Abstract
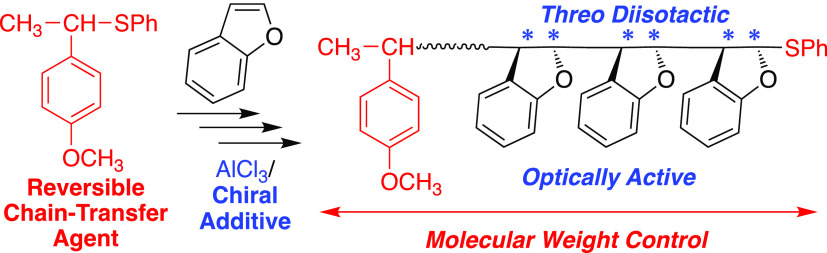
Benzofuran (BzF) is a prochiral, 1,2-disubstituted, unsymmetric cyclic olefin that can afford optically active polymers by asymmetric polymerization, unlike common acyclic vinyl monomers. Although asymmetric cationic polymerization of BzF was reported by Natta et al. in the 1960s, the polymer structure has not been clarified, and there are no reports on molecular weight control. Herein, we report dual control of the optical activity and molecular weight of poly(BzF) using thioether-based reversible chain-transfer agents for asymmetric cationic polymerization with β-amino acid derivatives as chiral additives and aluminum chloride as a catalyst. This asymmetric moderately living cationic polymerization leads to an increase in molecular weight and specific optical rotation with monomer conversion. In addition, asymmetric block polymers consisting of opposite absolute configurational segments were synthesized using both enantiomers sequentially as chiral additives. Finally, a comprehensive analysis of the polymerization products and the model reaction revealed that the optical activity of poly(BzF) originates from the threo-diisotactic structure, which occurs by regio-, trans-, and enantioselective propagation.
Introduction
Optically active natural macromolecules with highly controlled structures, such as proteins, deoxyribonucleic acids, and polysaccharides, possess unique and specialized functions that are indispensable for maintaining our lives. This constantly motivates research on the controlled synthesis of optically active polymers, which have unique structures and functions that can improve our modern life.1−3
Vinyl polymers are one of the largest families of synthetic polymers and are obtained by the chain-growth polymerization of a wide variety of olefinic compounds.4,5 They are generally synthesized from prochiral vinyl (CH2=CHX) or vinylidene (CH2=CXY) monomers and can possess asymmetric carbons (∼CH2–C*HX∼ or ∼CH2–C*XY∼) in the main chains.6−9 However, these vinyl polymers rarely show optical activity, even if their asymmetric carbons are structured to have high isotacticity with only one type of chiral center consisting of consecutive R or S sequences.1 This is because the whole polymer chain can be regarded as having a mirror plane, where the chain end groups are ignored due to the long polymer chains, i.e., the chiral center in the main chain is pseudoasymmetric. However, when the side chain has a very bulky substituent, the one-handed helical conformation of the main chain is conserved, resulting in optically active vinyl polymers as a special case.1−3
Benzofuran (BzF) is a prochiral, 1,2-disubstituted, and unsymmetric cyclic olefin with both vinyl ether- and styrene-like structures (Scheme 1). This monomer readily undergoes cationic polymerization to form rigid polymers with high glass-transition temperature (Tg) and high transparency, which is applicable to transparent thermoplastics.10−14 Recently, it attracted further attention as a biobased and chemically recyclable monomer from a sustainable viewpoint.13,14 In 1961, Natta et al. reported the synthesis of optically active polymers by the cationic polymerization of BzF using aluminum catalysts (AlCl3 or EtAlCl2) and asymmetric cocatalysts (β-phenylalanine or 10-camphorsulfonic acid) in toluene at −75 °C.15 The optical activity can be ascribed to main chain configurational chirality because in principle, an ideal structure of diisotactic poly(BzF) with both erythro- and threo-structures does not have a mirror plane, unlike the common vinyl polymers mentioned above.16−18 Since then, but at the latest in the 1960s, similar asymmetric (asymmetric synthesis, asymmetric induction, or asymmetric chirogenic)9 cationic polymerizations predominantly forming one type of chiral center in the main chain have been reported for BzF and related unsymmetrical cyclic olefins.19−21 However, it is still unknown whether threo- or erythro-structures are constructed as well as whether vinyl ether- or styrene-type cations form. Namely, cis- or trans-addition and 1,2- or 2,1-addition are not clarified. In addition, dual control of the chirality and molecular weight of poly(BzF) has not been attained. Later, in the 2010s, only moderate molecular weight control was reported using an achiral initiating system consisting of cumyl chloride and SnCl4 in CH2Cl2 at −78 °C.22 Novel polymerization methods for controlling both the chirality and molecular weight of poly(BzF) can not only clarify the asymmetric polymerization mechanisms but also lead to unique optically active polymer materials with highly controlled structures.
Scheme 1. Asymmetric Living Cationic Polymerization of Benzofuran (BzF) Based on Reversible Chain-Transfer Mechanism.
Recently, we developed a novel method to control molecular weight in the cationic polymerization of vinyl monomers using a reversible or degenerative chain-transfer (DT) mechanism, where the propagating cationic chain end dynamically interchanges with the dormant species originating from reversible chain-transfer agents (CTAs).23−29 The effective CTAs are thioesters and thioethers, which control the molecular weight of the resulting polymers as in radical reversible addition–fragmentation chain-transfer (RAFT) polymerization and work for various cationically polymerizable monomers, such as vinyl ethers and p-alkoxystyrenes. This cationic RAFT or DT process is applicable even for stereospecific cationic polymerization to enable simultaneous control of the molecular weight and tacticity of vinyl polymers. Indeed, stereospecific cationic RAFT and DT polymerizations have been achieved for vinyl ethers and α-methylstyrene by the judicious choice of counteranions in terms of bulkiness and/or chirality.30−33 However, no asymmetric RAFT or DT polymerization and no dual control of optical activity and molecular weight have been realized in cationic polymerization.28−36
Herein, we report the asymmetric living cationic polymerization of BzF, where both molecular weight and optical activity are controlled using thioether as CTA in conjunction with chiral N-substituted β-phenylalanines and AlCl3 as chiral catalysts. In particular, bulky chiral β-phenylalanine derivatives provide much higher specific optical rotation than that reported with native β-phenylalanine, while an appropriate thioether controls the polymer molecular weight up to several tens of thousands. A detailed analysis of the products obtained in the polymerization and its model reaction reveals that the asymmetric cationic polymerization proceeds via the selective trans-threo addition of vinyl ether-type cation and that the optical activity is a consequence of triple selectivities: in terms of regio-, trans-, and enantio- selectivity. Furthermore, an asymmetric block polymer is prepared using a pair of β-phenylalanine-based enantiomers sequentially as opposite chiral additives.
Results and Discussion
Asymmetric Cationic Polymerization Using Chiral Amino Acid Derivatives
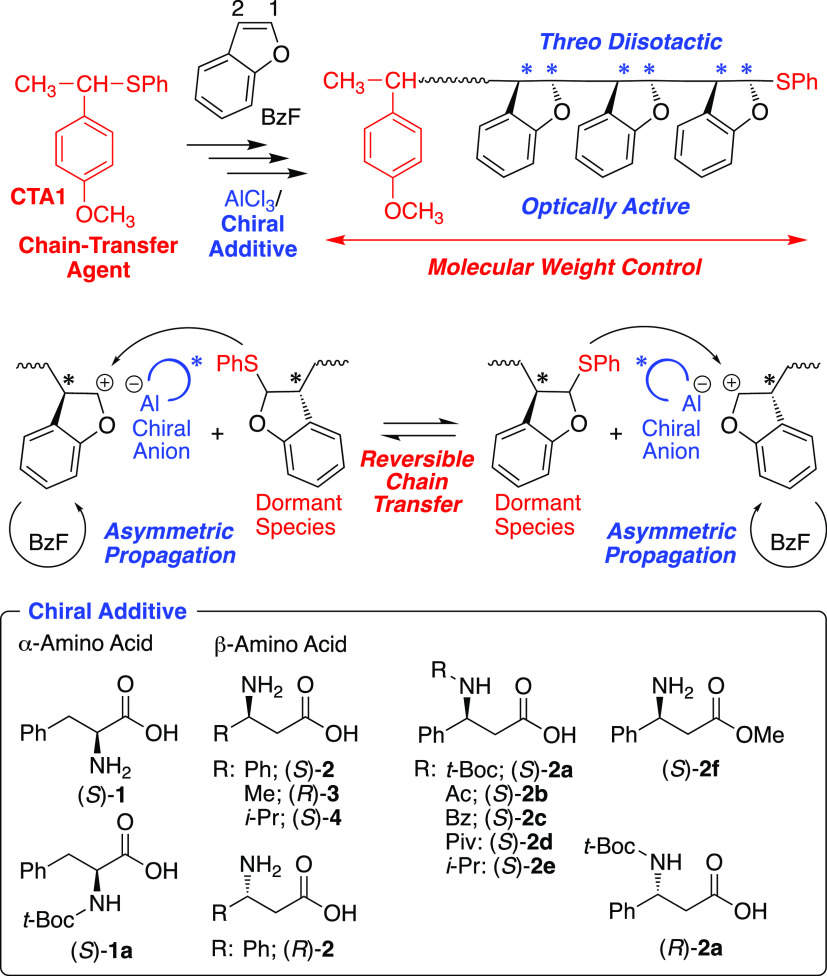
To reinvestigate and improve the asymmetric cationic polymerization of BzF, various chiral α- and β-amino acids and their derivatives were employed in conjunction with AlCl3 as a Lewis acid catalyst in toluene at −78 °C. Here, AlCl3 and a chiral additive were premixed at a 2:1 ratio in toluene at 20 °C for 24 h, according to a previous report.15 Although each of them was mostly insoluble in toluene, the mixed catalyst solution became homogeneous during aging, indicating the formation of aluminum compounds complexed with chiral amino acid derivatives. This solution was used as a mixed catalyst for the cationic polymerization of BzF.
With (S)- α-phenylalanine ((S)-1), an α-amino acid, almost no polymerization occurred, most likely due to the significant loss of the Lewis acidity of AlCl3 by its strong chelation (entry 1 in Table 1). Although protection of the amino group with tert-butoxycarbonyl (t-Boc) enabled the polymerization, the optical activity of the resulting polymer was very low ([α]D25 = −0.7) (entry 6). On the other hand, with the β-amino acid, i.e., (S)-β-phenylalanine ((S)-2), which was used in a previous report,15 polymerization proceeded and resulted in polymers with high molecular weight (Mn = 1.50 × 105) and low specific optical rotation ([α]D = +8.6) (entry 2). The optical activity was lower than the reported value ([α]D25 = +56.7),15 while the polymerization conditions were the same.
Table 1. Asymmetric Cationic Polymerization of BzF Using Various Chiral Additivesa.
| entry | chiral additive | time (h) | conv (%)b | Mnc | Mw/Mnc | [α]D25d |
|---|---|---|---|---|---|---|
| 1 | (S)-1 | 62 | 9 | |||
| 2 | (S)-2 | 44 | 92 | 150 000 | 2.48 | +8.6 |
| 3 | (R)-2 | 50 | 97 | 164 000 | 2.34 | –7.3 |
| 4 | (R)-3 | 63 | 82 | 130 000 | 2.17 | +15.9 |
| 5 | (S)-4 | 100 | 91 | 146 000 | 2.61 | +9.5 |
| 6 | (S)-1a | 4 | 99 | 183 000 | 2.65 | –0.7 |
| 7 | (S)-2a | 42 | 99 | 98 000 | 2.35 | +52.7 |
| 8 | (S)-2b | 6 | 99 | 227 000 | 3.86 | +65.0 |
| 9 | (S)-2c | 19 | 95 | 280 000 | 2.61 | +66.0 |
| 10 | (S)-2d | 2 | 93 | 516 000 | 2.29 | +93.4 |
| 11 | (S)-2e | 0.5 | 99 | 344 000 | 2.41 | +72.2 |
| 12 | (S)-2f | 6 | 94 | 306 000 | 2.48 | +0.8 |
Condition: [BzF]0/[AlCl3]0/[chiral additive]0 = 200/40/20 mM in toluene at −78 °C.
Determined by 1H NMR.
Determined by size-exclusion chromatography (SEC).
Measured in tetrahydrofuran (THF).
On the other hand, its enantiomer, (R)-β-phenylalanine ((R)-2), afforded polymers with almost the same molecular weight (Mn = 1.64 × 105) and the opposite optical rotation with a similar absolute value (−7.3) (entry 3), indicating that the optical rotation of the polymers was determined by the chirality of the β-amino acid. Furthermore, other β-amino acids with alkyl groups, (R)-aminobutyric acid ((R)-3) and (S)-β-homovaline ((S)-4), which have the same absolute configuration as that of (S)-2, also gave optically active polymers with similarly positive optical rotations, +15.9 and +9.5, respectively (entries 4 and 5). Thus, β-amino acids were effective chiral additives for the asymmetric cationic polymerization of BzF in conjunction with AlCl3.
To further improve the optical activity, substituted β-phenylalanine derivatives were synthesized and employed. When (S)-2a with a t-Boc-substituted amino group was used, the specific optical rotation of the resulting polymer dramatically increased (+52.7) (entry 7). On the other hand, the polymer obtained with (S)-2f, in which the carboxy group was esterified with a methyl group, showed almost no optical activity (entry 12). The carboxylic acid group in β-phenylalanine seemed essential for asymmetric induction into poly(BzF), and the substituents on the amino groups could improve the enantioselectivity.
Then, various (S)-β-phenylalanine derivatives with acetyl ((S)-2b), benzoyl ((S)-2c), pivaloyl ((S)-2d), and isopropyl ((S)-2e) substituents on the amino group were prepared. All of these N-substituted β-amino acids afforded high-molecular-weight polymers with high specific optical rotations over +50 (entries 8–11). In particular, (S)-2d with a bulky pivaloyl group showed the highest value (+93.4), indicating that bulkiness around the nitrogen atom improved the enantioselectivity during propagation. Thus, N-substituted β-amino acid derivatives functioned efficiently as chiral additives for the asymmetric cationic polymerization of BzF in the presence of AlCl3. In particular, bulky substituted groups on the amino group significantly improved the enantioselectivity.
The feed ratio of AlCl3 and chiral additives was also investigated (Table S1). The use of a higher amount of (S)-2a (AlCl3/(S)-2a = 1:2 and 1:1) deactivated the polymerization. In contrast, a very fast polymerization occurred at a smaller amount of (S)-2a (AlCl3/(S)-2a = 1:0.25) to give polymers with almost no optical activity. Although polymerization occurred at AlCl3/(S)-2a = 1:0.67, the optical activity was low. In addition, without premixing the catalysts, the optical activity became slightly lower. These results indicate that premixed catalyst at a 1:0.50 ratio of AlCl3 of (S)-2a is most efficient for the asymmetric cationic polymerization. Although the isolation and determination of aluminum complexes with β-amino acid derivatives are still under investigation, the enantioselectivity is governed by the chiral counteranions generated in situ from the chiral additives and Lewis acids.
Asymmetric Living Cationic Polymerization via Reversible Chain Transfer to Thioether
To achieve dual control of the optical activity and molecular weight of the resulting polymers, asymmetric living cationic polymerization of BzF was investigated using reversible CTAs in the presence of chiral catalysts. Here, an aromatic thioether (CTA1), a thiophenol adduct of p-methoxystyrene, was used as CTA since it has been reported that thioethers function efficiently for both vinyl ether and styrene derivatives.24,30 The chiral additives used herein were unsubstituted ((S)-2), t-Boc-substituted ((S)-2a), and pivaloyl-substituted ((S)-2d) (S)- β-phenylalanines, among which the last was the most effective in asymmetric cationic polymerization, as mentioned above.
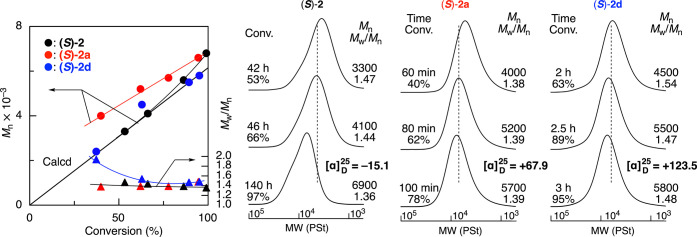
Even in the presence of CTA1, all polymerizations proceeded almost quantitatively without a significant decrease in the catalytic activity in toluene at −78 °C (Figure S1). Upon the addition of CTA1, the molecular weight of the obtained polymers drastically decreased from one hundred thousand to several thousands and agreed well with theoretical values, assuming that one polymer chain was generated from one CTA molecule (Figure 1 and entries 1, 6, and 9 in Table S2). In addition, a linear increase in the Mn values to the monomer conversion as well as relatively narrow and unimodal molecular weight distribution (MWD) curves (Mw/Mn = 1.3–1.5) was observed throughout the polymerizations, indicating that CTA1 could efficiently function as a reversible chain-transfer agent in the cationic polymerization of BzF.
Figure 1.
Mn and SEC curves of the polymers obtained in asymmetric living cationic polymerization of BzF using CTA1: [BzF]0/[CTA1]0/[AlCl3]0/[chiral additive]0 = 200/4.0/40/20 mM in toluene at −78 °C.
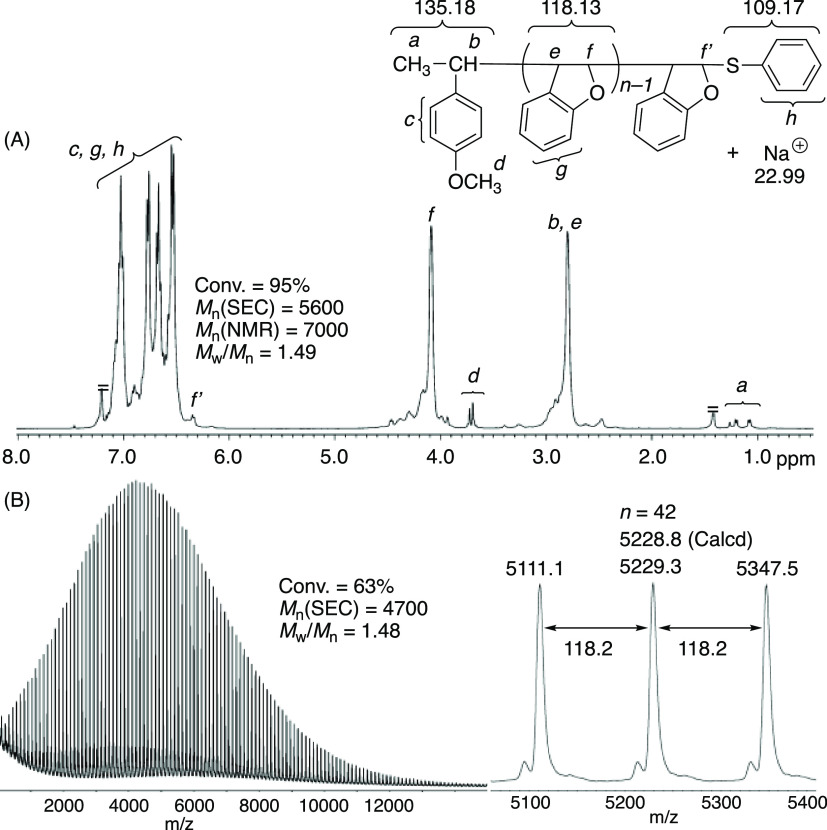
The polymer obtained with CTA1 and (S)-2d was then analyzed by 1H NMR and MALDI-TOF-MS (Figure 2). The proton signals ascribed to the repeating BzF units were relatively sharp, suggesting high stereoregularity, which will be discussed later. In addition, small but characteristic signals assignable to methoxy (d) and methyl (a) protons at the α-end originating from CTA1 were observed at 3.7 and 1.0–1.3 ppm, respectively. The molecular weight (Mn(NMR)) calculated using the integral ratios of the repeating units to the α-end was close to that obtained by SEC (Mn(SEC)). The MALDI-TOF-MS spectra showed almost only one main series of peaks separated by 118 Da, which corresponds to the molar mass of BzF. The absolute molar masses of the individual peaks were nearly the same as those of poly(BzF), with both α- and ω-chain ends derived from CTA1, indicating that almost all of the polymer chains were generated from CTA1 and reversibly terminated by the thiophenol moiety to possess high chain end fidelity, which can be used as macro CTA later.
Figure 2.
1H NMR (A) (CDCl3, 55 °C) and MALDI-TOF-MS (B) spectra of poly(BzF) obtained in asymmetric living cationic polymerization of BzF using CTA1 and (S)-2d.
To further investigate the controllability of molecular weight, the feed ratio of BzF to CTA1 was changed (Figure S2). The Mn of the obtained polymers increased in direct proportion to the product of the feed ratio ([BzF]0/[CTA1]0) and conversion by at least 30 000, where all polymers possessed relatively narrow MWDs. These results indicate that the moderately living cationic polymerization of BzF is achievable using a thioether as a reversible chain-transfer agent.
Then, we considered the optical activity of the polymers obtained in the presence of CTA1 and β-phenylalanine derivatives. All of the obtained polymers showed optical activity, and the absolute values of specific optical rotation were higher than those obtained in the absence of CTA (Figure 1; entries 6 and 9 in Table S2 vs entries 7 and 10 in Table 1, respectively), except for the case with (S)-2, in which opposite optical rotation was observed (Figure 1; entry 1 in Table S2 vs entry 3 in Table 1). In particular, the polymer obtained with (S)-2d in the presence of CTA1 showed the highest optical rotation (+123.5). In addition, the formation of chiral pol(BzF) was confirmed by circular dichroism (CD) (Figure S3). Similar CD spectra as well as positive specific optical rotations were also observed for those obtained with (S)-2a and (S)-2b (Figure S3 and entries 6 and 8 in Table S2).
Thus, dual control of the optical activity and molecular weight of poly(BzF), i.e., asymmetric living cationic polymerization of BzF, was achieved using thioether as a chain-transfer agent and β-amino acid derivatives as chiral additives in the presence of Lewis acid catalysts.
Asymmetric Block Polymerization
This success prompted us to examine the synthesis of asymmetric block copolymers. Prior to this, we used each enantiomer of 2a, i.e., (S)-2a and (R)-2a, for living cationic polymerization of BzF with CTA1 and AlCl3. The polymerizations proceeded at almost the same rate (Figure 3A) and produced polymers with almost the same molecular weight, which similarly increased with monomer conversions, maintaining a unimodal and narrow distribution (Figure S4). As in the polymers obtained without CTA, each polymer showed opposite optical activity with similar absolute values (entries 6 and 7 in Table S2). More interestingly, in both cases, the absolute values of the optical specific rotations increased with increasing the molecular weights, and the plots were almost mirror images (Figure 3B). Moreover, the obtained polymers exhibited mirror-imaged CD spectra (Figure 3C). In addition, the lack of dependence of the CD spectra on the measured temperature suggests that the optical activity results not from conformational chirality but from configurational chirality in the main chains (Figure S5). Thus, (S)-2a and (R)-2a gave moderately living polymers with opposite main chain configurational chirality when used with CTA1 and AlCl3.
Figure 3.
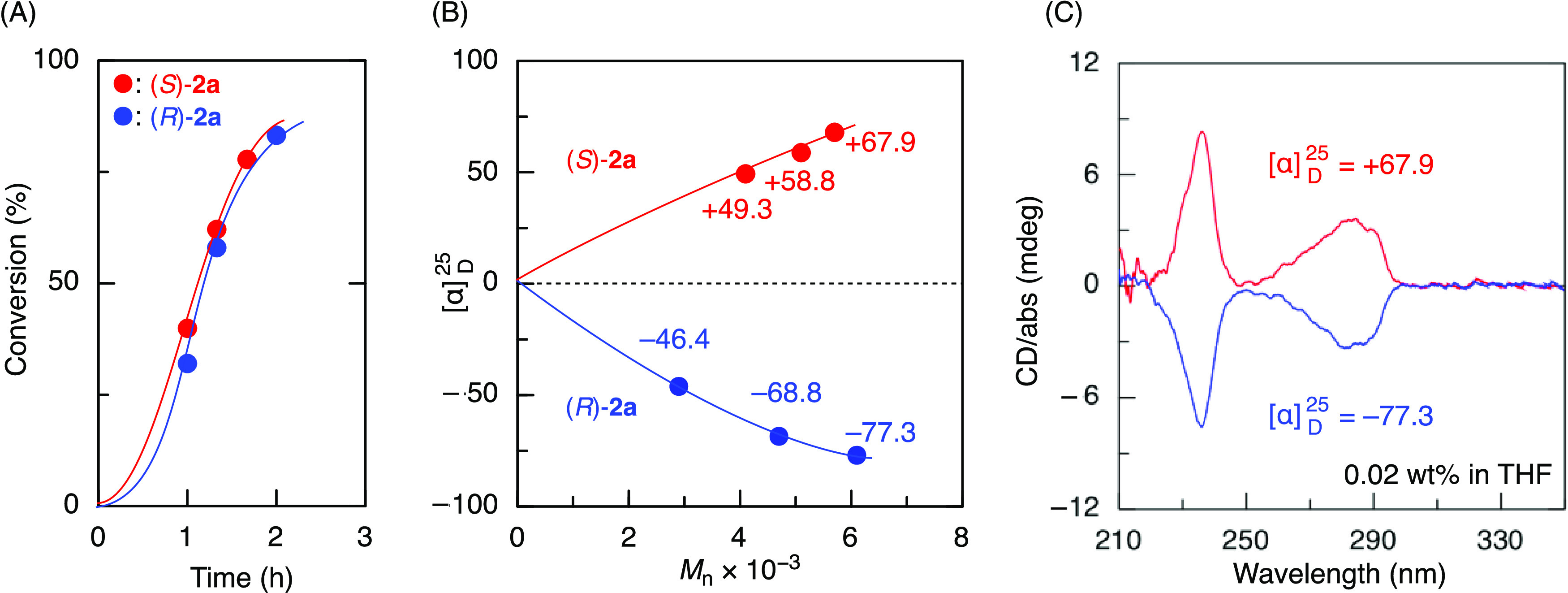
Time-conversion curves for asymmetric living cationic polymerization using enantiomer additives (S)-2a and (R)-2a (A), Mn-specific rotation ([α]D25) (B), and CD spectra (C) of the obtained polymers; [BzF]0/[CTA1]0/[AlCl]3/[(S)-2a or (R)-2a]0 = 200/4.0/40/20 mM in toluene at −78 °C.
The asymmetric block polymerization was then examined using (S)-2a and (R)-2a sequentially as chiral additives in the cationic DT polymerization based on the dormant thioether terminal. Poly(BzF) with thioether terminals could be isolated and used as a macro CTA because the thioether group was stable, and its amount was maintained quantitatively, as indicated by the 1H NMR and MALDI-TOF-MS (see above). The asymmetric moderately living cationic polymerization was thus conducted using (S)-2a in the presence of CTA1 and AlCl3 to obtain optically active polymers with a positive specific optical rotation (+49.3) and controlled molecular weight (Mn = 7100, Mw/Mn = 1.40) (Figure 4A).
Figure 4.
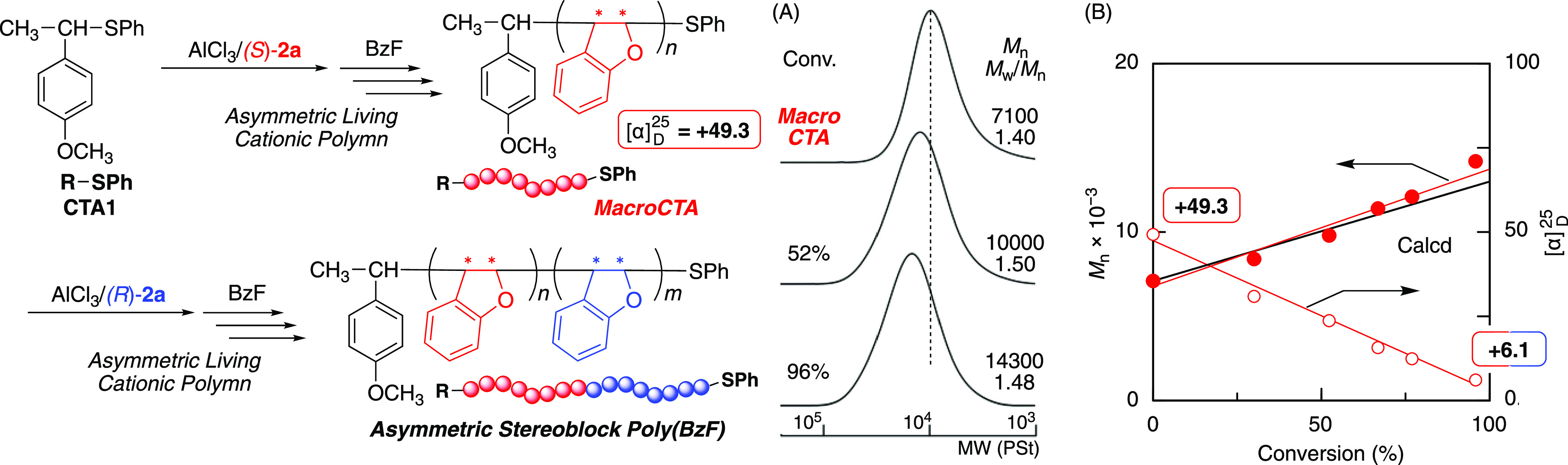
SEC curves (A) and Mn and specific rotation (B) of asymmetric stereoblock polymers obtained in asymmetric living cationic polymerization using macro CTA and (R)-2a: [BzF]0/[CTA1]0/[AlCl3]0/[(S)-2a]0 = [BzF]0/[macro CTA]0/[AlCl3]0/[(R)-2a]0 = 200/4.0/40/20 mM in toluene at −78 °C.
The obtained polymer, which was isolated by precipitation in methanol, was employed as a macro CTA for the next asymmetric cationic DT polymerization of BzF with (R)-2a as the enantiomer additive. The second polymerization also proceeded smoothly, leading to a further shift of the unimodal SEC curve to a high molecular weight (Figure 4A). The Mn values increased in direct proportion to monomer conversion and agreed well with the theoretical values (filled red circles in Figure 4B), indicating that block polymerization successfully proceeded using the macro CTA. On the other hand, the optical activity decreased in direct proportion to monomer conversion from +49.3 to +6.1 (open red circles in Figure 4B). With this change, the intensity of the CD signals gradually decreased and finally approached zero (Figure S6). Thus, the synthesis of an asymmetric stereoblock polymer, which consists of two segments with opposite absolute configurations of asymmetric carbon in the main chain, was successfully achieved using each enantiomer sequentially as chiral additives in the cationic DT polymerization.
Regio- and Stereospecificity in Asymmetric Cationic Polymerization
In this final part, regio- and stereospecificity in the asymmetric cationic polymerization of BzF were analyzed comprehensively. There are two possibilities: that the propagation of BzF occurs via a vinyl ether- or styryl-type cation, i.e., 2,1- or 1,2-addition, although the regioselectivity has not been clarified.10−12 To reveal this, we prepared two thioether regioisomers, CTA2 and CTA3, which are both thiophenol adducts of BzF and can generate a vinyl ether- and styryl-type cation of BzF, respectively. We employed them in the asymmetric cationic polymerization of BzF with (S)-2a and AlCl3 in toluene at −78 °C.
With CTA2, which can generate vinyl ether-type cations via chain transfer, the polymerization proceeded smoothly, although it became slower at the later stage in comparison to that with CTA1 (Figure 5A). The SEC curves shifted to high molecular weight, although a slight shoulder was observed at high monomer conversion (Figure 5B). On the other hand, polymerization did not occur using CTA3, suggesting that CTA3 could not generate the styryl-type cation and acted as an inhibitor rather than a chain-transfer agent by forming a stable sulfonium intermediate due to a stronger C–S bond of CTA3. These results indicate that CTA2, which can generate a more stable oxocarbenium cation, functions efficiently as a CTA and that asymmetric cationic propagation occurs by forming a vinyl ether-type cation via predominant 2,1-addition. Furthermore, model cationic addition reactions between CH3CH(OiBu)Cl and BzF with SnCl4/nBu4NCl indicated that 2,1-addition predominantly occurred in toluene at −78 °C (2.1-/1.2- = 79/21), although the regioselectivity was opposite in CH2Cl2 (2.1-/1.2- = 14/86) (see Figure S7).37
Figure 5.
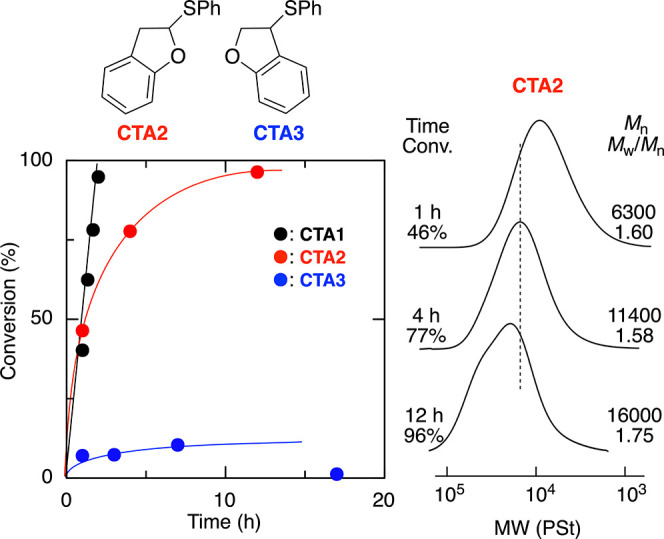
Time-conversion curves (A) and SEC curves (B) for asymmetric living cationic polymerization using CTA2 or CTA3: [BzF]0/[CTA2 or CTA3]0/[AlCl3]0/[(S)-2a]0 = 200/4.0/40/20 mM in toluene at −78 °C.
To examine solvent effects on the asymmetric moderately living cationic polymerization, CH2Cl2 was used for BzF in conjunction with CTA1, AlCl3, and (R)-2a at −78 °C. The polymerization was faster in CH2Cl2 than in toluene due to the higher polarity, which is generally true in cationic polymerizations (Figure S8). Although the molecular weights of the resulting polymers were close to the theoretical values at low monomer conversion, they became gradually higher at high monomer conversion, suggesting that Friedel–Crafts reactions between polymer chains occurred due to more dissociated cationic propagation species in CH2Cl2. Furthermore, the specific optical rotation of the polymer obtained in CH2Cl2 was lost (+1.0), whereas that obtained in toluene was −77.3. These results indicate that the choice of the solvent is important and that toluene is suitable for attaining dual control of the molecular weight and optical activity of the resulting polymers.
The polymers obtained in toluene and CH2Cl2 were analyzed in more detail by 1H and 13C NMR to clarify the stereostructures. The 1H NMR spectrum of the polymers obtained in CH2Cl2 showed broader peaks than those obtained in toluene (Figure 6A,B), indicating lower stereoregularity in CH2Cl2. The stereoregularity was evaluated in more detail by 13C NMR, in which the characteristic peak of methine carbon (47–50 ppm) in the main chain attached to the phenyl group was used (Figures 6C,D, and S9). In particular, the polymers obtained in toluene showed a very sharp peak at 49 ppm and a small distinctive peak at approximately 48 ppm. The peaks at 49 (X) and 48 ppm (Y) can be most probably ascribed to the trans-threo and cis-erythro enchainments of the BzF unit, respectively, according to the 13C NMR spectra of trans- and cis-2,3-dihydoro-2,3-dimethyl benzofuran as model compounds and poly(2,3-dihydrofuran) as similar cyclic vinyl ether polymers, where the peaks observed at a lower and higher magnetic filed were assigned to the trans- and cis-carbons, respectively.38−41 The peak intensity ratio of X/Y was 95:5, indicating a high trans-threo selectivity during cationic polymerization with AlCl3 and (R)-2a in toluene at −78 °C. This ratio was almost the same as that obtained without CTA1 (Figure S10). In contrast, in the 13C NMR spectrum of the polymers obtained in CH2Cl2, a peak assignable to the cis structure became more visible, and additional peaks at 49–50 ppm became larger, where the X/Y ratio was 89:11. Although the exact stereostructures of poly(BzF) are difficult to determine due to possible concurrent 2,1- and 1,2-progation, which could affect the peak shapes, it can be concluded that trans-threo addition is at least predominant in toluene at −78 °C. Thermal properties of the obtained polymers with different tacticities and different optical activities were evaluated by differential scanning calorimetry (DSC) (Figure S11). All polymers showed nearly the same high Tgs (184–189 °C) and no melting temperatures irrespective of their different stereostructures.
Figure 6.
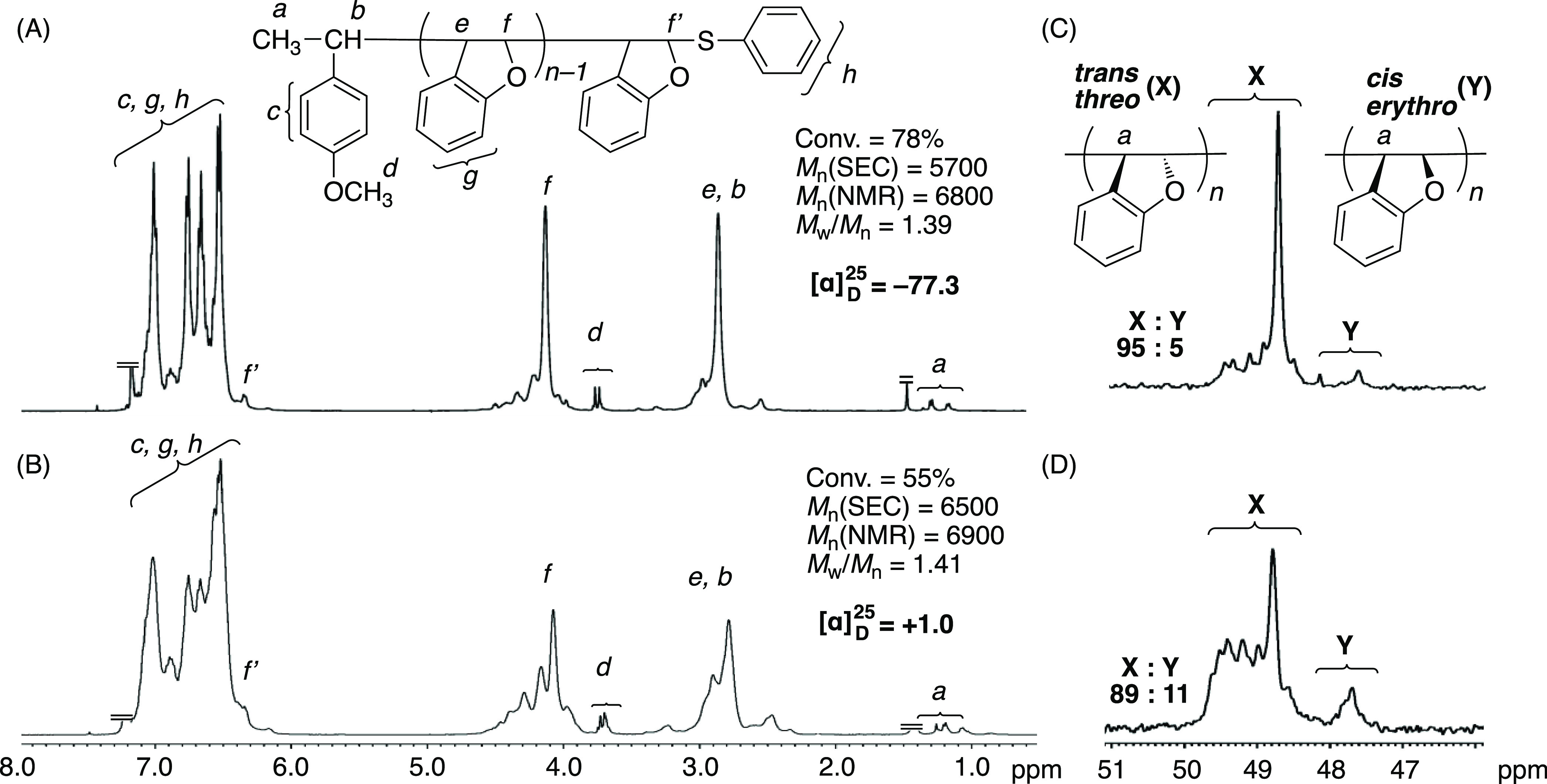
1H NMR (A, B) and 13C NMR (C, D) spectra (CDCl3, 55 °C) of the polymers obtained in asymmetric living cationic polymerization of BzF in toluene (A, C) and CH2Cl2 (B, D): [BzF]0/[CTA1]0/[AlCl3]0/[(R)-2a]0 = 200/4.0/40/20 mM in toluene or CH2Cl2 at −78 °C.
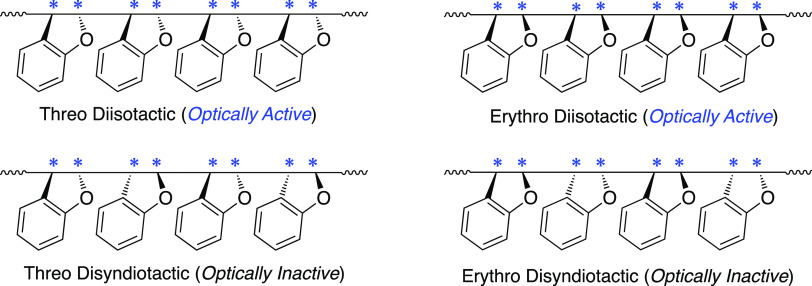
Based on these results, the stereostructures of the optically active poly(BzF) can be elucidated. Since BzF is a 1,2-disubstituted cyclic unsymmetric monomer, there are four possible tactic stereostructures for poly(BzF), i.e., threo-diisotactic, threo-disyndiotactic, erythro-diisotactic, and erythro-disyndiotactic (Figure 7).6,8,16−18 Although the four stereoregular structures are chiral due to the absence of mirror planes in the polymer chains, only threo-diisotactic and erythro-diisotactic structures can become optically active because they are composed of enantiomeric trans-threo and cis-erythro sequences, respectively. The threo-disyndiotactic and erythro-disyndiotactic poly(BzF)s are also chiral, but their optical activities may be negligibly small because they consist of racemic trans-threo and cis-erythro sequences, respectively. Considering that optically active poly(BzF) obtained in toluene consisted predominantly of trans-threo-structures, the main structure of poly(BzF) obtained in the asymmetric cationic polymerization is threo-diisotactic, which has been clarified since the first discovery of optically active poly(BzF) by Natta in 1961.
Figure 7.
Possible tactic stereostructures of poly(BzF).
Conclusions
Dual control of the optical activity and molecular weight of poly(BzF) was achieved using optically active β-amino acid derivatives as chiral additives and thioethers as reversible chain-transfer agents in the presence of AlCl3 as Lewis acid catalysts. The configurational chirality of the main chains was achieved by enantioselective propagation induced by the chiral catalysts, while the molecular weight was controlled by reversible chain transfer between the growing cationic species and dormant C–S terminals. The asymmetric living cationic polymerization enabled the synthesis of an asymmetric block polymer consisting of segments with opposite absolute configurations. Furthermore, a comprehensive analysis of the resulting polymers and model reactions revealed that the optical activity of poly(BzF) originates from a threo-diisotactic structure, which is a consequence of triple selectivity (enantio-, stereo-, and regioselectivity) during cationic propagation. Thus, the high versatility and applicability of the cationic DT mechanism for enabling molecular weight control is confirmed even in asymmetric polymerization and widens the scope of precision polymer synthesis for multiple-factor control of polymer structures.
Acknowledgments
This work was partially supported by JSPS KAKENHI Grant-in-Aid for Early-Career Scientists (Nos. JP18K14274 and JP20K15332) for M.U. and for Scientific Research (A) (No. JP22H00333) for M.K., the Asahi Glass Foundation for M.U., and Nagoya University Research Fund. The authors thank Prof. Yoshitaka Aramaki (Nagoya University) and Prof. Takashi Ooi (Nagoya University) for technical support with HRMS and IR measurements, and Prof. Daisuke Taura (Meijo University) and Prof. Eiji Yashima (Nagoya University) for technical support with CD measurements, fruitful discussions on chirality and optical activity of polymers, and valuable comments on the manuscript.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/jacs.2c02569.
Materials, experimental procedures, additional polymerization results, SEC, NMR, IR, and CD spectra (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Nakano T.; Okamoto Y.. Stereocontrolled Chiral Polymers. In Polymer Science: A Comprehensive Reference; Matyjazsewski K.; Möller M., Eds.; Elsevier: Amsterdam, 2012; Vol. 6, pp 629–687. [Google Scholar]
- Yashima E.; Ousaka N.; Taura D.; Shimomura Y.; Ikai T.; Maeda K. Supramolecular Helical Systems: Helical Assemblies of Small Molecules, Foldamers, and Polymers with Chiral Amplification and Their Functions. Chem. Rev. 2016, 116, 13752–13990. 10.1021/acs.chemrev.6b00354. [DOI] [PubMed] [Google Scholar]
- Worch J. C.; Prydderch H.; Jimaha S.; Bexis P.; Becker M. L.; Dove A. P. Stereochemical enhancement of polymer properties. Nat. Rev. Chem. 2019, 3, 514–535. 10.1038/s41570-019-0117-z. [DOI] [Google Scholar]
- Chain Polymerization of Vinyl Monomers. In Polymer Science: A Comprehensive Reference; Coates G. W.; Sawamoto M.; Matyjazsewski K.; Möller M., Eds.; Elsevier: Amsterdam, 2012; Vol. 3, pp 1–954. [Google Scholar]
- Teator A. J.; Varner T. P.; Knutson P. C.; Sorensen C. C.; Leibfarth F. A. 100th Anniversary of Macromolecular Science Viewpoint: The Past, Present, and Future of Stereocontrolled Vinyl Polymerization. ACS Macro Lett. 2020, 9, 1638–1654. 10.1021/acsmacrolett.0c00664. [DOI] [PubMed] [Google Scholar]
- Okamoto Y.; Nakano T. Asymmetric Polymerization. Chem. Rev. 1994, 94, 349–372. 10.1021/cr00026a004. [DOI] [Google Scholar]
- Wulff G. Main-Chain Chirality and Optical-Activity in Polymers Consisting of C–C Chains. Angew. Chem., Int. Ed. 1989, 28, 21–37. 10.1002/anie.198900211. [DOI] [Google Scholar]
- Ito S.; Nozaki K.. Asymmetric Polymerization. In Catalytic Asymmetric Synthesis, 3rd ed.; Ojima I., Ed.; John Wiley: New York, 2010; pp 931–985. [Google Scholar]
- Hatada K.; Kahovec J.; Barón M.; Horie K.; Kitayama T.; Kubisa P.; Moss G. P.; Stepto R. F. T.; Wilks E. S. Definitions Relating to Stereochemically Asymmetric Polymerizations (IUPAC Recommendations 2001). Pure Appl. Chem. 2002, 74, 915–922. 10.1351/pac200274060915. [DOI] [Google Scholar]
- Mizote A.; Tanaka T.; Higashimura T.; Okamura S. Cationic Polymerization of Cyclic Olefins. J. Polym. Sci., Part A-1: Polym. Chem. 1966, 4, 869–879. 10.1002/pol.1966.150040413. [DOI] [Google Scholar]
- Okuyama T.; Kunugiza K.; Fueno T. Substituent Effects in Benzofuran System. III. Cationic Polymerization. Bull. Chem. Soc. Jpn. 1974, 47, 1271–1273. 10.1246/bcsj.47.1271. [DOI] [Google Scholar]
- Lian B.; Ma H.; Spaniol T. P.; Okuda J. Neutral and Cationic Aluminum Complexes Containing a Chrial (OSSO)-Type Bis(phenolato) Ligand: Synthesis, Structures and Polymerization Activity. Dalton Trans. 2009, 9033–9042. 10.1039/b909287k. [DOI] [PubMed] [Google Scholar]
- Lin F.; Wang M.; Cui D. Renewable Benzofuran Initiated by Lewis Acid Al(C6F5)3 and Mechanism. Macromolecules 2017, 50, 8449–8455. 10.1021/acs.macromol.7b01928. [DOI] [Google Scholar]
- Lu L.; Hua R. A Monomer-polymer-monomer (MPM) Organic Synthesis Strategy: Synthesis and Application of Polybenzofuran for Funtionalizing Benzene Ring of Bnzofuran. Asian J. Org. Chem. 2021, 10, 2137–2142. 10.1002/ajoc.202100208. [DOI] [Google Scholar]
- Natta G.; Farina M.; Peraldo M.; Bressan G. Asymmetric Synthesis of Optically Active Di-isotactic Polymers from Cyclic Monomers. Makromol. Chem. 1961, 43, 68–75. 10.1002/macp.1961.020430106. [DOI] [Google Scholar]
- Yamada A.; Yanagita M.; Kobayashi E. Stereoregular Polycycloolefins. J. Polym. Sci. 1962, 61, S14–S16. 10.1002/pol.1962.1206117132. [DOI] [Google Scholar]
- Natta G.; Farina M. Optically Active Compounds: An Exception to the Usual Definition of Asymmetric Carbon Atom. Tetrahedron. Lett. 1963, 11, 703–709. [Google Scholar]
- Farina M.; Bressan G. Optically Active Polymers: Some New Results and Remarks on the Asymmetric Polymerization of Benzofuran. Makromol. Chem. 1963, 61, 79–89. 10.1002/macp.1963.020610108. [DOI] [Google Scholar]
- Takeda Y.; Hayakawa Y.; Fueno T.; Furukawa J. Studies on the Mechanism of the Stereospecific Polymerization. Asymmetric-induction Polymerization of Benzofuran by Use of Optically Active Organo-stannic Compounds. Makromol. Chem. 1965, 83, 234–243. 10.1002/macp.1965.020830121. [DOI] [Google Scholar]
- Bressan G.; Farina M.; Natta G. Optically Active Polymers: The Asymmetric Synthesis of Polynaphthofurans. Makromol. Chem. 1966, 93, 283–288. 10.1002/macp.1966.020930124. [DOI] [Google Scholar]
- Hayakawa Y.; Fueno T.; Furukawa J. Catalysts for Asymmetric-Induction Polymerization of Benzofuran. II. Properties and Catalysts of Some Binary Systems Containing the Menthoxy Group. J. Polym. Sci., Part A-1: Polym. Chem. 1967, 5, 2099–2109. 10.1002/pol.1967.150050826. [DOI] [Google Scholar]
- Yonezumi M.; Kanaoka S.; Aoshima S. Living Cationic Polymerization of Dihydrofuran and its Derivatives. J. Polym. Sci., Part A-1: Polym. Chem. 2008, 46, 4495–4504. 10.1002/pola.22785. [DOI] [Google Scholar]
- Uchiyama M.; Satoh K.; Kamigaito M. Cationic RAFT Polymerization Using ppm Concentrations of Organic Acid. Angew. Chem., Int. Ed. 2015, 54, 1924–1928. 10.1002/anie.201410858. [DOI] [PubMed] [Google Scholar]
- Uchiyama M.; Satoh K.; Kamigaito M. Thioether-Mediated Degenerative Chain-Transfer Cationic Polymerization: A Simple Metal-Free System for Living Cationic Polymerization. Macromolecules 2015, 48, 5533–5542. 10.1021/acs.macromol.5b01341. [DOI] [Google Scholar]
- Uchiyama M.; Satoh K.; Kamigaito M. A Phosphonium Intermediate for Cationic RAFT Polymerization. Polym. Chem. 2016, 7, 1387–1396. 10.1039/C5PY01879J. [DOI] [Google Scholar]
- Kamigaito M.; Satoh K.; Uchiyama M. Degenerative Chain-Transfer Process: Controlling All Chain-Growth Polymerizations and Enabling Novel Monomer Sequences. J. Polym. Sci., Part A: Polym. Chem. 2019, 57, 243–254. 10.1002/pola.29257. [DOI] [Google Scholar]
- Kamigaito M.; Sawamoto M. Synergistic Advances in Living Cationic and Radical Polymerizations. Macromolecules 2020, 53, 6749–6753. 10.1021/acs.macromol.0c01392. [DOI] [Google Scholar]
- Uchiyama M.; Satoh K.; Kamigaito M.. Cationic RAFT Polymerization. In RAFT Polymerization: Methods, Synthesis, and Applications; Moad G.; Rizzardo E., Eds.; Wiley-VCH GmbH: Weinheim, 2021; pp 1171–1194. [Google Scholar]
- Uchiyama M.; Satoh K.; Kamigaito M. Cationic RAFT and DT Polymerization. Prog. Polym. Sci. 2022, 124, 101485 10.1016/j.progpolymsci.2021.101485. [DOI] [Google Scholar]
- Uchiyama M.; Satoh K.; Kamigaito M. Diversifying Cationic RAFT Polymerization with Various Counteranions: Generation of Cationic Species from Organic Halides and Various Metal Salts. ACS Macro Lett. 2016, 5, 1157–1161. 10.1021/acsmacrolett.6b00541. [DOI] [PubMed] [Google Scholar]
- Uchiyama M.; Satoh K.; Kamigaito M. Stereospecific Cationic RAFT Polymerization of Bulky Vinyl Ethers and Stereoblock Poly(vinyl Alcohol) via Mechanistic Transformation to Radical RAFT Polymerization of Vinyl Acetate. Giant 2020, 5, 100047 10.1016/j.giant.2021.100047. [DOI] [Google Scholar]
- Knutson P. C.; Teator A. J.; Varner T. P.; Kozuszek C. T.; Jacky P. E.; Leibfarth F. A. Brønsted Acid Catalyzed Stereoselective Polymerization of Vinyl Ethers. J. Am. Chem. Soc. 2021, 143, 16388–16393. 10.1021/jacs.1c08282. [DOI] [PubMed] [Google Scholar]
- Zhang X.; Yang Z.; Jiang Y.; Liao S. Organocatalytic, Stereoselective, Cationic Reversible Addition–Fragmentation Chain-Transfer Polymerization of Vinyl Ethers. J. Am. Chem. Soc. 2022, 144, 679–684. 10.1021/jacs.1c11501. [DOI] [PubMed] [Google Scholar]
- Watanabe H.; Yamamoto T.; Kanazawa A.; Aoshima S. Stereoselective Cationic Polymerization of Vinyl Ethers by Easily and Finely Tunable Titanium Complexes Prepared from Tartrate-Derived Diols: Isospecific Polymerization and Recognition of Chiral Side Chains. Polym. Chem. 2020, 11, 3398–3403. 10.1039/D0PY00343C. [DOI] [Google Scholar]
- Teator A. J.; Leibfarth F. A. Catalyst-Controlled Stereoselective Cationic Polymerization of Vinyl Ethers. Science 2019, 363, 1439–1443. 10.1126/science.aaw1703. [DOI] [PubMed] [Google Scholar]
- Oishi M.; Yamamoto H. Polymerization of t-Butyl Vinyl Ether Mediated by an Aluminum Lewis Acid-TrF System and Its Complex Structure–Tacticity Correlation. Bull. Chem. Soc. Jpn. 2001, 74, 1445–1454. 10.1246/bcsj.74.1445. [DOI] [Google Scholar]
- A previous report in 1960s for cationic polymerization of BzF with conventional Lewis acid catalysts in CH2Cl2 concluded that the polymerization occurred possibly via the styryl-type cation, which was suggested by effects of substituents of BzF derivatives.11 On the other hand, the reported effective solvent for the asymmetric cationic polymerization of BzF was only toluene.15,17−21
- Barluenga J.; Fañanás F. J.; Sanz R.; Marcos C. Intramolecular Carbolithiation of Allyl o-Lithioaryl Ethers: A New Enantioselective Synthesis of Functionalized 2,3-Dihydrobenzofurans. Chem. Eur. J. 2005, 11, 5397–5407. 10.1002/chem.200500377. [DOI] [PubMed] [Google Scholar]
- Thapa S.; Basnet P.; Giri R. Copper-Catalyzed Dicarbofunctionalization of Unactivated Olefins by Tandem Cyclization/Cross-Coupling. J. Am. Chem. Soc. 2017, 139, 5700–5703. 10.1021/jacs.7b01922. [DOI] [PubMed] [Google Scholar]
- Xie W.-B.; Li Z. Bis(μ-oxo)–Dititanium(IV)–Chiral Binaphthyldisulfonate Complexes for Highly Enantioselective Intramolecular Hydroalkoxylation of Nonactivated Alkenes. ACS Catal. 2021, 11, 6270–6275. 10.1021/acscatal.1c01146. [DOI] [Google Scholar]
- Sanda F.; Matsumoto M. Cationic Polymerization of 2,3-Dihydrofuran. Study on the Relationship between Glass Transition Temperature and Tacticity of the Polymer. Macromolecules 1995, 28, 6911–6914. 10.1021/ma00124a029. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.