Abstract
Fundamental to all living organisms is the ability of proteins to interact with other biological molecules at the right time and location, with the proper affinity, and to do so reversibly. One well-established technique to study protein interactions is chemical cross-linking, a process in which proteins in close spatial proximity are covalently tethered together. An emerging technology that overcomes many limitations of traditional cross-linking methods is one in which photoactivatable cross-linking noncanonical amino acids are genetically encoded into a protein of interest using the cell’s native translational machinery. These proteins can then be used to trap interacting biomolecules upon UV illumination. Here, we describe a method for the site-specific incorporation of photoactivatable cross-linking amino acids into fluorescently tagged proteins of interest in E. coli. Photo-cross-linking and analysis by SDS-PAGE using in-gel fluorescence detection, which provides rapid, highly sensitive, and specific detection of cross-linked adducts even in impure systems, are also described. An example expression and cross-linking experiment involving transmembrane signaling of a bacterial second messenger receptor system that controls biofilm formation is shown. All reagents needed to carry out these experiments are commercially available, and do not require special or unique technology to perform, making this method tractable to a broad community studying protein structure and function.
Keywords: Photo-cross-linking, UV cross-linking, Para-azidophenylalanine, Para-benzoylphenylalanine, Genetic code expansion, Noncanonical amino acid
1. Introduction
Complex networks of protein–protein interactions are the foundation of a cell’s ability to send, receive, and respond to signals. Numerous strategies exist to delineate the language of these signaling networks. Cross-linking—the process of forming covalent bonds between two biological molecules, such as proteins and DNA—is a time-honored tool that can yield atomic resolution information about the interactions of biomolecules without the need for specialized equipment or technology [1]. Depending on the particular method, cross-linking crosslinkerphoto-activatable crosslinkers can be used to probe both specific and nonspecific interactions. Cross-linking is commonly performed by introducing small synthetic molecules that contain two functional groups separated by a linker of variable length and that are able to covalently connect amine groups (such as the side chain of lysines), sulfhydryl groups (cysteines), and carboxyl groups (aspartate and glutamate) [1]. Cross-linkers in which the functional cross-linking group is activated with light are also attractive, as it gives the researcher an additional level of temporal control over the cross-linking reaction [2–5].
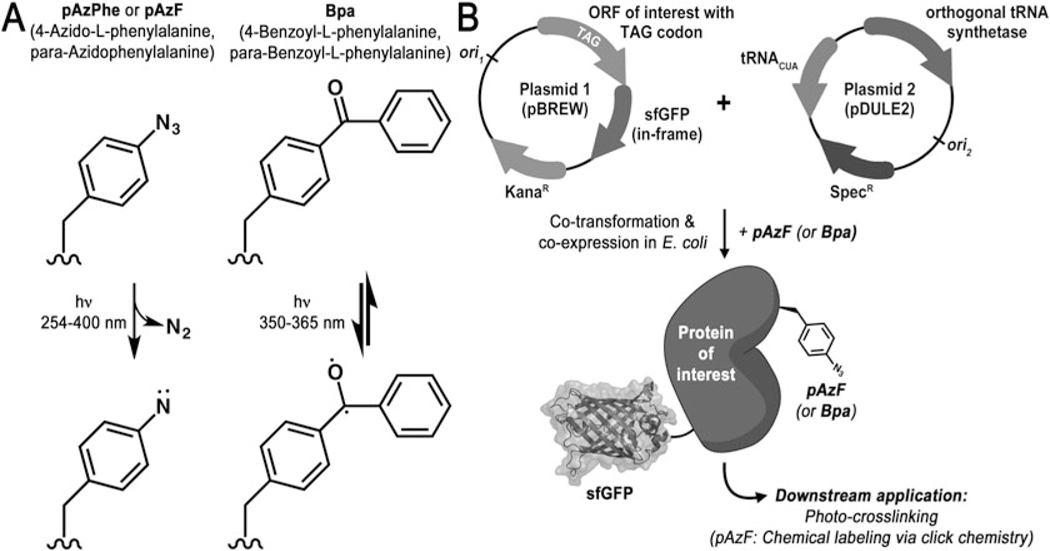
Here, we describe an increasingly popular cross-linking technique in which a UV photoactivatable cross-linker is introduced site-specifically into a protein in vivo via genetic code expansion [6, 7]. To do this, the protein of interest is coexpressed with an aminoacyl-tRNA syntetase that has been engineered to recognize a cross-linking noncanonical amino acid (ncAA), such as para-azidophenylalanine (pAzF) [8–10] or para-benzoylphenylalanine (Bpa) [11] (Fig. 1a). This synthetase then charges its cognate tRNA, which itself has been engineered to recognize the amber stop codon (TAG) so that when an amber stop codon is introduced at the desired site, the ncAA is incorporated at that site of the peptide chain during translation. Full-length protein, generated only if the amber stop codon is successfully supressed, can be purified using a C-terminally expressed affinity tag for in vitro cross-linking reactions. Alternatively, cross-linking reactions can be carried out in whole cells or cell lysates.
Fig. 1.
UV photo-cross-linking using genetic code expansion. (a) Structures and mechanism of photoactivation of pAzF and Bpa noncanonical amino acids. (b) Expression system and plasmids for the incorporation of noncanonical amino acids into a protein of interest. This sytem requires two plasmids: the expression plasmid (pBrew) in which the protein of interest containing the TAG amber stop codon is C-terminally fused to superfolder GFP, and the machinery plasmid (pDule2) that expresses the orthogonal tRNA synthetase that recognizes and charges its cognate amber-suppressing tRNA (also expressed on this plasmid) with the noncanonical amino acid. Only full-length protein containing the noncanonical amino acid is fluorescent
The methods below outline a general strategy for the incorporation for pAzF or Bpa into recombinantly expressed fluorescent proteins in E. coli (see Note 1), for subsequent cross-linking reactions, and for analysis by SDS-PAGE and detection by in-gel fluorescence. Though this chapter largely describes the use of pAzF as a cross-linker, Bpa is also widely used, and the choice of which to incorporate depends on the system being studied (see Note 2). As shown in Fig. 1a, pAzF is activated by UV light irreversibly, and will only cross-link if it is in the immediate proximity of a binding partner. Thus pAzF cross-linking efficiency, though variable (depending on the site of cross-linkage) and not stoichiometric, can be used as an analytical tool to reflect the amount of protein–protein binding at a particular point in time [12]. UV activation of Bpa, on the other hand, is reversible and so it can be continuously irradiated until a binding parter engages and thus Bpa-mediated cross-links in principle will continue to accumulate over time with increased UV flux. While both photo-cross-linkers can be used to assess inter-protein interactions, important to note is that intraprotein interactions and dynamics can be evaluated as well with photo-cross-linking (e.g., to analyze conformational states of multidomain proteins) (Fig. 2).
Fig. 2.
Applications and analysis of photo-cross-linking: inter-peptide vs. intra-peptide adducts. (a) Upon UV illumination, the cross-linker amino acid can form a covalent bond with an interacting protein when placed at a protein–protein interface (top), or to an amino acid in the same polypeptide chain distant in primary sequence but close in 3-dimensional space (< ∼4 Å ) (bottom). (b) SDS-PAGE analysis can be used to differentiate these two cross-linking phenomena. Inter-protein interactions will typically migrate slower as the cross-linked species is of larger moleculear weight (top), while intra-protein cross-linked species commonly migrate faster due to the formation of a more compact, circular polypeptide (bottom)
Here, we describe a general protocol for the incorporation of pAzF and Bpa into proteins using the popular T7-based expression system in BL21-type cells, as well as a protocol for the ensuing cross-linking reactions. The reader should be aware that many viable variations on the methods described here exist [13], and these protocols will almost always require optimization for the particular system being investigated. In addition, we describe the application of superfolder green fluorescence protein (sfGFP) for the specific and sensitive detection of cross-linked products by in gel-fluorescence imaging. The examples shown here demonstrate its utility for analyzing binding of the bacterial calcium-dependent periplasmic LapG protease (see Note 3) to its substrate LapA, an outer membrane anchored adhesin protein, a well-studied system involved in bacterial biofilm formation [12, 14–16].
2. Materials
2.1. The Machinery Plasmid
The appropriate genetic machinery plasmid(s) must first be obtained for ncAA incorporation.
The “pDule2” machinery plasmid conferring spectinomycinresistance expresses an engineered tRNA synthetase and its cognate amber-suppressing tRNA under constitutive promoters (the “pDule” plasmid is functionally identical but confers tetracycline resistance).
To incorporate a particular ncAA of interest, the correct synthetase must be expressed from this pDule2 (or pDule) plasmid.
To incorporate pAzF, the pDule2-pCNF (or pDule-pCNF) plasmid is required (see Note 4) while the pDule2-Bpa (or pDule-Bpa) plasmid is required for Bpa incorporation.
These plasmids (as well as others recommended for performing proper control experiments [see Note 5]) can be obtained by request from the Unnatural Protein Facility at Oregon State University (upfacility@oregonstate.edu).
2.2. The Expression Plasmids
- At a minimum, two expression plasmids are required:
- The first plasmid should express the protein that will be cross-linked, that is, the “Interacting protein” (IntP, Fig. 2). This protein will not have any ncAA’s incorporated into it. It should be expressed in the sytem best suited for this particular protein as optimized by the investigator. In the example below, this will be referred to as the pBAD-LapA plasmid, which confers ampicillin resistance.
- The second plasmid should express the protein in which pAzF (or Bpa) is to be incorporated site-specifically (Fig. 1).
The choice of plasmid for this expression system is important and it is essential that it not have a p15a origin of replication (use of such a vector will result in plasmid incompatbility issues as the pDule/pDule2 plasmids also have a p15a origin). Traditional pET (e.g., pET28a) and pBAD plasmids containing the pBR322 origin are suitable expression vectors for this purpose.
Note that if the protein is to be purified, it is adventageous that the expression construct contains a C-terminal affinity tag so that only full-length protein is isolated. Here, we describe methods for using the pET28a-based “pBrew” plasmid in which the protein of interest is expressed with a C-terminally fused monomeric superfolder green fluorescence protein (sfGFP) followed by a hexahistidine affinity tag (Fig. 1b) [12]. The sfGFP-His6 fusion protein is linked to the protein of interest by a TEV protease recognition sequence for convenient removal, if desired. We have observed several cases in which protein expression, as well as cross-linking analysis, was significantly facilitated by the use of the pBrew plasmid (see Note 6).
An amber stop codon (TAG) should be incorporated in the gene at the desired position of ncAA incorporation via standard mutagenesis protocols, while this codon shall not be used as a terminal stop codon to avoid secondary ncAA incorporation at the C-terminus of the protein. The example plasmid here will be referred to here as pBrew-LapGTAG, which has a TAG site located at position Y108 (see Note 7).
2.3. DNA Transformation into BL21 T7-Based Expression E. coli Cells
Escherichia coli BL21ai One Shot electrocompetent cells (see Note 8).
Electroporator.
0.1 cm electroporation cuvettes.
SOC medium: 0.5% (w/v) yeast extract, 2% (w/v) tryptone,10 mM NaCl, 2.5 mM KCl, 20 mM MgSO4, 20 mM glucose (see Note 9). Prepare 10 mL. Autoclave.
LB agar plates: 1% (w/v) yeast extract, 0.5% (w/v) tryptone,86 mM NaCl, 1.5% (w/v) Bacto agar with appropriate antibiotics. For the examples described here, one LB/agar plate should have 50 μg/mL kanamycin +100 μg/mL spectinomycin for the pBrew-LapGTAG/pDule2-pCNF double transformation, and the other plate should have 100 μg/mL ampicillin for the pBAD-LapA single transformation (see Note 10).
2.4. Protein Expression
LB medium: 1% (w/v) yeast extract, 0.5% (w/v) tryptone, 86 mM NaCl. Prepare 50 mL. Autoclave.
- Component solutions for making autoinduction media (adapted from [17]).
- ZY solution: 1% (w/v) yeast extract, 0.5% (w/v) tryptone. Prepare 500 mL. Autoclave.
- 1 M MgSO4: Prepare 50 mL. Autoclave.
- 25 M-salts: 0.625 M Na2HPO4, 0.625 M KH2PO4, 1 M NH4Cl, 125 mM (NH4)2SO4 (do not adjust pH). Prepare 500 mL. Autoclave.
- 5052 solution: 12.5 g α-D-glucose, 50 g α-lactose, 125 mL glycerol. Autoclave. Prepare 500 mL (see Note 11).
- 20% (w/v) L-(+)-arabinose. Prepare 50 mL. Sterile filter.
- 5000 trace metal solution (see Note 12): 20 mM CaCl2, 10 mM MnCl2, 10 mM ZnSO4, 2 mM CoCl2, 2 mM CuCl2, 2 mM NiCl2, 2 mM NaMoO4, 2 mM Na2SeO3, 2 mM H3BO3, 5 mM FeCl3. Prepare 100 mL. Sterile filter.
- 1000× antibiotic stock solutions. Dissolve with sterile water. Freeze at –20 °C until use. For the examples described here, prepare 1 mL of each of the following:
- 50 mg/mL kanamycin.
- 100 mg/mL spectinomycin.
- 100 mg/mL ampicillin.
100 mM para-azido-phenylalanine (pAzF) or para-benzoyl-phenylalanine (Bpa) stock in water (see Note 13).
15 mL culture tubes, sterile.
500 mL baffled culture flasks, sterile.
2.5. Protein Purification
2 mL Ni-NTA resin (Qiagen).
PD-10 disposable desalting columns, bed dimensions 14.5 × 50 mm, bed volume 8.3 mL of sephadex G-25 resin (GE Healthcare, product number 17–0851-01).
Disposable columns that hold up to 2 mL of resin and 10 mL ofsample.
Sonicator with 0.5 in. wide probe, or French Press cell disruptor.
UV-Visible spectrophotometer.
Quartz cuvettes.
Wash buffer: 25 mM HEPES, 500 mM NaCl, 20 mM imidazole pH 7.5. Prepare 1 L.
Elution buffer: 25 mM HEPES, 500 mM NaCl, 300 mMimidazole pH 7.5. Prepare 50 mL.
Reaction buffer: 25 mM HEPES, 250 mM NaCl pH 7.5. Prepare 500 mL.
2.6. Cross-Linking and SDS-PAGE Analysis
Clear 96-well microplate.
Handheld UV-lamp with short and long wave UV-light illumination capability.
Power supply for SDS-PAGE.
SDS-PAGE gel running setup (e.g., Bio-Rad Mini-PROTEAN Tetra-cell).
0.25 M Tris base, pH to 6.8 with 6 M HCl.
5 ×SDS-sample buffer (50 mL): 0.25% (w/v) bromophenol blue, 0.25 M Tris base, pH 6.8, 10% (w/v) SDS, 50% (v/v) glycerol.
5 SDS-sample reducing buffer To make the 5 SDS sample buffer a sample buffer reducing, add 100 μL of β-mercaptoethanol (see Note 14) to 400 μL of 5 SDS sample buffer. 10 SDS-PAGE running buffer: 0.25 M Tris base, 1.92 M glycine, 1% (w/v) SDS. Do not adjust pH. Dilute to 1× concentration before use
Precast acrylamide SDS-PAGE gel (see Note 15).
In-gel fluorescence imager (e.g., Bio-Rad GelDoc system) withblue light LED excitation and emission filter able to detect ∼510–530 nm fluorescence. If not available, or if not using fluorescently tagged proteins like those expressed in the pBrew plasmid, proteins may be visualized via alternative methods including Coomassie Blue stain, silver stain, SYPRO ruby protein stain, or by Western blot.
3. Methods
The methods below discuss the incorporation of pAzF into a protein of interest and subsequent cross-linking to a binding partner protein. The incorporation of Bpa follows the identical method except where noted.
3.1. Plasmid Transformation
We recommend performing a fresh transformation before each expression rather than growing from strains frozen in glycerol stocks (see Note 16).
Transfer 50 μL of freshly thawed electrocompetent BL21ai cells to a prechilled 0.1 cm electroporation cuvette. Add ∼1–10 ng of the pBrew-LapGTAG plasmid and ∼1–10 ng of the pDule2-pCNF plasmid (see Note 17).
Pulse the cells at 1.8 kV (200 Ω, 25 μF). If the electrocuvette generates an arc, redo the transformation with fresh cells using 1/10th the amount of DNA.
Immediately resuspend cells with 1 mL of room temperatureSOC media and transfer suspension to a 1.5 mL eppendorf tube.
Perform a second, separate transformation with only the pBAD-LapA plasmid by repeating above steps 1–3.
Incubate cells at 37 °C for 1 h with shaking at 200–250 rpm.
Plate ∼200 μL of recovered cells onto LB/agar plates with the appropriate antibiotics (50 μg/mL kanamycin + 100 μg/mL spectinomycin for the pBrew-LapGTAG/pDule2-pCNF double transformation, 100 μg/mL ampicillin for the pBAD-LapA single transformation). Discard remaining cells.
Incubate plates at 37 °C overnight (14–20 h).
3.2. Protein Expression
Outlined here is a general strategy for expressing proteins with autoinduction media at 37 °C as a reasonable starting point since these are the conditions where ncAA incorporation is most efficient. The ideal expression conditions of the reader’s protein may require optimization or alterations.
Scrape several colonies of the pBrew-LapGTAG/pDule2-pCNF strain and use them to inoculate a 3 mL LB culture containing 50 μg/mL kanamycin and 100 μg/mL spectinomycin.
Repeat for pBAD-LapA except media should contain 100 μg/mL ampicillin.
Grow these two starter cultures at 37 °C for 3–4 h with shaking at 200–250 rpm or until visibly turbid (OD600> ∼1).
Divide autoinduction medium into two 500 mL culture flaskseach containing 100 mL of medium.
Add appropriate antibiotics to each flask. For pBrew-LapGTAG/pDule2-pCNF, use 50 μg/mL kanamycin and 100 μg/mL spectinomycin. For pBAD-LapA use 100 μg/mL ampicillin.
Once starter cultures are visually turbid (prepared in Subheading 3.2, step 2), add 1 mL of starter culture to each flask containing autoinduction media prepared in Subheading 3.2, step 6.
Grow at 37 °C for 1–2 h until cells are visible (OD ∼0.1–0.3) to ensure cells are growing properly before addition of ncAA.
Once cell growth is confirmed (∼1–2 h after inoculation), tothe pBrew-LapGTAG/pDule2-pCNF culture, add 1 mL of 100 mM pAzF stock for a final concentration of 1 mM in the medium (see Note 20).
Continue shaking at 37 °C for 16–24 h (see Note 21).
3.3. Preparation of Cell-Free Soluble Lysates
Centrifuge cells at 4000 × g for 15 min at 4 °C.
Pour off supernatant and gently resuspend pelleted cells with30 mL of wash buffer (see Note 22). At this point, cells may be frozen by rapid immersion in liquid nitrogen and stored at 80 °C.
Lyse each set of cells via sonication for 2 × 30 s intervals at 30–80% power. Appropriate power settings and length of sonication will vary with sonicator manufacturer, probe size, sample size and density of cell suspension (see Note 23).
Centrifuge lysed cells at 20,000 × g for 45 min at 4 °C to pellet membranes and insoluble debris.
After centrifugation, collect supernatant of each sample. The supernatant contains the soluble fraction of cell lysate with the expressed protein.
3.4. Protein Purification
These steps should all be performed at 4 °C. When working withproteins containing pAzF, light exposure should be minimized (see Note 24)
Equilibrate ∼2 mL bed volume of Ni-NTA resin with 10 column volumes (i.e., 20 mL) of wash buffer. This can be done by removing 4 mL of a 50% slurry suspesion and pipetting into column. Allow the storage solution (usually 20% ethanol) to flow through completely. Discard this flow through. Then gently add 10 mL of wash buffer without disturbing resin bed, and allow all buffer to flow through.
Resuspend resin with 2 mL of wash buffer by gentle pipetting.The final volume of this resin solution will be 4 mL.
Add 2 mL of this 50% slurry to each freshly prepared cell lysate.
Gently rotate (or stir) cell-free lysate with resin for 30 min at 4 °C.
Collect resin by pouring lysate–resin mixture through column(see Note 25).
Once lysate has flowed through, wash resin with 20 column volumes (40 mL) of wash buffer.
Elute protein from resin by adding 2.5 mL of elution buffer.
Collect all 2.5 mL of eluted protein in a single 15-mL conical tube.
Equilibrate two PD-10 desalting columns each with 30 mL of reaction buffer.
Add the protein (2.5 mL, obtained in Subheading 3.4, step 7) that is currently in elution buffer to the PD-10 column. Discard eluate.
Add 3.5 mL of reaction buffer, and collect eluate. This eluate contains the purified protein, now in reaction buffer.
- Quantitate the amount of purified protein.
- For the sfGFP fusion proteins, measure the absorbance at 488 nm and calculate protein concentration using Beer’s law (sfGFP extinction coefficient at 488 nm is 83,300/M/cm).
- For nonfluorescent proteins, this can be performed by measuring the absorbance at 280 nm and using the primary sequence to calculate molar extinction coefficients (for LapG, use 11,000/M/cm) (see Note 26).
3.5. Protein Photo-Cross-Linking
The concentrations, additives, and conditions in which the cross-linking reactions occur will vary and will require optimizing for each individual application.
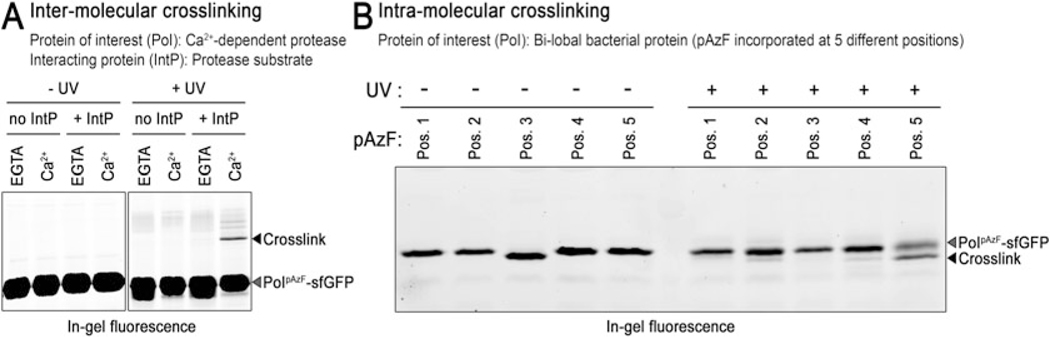
In 25 μL total volume, dilute into reaction buffer the sfGFP fusion protein of interest containing the pAzF (or Bpa) to 2 μM along with 10–100 μM interacting partner (obtained upon completion of the purification outlined in Subheading 3.4). In the example described here, we diluted LapG Y108pAzF-sfGFP (expressed from the pBrew-LapGTAG/pDule2-pCNF expression system) to 2 μM with 50 μM LapA (expressed from the pBAD-LapA system) in the presence of calcium or the calcium chelator EGTA (see Note 3), allowing us to ascertain the effect calcium has on the binding of LapG to its substrate, LapA (Fig. 3).
Allow protein interactions to equilibrate for 20 min at roomtemperature.
Take 12 μL out of each reaction, and place into a well of a clear 96-well microplate. Save the remainder of each reaction on ice.
- Once all samples are in the 96-well plate, place a dual wavelength handheld UV lamp directly on top of the plate so that the lamp is as close to the samples as possible. Ensure that during the course of the cross-linking process, UV light is evenly distributed across all samples. Wear protective eyewear and/or cover samples with aluminum foil to avoid UV damage to eyes.
- For pAzF cross-linking, illuminate samples with short wave (∼254 nm) UV light for 5 min or less (see Note 27).
- For Bpa cross-linking, use longer wavelength light (∼360 nm), and shine light for 10 min–2 h (time may need to be optimized).
Once cross-linking reaction is complete, remove 8 μL from each UV-exposed well to a fresh microcentrifuge tube.
Add 2 μL of 5 × reducing SDS sample buffer and mix well.
In a similar manner, remove 8 μL of each non-UV-exposed sample (saved in step 3, Subheading 3.5) and mix with 2 μL of 5 × reducing SDS sample buffer. This will act as a negative control in which no cross-linking should occur.
To maintain sfGFP fluorescence, do not boil these samples (see Note 28).
Run all 10 μL on a 12% SDS-PAGE according to standard protocols.
Image gels by fluorescence using a Bio-Rad GelDoc system using blue light emission and a 525/10 (or equivalent) filter in order to visualize fluorescent bands from the sfGFP fusion proteins (Fig. 3). When two proteins are inter-molecularly cross-linked, one should observe a new band in the +UV sample with an apparent molecular weight corresponding to roughly the sum of both protein proteins (Fig. 3a). On the other hand, intra-molecularly cross-linked peptides will commonly migrate with faster electrophoretic mobility than their non-cross-linked counterparts due to increased compactness (Fig. 3b).
Fig. 3.
SDS-PAGE analysis of LapG photo-cross-linking. (a) Inter-peptide cross-linking: a fluorescently labeled, covalently cross-linked adduct with slower electrophoretic mobility is observed only when the LapG proteasesfGFP containing pAzF is illuminated with UV light in the presence of its substrate LapA (i.e., “IntP”) and calcium. This observation demonstrates calcium is required for protease–substrate interaction. (b) Intra-peptide cross-linking: pAzF was incorported at 5 different positions into a protein containing two structural lobes. Only when AzF was located as “position 5” (Pos. 5) was a new intra-peptide cross-linked species with faster electrophoretic mobility observed upon UV light illumination, indicating this residue (and not the others) is at the interface of two folded sub-domains
4. Notes
The synthetase/tRNA system described here, which was derived from the archaeon Methanocaldococcus janasheii, works only in E. coli. However, systems have been developed to incorporate pAzF, Bpa, and other cross-linking ncAAs into yeast and mammalian cell lines.
Amino acids with diazirine functional groups have also beengenetically incorporated into proteins and successfully used as a photo-cross-linker. To our knowledge, these reagents are not commercially available and therefore their use is less accessible to the broader community than pAzF or Bpa.
In its native host, the LapG protease is targeted to the periplasm, however here we recombinantly express the LapG gene without its leader periplasmic signal sequence so that LapG is expressed in the cytoplasm. Calcium is required for LapG proteolytic activity, and when this protein is incubated with calcium chelators (such as EGTA) the LapG protease is unable to proteolyze substrates [18].
Though tRNA synthetases have been engineered to specificallyrecognize pAzF, reports have serendipitously shown a different synthetase originally selected for para-cyanophenylalanine (pCNF) actually works better for pAzF incorporation. This is why the pDule-pCNF plasmid should be used for pAzF incorporation.
Also worthwhile obtaining are control plasmids that expresswild-type superfolder GFP (e.g., pET28a-sfGFP) and sfGFP that has a TAG site incorporated (e.g., pET28-sfGFP 150TAG). These allow the researcher to ensure that the ncAA incorporation technology is working in their hands. Perform these controls as outlined for the system described here by substituting the pBrew-LapGTAG plasmid for pET28-sfGFP150TAG plasmid. In these controls, expressions with for example pET28a-sfGFP150TAG/pDule2-pCNF in the presence of pAzF in the media should produce full-length protein and the cells should be notably fluorescent to the eye. In the absence of ncAA, the cells should not be colored.
Ideal expression construct for ncAA incorporation will varydepending on the protein being expressed.
Expressing the wild-type protein (i.e., protein with no ncAA incorporated) is also an important negative control to include, but omitted here in the methods for brevity. This can be particularly useful to assess the affect of UV light on the protein of interest as some proteins (those with redox cofactors, for example) may have unexpected responses to UV light exposure.
Chemically competent cells may also be used for double plasmid transformations provided they are of sufficient competency (>108 cfu/μg pUC19 DNA). Also, other BL21 T7-based expressing strains may be used as long as they are compatible with autoinduction media (e.g., BL21(DE3)).
Autoclave 2 M glucose and 2 M MgSO4 stocks separately from other components and add to proper concentration once all solutions have cooled to room temperature.
After the LB–agar mix is prepared and poured into an autoclave compatible bottle, a magnetic stir bar should be added to the bottle before autoclaving. Once sterilization is complete, the LB/agar solution will be a clear liquid. Do not add antibiotics to the hot medium. Gently stir hot medium until cooled to <65 °C (comfortable to touch). Antibiotics can be added to the cooled medium at this point and the medium poured onto sterile plates to solidify.
To get the lactose to dissolve, heat gently (microwave works well). Once dissolved, the lactose will stay in solution indefinitely.
When using the described autoinduction media containing richZY media, trace metals may not be necessary but they can facilitate cellular growth to higher densities and expression of metal-containing proteins. When making the 5000× trace metal solution, it may be advantageous to first make individual concentrated stocks of each metal, and then mix these stocks together with the appropriate dilution to the desired 5000× concentration.
Make fresh immediately before use. To make, weigh appropriate amount of amino acid (∼200 mg/L of culture for pAzF). Add sterile water to make a 100 mM solution. At this point the amino acid will not dissolve. To dissolve amino acid, add NaOH to 100–200 mM final concentration from an 8 M stock. The deprotonated amino acid will go readily into solution.
Mercaptoethanol must be handled with care and in a fume hood at all times.
Alternatively, acrylamide gels for SDS-PAGE may be hand-castrather than purchasing commercially precast gels.
BL21 cells containing expression plasmids often do not express protein as well or at all after being frozen even though they grow normally and are resistant to the correct antibiotic(s). Reasons for this are unclear, and some strains may be more tolerant than others depending on the expression plasmid and protein of interest, however the only way to guarantee reliable expression from BL21 cells is fresh transformation for each expression.
If Bpa is to be incorporated, use the pDule2-Bpa machineryplasmid.
For proteins that do not express very well, or if more protein isrequired, the volume of culture can be scaled accordingly. Ensure autoinduction cultures are well aerated. Insufficient aeration will significantly compromise expression.
When using BL21ai cells, arabinose is required even when using non-arabinose inducible protein expression vectors because the T7 polymerase was inserted into the genome under the control of an arabinose promoter. When using other BL21 cells lines (e.g., BL21(DE3)) and expressing from a non-arabinose inducible vector (e.g., pET28a), arabinose may be omitted from the media.
If Bpa is to be incorporated, add 1 mL of 100 mM Bpa stockinstead of pAzF.
For proteins that require lower temperatures for expression,grow cultures at 37 °C until OD600 ∼ 1–1.5, at which point expression will begin and the temperature should be reduced for the duration of the expression. Lower temperature expressions may require incubation times longer than 24 h.
Because pAzF can readily react with primary amines uponactivation with UV light, Tris-based buffers should be avoided.
Care should be taken to ensure cells are immersed in an ice-water bath during sonication to prevent overheating. Keep sonication probe immersed in sample to avoid excessive frothing of the cell lysate.
If using an FPLC to purify the protein containing pAzF, turnoff the UV detector as this will inactivate the cross-linker.
Alternatively, the resin–lysate suspension can be centrifuged at 500 × g for 5 min to sediment the resin. Aspirate the supernatant, with care taken not to evacuate the resin beads. The resin can be resuspended in slurry with buffer and applied to column.
If no or low (<0.1 mg/mL) protein concentrations are observed, expression may not be optimal. Try different expression constructs and conditions, or a different site of ncAA incorporation.
Placing the 96-well plate on a bed of ice may help prevent samples from becoming too warm during exposures to UV light.
When the sample is not boiled, the sfGFP remains folded inSDS-PAGE. As a result, these protein fusions commonly have faster electrophoretic mobility than expected, however it can also result in unusual and unpredictable electrophoretic mobility. If problematic, gel visualization techniques other than ingel fluorescence may be used, including Western blot, Coo-massie Blue staining, silver staining, or Sypro Ruby staining. We prefer in-gel fluorescence when applicable due to its high sensitivity and specificity even in non-purified systems.
Acknowledgments
This work was supported by the NIH under grants R01-AI097307 (H.S.) and F32-GM108440 (R.B.C.)
References
- 1.Melcher K, Chen HT (2007) Identification and analysis of multiprotein complexes through chemical crosslinking. Curr Protoc Cell Biol Chapter 17:Unit 17 10 [DOI] [PubMed] [Google Scholar]
- 2.Lowder MA, Appelbaum JS, Hobert EM, Schepartz A (2011) Visualizing protein partnerships in living cells and organisms. Curr Opin Chem Biol 15(6):781–788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tanaka Y, Bond MR, Kohler JJ (2008) Photo-crosslinkers illuminate interactions in living cells. Mol BioSyst 4(6):473–480 [DOI] [PubMed] [Google Scholar]
- 4.Mohibullah N, Hahn S (2008) Site-specific cross-linking of TBP in vivo and in vitro reveals a direct functional interaction with the SAGA subunit Spt3. Genes Dev 22(21):2994–3006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rannversson H, Andersen J, Sorensen L, Bang-Andersen B, Park M, Huber T, Sakmar TP, Stromgaard K (2016) Genetically encoded photocrosslinkers locate the high-affinity binding site of antidepressant drugs in the human serotonin transporter. Nat Commun 7:11261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang L, Brock A, Herberich B, Schultz PG(2001) Expanding the genetic code of Escherichia coli. Science 292(5516):498–500 [DOI] [PubMed] [Google Scholar]
- 7.Dumas A, Lercher L, Spicer CD, Davis BG(2015) Designing logical codon reassignment–expanding the chemistry in biology. Chem Sci 6(1):50–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chin JW, Santoro SW, Martin AB, King DS,Wang L, Schultz PG (2002) Addition of pazido-L-phenylalanine to the genetic code of Escherichia coli.J Am Chem Soc 124 (31):9026–9027 [DOI] [PubMed] [Google Scholar]
- 9.Young DD, Young TS, Jahnz M, Ahmad I,Spraggon G, Schultz PG (2011) An evolved aminoacyl-tRNA synthetase with atypical poly-substrate specificity. Biochemistry 50 (11):1894–1900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Miyake-Stoner SJ, Miller AM, Hammill JT,Peeler JC, Hess KR, Mehl RA, Brewer SH (2009) Probing protein folding using site-specifically encoded unnatural amino acids as FRET donors with tryptophan. Biochemistry 48(25):5953–5962 [DOI] [PubMed] [Google Scholar]
- 11.Chin JW, Martin AB, King DS, Wang L,Schultz PG (2002) Addition of a photocrosslinking amino acid to the genetic code of Escherichia coli. Proc Natl Acad Sci U S A 99 (17):11020–11024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chatterjee D, Cooley RB, Boyd CD, Mehl RA,O’Toole GA, Sondermann H (2014) Mechanistic insight into the conserved allosteric regulation of periplasmic proteolysis by the signaling molecule cyclic-di-GMP. eLlife 3: e03650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Peeler JC, Mehl RA (2012) Site-specific incorporation of unnatural amino acids as probes for protein conformational changes. Methods Mol Biol 794:125–134 [DOI] [PubMed] [Google Scholar]
- 14.Cooley RB, Smith TJ, Leung W, Tierney V,Borlee BR, O’Toole GA, Sondermann H (2016) Cyclic di-GMP-regulated Periplasmic proteolysis of a Pseudomonas aeruginosa type Vb secretion system substrate. J Bacteriol 198 (1):66–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Navarro MV, Newell PD, Krasteva PV, Chatterjee D, Madden DR, O’Toole GA, Sondermann H (2011) Structural basis for c-di-GMP-mediated inside-out signaling controlling periplasmic proteolysis. PLoS Biol 9(2):e1000588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Newell PD, Boyd CD, Sondermann H, O’Toole GA (2011) A c-di-GMP effector system controls cell adhesion by inside-out signaling and surface protein cleavage. PLoS Biol 9 (2):e1000587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Studier FW (2005) Protein production byauto-induction in high density shaking cultures. Protein Expr Purif 41(1):207–234 [DOI] [PubMed] [Google Scholar]
- 18.Boyd CD, Chatterjee D, Sondermann H,O’Toole GA (2012) LapG, required for modulating biofilm formation by Pseudomonas fluorescens Pf0–1, is a calcium-dependent protease. J Bacteriol 194(16):4406–4414 [DOI] [PMC free article] [PubMed] [Google Scholar]