Abstract
Bisphenol a (BPA) is a high production volume chemical that is frequently used to manufacture epoxy resins and polycarbonate plastics. BPA-containing products are now pervasive, and as a result, biomonitoring studies report widespread exposure in > 90% of adults, adolescents, and children. Both epidemiological and experimental studies have reported associations between BPA exposure and adverse cardiovascular health outcomes. With increasing concerns regarding BPA exposure, a few structurally similar bisphenol chemicals have been introduced as replacements, including bisphenol s (BPS) and bisphenol f (BPF). In accordance with the recently established “Key characteristics of cardiovascular toxicants”, we reviewed the literature to highlight the immediate effects of bisphenol chemicals on (1) cardiac excitability, and (2) contractility and relaxation. BPA inhibits key cardiac ion channels, impairs cardiac excitability, and acts as a more potent inhibitor as compared to BPF and BPS. Through the inhibition of calcium current, some studies report that bisphenol chemicals can act as negative inotropic agents. Yet, others suggest that low dose exposures may increase contractility and precipitate triggered arrhythmias via the phosphorylation of key calcium handling proteins. Accordingly, we propose additional considerations for future work to comprehensively address the cardiac safety profile of BPA, as compared to replacement chemicals.
Keywords: Bisphenol, Cardiotoxicity, Excitability, Contractility, Relaxation
Introduction
Bisphenol A (BPA) is a synthetic chemical that was first discovered in 1891, and later explored as a potential pharmaceutical hormone due to its estrogenic properties [1]. BPA gained prominence once again in the 1950s, when the chemical industry started using BPA for epoxy resins in protective coatings and canned food liners and using polymerized BPA to manufacture polycarbonate plastic. Notably, polycarbonate plastic offered several advantages – it was clear and transparent enough to replace glass, while also lightweight and durable enough to replace metal materials. Accordingly, BPA quickly became a high production volume chemical, with more than 8 billion pounds produced globally each year [2, 3].
BPA-containing products are now pervasive, and as a result, biomonitoring studies report widespread BPA exposure in > 90% of adults, adolescents, and children [4-7]. The latter is largely attributed to inadvertent ingestion. Since measurable amounts of BPA migrate from food packaging materials, it is considered an indirect food additive by the Food and Drug Administration. Initial safety assessments assumed that BPA followed a monotonic dose–response—wherein high dose toxicity testing may be used to extrapolate and predict the safety of low dose exposure. Although more recent evidence suggests harmful health effects from BPA exposure even at low nanomolar concentrations, which may be attributed to its (estrogenic) endocrine-disrupting properties [8, 9]. Notably, approximately 20% of experimental BPA studies report a non-monotonic dose response [10], a characteristic commonly observed with natural hormones [11]. This property can complicate risk assessment, as BPA toxicity may involve direct interactions with hormone receptors in specific cell types, and/or disruption of multiple biological endpoints with linear dose-responses that collectively produce a non-monotonic dose–response relationship.
Given its ubiquity and endocrine-disrupting properties, daily exposure to BPA has become a major public health concern. Both epidemiological and experimental studies have reported associations between BPA exposure and an increased risk of all-cause mortality, cardiovascular disease, myocardial infarction, hypertension, and altered cardiac electrophysiology [12-16]. With mounting evidence that BPA exposure contributes to adverse health outcomes, a few structurally similar bisphenol chemicals have been introduced as replacements, including bisphenol s (BPS) and bisphenol f (BPF). As manufacturers adopted replacement chemicals for (some) consumer products, the Food and Drug Administration revised its regulation on BPA use in baby bottles, sippy cups, and infant formula packaging—but only after BPA-based products were voluntarily phased out [17]. In 2015, the State of California’s Developmental and Reproductive Toxicant Identification Committee added BPA to the Proposition 65 list of chemicals due to its harmful effects on the female reproductive system [18]—albeit, no additional regulation has since occurred in the USA. Although BPA exposure has decreased slightly over time in the USA [19], widespread human exposure to bisphenol chemicals persists, with detection in 96% (BPA), 89% (BPS), and 67% (BPF) of analyzed samples [7], Notably, these alternatives have been dubbed “regrettable substitutions” as their toxicity profile is largely unknown and recently developed replacement chemicals could exert effects that are comparable to BPA [20].
In the presented mini-review, we utilized the recently established “Key characteristics of cardiovascular toxicants” [21] to summarize ongoing investigations into bisphenol-induced toxicity with a specific focus on (1) regulation of cardiac excitability, and (2) impaired cardiac contractility and relaxation (Fig. 1). Given the known estrogenic properties of BPA, we also highlight research findings that explore sexually dimorphic cardiac endpoints. When applicable, we evaluate and compare the cardiotoxicity profile of BPA with potential replacement chemicals, including BPS and BPF. Collectively, this mini-review aims to summarize our current understanding of bisphenol-induced cardiotoxicity and highlight important areas for future research.
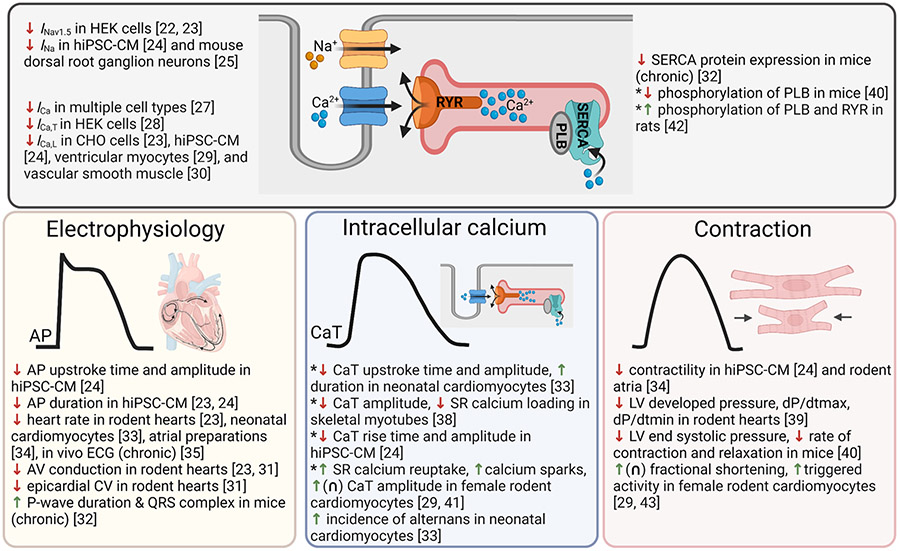
Fig. 1.
Summary of BPA’s effects on cardiac physiology. Top: effects of BPA on ion channels and intracellular signaling. Bottom: phenotypic effects of BPA on cardiac electrophysiology, intracellular calcium handling, or contraction. Key: ↑ increases, enhances, lengthens; ↓ decreases, blocks, slows, reduces; ∩ non-monotonic dose response; *contradictory results. AP action potential, AV atrioventricular, CaT calcium transient, CV conduction velocity, PLB phospholamban, RYR ryanodine receptor, SERCA SR ATPase. Created with BioRender.com
Cardiac Excitability
Cardiac excitability is characterized by the ease with which cardiomyocytes depolarize, repolarize, and propagate an electrical signal. Nodal action potentials are defined by a spontaneous depolarization phase, followed by the opening of voltage-gated calcium channels to generate the upstroke phase, and then the opening of potassium channels during the repolarization phase. Following sinoatrial nodal depolarization, electrical activity spreads to the atria, atrioventricular node, Purkinje fibers, and then the ventricles. In comparison, non-pacemaker action potentials (e.g., ventricular cardiomyocytes) are defined by a resting membrane phase, followed by the opening of fast voltage-gated sodium channels in the upstroke phase, a plateau phase governed by outward potassium and inward calcium currents, and finally a repolarization phase. Disruption of cardiac excitability can manifest as either an alteration in heart rate and/or electrical conduction, both of which have been reported following bisphenol chemical exposure.
Multiple studies have shown that BPA inhibits individual ion channels that play a role in the cardiac action potential. Using an HEK-transfected cell line and whole-cell patch clamp experiments, O’Reilly et al. reported that BPA induced a dose-dependent tonic block of the human Nav1.5 sodium channel (1–100 μM), by localizing to the local anesthetic receptor site in the sodium channel pore [22]. Similarly, Prudencio et al. performed sodium channel recordings in an HEK-transfected cell line and reported a half-maximal inhibitory concentration (IC50) of 55.3 μM and 23.6 μM BPA for fast/peak and late sodium channel currents, respectively [23]. These results were recently replicated in human-induced pluripotent stem cell derived cardiomyocytes (hiPSC-CM), with a significant reduction in sodium channel current observed at 1–100 μM BPA (IC50 = 56.5 μM) [24]. Albeit a different cell line (mouse dorsal root ganglion neurons), Wang et al. also reported that BPA reduces TTX-sensitive (Nav1.1, Nav1.6, Nav1.7) and TTX-resistant (Nav1.8, Nav1.9, Nav1.5) sodium current in a rapid, yet reversible manner [25]. In cardiac tissue, the fast voltage-gated sodium channel is responsible for the action potential upstroke in ventricular cardiomyocytes and blockade is likely to slow depolarization and conduction velocity. While late sodium channel current is active during the action potential plateau phase, BPA-induced blockade is expected to shorten the repolarization time, and in turn, reduce calcium channel current [26]. Of interest, Prudencio et al. found that BPA was a more potent inhibitor of both fast/peak and late sodium currents compared with other bisphenol chemicals, as higher IC50 values were reported for both BPF (232 and 100 μM) and BPS (1090 and 369 μM) [23].
BPA has also been shown to inhibit calcium current, which plays a critical role in nodal cell automaticity, atrioventricular conduction, and the plateau phase of the ventricular action potential. Deutschmann et al. demonstrated that BPA is a potent blocker of multiple (L-, T-, N-, P/Q-type) voltage-activated calcium channels expressed in a variety of cell types, including cardiac myocytes, neurons, and HEK cells [27]. Across multiple experiments, the inhibitory effects of BPA were rapid, reversible, and of similar potency (IC50 = 26–35 μM)—and pharmacological challenges suggest that BPA interacts directly with the extracellular component of the channel protein. These results were supported by Michaela et al., which documented the potent inhibitor effects of BPA (0.01–100 μM) on T-type calcium channels that are expressed in nodal and conduction cells [28]. Further, BPA exposure has been shown to significantly reduce L-type calcium current in ventricular myocytes (IC50 = 27.4 nM) [29], hiPSC-CM (IC50 = 6.9 μM) [24], and vascular smooth muscle [30]. Using a transfected CHO cell line, Prudencio et al. reported that BPA was the most potent inhibitor of L-type calcium channels (IC50 = 30.8 μM), with greater concentrations required for BPF (76 μM) and BPS (333 μM) to attain the same level of inhibition [23]. This experimental finding is in agreement with structure-effect analyses, which suggest that bisphenol potency is influenced by the chemical structure and bridge between the two phenol rings, with less inhibitory effects anticipated for BPF and BPS [27]. Considering the importance of calcium channels in both cardiac excitability and contractility, it is highly probable that BPA’s effects on cardiac physiology are mediated through a calcium-dependent mechanism.
Individual channel measurements are a valuable tool to measure the effects of bisphenol chemicals on ionic currents in the cardiac action potential, which are then validated using either cardiomyocyte or intact heart preparations. Using a hiPSC-CM model, Hyun et al. reported significant slowing of the action potential upstroke (1–100 μM BPA) and a reduction in the action potential amplitude (30–100 μM BPA), likely due to sodium channel inhibition, using both optical and microelectrode array methodologies [24]. This finding is in agreement with Posnack et al., which reported a dose-dependent (0.1–100 μM BPA) slowing of epicardial conduction velocity in excised, intact heart preparations [31]. Likewise, mice subjected to chronic BPA treatment (2.5 ng/ml drinking water, 15 weeks) presented with a longer P-wave duration and QRS complex, compared with control animals [32]. Given the inhibitory effects of BPA on L-type calcium channels, and the role of this channel in the plateau phase of the action potential, one would also expect shortening of the action potential duration (APD). Indeed, optical action potential (10–100 μM) [24] and microelectrode array recordings (30–100 μM) [23] revealed APD shortening following acute BPA exposure, but no effect was observed after BPS treatment. Collectively, these results support the finding that BPA interferes with voltage-gated sodium and calcium channels in a monotonic dose-dependent manner, which can have an immediate impact on cardiac electrophysiology.
As further evidence that BPA interferes with L-type calcium channels, intact Langendorff-perfused heart preparations demonstrate considerable heart rate slowing after acute BPA exposure (16% at 10 μM BPA and by 85% at 100 μM BPA [23]). Similar effects on automaticity have been observed in neonatal cardiomyocytes [33], atrial preparations [34], and in vivo electrocardiogram recordings [35]. Although the latter was attributed to chronic BPA exposure increasing parasympathetic activity in male mice or exaggerating reflex bradycardia in female mice in the presence of phenylephrine. Dose-dependent slowing of atrioventricular (AV) conduction and increased AV node refractoriness have also been observed after exposure to BPA (1 nM to 100 μM) and BPF (100 μM), but not BPS [23, 31]. As calcium channel blockers precipitate similar outcomes, these results fortify the hypothesis that the electrophysiology effects of BPA are likely mediated by dose-dependent interactions with voltage-gated sodium and calcium channels, with reduced effects observed after BPF or BPS exposure.
Calcium Handling, Contractility, and Relaxation
Calcium ions are not only integral to cardiac automaticity and electrical conduction, but also excitation–contraction coupling—the process by which an action potential triggers contraction. As an action potential depolarizes the cardiomyocyte, voltage-gated L-type calcium channels open and permit calcium influx, which triggers calcium release from the sarcoplasmic reticulum into the cytosol. Intracellular calcium ions associate with the troponin-tropomyosin complex, causing a conformational change that permits actin and myosin filament interaction and shortening of the sarcomere to facilitate muscle contraction. Subsequently, calcium is shuttled back into storage by the sarcoplasmic reticulum (SR) calcium ATPase (SERCA) and transported out of the cell via the sodium/calcium exchanger (NCX). Phospholamban (PLB) regulates the SERCA pump and calsequestrin (CASQ), a calcium binding protein, facilitates calcium storage within the SR. As the intracellular calcium concentration decreases, calcium dissociates from troponin and the initial sarcomere length is restored. Dysregulation of intracellular calcium handling can impair cardiac contraction, relaxation, and/or promote triggered arrhythmias.
BPA exposure has been shown to exert immediate effects on intracellular calcium handling, contractility, and relaxation—likely through the inhibition of ionic currents and/or phosphorylation of key regulatory proteins. As previously highlighted, BPA inhibits calcium influx through its interaction with voltage-gated calcium channels. Since cardiomyocyte contractility is proportional to the magnitude of this slow inward current [36], BPA can act as a negative inotropic agent. Using neonatal cardiomyocyte preparations, Ramadan et al. reported that acute BPA exposure slowed the calcium transient upstroke time (indicator of synchronized calcium release from the SR), reduced the calcium transient amplitude (indicator of the intracellular calcium concentration), and prolonged the calcium transient duration (indicator of calcium reuptake back into the SR) in a monotonic dose-dependent manner (1 nM to 100 μM) [33]. Further, BPA exposure was associated with an increased incidence of alternans, which can be linked to disturbances in both calcium release and/or sequestration back into the SR [37]. Similar findings have been reported using skeletal myotubes, wherein acute BPA exposure decreased the calcium transient amplitude and reduced total SR calcium loading as measured by caffeine application [38]. BPA exposure has also been shown to slow the calcium transient rise time and decrease the calcium transient amplitude of hiPSC-CM in a dose-dependent manner [24]. Further, BPA treatment decreased cardiomyocyte contractility, as assessed by microelectrode array impedance-based measurements. These results align with studies in rodent atrial preparations, wherein BPA dose-dependently (100 nM to 100 μM) decreased the rate and force of contractions, up to a 90% reduction after exposure to the highest concentration tested [34]. Notably, these changes were blocked by L-NAME, a nitric oxide synthase inhibitor, suggesting that BPA may act (in part) via a nitric oxide dependent signaling pathway.
The immediate negative inotropic effects of BPA are most evident in Langendorff-perfused heart preparations, wherein BPA exerts a monotonic dose-dependent reduction in left ventricular developed pressure (LVDP), contractility (dP/dtmax), and lusitropy (dP/dtmin) [39]. During normal sinus rhythm, a decrease in LVDP was observed beginning at 100 nM BPA and dP/dtmax slowing began at 1 μM BPA. Notably, these effects became exacerbated at faster pacing rates, reducing the rate of contraction and relaxation (100 nM to 100 μM), and the peak developed pressure in response to pacing or the potentiated peak pressure after a period of rest (1 nM to 100 μM). Of interest, chronic BPA exposure (2.5 ng/mL) has been shown to decrease SERCA protein expression in male and female mice, which is likely to decrease total SR calcium loading [32]. Ferguson et al. also reported a significant reduction in left ventricular end systolic pressure (12%) in female mice and slowed rate of relaxation (18%) in male mice after acute, low dose 1 nM BPA exposure [40]. Notably, the authors found that low dose (1 nM) BPS exposure decreased the left ventricular end systolic pressure, the rate of contraction, and the rate of relaxation in both male and female mice – and these effects were blunted when hearts were pretreated with an estrogen receptor beta antagonist, suggesting dysregulation of estrogen receptor signaling pathways. Further, molecular analysis revealed that BPA treatment decreased phospholamban phosphorylation, which is expected to increase SERCA inhibition and reduce calcium reuptake into the SR. Collectively, these findings reveal that bisphenol chemicals may act as negative inotropic agents by reducing inward calcium current and/or disrupting intracellular calcium signaling through impaired SR calcium release and reuptake.
Nevertheless, the effects of bisphenol chemicals on cardiac contraction and relaxation remain uncertain—as a few experimental studies have reported increased contractility at low nanomolar concentrations in isolated cardiomyocytes. Using isolated adult ventricular myocytes, Yan et al. observed that BPA-treated (1 nM) female cardiomyocytes had increased calcium transient amplitudes, faster SR calcium reuptake, and were prone to spontaneous aftercontractions [41]. Mechanistic studies revealed that BPA exposure resulted in increased phosphorylation of both phospholamban and ryanodine receptors, which can increase SERCA activity, hasten SR calcium reuptake, and precipitate SR calcium leak [42]. Of interest, the effects of BPA followed a non-monotonic dose response that was sex-specific, wherein low nanomolar concentrations maximally increased fractional shortening in female but not male cardiomyocytes [29, 43]. Further, this group reported that the incidence of calcium sparks or spontaneous aftercontractions were reduced in cardiomyocytes isolated from female estrogen receptor beta knockout animals [41], cells treated with an estrogen receptor beta antagonist [29], or cells pretreated with progesterone [44]. Collectively, these authors suggest that BPA’s interactions with estrogen receptors prevail at low nanomolar concentrations, resulting in increased contractility via the phosphorylation of calcium handling proteins, which in turn promotes SR calcium leak and triggered arrhythmias. While at higher concentrations, BPA inhibits inward calcium current and impairs contractility, which collectively produces a non-monotonic dose response [29, 45]. Notably, this group reported similar effects with BPS exposure—wherein low nanomolar concentrations increase calcium transient amplitude, fractional shortening, and calcium spark frequency via a similar mechanism [46]. To the best of our knowledge, the immediate effects of BPA on cardiac contractility have not been investigated in vivo. To elucidate the acute effects of BPA on contractility and relaxation, future studies should take into account both the route of administration and estimated timing for peak serum levels [47].
Future Directions
We aimed to review ongoing investigations into bisphenol-induced cardiotoxicity, in accordance with the recently established “Key characteristics of cardiovascular toxicants” to summarize experimental results related to 1) cardiac excitability, and 2) contractility and relaxation. Bisphenol chemical exposures can impair cardiac electrophysiology and mechanical function through disrupted calcium signaling, with some bisphenol chemicals (e.g., BPA) exerting more dramatic effects compared to others (e.g., BPS, BPF). Bisphenol-induced cardiotoxicity can result in decreased automaticity, slowed electrical conduction, and alterations in intracellular calcium handling that can precipitate triggered arrhythmias and/or impair contraction and relaxation. While the presented studies have expanded our understanding of BPA’s deleterious effects on cardiac physiology, significant knowledge gaps remain. As a guide for future research endeavors, we have identified additional considerations that can help to facilitate a scientific consensus on the risk of bisphenol-induced cardiotoxicity.
Can More Translational Models be Used to Validate Existing Experimental Findings?
Our current understanding of bisphenol-induced cardiotoxicity is largely based on the use of experimental rodent models. Species differences in cardiac electrophysiology, calcium handling, and mechanical function are well-documented [48-52] and emphasize the importance of evaluating cardiac endpoints using more human-relevant models. Future experimental work should utilize both non-rodent animal models and hiPSC-CM. Although hiPSC-CM display a more immature phenotype compared to adult cardiomyocytes [53], this cell model has proven to be a useful tool in both drug discovery and toxicity screening [54-56]. In addition to species-specific effects, future work should employ animal models or hiPSC-CM derived from both sexes, as some studies suggest that bisphenol chemicals may exert sex-specific effects that are mediated via estrogen receptor signaling. Finally, investigative studies should further explore the effects of bisphenol exposures on human cardiac electrophysiology through electrocardiogram recordings with a focus on resting heart rate, atrioventricular conduction, and adaptive responses to stress or exercise.
Are Vulnerable Groups More Susceptible to Adverse Cardiac Effects?
It is plausible that an acute high dose exposure and/or chronic low dose exposure to bisphenol chemicals may exacerbate conduction and contractile abnormalities in vulnerable populations—including neonates, the elderly, or those with pre-existing heart conditions. Intensive care patients may be exposed to elevated bisphenol chemical concentrations through contact with plastic medical devices, while neonatal and pediatric intensive care patients may also be exposed over longer periods of time due to immature metabolism. Further, patients with congenital heart defects, cardiomyopathies, or arrhythmias should also be considered, as bisphenol chemical exposures may exacerbate existing conditions. Notably, many of the low dose BPA effects are more readily observed in combination with external stressors (e.g., isoproterenol or fast pacing rates). Accordingly, patients with heart disease or cardiovascular conditions may experience exaggerated effects with even low dose exposures—particularly when cardiodepressive drugs are administered (e.g., L-type calcium channel blockers that slow heart rate and atrioventricular conduction). The timing of these measurements is critically important—as ion channel inhibition occurs rapidly and is likely to coincide with peak serum levels, which in turn, are influenced by both the route of exposure (e.g., oral vs intravenous) and metabolic maturation [47, 57].
Do Replacement Chemicals Have Comparable Adverse Cardiac Effects?
As health risks of BPA exposure have become more apparent, alternative chemicals (e.g., BPF, BPS) are being used as replacement chemicals to manufacture plastic products. While products manufactured with these chemicals can be marketed as “BPA-free”, the effects of these replacements on cardiovascular health are largely unknown and untested. Recent work by Prudencio et al. suggests that these chemical replacements may be less potent as compared with BPA, but this work was limited to electrophysiology recordings and additional studies are still needed to fully elucidate their cardiac safety profile. Conversely, studies by Ferguson et al. [40] and Gao et al. [46] suggest that BPS may exert effects on cardiac contractility that are comparable to BPA. Experimental results can differ due to the type of cardiac model (e.g., cell vs intact heart preparations, neonatal vs adult, atrial vs ventricular myocytes, rodent vs human, male vs female), the cardiac endpoints tested, the length of exposure time, and the dose tested. As previously highlighted, BPA may exert a non-monotonic dose response depending on the cardiac endpoint tested and experimental studies are likely to require the use of multiple doses to comprehensively address dose–response relationships [10]. Finally, recent biomonitoring studies suggest that humans are frequently exposed to multiple bisphenol chemicals [7], which underscores the importance of addressing co-exposure patterns and/or the ‘exposome’ and the collective impact of environmental chemicals on cardiac health [58, 59].
Conclusion
Epidemiological studies have highlighted associations between bisphenol chemical exposure and adverse cardiovascular health outcomes, and experimental research findings have revealed direct adverse effects of bisphenol chemical exposures on cardiac excitability, contractility, and relaxation. Such inhibitory effects on ionic currents follow a monotonic dose response—whereby increasing bisphenol chemical concentrations depress heart rate and slow electrical conduction. Yet, the consequences on cardiac contractility and relaxation are uncertain, and may be attributed to variable experimental approaches in dose, length of exposure, and the type of experimental model used. Although additional work is needed to fully elucidate the cardiac safety profile of replacement chemicals, to date, studies suggest that BPA exerts more potent inhibitory effects on cardiac ionic currents, as compared with BPF and BPS. Future experimental work can clarify the direct mechanistic effects of bisphenol chemicals on cardiac electrophysiology and mechanical function, while public health approaches can inform on best practices to reduce exposure. Complete avoidance of bisphenol exposure is improbable, but lifestyle changes (e.g., utilizing glass food storage containers, reduced use of canned goods or plastic containers) can help to minimize daily exposure to bisphenol chemicals.
Funding
This work was supported by the NIH (R01HL139472 to NGP).
Footnotes
Consent for Publication BC and NGP approved the manuscript for publication.
Conflict of interest The authors declare that they have no conflict of interest.
References
- 1.Dodds EC, & Lawson W (1936). Synthetic œstrogenic agents without the phenanthrene nucleus. Nature. 10.1038/137996a0 [DOI] [Google Scholar]
- 2.Vandenberg LN, Chahoud I, Heindel JJ, Padmanabhan V, Paumgartten FJR, & Schoenfelder G (2010). Urinary, circulating, and tissue biomonitoring studies indicate widespread exposure to bisphenol A. Environmental Health Perspectives, 118(8), 1055–1070. 10.1289/ehp.0901716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Betts KS (2010). Body of proof: Biomonitoring data reveal widespread bisphenol A exposures. Environmental Health Perspectives, 118(8), A353. 10.1289/ehp.118-a353a [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Calafat AM, Kuklenyik Z, Reidy JA, Caudill SP, Ekong J, & Needham LL (2005). Urinary concentrations of bisphenol A and 4-Nonylphenol in a human reference population. Environmental Health Perspectives, 113(4), 391–395. 10.1289/ehp.7534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Calafat AM, Ye X, Wong LY, Reidy J. a., & Needham LL (2008). Exposure of the U.S. population to Bisphenol A and 4-tertiary-octylphenol: 2003–2004. Environmental Health Perspectives, 116(1),39–44. 10.1289/ehp.10753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ye X, Wong LY, Kramer J, Zhou X, Jia T, & Calafat AM (2015). Urinary concentrations of bisphenol A and three other bisphenols in convenience samples of U.S. adults during 2000–2014. Environmental Science and Technology, 49(19), 11834–11839. 10.1021/acs.est.5b02135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lehmler HJ, Liu B, Gadogbe M, & Bao W (2018). Exposure to bisphenol A, bisphenol F, and bisphenol S in U.S. adults and children: The National Health and Nutrition Examination Survey 2013–2014. ACS Omega, 3(6), 6523–6532. 10.1021/acsomega.8b00824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.La Merrill MA, Vandenberg LN, Smith MT, Goodson W, Browne P, Patisaul HB, et al. (2020). Consensus on the key characteristics of endocrine-disrupting chemicals as a basis for hazard identification. Nature Reviews Endocrinology, 16(1), 45–57. 10.1038/s41574-019-0273-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rubin BS (2011). Bisphenol A: An endocrine disruptor with widespread exposure and multiple effects. Journal of Steroid Biochemistry and Molecular Biology, 127(1–2), 27–34. 10.1016/j.jsbmb.2011.05.002 [DOI] [PubMed] [Google Scholar]
- 10.Vandenberg LN (2014). Non-monotonic dose responses in studies of endocrine disrupting chemicals: Bisphenol a as a case study. Dose-Response, 12(2), 259–276. 10.2203/dose-response.13-020.Vandenberg [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vandenberg LN, Colborn T, Hayes TB, Heindel JJ, Jacobs DR, Lee D-H, et al. (2012). Hormones and endocrine-disrupting chemicals: Low-dose effects and nonmonotonic dose responses. Endocrine Reviews, 33(3), 378–455. 10.1210/ER.2011-1050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bao W, Liu B, Rong S, Dai SY, Trasande L, & Lehmler HJ (2020). Association between bisphenol a exposure and risk of all-cause and cause-specific mortality in US Adults. JAMA network open, 3(8), e2011620. 10.1001/jamanetworkopen.2020.11620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bae S, & Hong Y-C (2015). Exposure to bisphenol A from drinking canned beverages increases blood pressure: Randomized crossover trial. Hypertension, 65(2), 313–319. 10.1161/HYPERTENSIONAHA.114.04261 [DOI] [PubMed] [Google Scholar]
- 14.Bae S, Kim JH, Lim Y-H, Park HY, & Hong Y-C (2012). Associations of bisphenol A exposure with heart rate variability and blood pressure. Hypertension, 60(3), 786–793. 10.1161/HYPERTENSIONAHA.112.197715 [DOI] [PubMed] [Google Scholar]
- 15.Melzer D, Rice NE, Lewis C, Henley WE, & Galloway TS (2010). Association of urinary bisphenol a concentration with heart disease: Evidence from NHANES 2003/06. PLoS ONE, 5(1), e8673–e8673. 10.1371/journal.pone.0008673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Monica Lind P, & Lind L (2011). Circulating levels of bisphenol A and phthalates are related to carotid atherosclerosis in the elderly. Atherosclerosis, 218(1), 207–213. 10.1016/j.atherosclerosis.2011.05.001 [DOI] [PubMed] [Google Scholar]
- 17.Food and Drug Administration. (2014). Bisphenol A (BPA): Use in Food Contact Application. Retrieved October 12, 2021, from https://www.fda.gov/food/food-additives-petitions/bisphenol-bpa-use-food-contact-application [Google Scholar]
- 18.State of California, Office of Environmental Health Hazard Assessment, Proposition 65. (2015). Retrieved from https://oehha.ca.gov/media/downloads/proposition-65/transcript/may72015transcript.pdf [Google Scholar]
- 19.Gyllenhammar I, Glynn A, Jönsson BAG, Lindh CH, Darnerud PO, Svensson K, & Lignell S (2017). Diverging temporal trends of human exposure to bisphenols and plastizisers, such as phthalates, caused by substitution of legacy EDCs? Environmental research, 153, 48–54. 10.1016/J.ENVRES.2016.11.012 [DOI] [PubMed] [Google Scholar]
- 20.Trasande L (2017). Exploring regrettable substitution: Replacements for bisphenol A. The Lancet Planetary Health, 1(3), e88–e89. 10.1016/S2542-5196(17)30046-3 [DOI] [PubMed] [Google Scholar]
- 21.Lind L, Araujo JA, Barehowsky A, Belcher S, Berridge BR, Chiamvimonvat N, et al. (2021). Key characteristics of cardiovascular toxicants. Environmental Health Perspectives, 129(9), 95001–95001. 10.1289/EHP9321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.O’Reilly AO, Eberhardt E, Weidner C, Alzheimer C, Wallace BA, & Lampert A (2012). Bisphenol a binds to the local anesthetic receptor site to block the human cardiac sodium channel. PLoS ONE, 7(7), e41667–e41667. 10.1371/journal.pone.0041667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Prudencio TM, Swift LM, Guerrelli D, Cooper B, Reilly M, Ciccarelli N, et al. (2021). Bisphenol S and bisphenol F are less disruptive to cardiac electrophysiology, as compared with bisphenol A. Toxicological Sciences, 183(1), 214–226. 10.1093/toxsci/kfab083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hyun S-A, Lee CY, Ko MY, Chon S-H, Kim Y-J, Seo J-W, et al. (2021). Cardiac toxicity from bisphenol A exposure in human-induced pluripotent stem cell-derived cardiomyocytes. Toxicology and Applied Pharmacology, 428, 115696. 10.1016/j.taap.2021.115696 [DOI] [PubMed] [Google Scholar]
- 25.Wang Q, Cao J, Zhu Q, Luan C, Chen X, Yi X, et al. (2011). Inhibition of voltage-gated sodium channels by bisphenol A in mouse dorsal root ganglion neurons. Brain Research, 1378, 1–8. 10.1016/j.brainres.2011.01.022 [DOI] [PubMed] [Google Scholar]
- 26.Horváth B, Hézső T, Kiss D, Kistamás K, Magyar J, Nánási PP, & Bányász T (2020). Late sodium current inhibitors as potential antiarrhythmic agents. Frontiers in Pharmacology, 11, 413. 10.3389/fphar.2020.00413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Deutschmann A, Hans M, Meyer R, Häberlein H, & Swandulla D (2013). Bisphenol A inhibits voltage-activated Ca(2+) channels in vitro: Mechanisms and structural requirements. Molecular pharmacology, 83(2), 501–511. 10.1124/mol.112.081372 [DOI] [PubMed] [Google Scholar]
- 28.Michaela P, Mária K, Silvia H, L’ubica L, & L’ubica L (2014). Bisphenol A differently inhibits CaV3.1, Ca V3.2 and Ca V3.3 calcium channels. Naunyn Schmiedebergs Archives of Pharmacology. 10.1007/s00210-013-0932-6 [DOI] [PubMed] [Google Scholar]
- 29.Liang Q, Gao X, Chen Y, Hong K, & Wang H-S (2014). Cellular mechanism of the nonmonotonic dose response of bisphenol A in rat cardiac myocytes. Environmental Health Perspectives, 122(6), 601–608. 10.1289/ehp.1307491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Feiteiro J, Mariana M, Glória S, & Cairrao E (2018). Inhibition of L-type calcium channels by Bisphenol A in rat aorta smooth muscle. Journal of Toxicological Sciences, 43(10), 579–586. 10.2131/jts.43.579 [DOI] [PubMed] [Google Scholar]
- 31.Posnack NG, Jaimes R, Asfour H, Swift LM, Wengrowski AM, Sarvazyan N, et al. (2014). Bisphenol A exposure and cardiac electrical conduction in excised rat hearts. Environmental Health Perspectives, 122(4), 384–390. 10.1289/ehp.1206157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Patel BB, Raad M, Sebag IA, & Chalifour LE (2015). Sex-specific cardiovascular responses to control or high fat diet feeding in C57bl/6 mice chronically exposed to bisphenol A. Toxicology Reports, 2, 1310–1318. 10.1016/j.toxrep.2015.09.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ramadan M, Sherman M, Jaimes R, Chaluvadi A, Swift L, & Posnack NG (2018). Disruption of neonatal cardiomyocyte physiology following exposure to bisphenol-a. Scientific Reports. 10.1038/s41598-018-25719-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pant J, Ranjan P, & Deshpande SB (2011). Bisphenol A decreases atrial contractility involving NO-dependent G-cyclase signaling pathway. Journal of Applied Toxicology, 31(7), 698–702. 10.1002/JAT.1647 [DOI] [PubMed] [Google Scholar]
- 35.Belcher SM, Gear RB, & Kendig EL (2015). Bisphenol a alters autonomic tone and extracellular matrix structure and induces sex-specific effects on cardiovascular function in male and female CD-1 mice. Endocrinology, 156(3), 882–895. 10.1210/en.2014-1847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Katz AM, & Lorell BH (2000). Regulation of Cardiac Contraction and Relaxation. Circulation, 102(suppl_4), 69–74. 10.1161/circ.102.suppl_4.iv-69 [DOI] [PubMed] [Google Scholar]
- 37.Edwards JN, & Blatter LA (2014). Cardiac alternans and intracellular calcium cycling. Clinical and Experimental Pharmacology & Physiology, 41(7), 524–532. 10.1111/1440-1681.12231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang R, & Pessah IN (2017). Divergent mechanisms leading to signaling dysfunction in embryonic muscle by bisphenol a and tetrabromobisphenol A. Molecular Pharmacology, 91(4), 428–436. 10.1124/mol.116.107342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Posnack NG, Brooks D, Chandra A, Jaimes R, Sarvazyan N, & Kay MW (2015). Physiological response of cardiac tissue to Bisphenol A: Alterations in ventricular pressure and contractility. American Journal of Physiology. Heart and Circulatory Physiology, 309(2), H267–H275. 10.1152/ajpheart.00272.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ferguson M, Lorenzen-Schmidt I, & Pyle WG (2019). Bisphenol S rapidly depresses heart function through estrogen receptor-β and decreases phospholamban phosphorylation in a sex-dependent manner. Scientific Reports, 9(1), 15948. 10.1038/s41598-019-52350-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yan S, Chen Y, Dong M, Song W, Belcher SM, & Wang HS (2011). Bisphenol A and 17β-estradiol promote arrhythmia in the female heart via alteration of calcium handling. PLoS ONE, 6(9), e25455. 10.1371/journal.pone.0025455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gao X, Liang Q, Chen Y, & Wang H-SS (2013). Molecular mechanisms underlying the rapid arrhythmogenic action of bisphenol A in female rat hearts. Endocrinology, 154(12), 4607–4617. 10.1210/en.2013-1737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Belcher SM, Chen Y, Yan S, & Wang H-SS (2011). Rapid estrogen receptor-mediated mechanisms determine the sexually dimorphic sensitivity of ventricular myocytes to 17beta-estradiol and the environmental endocrine disruptor bisphenol A. Endocrinology, 153(2), 712–720. 10.1210/en.2011-1772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ma J, Hong K, & Wang H-SS (2017). Progesterone protects against bisphenol a-induced arrhythmias in female rat cardiac myocytes via rapid signaling. Endocrinology, 158(4), 778–790. 10.1210/en.2016-1702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gao X, & Wang H-S (2014). Impact of bisphenol A on the cardiovascular system—Epidemiological and experimental evidence and molecular mechanisms. International Journal of Environmental Research and Public Health, 11(8), 8399–8413. 10.3390/ijerph110808399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gao X, Ma J, Chen Y, & Wang H-S (2015). Rapid responses and mechanism of action for low-dose bisphenol S on ex vivo rat hearts and isolated myocytes: Evidence of female-specific proarrhythmic effects. Environmental health perspectives, 123(6), 571–578. 10.1289/ehp.1408679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Doerge DR, Twaddle NC, Vanlandingham M, & Fisher JW (2010). Pharmacokinetics of bisphenol A in neonatal and adult Sprague-Dawley rats. Toxicology and Applied Pharmacology, 247(2), 158–165. 10.1016/j.taap.2010.06.008 [DOI] [PubMed] [Google Scholar]
- 48.Rudy Y, Ackerman MJ, Bers DM, Clancy CE, Houser SR, London B, et al. (2008). Systems approach to understanding electromechanical activity in the human heart a national heart, lung, and blood institute workshop summary. Circulation, 118(11), 1202–1211. 10.1161/CIRCULATIONAHA.108.772715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Edwards AG, & Louch WE (2017). Species-dependent mechanisms of cardiac arrhythmia: A cellular focus. Clinical Medicine Insights. Cardiology 10.1177/1179546816686061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bers DM (1991). Species differences and the role of sodiumcalcium exchange in cardiac muscle relaxation. Annals of the New York Academy of Sciences, 639(1), 375–385. 10.1111/j.1749-6632.1991.tb17326.x [DOI] [PubMed] [Google Scholar]
- 51.Tanaka H, Komikado C, Namekata I, Nakamura H, Suzuki M, Tsuneoka Y, et al. (2008). Species difference in the contribution of T type calcium current to cardiac pacemaking as revealed by R(−)-efonidipine. Journal of Pharmacological Sciences, 107(1), 99–102. 10.1254/jphs.SC0070405 [DOI] [PubMed] [Google Scholar]
- 52.Cooper BL, Gloschat C, Swift LM, Prudencio T, McCullough D, Jaimes R 3rd., & Posnack NG (2021). KairoSight: Open-source software for the analysis of cardiac optical data collected from multiple species. Frontiers in Physiology. 10.3389/FPHYS.2021.752940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Karbassi E, Fenix A, Marchiano S, Muraoka N, Nakamura K, Yang X, & Murry CE (2020). Cardiomyocyte maturation: Advances in knowledge and implications for regenerative medicine. Nature Reviews Cardiology. 10.1038/s41569-019-0331-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Magdy T, Schuldt AJT, Wu JC, Bernstein D, & Burridge PW (2018). Human induced pluripotent stem cell (hiPSC)-derived cells to assess drug cardiotoxicity: Opportunities and problems. Annual Review of Pharmacology and Toxicology. 10.1146/annurev-pharmtox-010617-053110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Harding SE (2011). Human stem cell-derived cardiomyocytes for pharmacological and toxicological modeling. Annals of the New York Academy of Sciences, 1245(1), 48–49. 10.1111/J.1749-6632.2011.06328.X [DOI] [PubMed] [Google Scholar]
- 56.Pang L, Sager P, Yang X, Shi H, Sannajust F, Brock M, et al. (2019). Workshop report: FDA workshop on improving cardiotoxicity assessment with human-relevant platforms. Circulation Research, 125(9), 855–867. 10.1161/CIRCRESAHA.119.315378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Thayer KA, Doerge DR, Hunt D, Schurman SH, Twaddle NC, Churchwell MI, et al. (2015). Pharmacokinetics of bisphenol A in humans following a single oral administration. Environment International, 83, 107–115. 10.1016/j.envint.2015.06.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.DeBord DG, Carreón T, Lentz TJ, Middendorf PJ, Hoover MD, & Schulte PA (2016). Use of the “exposome” in the practice of epidemiology: A primer on -omic technologies. American Journal of Epidemiology, 184(4), 302–314. 10.1093/aje/kwv325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chung MK, Kannan K, Louis GM, & Patel CJ (2018). toward capturing the exposome: Exposure biomarker variability and coexposure patterns in the shared environment. Environmental Science & Technology, 52(15), 8801. 10.1021/ACS.EST.8B01467 [DOI] [PMC free article] [PubMed] [Google Scholar]