408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Reinmuth N., Dvorkin M., Garassino M.C., et al. P48.03 first-line durvalumab plus platinum-etoposide in ES-SCLC: exploratory analyses based on extent of disease in CASPIAN. J Thorac Oncol. 2021;16(suppl 3):S500. [Google Scholar]
- 12.Tsui D.C.C., Polito L., Madhavan S., Adler L., Ogale S., Camidge D.R. 1650P - Adoption and early clinical outcomes of atezolizumab (atezo) + carboplatin and etoposide (CE) in patients with extensive-stage small cell lung cancer (ES-SCLC) in the real-world (RW) setting. Ann Oncol. 2021;32(suppl 5):S1164–S1174. [Google Scholar]
- 13.Pacheco J., Bunn P.A. Advancements in small-cell lung cancer: the changing landscape following IMpower-133. Clin Lung Cancer. 2019;20:148–160.e2. doi: 10.1016/j.cllc.2018.12.019. [DOI] [PubMed] [Google Scholar]
- 14.Le Rhun E., Guckenberger M., Smits M., et al. EANO-ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up of patients with brain metastasis from solid tumours. Ann Oncol. 2021;32:1332–1347. doi: 10.1016/j.annonc.2021.07.016. [DOI] [PubMed] [Google Scholar]
- 15.Rudin C.M., Awad M.M., Navarro A., et al. Pembrolizumab or placebo plus etoposide and platinum as first-line therapy for extensive-stage small-cell lung cancer: randomized, double-blind, Phase III KEYNOTE-604 study. J Clin Oncol. 2020;38:2369–2379. doi: 10.1200/JCO.20.00793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pathak R., Amini A., Hill A., Massarelli E., Salgia R. Immunotherapy in non-small cell lung cancer patients with brain metastases: clinical challenges and future directions. Cancers (Basel) 2021;13:3407. doi: 10.3390/cancers13143407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goldberg S.B., Schalper K.A., Gettinger S.N., et al. Pembrolizumab for management of patients with NSCLC and brain metastases: long-term results and biomarker analysis from a non-randomized, open-label, phase 2 trial. Lancet Oncol. 2020;21:655–663. doi: 10.1016/S1470-2045(20)30111-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vilariño N., Bruna J., Bosch-Barrera J., Valiente M., Nadal E. Immunotherapy in NSCLC patients with brain metastases. Understanding brain tumor microenvironment and dissecting outcomes from immune checkpoint blockade in the clinic. Cancer Treat Rev. 2020;89:102067. doi: 10.1016/j.ctrv.2020.102067. [DOI] [PubMed] [Google Scholar]
- 19.Eguren-Santamaria I., Sanmamed M.F., Goldberg S.B., et al. PD-1/PD-L1 blockers in NSCLC brain metastases: challenging paradigms and clinical practice. Clin Cancer Res. 2020;26:4186–4197. doi: 10.1158/1078-0432.CCR-20-0798. [DOI] [PubMed] [Google Scholar]
- 20.Dingemans A.C., Früh M., Ardizzoni A., et al. Small-cell lung cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up☆. Ann Oncol. 2021;32:839–853. doi: 10.1016/j.annonc.2021.03.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.