Abstract
Introduction:
Physical activity (PA) is recognized as one of the key lifestyle behaviors that reduces risk of developing dementia late in life. However, PA also leads to increased respiration, and in areas with high levels of air pollution, PA may increase exposure to pollutants linked with higher risk of developing dementia. Here, we investigate whether air pollution attenuates the association between PA and dementia risk.
Methods:
This prospective cohort study included 35,562 adults 60 and older from the UK Biobank. Average acceleration magnitude (ACCave) from wrist-worn accelerometers was used to assess PA levels. Air pollution levels (NO, NO2, PM10, PM2.5, PM2.5–10, and PM2.5 absorbance) were estimated with land use regression methods. Incident all-cause dementia was derived from inpatient hospital records and death registry data.
Results:
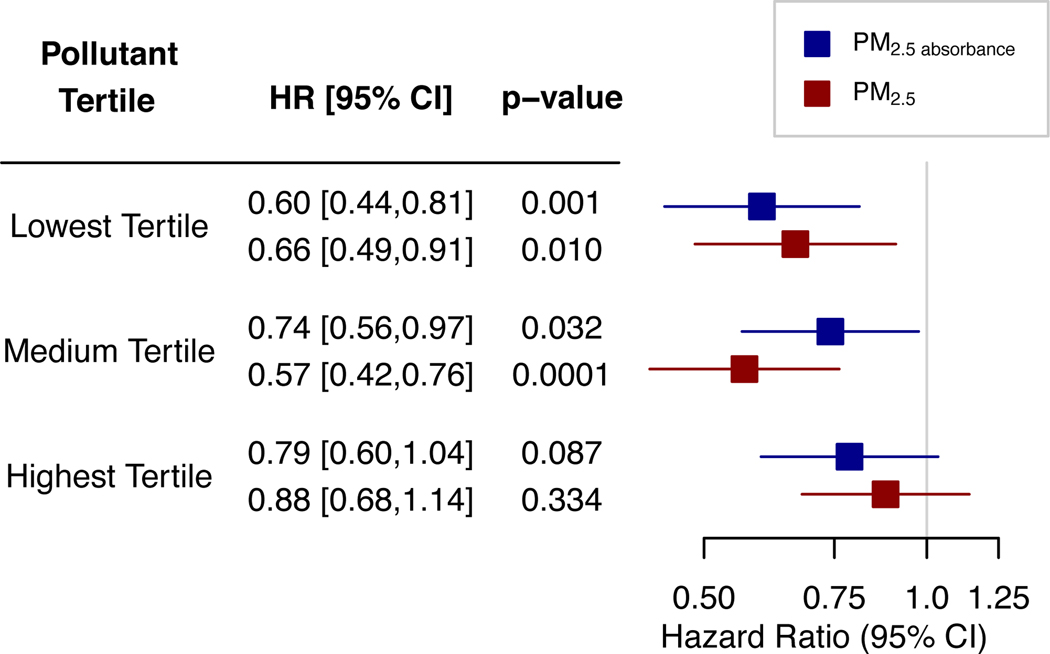
In adjusted models, ACCave was associated with reduced risk of developing dementia (HR [CI]=0.71 [0.60,0.83]), while air pollution variables were not associated with dementia risk. There were significant interactions between ACCave and PM2.5 (HRinteraction [CI]=1.33 [1.13,1.57]) and PM2.5 absorbance (HRinteraction [CI]=1.24 [1.07,1.45]) on incident dementia. At the lowest tertiles of pollution, ACCave was associated with reduced risk of incident dementia (HRPM 2.5 [CI]=0.66 [0.49,0.91], HRPM 2.5 absorbance [CI]=0.60 [0.44,0.81]). At the highest tertiles of these pollutants, there was no significant association of ACCave with incident dementia (HRPM 2.5 [CI]=0.88 [0.68,1.14], HRPM 2.5 absorbance [CI]=0.79 [0.60,1.04]).
Conclusions:
PA is associated with reduced risk of developing all-cause dementia. However, exposure to even moderate levels of air pollution attenuates the benefits of PA on risk of dementia.
Keywords: PARTICULATE MATTER, ALZHEIMER’S DISEASE, EXERCISE, ACCELEROMETER
INTRODUCTION
Engagement in physical activity (PA) is one of the key lifestyle behaviors often associated with reduced risk of developing dementia or Alzheimer’s disease in meta-analyses of prospective cohorts (1–4). Although the underlying pathways remain unclear, researchers have suggested that PA improves vascular and metabolic health, reduces age-related cognitive and brain structural decline, increases production of neurotransmitters and growth factors related to brain structural health, and alters key biomarkers associated with neurodegenerative disease including reduced amyloid-b levels in the brain (5). The weight of available evidence has led the Lancet commission on dementia prevention and the Committee on Preventing Dementia and Cognitive Impairment from the U.S. National Academies of Sciences, Engineering, and Medicine to include PA as a recommended behavioral modification that may reduce the risk of developing dementia or Alzheimer’s disease (3, 6).
While epidemiological evidence suggests that PA improves brain health and disease prevention, there may be circumstances in which the benefits of PA are inconsistent. For example, exposure to air pollution is increasingly recognized as a potential risk factor for the development of dementia in older adults (3). Specifically, exposure to particulate matter (PM2.5) and other pollutants (including NO and NO2) has been linked to age-related cognitive decline and increased risk of neurodegenerative disease (7, 8). There is some evidence that inhalation of gases or particulate matter induces inflammatory responses, microglial activation, and the deposition of amyloid plaques in the brain (9, 10). While PA generally improves cardiovascular and respiratory health through several mechanisms including beneficial changes in inflammatory status, improved blood flow, and decreases in myocardial oxygen demand (11), PA may also exacerbate the effects of pollution on health because it effectively increases exposure to pollutants due to higher respiration rates (higher minute ventilation: the volume of air displaced per minute of respiration) compared to rest (12). For example, during cycling commutes, individuals have minute ventilation two to five times higher than at rest, leading to a larger volume of lung exposure to air pollution, and higher fractions of particulate matter (PM) deposited in the respiratory tract or bloodstream (12). Acute exposure to air pollution during exercise is associated with altered blood biomarkers of inflammation and cardiovascular risk (13), reduced pulmonary function and airway inflammation (14), and attenuated exercise-induced increases in circulating brain-derived neurotrophic factor, a protein thought to be at least partially responsible for the brain benefits of exercise (15).
Previous work has shown that PA in areas of high pollution may diminish the benefits of exercise for overall health. For example, Lin et al. (16) showed that high levels of PA exacerbated the effects of air pollution on stroke risk. In addition, a recent meta-analysis demonstrated that exercise in areas of high air pollution was associated with decreased pulmonary function and increased vascular damage that are both linked with negative health outcomes (14). Similarly, exposure to black carbon, a constituent of PM2.5, was found to reduce the benefits of PA for lung function (17). However, many studies find no significant interaction between PA and pollution on risk of myocardial infarction (11), arterial stiffness (18), hypertension (19), diabetes (20), and cardiovascular or respiratory mortality (21).
While the balance of these results suggest that pollution may not impact the health benefits of PA, these studies all used self-reported questionnaires to assess activity levels, which are often prone to recall and social-desirability biases (22). To date, we are not aware of any study that has examined the interaction between PA as measured by accelerometry and air pollution exposure on the risk of developing dementia. The goal of this study is to determine how objectively measured PA and air pollution interact to alter or attenuate the risk of developing all-cause dementia in a large prospective study, the UK Biobank. We predict that PA will be associated with reduced risk of incident dementia in low and moderate, but not in the highest levels of pollution exposure.
METHODS
Sample
The UK Biobank, funded by the Wellcome Trust and other charity agencies in the UK, set out in 2006 to collect extensive baseline and follow-up data on a large sample of participants in the UK, ages 40–69 (23). Individuals were invited to participate in the study if they were aged 40–69, lived within 40km of one of 22 assessment centers in England, Scotland, and Wales, and were registered with the National Health Service (23). Over 500,000 volunteered and consented to join this community-dwelling study cohort between 2006 and 2010 (23). For this study, we restricted our primary analyses to individuals without prevalent dementia who were 60 years of age and older at start of the follow-up period (see below). This study was approved by the University of Southern California IRB, #UP-20–00453.
Physical activity
Approximately 100,000 participants were asked to wear, over the course of seven days, an Axivity AX3 wrist-worn accelerometer (24) in a follow-up sub-study from 2013–2015. Devices began collecting data at 10am two working days after mailing (24). Accelerometers were set to record tri-axial accelerations at 100 Hz for a seven day period and had a dynamic range of ±8g (24). Individuals were asked to wear the accelerometer on their dominant wrist beginning immediately after they received the device in the mail, and to continue with their normal activities throughout the duration of accelerometer wear (24). Raw data were processed by the UK Biobank team with methods previously described in Doherty et al. (24). Briefly, 3D accelerometer data were converted to vector magnitudes (VM), and 1g was subtracted from the VMs (negative values rounded to zero) to calculate the acceleration magnitude (mg). We used average acceleration magnitude (ACCave) as our PA measure here, and excluded individuals with less than 72 hours of data, those not having data in each one-hour period of the 24-hour cycle, and individuals whose device could not be calibrated, using quality control (QC) variables provided by the UK Biobank (n=6,997). ACCave was standardized with a mean of 0 and standard deviation of 1.0 for analyses.
Air pollution exposure
Estimated levels of NO, NO2, PM10, PM2.5 (fine particulate matter with diameter < 2.5 μm), coarse particulate matter (diameters between 2.5 and 10 μm), and PM2.5 absorbance (an estimate of black carbon) were determined by land use regression methods (LUR) and were scaled to interquartile ranges (IQR). NO and NO2 are highly reactive gases that are generated primarily from burning of fuel in motor vehicles, power plants, and other motorized equipment, and these pollutants act as an irritant of the respiratory system with deep penetration into the lungs (25). Likewise, PM is linked with combustion engines and power plants, as well as with combustion of natural fuel during wildfires (25). While the constituent elements of PM can vary, particles less than 10 μm can invade the lungs and bloodstream, with smaller particles (< 2.5 μm) penetrating deeper and potentially conferring greater health risks than larger particles (25). Exposure to these pollutants are often linked with both respiratory and cardiovascular disease risks (25). Values were generated for 2010 as part of ESCAPE (European Study of Cohorts for Air Pollution Effects) (26, 27). For UK Biobank participants, values were linked to their geocoded residential addresses at baseline. PM and gases (NO and NO2) were developed using separate LUR models (26, 27). Detailed descriptions of LUR methods and sites used are found in Beelen et al. (26) and Eeftens et al. (27). LUR models performed well across much of the study area. R2 values for NO and NO2 in LUR models in the study region ranged from 0.83 to 0.91 (27), and the r2 for PM2.5 and PM2.5 absorbance ranged from 0.82–0.96 in included study regions (26). Models for PM measures that were >400 km from the center of London had metrics that were below the ESCAPE threshold, and air pollution for participants in these areas are not reported by the UK Biobank.
Dementia diagnosis
Following Lourida et al. (28), hospital inpatient records were used to determine all-cause dementia diagnoses. Hospital records were drawn from the Hospital Episode Statistics for England, the Scottish Morbidity Record data for Scotland, and the Patient Episode Database for Wales. These databases contained information on hospital admissions dates and diagnoses. Linkages with death register datasets for England, Scotland, and Wales provided additional cases. We used the International Classification of Diseases (ICD) coding system to determine primary or secondary diagnoses of all-cause dementia from hospital records, or as underlying or contributing cause of death from death register linkages (see Lourida et al. (28) for ICD9 and ICD10 codes used for diagnoses).
Statistical Analyses
We conducted complete case analyses and restricted these analyses to participants 60 years and older at the start of follow-up with complete covariate, exposure, and outcome data (n=35,562). We used single-pollutant Cox proportional hazard regression models to examine the associations of ACCave and air pollution with incident all-cause dementia. To determine whether high levels of air pollution exposure attenuate the association of PA with incident dementia risk, we included an interaction term of ACCave × air pollution. To maximize time to follow-up and incident cases, we considered participants to be at risk for dementia from the end of the year of air pollution exposure measurement (December 31, 2010; see Supplementary Tables, SDC 1, for alternative start-time analyses). Participants were followed up until their first dementia diagnosis, death, loss to follow-up, or to the last date of hospital admission from the respective database (England: December 31, 2020, Wales: March 6, 2018, Scotland: February 5, 2021). We tested the proportionality of hazards assumption using Schoenfeld residuals (29) (p > 0.05 for all models).
Models were adjusted for a range of covariates, all measured at the baseline visit, that accounted for demographic, lifestyle, and health characteristics. First, models were adjusted for demographic characteristics including age at the start of follow-up, sex, education (higher education/no higher education), socioeconomic status using the Townsend deprivation index (higher score is associated with higher deprivation), presence of the APOE ε4 allele (genetic risk for dementia; 0, 1, or 2 ε4 alleles), and ethnicity (white/non-white). Fully adjusted models were then evaluated which included the following additional covariates: chronic conditions (received a diagnosis of cardiovascular disease, diabetes, or cancer), self-reported general health (excellent/good/fair/poor), smoking status (never smoker, former smoker, current smoker), alcohol use (none, moderate alcohol consumption, high alcohol consumption following Lourida et al. (28)), body mass index (BMI), depression (self-report or doctor diagnosed), adherence to a healthy diet (derived from Lourida et al. (28)), and accelerometer wear duration (measured at time of accelerometer sub-study). Because the start of the follow up occurred prior to accelerometer data collection, we also included the difference in time from start of follow-up to the start of accelerometer wear as a covariate in fully adjusted models. Correction for multiple testing was implemented using the Benjamini-Hochberg false discovery rate (FDR) method (30), with FDR<0.05 considered as statistically significant. In sensitivity analyses, we excluded participants who developed dementia within seven years of the start of follow-up, included all participants aged 40 and older, and examined models using the date of accelerometer wear as the start of follow-up periods in Cox proportional hazards models.
RESULTS
Among the participants with accelerometer data available, there was a total of 35,562 participants after excluding participants younger than 60 years, those with prevalent dementia at the start of follow-up, and those that did not have complete covariate data. Baseline characteristics of participants included in this study are shown in Table 1. Over 350,978 person years of follow-up, there were 283 cases of incident dementia. Incidence rates for this sample are similar to those found in the full UK Biobank cohort by Lourida et al. (28). Participants that developed dementia were significantly more likely to be older, male, to have lower ACCave, to have APOE ε4 alleles, to have smoked, to have consumed less alcohol, and to self-report poorer general health (Table 1).
Table 1.
Baseline characteristics of study participants and levels of PA during accelerometer wear.
| No Incident Dementia (n = 35,279) | Incident Dementia (n = 283) | ||
|---|---|---|---|
|
|
|||
| Variables | Mean (SD) or n (%) | Mean (SD) or n (%) | p-value |
| Age (years) | 64.96 (3.28) | 67.43 (3.17) | <0.001 |
| Sex (Female) | 17,767 (50.36) | 124 (43.82) | 0.03 |
| Education (college or higher) | 14,116 (40.01) | 119 (42.05) | 0.53 |
| Townsend deprivation index | −2.04 (2.61) | −1.89 (2.73) | 0.39 |
| Ethnicity (white) | 34,765 (98.54) | 277 (97.88) | 0.50 |
| APOE genotype | <0.001 | ||
| 1 ε4 allele | 8,916 (25.27) | 120 (42.40) | |
| 2 ε4 alleles | 713 (2.02) | 29 (10.25) | |
| BMI (kg/m2) | 26.77 (4.2) | 26.88 (4.92) | 0.70 |
| Smoking status | <0.001 | ||
| never | 18,114 (51.34) | 129 (45.58) | |
| former | 15,335 (43.47) | 140 (49.47) | |
| current | 1,830 (5.19) | 14 (4.95) | |
| Alcohol | <0.001 | ||
| never | 4,640 (13.15) | 54 (19.08) | |
| moderate | 18,665 (52.91) | 143 (50.53) | |
| excessive | 11,974 (33.94) | 86 (30.39) | |
| Any Chronic condition (present) | 14,446 (40.95) | 125 (44.17) | 0.30 |
| General health | <0.001 | ||
| excellent | 7,433 (21.07) | 46 (16.25) | |
| good | 21,938 (62.18) | 158 (55.83) | |
| fair | 5,202 (14.75) | 57 (20.14) | |
| poor | 706 (2) | 22 (7.77) | |
| Depression (yes) | 12,124 (34.37) | 103 (36.4) | 0.51 |
| Healthy diet score (yes) | 20,540 (58.22) | 159 (56.18) | 0.53 |
| ACCave (mg) | 26.29 (7.38) | 23.29 (7.19) | <0.001 |
| PM2.5 absorbance | 1.09 (0.31) | 1.09 (0.32) | 0.42 |
| PM2.5 | 9.77 (1.28) | 9.85 (1.2) | 0.15 |
| PM10 | 15.95 (1.82) | 16.04 (1.87) | 0.02 |
| PM2.5-PM10 | 6.08 (0.75) | 6.07 (0.82) | 0.19 |
| NO | 39.99 (16.34) | 39.91 (15.85) | 0.32 |
| NO2 | 24.83 (10.11) | 24.88 (8.76) | 0.34 |
Note: air pollution variables are presented as median (interquartile range). P-values from t-tests or chi square tests comparing baseline characteristics in samples with and without incident dementia.
In models controlling for demographic factors, ACCave was significantly associated with reduced risk of incident dementia after controlling for air pollution exposure and FDR correction for multiple comparisons (HR [95%CI] = 0.67 [0.57–0.79]; Table 2). Controlling for ACCave, no measure of air pollution was significantly associated with incident dementia after FDR correction (Table 2). Additional adjustment for health and lifestyle factors did not change these results (Table 2). In minimally adjusted models, interactions between ACCave and air pollution exposures were positive and significant for all pollutants except NO2 (Table 2). Following FDR correction, interactions were significant for PM2.5 absorbance (HR [95%CI] = 1.26 [1.08–1.47]) and PM2.5 (HR [95%CI] = 1.35 [1.14–1.60]), but not for other air pollution exposures (Table 2). These results remained significant in fully adjusted models following FDR correction (PM2.5 absorbance: HR [95%CI] = 1.24 [1.07–1.45]; PM2.5 HR [95%CI] = 1.33 [1.13–1.57]; Table 2). When participants were split into tertiles according to their level of air pollution exposure, the associations of ACCave with reduced risk of incident dementia were strongest among those at low levels of pollution, but were attenuated towards the null among those in the 3rd tertile for PM2.5 absorbance and PM2.5 (Fig. 1).
Table 2.
Risk of incident dementia (n=283) according to ACCave and air pollution exposure
| Model 1a | Model 2b | |||||
|---|---|---|---|---|---|---|
| variable | HR (95% confidence limits) | p-value | FDR p-value | HR (95% confidence limits) | p-value | FDR p-value |
| ACCave | 0.67 (0.57,0.79) | 6.26E-07 | 1.63E-05 | 0.71 (0.60,0.83) | 3.76E-05 | 4.89E-04 |
| PM2.5 absorb | 1.04 (0.90,1.20) | 0.616 | 0.616 | 1.04 (0.90,1.21) | 0.602 | 0.616 |
| PM2.5 | 1.09 (0.93,1.27) | 0.287 | 0.373 | 1.1 (0.94,1.28) | 0.253 | 0.346 |
| PM10 | 1.13 (1.02,1.26) | 0.022 | 0.073 | 1.14 (1.02,1.26) | 0.020 | 0.073 |
| PM2.5-PM10 | 1.07 (0.98,1.18) | 0.141 | 0.207 | 1.08 (0.98,1.18) | 0.135 | 0.207 |
| NO | 1.04 (0.91,1.19) | 0.554 | 0.600 | 1.04 (0.92,1.19) | 0.517 | 0.600 |
| NO2 | 1.05 (0.89,1.25) | 0.545 | 0.600 | 1.06 (0.89,1.25) | 0.528 | 0.600 |
| PM2.5 absorb*ACCave | 1.26 (1.08,1.47) | 0.004 | 0.021 | 1.24 (1.07,1.45) | 0.005 | 0.023 |
| PM2.5 * ACCave | 1.35 (1.14,1.60) | 3.95E-04 | 0.003 | 1.33 (1.13,1.57) | 0.001 | 0.004 |
| PM10*ACCave | 1.15 (1.01,1.32) | 0.032 | 0.077 | 1.15 (1.01,1.31) | 0.040 | 0.077 |
| PM2.5 – PM10*ACCave | 1.13 (1.01,1.26) | 0.036 | 0.077 | 1.12 (1.00,1.26) | 0.041 | 0.077 |
| NO*ACCave | 1.17 (1.01,1.36) | 0.033 | 0.077 | 1.16 (1.01,1.34) | 0.039 | 0.077 |
| NO2*ACCave | 1.17 (0.96,1.42) | 0.117 | 0.203 | 1.16 (0.95,1.40) | 0.144 | 0.207 |
Model 1: Cox proportional hazards regression adjusted for age, sex, education, Townsend deprivation index, ethnicity, and APOE ε4 genotype. Models for main effects are mutually adjusted for PA and pollution variable and did not include interaction terms. Results for ACCave are from model that includes PM2.5 absorbance as a covariate (results are similar for mutual adjustment for other pollution variables). Air pollution exposures are scaled so that a one unit increase equals one interquartile range increase.
Model 2: Cox proportional hazards regression adjusted for Model 1, alcohol consumption, smoking status, chronic disease, general health, depression, healthy diet score, BMI, difference in date between accelerometer wear and pollution measure, and accelerometer wear duration. Air pollution exposures are scaled so that a one unit increase equals one interquartile range increase.
Figure 1.
Risk of incident dementia according to ACCave within pollution exposure tertiles. Hazard ratios are for ACCave within each tertile of air pollution exposure. Models are fully adjusted (see Table 2 for full list of covariates).
In sensitivity analyses, we found similar results with follow-up beginning at accelerometer wear (see Supplementary Table 1, SDC 1, sensitivity analysis). We additionally excluded individuals who developed dementia within seven years of the start date and results did not change (Supplementary Table 1, SDC 1). We also included all participants (ages 40 and over), and observed consistent results (Supplementary Table 1, SDC 1).
DISCUSSION
Objectively measured PA was associated with reduced risk for all-cause dementia in this prospective cohort. Despite these overall PA-related benefits, we found significant positive interactions between PA and two pollutants (PM2.5 absorbance and PM2.5 exposure) on the risk of incident dementia. For these pollution measures, exposure to higher air pollution levels attenuated the benefits of PA on risk of developing all-cause dementia. Our results suggest that not all pollutants have strong effects on the relationship between PA and dementia risk. PM2.5 and PM2.5 absorbance both showed positive interactions with PA on dementia risk, while interactions between larger particulate matter (PM10 and PM2.5–10) and gases (NO and NO2) with PA to modify dementia risk were no longer significant after adjusting results for multiple testing. Importantly, PM2.5 levels in this cohort were within US Environmental Protection Agency (EPA) guidelines for health and welfare with ~97% of the sample falling below the primary (health-based) target of 12 μg/m3 and ~99% of the sample falling below the secondary (welfare-based) target of 15 μg/m3. In addition, median annual average PM2.5 in this cohort was below the healthy air guidelines for the World Health Organization (WHO; guideline level: 10 μg/m3). Thus, our findings suggest that attenuation of PA-related brain benefits occur at air pollution exposures that are often experienced in urban environments around the world.
These air pollutants have been linked with neurodegenerative disease in prior research. For example, several studies have shown significant associations between PM2.5 and increased risk of dementia (31). While less studied, recent work also suggests that black carbon (BC) may be a specific component of PM2.5 that has a strong impact on dementia risk (32) and cognitive function (33). BC is generally associated with local combustion of particles, usually through traffic emissions, but is also associated with fire combustion (both residential wood combustion and from vegetation combustion through fires) (34).
Mechanisms linking pollution exposure and dementia suggest both indirect and direct effects. For example, it is possible that particulate matter, including BC, reach the brain directly via the olfactory bulb (35) or by entering the bloodstream and crossing the blood-brain-barrier (36). Exposure to air pollution may also induce indirect effects that include damage to vasculature and associated cerebral ischemia, as well as inflammatory responses to inhaled pollutants that may trigger chronic microglia activation (10, 37, 38). Recent work supports an association between air pollution exposure (PM2.5) and amyloid-b plaques in animal models (39–42) and in humans with positron emission tomography (PET) imaging (41), although others have found inconclusive associations between 10-year exposure to PM2.5 and Alzheimer’s disease neuropathology on autopsy (43). As described earlier, PA increases minute ventilation, and leads to higher deposition of particulate matter in the airways and bloodstream (12), which may help explain the significant attenuation of PA benefits for incident dementia found here.
Previous work has identified the potential for PA in environments with high levels of pollution to have short-term effects on cardiopulmonary function (14, 17). However, many studies have failed to find a significant health impact of PA in polluted environments. For example, no evidence of interactions between PA and PM2.5 on risk of mortality were found in a prospective study of Chinese adults (21). However, Lin et al. (16) found a positive interaction between PA and PM2.5, such that PA in highly polluted environments may increase odds of stroke. It is possible that brain health is especially sensitive to the increased small particulate deposition associated with PA in polluted environments. While the potential mechanisms remain somewhat unclear, and more study is needed, the results of this study suggest that exposure to levels of air pollution that generally fall within WHO and EPA guidelines may attenuate the association between PA and reduced dementia risk.
Strengths and Limitations
Our study has several strengths, including the size of the UK Biobank cohort, with the largest prospective sample analyzed to date. A second key strength of our study design is the use of objective measures of PA from wearable devices. Self-reported PA is often less reliable than objectively measured PA, leading to potential over-estimates of activity levels (22). In addition, overall pollution exposures in the UK sample are lower than in many cities around the world (44), and our findings may be particularly relevant to urban settings that experience moderate levels of air pollution which fall within health and general welfare guidelines. Finally, the inclusion of a wide range of covariates linked with increased risk of developing dementia, along with sensitivity analyses that test assumptions relating exposures across different time points, strengthens our conclusions.
While our study has several strengths, there are limitations that may form the foundation for future studies of exercise and air pollution interactions in relation to brain health. First, air pollution data were estimated from a single year (2010) and PA measures were collected after this date. It is possible that PA patterns changed within the relatively short time between these events, which would alter the PA-related exposure to air pollution used here. However, objectively measured PA is relatively stable over short periods (1–6 years) (45), and while PA levels do tend to decrease over time, levels at baseline measurement are highly correlated with follow-up measures (46). To account for possible changes in PA levels over time, we included the difference in time between measurement of air pollution and PA in our fully adjusted models, and we performed sensitivity analyses using accelerometer wear date as the start time for follow up. In these sensitivity analyses, we assume that pollution levels showed minimal change from measure date to accelerometer wear date. This assumption is supported both by data collected in the UK Biobank (pollution measures are highly correlated across years: NO r2 all > 0.98; see Supplementary Table 2, SDC 1, correlation matrix of NO2 measures over time), and by several studies in Europe and in North America that have demonstrated that LUR models are temporally stable over both short time frames and over time frames of 7–12 years (47–50). While these sensitivity analyses support the reported results, closer alignment between pollution exposure and direct measures of PA should be done in future work. In addition, some of our participants may have moved during the follow-up period, and it is possible that participants who did not develop dementia were more likely to move, while those developing dementia may have moved to care homes or other living situations with increased assistance. Previous work examining the effects of mobility on AP exposure and health suggest that ignoring mobility generally biases results towards the null (51). However, future work focused on the timing of AP exposure relative to the development of dementia will help clarify the effects of residential mobility on our results. Our analyses also assume that PA occurs in areas where air pollution exposures are representative of the LUR-modelled air pollution levels (e.g., outdoors). While this is a limitation of the study design, previously published data show strong correlations among indoor, outdoor, and personal air pollution levels, and therefore, ambient air pollution measures are likely a good proxy for personal exposures assumed here (52, 53). While we incorporated a wide range of covariates that included risk factors for dementia, other risk factors were not included here and should be incorporated into future work, including biomarkers of cardiovascular disease regardless of disease diagnosis, as well as aspects of lifestyle including social isolation or sleep disorders. The reliance on hospital records and death registry for dementia diagnosis is a limitation of this study since it may underestimate cases with dementia in this cohort. However expert adjudication of cases is not possible in a cohort this large, and a previous validation study supported these methods for determining all-cause dementia and demonstrated agreement with primary care records (54). ACCave is a measure of the average volume of PA for an individual and does not directly equate to specific activities or intensities. Thus, our use of average acceleration magnitude to define PA limits our ability to determine the specific types and intensities of PA that may most strongly impact dementia risk in relation to AP exposure. Future work that includes behaviors derived from accelerometers will help further clarify how PA type and intensity in different levels of AP impact dementia risk. In addition, wrist-worn accelerometers measure movements of the wrist, which, while assumed to more generally assess body movement, can also pick up movements isolated to the upper limbs, and may not adequately measure some types of low-impact activity. Finally, the UK Biobank is ethnically and racially homogeneous, which limits generalizability of our findings to more diverse populations, though researchers have argued that, generally, results from this cohort could be externally valid for linking exposures with health outcomes (23). Future studies that include a more diverse sample would enhance the generalizability of these results.
CONCLUSIONS
In a sample of middle-aged to older adults, we find evidence that air pollution attenuates the beneficial association between PA and risk of all-cause dementia. The effects are strongest for PM2.5 and PM2.5 absorbance, which may be related to traffic-induced black carbon. Overall levels of pollution in this sample are lower than in many cities around the world (44) and generally fall within PM2.5 guideline levels from the US EPA and WHO. Thus, our results suggest that increased exposure to particulate matter associated with PA may have effects on brain health.
Supplementary Material
SDC 1: SUPPLEMENTARY MATERIALS_Final.docx – Supplementary Tables
ACKNOWLEDGEMENTS
This work was conducted using the UK Biobank dataset under application number 21259. Study authors are supported by the NIH (P30AG019610, P30AG072980, R56AG067200, R01AG049464, R00ES028743, P30ES006694), the state of Arizona and Arizona Department of Health Services, and the McKnight Brain Research Foundation.
Funding Source:
The results of the present study do not constitute endorsement by ACSM. The results of the study are presented clearly, honestly, and without fabrication, falsification, or inappropriate data manipulation. Study authors are supported by the NIH (P30AG019610, P30AG072980, R56AG067200, R01AG049464, R00ES028743, P30ES006694), the state of Arizona and Arizona Department of Health Services, and the McKnight Brain Research Foundation.
Footnotes
DECLERATION OF INTERESTS
The authors declare no competing interests. The results of the present study do not constitute endorsement by ACSM. The results of the study are presented clearly, honestly, and without fabrication, falsification, or inappropriate data manipulation.
Conflict of Interest
The authors declare no competing interests.
REFERENCES
- 1.Guure CB, Ibrahim NA, Adam MB, Said SM. Impact of physical activity on cognitive decline, dementia, and its subtypes: meta-analysis of prospective studies. Biomed Res. Int. 2017;2017:9016924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hersi M, Irvine B, Gupta P, Gomes J, Birkett N, Krewski D. Risk factors associated with the onset and progression of Alzheimer’s disease: A systematic review of the evidence. Neurotoxicology. 2017;61:143–87. [DOI] [PubMed] [Google Scholar]
- 3.Livingston G, Huntley J, Sommerlad A et al. Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet. 2020;396(10248):413–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Livingston G, Sommerlad A, Orgeta V et al. Dementia prevention, intervention, and care. Lancet. 2017;390(10113):2673–734. [DOI] [PubMed] [Google Scholar]
- 5.Brown BM, Peiffer J, Taddei K et al. Physical activity and amyloid-β plasma and brain levels: results from the Australian Imaging, Biomarkers and Lifestyle Study of Ageing. Mol. Psychiatry. 2013;18(8):875–81. [DOI] [PubMed] [Google Scholar]
- 6.National Academies of Sciences Engineering and Medicine. Preventing cognitive decline and dementia: A way forward. Washington, DC: The National Academies Press; 2017. [PubMed] [Google Scholar]
- 7.Costa LG, Cole TB, Dao K, Chang Y-C, Coburn J, Garrick JM. Effects of air pollution on the nervous system and its possible role in neurodevelopmental and neurodegenerative disorders. Pharmacol. Ther. 2020;210:107523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Paul KC, Haan M, Mayeda ER, Ritz BR. Ambient air pollution, noise, and late-life cognitive decline and dementia risk. Annu. Rev. Public Health. 2019;40:203–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Calderon-Garciduenas L, Reed W, Maronpot RR et al. Brain inflammation and Alzheimer’s-like pathology in individuals exposed to severe air pollution. Toxicol. Pathol. 2004;32(6):650–8. [DOI] [PubMed] [Google Scholar]
- 10.Mumaw CL, Levesque S, McGraw C et al. Microglial priming through the lung—brain axis: the role of air pollution-induced circulating factors. FASEB J. 2016;30(5):1880–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kubesch NJ, Therming Jørgensen J, Hoffmann B et al. Effects of leisure-time and transport-related physical activities on the risk of incident and recurrent myocardial infarction and interaction with traffic-related air pollution: A cohort study. J Am Heart Assoc. 2018;7(15):e009554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bigazzi AY, Figliozzi MA. Review of urban bicyclists’ intake and uptake of traffic-related air pollution. Transp. Rev. 2014;34(2):221–45. [Google Scholar]
- 13.Pasqua LA, Damasceno MV, Cruz R et al. Exercising in the urban center: Inflammatory and cardiovascular effects of prolonged exercise under air pollution. Chemosphere. 2020;254:126817. [DOI] [PubMed] [Google Scholar]
- 14.Qin F, Yang Y, Wang S-t et al. Exercise and air pollutants exposure: A systematic review and meta-analysis. Life Sci. 2019;218:153–64. [DOI] [PubMed] [Google Scholar]
- 15.Bos I, Jacobs L, Nawrot T et al. No exercise-induced increase in serum BDNF after cycling near a major traffic road. Neurosci Lett. 2011;500(2):129–32. [DOI] [PubMed] [Google Scholar]
- 16.Lin H, Guo Y, Di Q et al. Ambient PM2. 5 and stroke: effect modifiers and population attributable risk in six low-and middle-income countries. Stroke. 2017;48(5):1191–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Laeremans M, Dons E, Avila-Palencia I et al. Short-term effects of physical activity, air pollution and their interaction on the cardiovascular and respiratory system. Environ. Int. 2018;117:82–90. [DOI] [PubMed] [Google Scholar]
- 18.Endes S, Schaffner E, Caviezel S et al. Is physical activity a modifier of the association between air pollution and arterial stiffness in older adults: the SAPALDIA cohort study. Int J Hyg Environ Health. 2017;220(6):1030–8. [DOI] [PubMed] [Google Scholar]
- 19.Guo C, Zeng Y, Chang L-y et al. Independent and Opposing Associations of Habitual Exercise and Chronic PM2. 5 Exposures on Hypertension Incidence. Circulation. 2020;142(7):645–56. [DOI] [PubMed] [Google Scholar]
- 20.Guo C, Yang HT, Chang L-y et al. Habitual exercise is associated with reduced risk of diabetes regardless of air pollution: a longitudinal cohort study. Diabetologia. 2021;64(6):1298–1308. [DOI] [PubMed] [Google Scholar]
- 21.Sun S, Cao W, Qiu H et al. Benefits of physical activity not affected by air pollution: a prospective cohort study. Int J Epidemiol. 2020;49(1):142–52. [DOI] [PubMed] [Google Scholar]
- 22.Prince SA, Adamo KB, Hamel ME, Hardt J, Gorber SC, Tremblay M. A comparison of direct versus self-report measures for assessing physical activity in adults: a systematic review. Int J Behav Nutr Phys Act. 2008;5(1):1–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fry A, Littlejohns TJ, Sudlow C et al. Comparison of sociodemographic and health-related characteristics of UK Biobank participants with those of the general population. Am J Epidemiol. 2017;186(9):1026–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Doherty A, Jackson D, Hammerla N et al. Large scale population assessment of physical activity using wrist worn accelerometers: The UK Biobank Study. PLoS One. 2017;12(2):e0169649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Manisalidis I, Stavropoulou E, Stavropoulos A, Bezirtzoglou E. Environmental and health impacts of air pollution: a review. Front Public Health. 2020;8:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Beelen R, Hoek G, Vienneau D et al. Development of NO2 and NOx land use regression models for estimating air pollution exposure in 36 study areas in Europe–the ESCAPE project. Atmos Environ. 2013;72:10–23. [Google Scholar]
- 27.Eeftens M, Beelen R, de Hoogh K et al. Development of land use regression models for PM2. 5, PM2. 5 absorbance, PM10 and PMcoarse in 20 European study areas; results of the ESCAPE project. Environ Sci Technol. 2012;46(20):11195–205. [DOI] [PubMed] [Google Scholar]
- 28.Lourida I, Hannon E, Littlejohns TJ et al. Association of lifestyle and genetic risk with incidence of dementia. JAMA. 2019;322(5):430–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schoenfeld D. Partial residuals for the proportional hazards regression model. Biometrika. 1982;69(1):239–41. [Google Scholar]
- 30.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Ser B Methodol. 1995;57(1):289–300. [Google Scholar]
- 31.Peters R, Ee N, Peters J, Booth A, Mudway I, Anstey KJ. Air pollution and dementia: a systematic review. J Alzheimers Dis. 2019;70(s1):S145–S63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Oudin A, Segersson D, Adolfsson R, Forsberg B. Association between air pollution from residential wood burning and dementia incidence in a longitudinal study in Northern Sweden. PLoS One. 2018;13(6):e0198283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Power MC, Adar SD, Yanosky JD, Weuve J. Exposure to air pollution as a potential contributor to cognitive function, cognitive decline, brain imaging, and dementia: a systematic review of epidemiologic research. Neurotoxicology. 2016;56:235–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Grahame TJ, Klemm R, Schlesinger RB. Public health and components of particulate matter: the changing assessment of black carbon. J Air Waste Manag Assoc. 2014;64(6):620–60. [DOI] [PubMed] [Google Scholar]
- 35.Oberdörster G, Elder A, Rinderknecht A. Nanoparticles and the brain: cause for concern? J Nanosci Nanotechnol. 2009;9(8):4996–5007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Banks WA, Kastin AJ, Broadwell RD. Passage of cytokines across the blood-brain barrier. Neuroimmunomodulation. 1995;2(4):241–8. [DOI] [PubMed] [Google Scholar]
- 37.Brockmeyer S, d’Angiulli A. How air pollution alters brain development: the role of neuroinflammation. Transl Neurosci. 2016;7(1):24–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Delgado-Saborit JM, Guercio V, Gowers AM, Shaddick G, Fox NC, Love S. A critical review of the epidemiological evidence of effects of air pollution on dementia, cognitive function and cognitive decline in adult population. Sci Total Environ. 2021;757:143734. [DOI] [PubMed] [Google Scholar]
- 39.Cacciottolo M, Morgan TE, Saffari AA et al. Traffic-related air pollutants (TRAP-PM) promote neuronal amyloidogenesis through oxidative damage to lipid rafts. Free Radic Biol Med. 2020;147:242–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hullmann M, Albrecht C, van Berlo D et al. Diesel engine exhaust accelerates plaque formation in a mouse model of Alzheimer’s disease. Part Fibre Toxicol. 2017;14(1):1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Iaccarino L, La Joie R, Lesman-Segev OH et al. Association between ambient air pollution and amyloid positron emission tomography positivity in older adults with cognitive impairment. JAMA Neurol. 2021;78(2):197–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jang S, Kim EW, Zhang Y et al. Particulate matter increases beta-amyloid and activated glial cells in hippocampal tissues of transgenic Alzheimer’s mouse: Involvement of PARP-1. Biochem Biophys Res Commun. 2018;500(2):333–8. [DOI] [PubMed] [Google Scholar]
- 43.Shaffer RM, Li G, Adar SD et al. Fine Particulate Matter and Markers of Alzheimer’s Disease Neuropathology at Autopsy in a Community-Based Cohort. J Alzheimers Dis. 2021;79(4):1761–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rodríguez-Urrego D, Rodríguez-Urrego L. Air quality during the COVID-19: PM2. 5 analysis in the 50 most polluted capital cities in the world. Environ Pollut. 2020;266(Pt 1):115042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hagströmer M, Kwak L, Oja P, Sjöström M. A 6 year longitudinal study of accelerometer-measured physical activity and sedentary time in Swedish adults. J Sci Med Sport. 2015;18(5):553–7. [DOI] [PubMed] [Google Scholar]
- 46.Hamer M, Kivimaki M, Steptoe A. Longitudinal patterns in physical activity and sedentary behaviour from mid-life to early old age: a substudy of the Whitehall II cohort. J Epidemiol Community Health. 2012;66(12):1110–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cesaroni G, Porta D, Badaloni C et al. Nitrogen dioxide levels estimated from land use regression models several years apart and association with mortality in a large cohort study. Environ Health. 2012;11(1):1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cullen B, Newby D, Lee D et al. Cross-sectional and longitudinal analyses of outdoor air pollution exposure and cognitive function in UK Biobank. Sci Rep. 2018;8(1):1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.De Hoogh K, Chen J, Gulliver J et al. Spatial PM2. 5, NO2, O3 and BC models for Western Europe–evaluation of spatiotemporal stability. Environ Int. 2018;120:81–92. [DOI] [PubMed] [Google Scholar]
- 50.Eeftens M, Beelen R, Fischer P, Brunekreef B, Meliefste K, Hoek G. Stability of measured and modelled spatial contrasts in NO2 over time. Occup Environ Med. 2011;68(10):765–70. [DOI] [PubMed] [Google Scholar]
- 51.Pennington AF, Strickland MJ, Klein M et al. Measurement error in mobile source air pollution exposure estimates due to residential mobility during pregnancy. J Expo Sci Environ Epidemiol. 2017;27(5):513–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Janssen NA, Hoek G, Brunekreef B, Harssema H, Menswik I, Zuidhof A. Personal sampling of particles in adults: relation among personal, indoor, and outdoor air concentrations. Am J Epidemiol. 1998;147(6):537–47. [DOI] [PubMed] [Google Scholar]
- 53.Kingham S, Briggs D, Elliott P, Fischer P, Lebret E. Spatial variations in the concentrations of traffic-related pollutants in indoor and outdoor air in Huddersfield, England. Atmos Environ. 2000;34(6):905–16. [Google Scholar]
- 54.Wilkinson T, Schnier C, Bush K et al. Identifying dementia outcomes in UK Biobank: a validation study of primary care, hospital admissions and mortality data. Eur J Epidemiol. 2019;34(6):557–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
SDC 1: SUPPLEMENTARY MATERIALS_Final.docx – Supplementary Tables