Abstract
Background
Smartwatches can be used for atrial fibrillation (AF) detection, but little is known about how older adults at risk for AF perceive their usability.
Methods
We employed a mixed-methods study design using data from the ongoing Pulsewatch study, a randomized clinical trial (NCT03761394) examining the accuracy of a smartwatch-smartphone app dyad (Samsung/Android) compared to usual care with a patch monitor (Cardea SOLO™ ECG System) for detection of AF among older stroke survivors. To be eligible to participate in Pulsewatch, participants needed to be at least 50 years of age, have had an ischemic stroke, and have no major contraindications to anticoagulation therapy should AF be detected. After 14 days of use, usability was measured by the System Usability Scale (SUS) and investigator-generated questions. Qualitative interviews were conducted, transcribed, and coded via thematic analysis.
Results
Ninety participants in the Pulsewatch trial were randomized to use a smartwatch-smartphone app dyad for 14 days (average age: 65 years, 41% female, 87% White), and 46% found it to be highly usable (SUS ≥68). In quantitative surveys, participants who used an assistive device (eg, wheelchair) and those with history of anxiety or depression were more likely to report anxiety associated with watch use. In qualitative interviews, study participants reported wanting a streamlined system that was more focused on rhythm monitoring and a smartwatch with a longer battery life. In-person training and support greatly improved their experience, and participants overwhelmingly preferred use of a smartwatch over traditional cardiac monitoring owing to its comfort, appearance, and convenience.
Conclusion
Older adults at high risk for AF who were randomized to use a smartwatch-app dyad for AF monitoring over 14 days found it to be usable for AF detection and preferred their use to the use of a patch monitor. However, participants reported that a simpler device interface and longer smartwatch battery life would increase the system’s usability.
Keywords: Acceptability, Atrial fibrillation, Smartwatch, Stroke, Usability
Key Findings.
-
•
Older stroke survivors find a smartwatch to be generally usable for detecting atrial fibrillation.
-
•
Some patients may experience anxiety from using a smartwatch for rhythm monitoring, especially older patients, those who need assistive devices, or those who have mood comorbidities at baseline.
-
•
Further developments in industry are necessary to streamline functionalities and improve battery life to fully realize the potential of smartwatches for atrial fibrillation detection.
Introduction
Atrial fibrillation (AF) is a highly prevalent cardiac rhythm disorder that affects more than 6 million people in the United States and more than 30 million individuals worldwide, though a large portion of individuals with the condition go undiagnosed owing to AF’s often asymptomatic and paroxysmal nature.1, 2, 3 However, even brief episodes of AF are associated with a greatly increased risk of stroke, and this additional risk is especially magnified in older adults.4 Indeed, it is estimated that ischemic stroke is the initial clinical manifestation of about 1 in 5 cases of AF.5 Furthermore, strokes associated with AF are also more severe than non-AF-related ones, and stroke in general contributes to a significant portion of the morbidity and mortality associated with the condition.6 Fortunately, treatment options for stroke prevention in AF patients have been shown to be highly effective and safe, which further highlights the importance of timely diagnosis and treatment of the arrhythmia.7,8 This is especially important in patients who have already experienced a stroke, as they are a particularly vulnerable population already at higher risk for recurrent stroke, and risk is further exacerbated if they have AF.9
Standard of care in patients after a stroke, and especially in patients whose strokes are of undetermined origin, entails routine cardiac rhythm monitoring, which most often takes the form of a device with several wires affixed to the patient’s chest with adhesive gel.10 This burdensome modality complicates many activities of daily living, such as exercise or even bathing, and thus suffers from poor patient satisfaction and use.11 In recent years, a variety of wearable biosensors, and specifically smartwatches, have become available for AF detection. Currently, there are many consumer-available smartwatches that are cleared by the Food and Drug Administration for use in detection of AF.12,13 However, despite tremendous advancements in sensor and computing technologies that have enabled relatively high accuracy of these smartwatches for AF detection, there has been little consideration of their usability in the older adult population who would benefit most from AF monitoring, particularly for patients who have experienced a stroke. Though smartwatch use in the general population has steadily risen over the last few years, they are by no means ubiquitous, especially among older adults, who traditionally have been relatively late adopters of nascent technology.14 Additionally, the poststroke population faces an additional host of challenges that may further complicate smartwatch use, such as potential residual neurological deficits or resultant cognitive or psychiatric challenges from their stroke.15
Relatively few existing mobile and digital health studies are focused on the older adults, and only a portion of them examine device usability. However, advancements in the technological capabilities of wearable sensors have fueled interest in the conversation. The American Heart Association recently published a scientific statement emphasizing the promise of mobile health technologies for preventing cardiovascular disease in older adults, as well as the dire need for a more comprehensive understanding of the barriers that older adults face in using them.16 Emerging evidence of smartwatch use in older adults has identified potential usability considerations unique to this population, such as accessibility (larger font and icons), intuitive interface design, and potential need for assistance.17, 18, 19 These studies have often been exploratory in nature, and none thus far have focused on patients who have suffered a stroke. We used a mixed-methods approach to gain a multifaceted understanding of the usability challenges faced by older adults in using smartwatches for AF detection, which would be crucial for realizing their full potential as a heart rhythm monitoring modality.
Methods
Study design and population
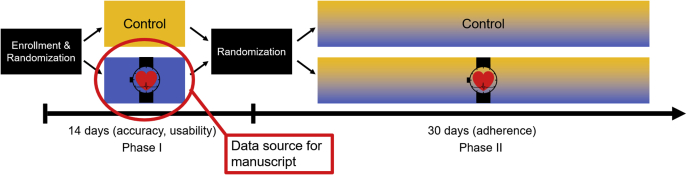
This study uses data from the Pulsewatch study, a randomized controlled trial in which participants were randomized in a 3:1 fashion into intervention and control groups. The intervention group received the Pulsewatch system: a smartwatch-smartphone app dyad capable of AF detection via pulse plethysmography, while both the control and intervention groups received standard-of-care electrocardiogram (ECG) patch monitoring (ie, no smart devices) for a 14-day monitoring period. Although the Pulsewatch study consists of a second phase following this 14-day period, because the focus of the present study is on smartwatch usability we used data only from the intervention group from the first phase of the study (Figure 1). Eligible participants were recruited from the neurology and cardiology clinics of a single tertiary care center in central Massachusetts, and were over 50 years old, had an ischemic stroke or transient ischemic attack in the last decade, and were willing to use the Pulsewatch system over the course of the study. Exclusion criteria included prior diagnosis of AF having any major contraindications to anticoagulation therapy, inability to provide informed consent, contraindication for wearing an ECG patch monitor (eg, sensitivity or allergy to medical adhesives, implantable pacemaker), or a life-threatening arrhythmia requiring immediate analysis and in-patient monitoring.
Figure 1.
Pulsewatch study structure. Data from participants in the Phase I intervention group of the Pulsewatch trial.
Study procedures
Eligible patients were identified through the electronic medical record and mailed an invitation letter briefly describing the study and including a study number to call if they had any questions regarding the study or if they wanted to opt out of further contact. These potential participants were then approached at the time of their clinic appointment. If they chose to enroll in the study, they provided informed consent and filled out a baseline study questionnaire assessing a multitude of sociodemographic and psychosocial domains. The participant was then randomized into either the control or intervention groups. Both groups received standard-of-care cardiac rhythm monitoring in the form of an ECG patch monitor (Cardea SOLO Wireless ECG Patch; Cardiac Insight, Bellevue, WA). In addition, participants randomized to the intervention group received a smartwatch (Samsung Gear S3 or Samsung Galaxy Watch 3; Samsung, Suwon-si, South Korea) and an accompanying smartphone with the Pulsewatch app installed. This pair of smartwatch and smartphone apps were developed by the study team with significant input from patients who have had a stroke, their at-home caregivers, neurologists, and cardiologists. All of these stakeholders provided their perspectives on how best to optimize a study app designed for AF detection to be deployed in older patients who have experienced a stroke. This end-user input was incorporated into the app prior to deployment in the trial and included considerations ranging from app interface, including adequately large font size and intuitive menu organization, to physical comfort of device wear, such as using easily adjustable magnetic or Velcro watchbands. The final version of the Pulsewatch system consisted of a smartwatch app that near-continuously monitored a participant’s pulse for rhythm abnormalities, informing them if potential AF was detected, as well as a phone app that serves as a dashboard for participants to view their data and resources to learn more about AF and strokes. Participants who were randomized to use the Pulsewatch system were asked to consistently wear the watch as much as possible and to engage with the phone app as much as they liked. Research staff called intervention participants on the third and seventh days of the study to encourage watch wear and help troubleshoot any technical challenges that arose. Additionally, participants were provided a comprehensive user guide (Appendix) as well as in-person training at enrollment on general device use. This training involved demonstrating all of the smartphone app’s functionalities by explaining each screen in detail and how to navigate between them. All participants were also shown how to properly wear the smartwatch (specifically the importance of ensuring that the watchstrap is tight) and charging the devices.
At the conclusion of the 14-day study period, participants completed a questionnaire similar to the baseline assessment, with additional questions regarding their experience using the Pulsewatch system over the past 2 weeks. Ten purposively selected participants (to represent a range of demographic factors) were additionally asked to participate in an in-depth interview to garner a more comprehensive understanding of the needs and barriers they faced in their user experience. These interviews were recorded and consisted of 2 parts. In the first portion of the interview, participants were asked to simulate daily use of the Pulsewatch system, namely putting on and removing the smartwatch, charging the devices, and demonstrating how they have been interacting with the phone app, if at all. The interviewer deemed whether each action was successfully completed, and if yes, whether any assistance was required. The second half followed the format of a semi-structured interview, during which participants are asked about their user experience. Examples of usability domains explored with participants include any barriers or challenges they faced and potential ways to circumvent these barriers, motivations for device use, and user experience compared to traditional cardiac rhythm monitoring modalities.
Study measures
This mixed-methods study consists of both quantitative and qualitative data elements. The primary outcome of the quantitative portion of this study was the System Usability Scale (SUS),20 a validated measure of usability utilized by numerous studies of emerging mobile and digital health tools. Additionally, we generated smartwatch-specific Likert scale questions (5 points: 1 = “strongly disagree,” 2 = “disagree,” 3 = “neutral,” 4 = “agree,” 5 = “strongly agree”) to better understand facets of use that the more generalized SUS potentially may not cover. These questions were developed through an iterative process by a panel of content experts, including digital health researchers (ED, DDM), biomedical engineers (DH, KC), a cardiac electrophysiologist (DDM), and a gerontologist (JSS). The final iteration of questions addressed the participants’ perceived ease of use of both the phone and watch, their feeling connected to health care providers, any potential anxiety as a result of using the system, and their general use experience. Participants’ medical characteristics were abstracted from the electronic medical record by trained research staff. Cognitive status was assessed with the Montreal Cognitive Assessment,21 depressive symptoms were examined using the Patient Health Questionnaire (PHQ-9),22 and anxiety symptoms were assessed with the Generalized Anxiety Disorder (GAD-7)23 instruments.
Analytic approach
Descriptive statistics were calculated to summarize participant-level factors for the overall cohort as well as for the 10 participants who also completed in-depth interviews. Linear regression models were used to examine associations between individual participant-level characteristics and SUS score. Independent variables in the models were chosen based on prior literature, as well as other factors that are likely to be associated with usability. We also conducted secondary analyses using logistic regression models with SUS as a binary outcome (dichotomizing with a previously validated cutoff of 68 for high vs low usability20). The investigator-generated Likert scale questions are presented as the proportion of participants who responded with a 4 or 5 (ie, “agree” or “strongly agree”). Additionally, each question is used in an ordinal logistic regression model with the full “strongly disagree” to “strongly agree” scale as the dependent variable, with the same predictor variables as the models described above. Interview recordings were transcribed and reviewed for quality control. Qualitative coding via directed content analysis was completed by 2 independent researchers (ED, MCA) and any differences were resolved through discussion and mutual agreement. Themes from the analysis originate from concepts outlined in previous literature, and the coding guide was developed after an initial review of transcriptions through an iterative process, and was refined through discussion between research team members (ED, KM). The quantitative and qualitative results were triangulated, and the qualitative outcomes were used to contextualize the quantitative findings from the study.
Results
Quantitative results
A total of 90 participants were randomized to receive the Pulsewatch system. The average age of the cohort was 65 years old (standard deviation ±9 years), 41% were women, and most were White (87%). A large proportion of participants were of high socioeconomic status, with 52% having a college degree and 33% who had an annual income of over $100,000. Thirty percent were cognitively impaired, though there was relatively low burden of depressive and anxiety symptoms in the sample. Our participants were highly engaged with technology, especially given the age group, with 83% of participants owning a smartphone, 25% already owning a smartwatch, and two-thirds of participants using apps on their devices on a daily basis (Table 1).
Table 1.
Baseline characteristics of participants
| Demographics (N = 90 participants) | Result |
|---|---|
| Age, mean, y (SD) | 65.1 (9.3) |
| Female | 37 (41%) |
| Race | |
| White | 78 (87%) |
| More than 1 race | 6 (7%) |
| Black | 1 (1%) |
| Asian/Pacific Islander | 1 (1%) |
| Other | 4 (4%) |
| Non-Hispanic ethnicity | 87 (97%) |
| Married/living as married | 61 (69%) |
| Education | |
| Less than high school | 4 (5%) |
| High school degree or equivalent | 38 (43%) |
| College degree | 28 (32%) |
| Postgraduate degree | 18 (20%) |
| Income | |
| <$50,000 | 29 (35%) |
| $50,000–$99,999 | 27 (33%) |
| ≥$100,000 | 27 (33%) |
| Medical history | |
| History of ischemic stroke | 71 (79%) |
| History of TIA | 26 (29%) |
| Congestive heart failure | 6 (7%) |
| Cardiac arrhythmias | 12 (13%) |
| Valvular disease | 9 (10%) |
| Hypertension | 70 (78%) |
| Chronic pulmonary disease | 7 (8%) |
| Diabetes | 25 (28%) |
| Vascular disease | 24 (27%) |
| Renal disease | 4 (4%) |
| Prior major bleed | 5 (6%) |
| Prior MI | 16 (18%) |
| Hyperlipidemia | 77 (86%) |
| Sleep apnea | 25 (28%) |
| Percutaneous coronary intervention | 11 (12%) |
| Residual neurological deficit | 28 (31%) |
| Medication use | |
| Antiarrhythmic | 2 (2%) |
| Beta blocker | 40 (44%) |
| Calcium channel blocker | 62 (69%) |
| Anticoagulant | 11 (12%) |
| Antihypertensive | 51 (57%) |
| Antiplatelet | 79 (88%) |
| Statin | 82 (91%) |
| Vital signs | |
| BMI, mean (SD) | 32.0 (21.0) |
| Systolic BP, mm Hg, mean (SD) | 131.4 (16.7) |
| Diastolic BP, mm Hg, mean (SD) | 76.0 (8.6) |
| Heart rate, mean (SD) | 73.1 (14.7) |
| Psychosocial variables | |
| Alcohol use | 7 (8%) |
| Cognitive impairment, MoCA <23 for in-person, <17 for phone | 26 (30%) |
| Vision impairment | 48 (54%) |
| Hearing impairment | 26 (29%) |
| Depressive symptoms | |
| None (0–4) | 49 (54%) |
| Mild (5–9) | 27 (30%) |
| Moderate (10–14) | 8 (9%) |
| Moderately severe (15–19) | 3 (3%) |
| Severe (>20) | 3 (3%) |
| Anxiety symptoms | |
| None/minimal (0–4) | 62 (69%) |
| Mild (5–9) | 16 (18%) |
| Moderate (10–14) | 7 (8%) |
| Severe (>15) | 5 (6%) |
| Technology engagement | |
| Device ownership | |
| Tablet | 60 (67%) |
| Smartphone | 74 (83%) |
| Smartwatch | 22 (25%) |
| Basic cellphone (SMS enabled) | 30 (34%) |
| App use frequency (excluding call/text) | |
| Daily | 54 (68%) |
| A few days a week | 12 (15%) |
| At least once a week | 5 (6%) |
| Less than once a week | 2 (3%) |
| Once a month | 3 (4%) |
| Never | 4 (5%) |
Values are n (%) unless otherwise specified.
BMI = body mass index; BP = blood pressure; MI = myocardial infarct; MoCA = Montreal Cognitive Assessment; TIA = transient ischemic attack.
With regard to the SUS, 39% of participants deemed the system highly usable (SUS ≥68). More than half of participants either “agreed” or “strongly agreed” with both the watch and phone app being easy to use, and 42% endorsed that using the system made them feel more connected to their health care providers. About one-eighth of participants experienced anxiety from using the devices, and more than half of the cohort agreed that they enjoyed their experience using the devices (Table 2).
Table 2.
Participant perceptions of the Pulsewatch system
| Usability domain | Percent of participants in agreement (N = 90) |
|---|---|
| Easy to use (smartwatch) | 56 (63.6%) |
| Easy to use (smartphone app) | 46 (52.3%) |
| More connected to healthcare providers | 37 (42.5%) |
| Anxiety or worry from use | 11 (12.5%) |
| Overall enjoyed user experience | 45 (51.1%) |
Age was associated with increased likelihood to experience anxiety or worry with use of the system, and every additional year is associated with approximately 6% odds (odds ratio 1.06, 95% confidence interval: 1.01–1.11) of moving up 1 point on a 5-point Likert scale (with 1 being “strongly disagree” with the statement that using the system caused anxiety and 5 being “strongly agree”). Participants who reported using an assistive device (defined as cane, walker, wheelchair) or having anxiety or depression at baseline were more likely to report anxiety or worry with use of the system (Table 3). No other demographic, medical history, psychosocial, or technologic familiarity variables investigated were associated with either the SUS or any investigator-generated usability questions.
Table 3.
Factors associated with smartwatch causing anxiety
| Characteristics | Unadjusted OR |
|---|---|
| Age | 1.05 (95% CI: 1.01–1.11) |
| Use of assistive device† | 3.34 (95% CI: 1.12–9.96) |
| Baseline depression symptoms | 2.31 (95% CI: 1.01–5.25) |
| Baseline anxiety symptoms | 3.85 (95% CI: 1.56–9.48) |
CI = confidence interval; OR = odds ratio.
Assistive device defined as cane, walker, and wheelchair.
Qualitative results
Characteristics of the sub-cohort of participants who were selected to do an in-depth interview are presented in Table 4. Interview lengths ranged from 16 to 51 minutes. In the demonstration portion of the interview, all participants were successful in independently placing the watch on their wrists and adequately tightening the watchstrap, removing the watch, and charging devices with no external assistance. Eight of the 10 participants engaged with the accompanying phone app to varying degrees, and all 8 were able to successfully navigate through the app to visualize their own heart rate and rhythm data, though multiple participants beyond those selected for the qualitative portion noted to study staff that they rarely engaged with the phone app.
Table 4.
Characteristics of participants who completed an in-depth interview
| Demographics (N = 10 participants) | Result |
|---|---|
| Age, mean (SD) | 62.8 (11.6) |
| Female | 7 (70%) |
| Race | |
| White | 6 (60%) |
| Black | 1 (10%) |
| American Indian/Alaska Native | 1 (10%) |
| More than 1 race | 1 (10%) |
| Other | 1 (10%) |
| Married/living as married | 4 (40%) |
| Education | |
| Less than high school | 1 (10%) |
| High school degree | 5 (50%) |
| College degree | 1 (10%) |
| Postgraduate degree | 3 (30%) |
| Income† | |
| Less than $50,000 | 5 (56%) |
| $50,000–$99,999 | 2 (22%) |
| More than $100,000 | 2 (22%) |
| Device ownership | |
| Tablet computer | 8 (80%) |
| Smartphone | 8 (80%) |
| Smartwatch | 2 (20%) |
| Basic cellphone | 4 (40%) |
Values are n (%) unless otherwise specified.
One participant chose not to disclose income (percentages based on N = 9 participants).
Device training and resources
Participants indicated that the training they received initially upon receiving the devices from study staff was instrumental to successfully using the devices; this was particularly true for those who do not own smartwatches. Demonstration of use of the watch and phone apps appeared to remove a major usability barrier by familiarizing the participant prior to individual use. This live session with research staff was perceived as far superior to the detailed written instructions with images that were also provided. Some participants preferred a more brief, bulleted list in lieu of the detailed instructions provided, and those who did find the detailed written instructions helpful mentioned that they generally used the document as a back-up reference, and did not often use the guide.
Training made it way easier, because she [research staff] explained everything in full, and I don’t know, I probably wouldn’t have done it if she didn’t tell me exactly what to expect, and what I was going to do, you know? The training is a necessity. Absolutely. Especially the older people, because they're going be so confused [otherwise].
- Female, age 53, owns a smartphone
This preference for support in the form of dedicated personnel extended beyond the initial encounter, and participants found the ability to reach study staff any time they encountered any issues to be tremendously useful. This was true even for participants who did not use the study phone number for troubleshooting, as they reported that just the knowledge that they could contact study personnel for assistance if needed contributed positively to their experience.
Most participants had family members at home who they perceived to be more technologically adept than they themselves were, and who were able to help them with devices if needed, though it was often not necessary owing to the constant availability of study staff and the perceived ease of use of the system. This ease of use was attributed to the passivity of monitoring and the relatively low amount of active engagement asked of participants.
I have people that would be more technically advanced than me, if there was something I needed. But I mean, I just saw that I was supposed to turn it on [the watch], and have that icon come up and see that it [the phone app] was open once a day, so I could see that my heart rate and everything else. I had nothing else that I needed to do.
- Female, age 61, owns a smartphone
Streamlining rhythm monitoring and minimizing patient burden
While participants generally indicated that the Pulsewatch system was easy to use, many cited streamlining the system to be a crucial factor for any digital health tools to be deployed in this population. Although newer-generation smart devices are being embedded with an increasingly wider array of functionalities, from texting and calling to mobile payment, participants indicated that these additional functions only served to confound the user experience and detracted from the main goal of cardiac rhythm monitoring:
There were just too many other things that you could do … the next thing you know, you’re on the weather [app] – “wait a minute, I don’t want that.” So, it would be better if they had a [system] that was not as complete as one of these, just having the ability to do the heart recording and monitoring, but not having all these other functions. Without having all the extraneous applications and stuff on there, it would make it so much easier to function, to focus on.
- Male, age 61, owns a smartphone
While streamlining and focusing the functionalities of the watch was a recurrent theme, several participants also noted that the general health monitoring provided by smartwatches, such as physical activity or sleep metrics, are potentially helpful to visualize as well, and may be acceptable to include in a cardiac rhythm monitoring system. However, among all participants, including those who noted the potential helpfulness of these additional functionalities, there was still particular emphasis on the necessity of a passive system that does not require extensive active engagement on the part of the patient:
If your goal is to have an app that is all-encompassing, then a step tracker and some of those other features would be great. But if it’s really to try to replace an invasive loop monitor, it needs to be super passive. It needs to be a “set it and forget it.”
- Male, age 53, owns smartphone and smartwatch
A passive system would be perfect. Because I wouldn’t have to [actively] record anything myself, like everything is there. So, it would be pretty easy and … I do not want to touch it.
- Female, age 51, does not own smartphone or watch
Comparison to traditional monitoring modalities
Most participants interviewed had prior experience with either a Holter monitor, external event monitor, or implantable loop recorder. Participants overwhelmingly indicated a strong preference for the Pulsewatch system if given the choice of cardiac monitors, provided that the watch could attain the same diagnostic accuracy as clinical gold-standard monitors. The appearance of the watch was a factor for some participants who were dissatisfied with the obtrusive and bulky appearance of Holter monitors. Comfort was also the most commonly cited reason, specifically given the various ways that external cardiac monitoring significantly impacts quality of life and daily activities.
This [watch] would be better. Well, it’s not as intrusive. I mean, I’m wearing this big thing [event monitor] and every time I turn over in bed, it’ll pull on me and wake me up, so it’s very tough to deal with when you’re wearing it all the time. I had to cover it [event monitor] up to take a shower and stuff, so this [watch] is great, it really was good.
- Male, age 87, owns a smartphone
Oh absolutely. I could go out [with the watch] and no one would notice it. There wouldn’t be wires hanging out of my shirt, people staring at me like what the hell is wrong with her, you know?
- Female, age 51, owns smartphone
An additional advantage of the Pulsewatch system that some participants noted over traditional monitoring modalities is the ability to visualize their own data in near–real time on the accompanying phone app. One participant reported that her heart rate dropped to 37 beats per minute while wearing the smartwatch, and while it was not AF related, she was able to monitor her heart rate displayed on the watch in real time in order to make a conscious and informed decision on whether or not to seek urgent medical care.
Sense of security
Participants viewed wearing the smartwatch as providing them with a sense of security and peace of mind. The Pulsewatch system was often cited as a “safety net” by participants, especially by those who have had acute cardiac events. This aspect of the system was the biggest incentive to use a commercial wearable device for them. Conversely, several participants experienced anxiety while using the Pulsewatch system attributable to the stress of potentially missing AF episodes during times when they were not wearing the smartwatch—for example, while it is charging or when they were in the shower. One participant cited the waterproof feature of the watch to be useful specifically for the purpose of being able to wear it in the shower, to minimize the duration of nonwear. Participants recognized the value of continuous AF monitoring, and any gaps in this monitoring was perceived as a major issue. This was especially salient given that owing to the near-continuous nature of pulse monitoring by the smartwatch (and therefore battery expenditure), it ran out of power in about 6–8 hours, and needed to be charged multiple times a day. These periods of nonwear therefore conferred anxiety and was a major source of frustration for participants:
Battery life was definitely a big issue. It was on the charger more than my hand! But I was more relaxed wearing the watch actually, because of my heart thing. Like maybe it [the watch] would be able to show something. I felt more comfortable with it on, when it had power. Because if it’s not going to be on, then what’s the point?
- Female, age 73, does not own smartphone or watch
Health as a motivator for system use
The major motivation for participants to use a wearable system for heart rhythm monitoring was to maintain their health, whether it was intrinsically motivated or to generate data for their health care providers. Having this information contributed to the sense of security described. One participant cited the Pulsewatch system as helping normalize her life after her stroke, as the constant feedback on her heart rate and rhythm gradually restored her confidence to engage in exertional daily tasks, such as gardening, that she felt anxious about doing immediately following the stroke. Additionally, some participants noted the novelty of a smartwatch for rhythm monitoring may help motivate them to use the device:
Just having it monitoring my heart and noticing if anything goes wrong, or if everything is just normal. And also, the novelty factor, having new stuff to play with.
- Male, age 61, owns a smartphone
Integrating quantitative and qualitative results
Quantitative and qualitative data elements in the study were integrated based on triangulation protocol to describe whether data elements were convergent (where findings from both methods agree), complementary (where results from qualitative and quantitative methods enhance understanding of a particular phenomenon), or divergent (where findings appear to contradict one another).24 We observed overall agreement, and the qualitative component of this study contextualized the quantitative observations (Table 5).
Table 5.
Triangulation of quantitative and qualitative usability results
| Outcome of interest | Quantitative results | Qualitative results | Triangulation |
|---|---|---|---|
| Ease of use | 63.7% of participants found watch easy to use 56.5% found the phone app to be easy to use |
- The devices were simple to use because there was little interaction required—passivity is an important facet of making the devices easier to use for older adults - Training and support for dedicated personnel greatly enhanced user experience and made the system easier to use for participants |
Convergent / complementary |
| Anxiety from use | 10.2% of participants reported experiencing anxiety or worry from using the system | - Anxiety from use stemmed from not being monitored continuously owing to having to take off the smartwatch for charging or other reasons - Extending the battery life of the smartwatches would have severely curbed feelings of anxiety in participants |
Complementary |
Discussion
In this usability study of smartwatch-based AF detection in older adults after stroke, we observed that participants found the Pulsewatch system generally easy to use owing to its passivity, though a more streamlined approach focused only on cardiac rhythm monitoring may have been preferable to a multifunctional commercial smartwatch. A small portion of participants experienced anxiety from using the system. Those who are older, use assistive devices, or report anxiety or depression at baseline endorsed higher agreement with the system causing them anxiety, suggesting that they may benefit from more traditional methods of AF detection. Finally, participants overwhelmingly preferred smartwatches as a heart monitoring modality to traditional clinical monitors. Their preference stemmed from logistical reasons related to wear factors such as comfort, adherence, and convenience.
Contextualizing usability score
Usability of the Pulsewatch system was rated very similarly to other commercially available smartwatches, though there is limited research conducted in older patients with stroke. A usability study of 7 different models of commercially available smartwatches, including the Samsung Gear S3 used in the present study, found that average SUS scores of the devices ranged from 61.4 to 67.6 (vs 65.1 in this study), though notably, the study recruited existing smartwatch users, and only 1 of the nearly 400 participants was over the age of 60.25 Additionally, a recent mixed-methods study of 8 older adults (mean age = 62 years) reported a median SUS score of 60 for using a wrist-based activity tracker.26 The stability of SUS score ratings of smartwatches across drastically different populations may indicate that though older adults may be initially less willing to embrace emerging technologies, their actual use of these devices may not differ significantly from the general population. Additionally, even the specific challenges associated with a stroke do not necessarily preclude the use of smartwatches for cardiac rhythm monitoring. This is further exemplified by our finding that no demographic, medical history, psychosocial, or technological familiarity factors were significantly associated with SUS score. The majority of participants indicated that the system was easy to use, but characteristics such as education, cognitive impairment, or previous device ownership were, interestingly, not associated with any usability domains examined. This suggests that a simple and passive smartwatch-based system for cardiac monitoring may be accessible for many older patients after stroke, irrespective of many individual-level factors.
Facilitators of smartwatch usability
However, though the Pulsewatch system was generally rated as fairly usable, there are many challenges unique to this population that impact their user experience. An important and recurring theme was the need for streamlining the system to focus primarily on measuring health metrics without convoluting the process with extraneous functionalities. While manufacturers in the industry sector race to integrate new functionalities into successive generations of devices, it would be prudent to more thoroughly consider the needs of older populations, for whom many of these health features are designed, and devote more resources to streamlining the user interfaces of these devices to be more intuitive for older individuals. Additionally, passivity was deemed crucial to ensuring that the devices were easy to use for participants. A usability study of Fitbit devices among adults over the age of 65 found that when asked to set up their own Fitbit accounts and entering their own data through the app, participants were reluctant to use the devices and deemed the process to be not worth the effort.27 Decreasing participant burden by minimizing their active role appears to be an important factor in smartwatch usability for older adults. This is further corroborated by other studies indicating that older adults found a wrist-based activity tracker to be simple to use specifically owing to the lack of interaction required on their part.26 Finally, dedicated training and support personnel appears to drastically improve the user experience for the system among participants. This initial training was perceived as especially important, and not something that could be replaced by written instructions. The interpersonal nature of this interaction is likely key to successful use, as older adults have been shown to perceive wearable devices to be less complex when they are able to observe others using it.27
One issue noted by several participants was the short battery life on the smartwatches. Previous research suggests that a majority of older adults consider long battery life to be essential for ensuring long-term use of wearable devices, indicating that 1 week would be ideal and that having to charge the device daily was unacceptable.26 While the Pulsewatch cohort had lower expectations of the devices with respect to being able to last a week without having to charge, limited battery life was nonetheless a common complaint among participants. For most participants, having to frequently charge the devices seemed to be a mere annoyance, but several experienced anxiety as a result of having gaps in their cardiac rhythm monitoring. This impacts quality of life for potential smartwatch users and should be taken into consideration by manufacturers of these devices. A potential method to prolong smartwatch battery life may be to reduce its functionalities to the core components necessary for rhythm monitoring, thus eliminating additional demands for processing power and battery expenditure. Furthermore, this would be in line with the ease-of-use considerations mentioned above in streamlining the functionalities and interfaces of these devices, thereby facilitating use.
Strengths and limitations
Our study has multiple strengths. It fills a critical research gap in studying older adults’ perceptions of and willingness to use a smartwatch-based cardiac rhythm monitoring system when a majority of mobile and digital health studies focus on younger populations. Additionally, our mixed-methods approach enabled a comprehensive and multifaceted investigation, allowing for a synergistic understanding of crucial considerations with regard to usability of smartwatches in this population. This study also has several weaknesses. The study cohort is homogenous with regard to race and ethnicity and had experience with technology use at baseline, so the findings of this study may not be generalizable across different populations. Generalizability may be further limited by the use of 1 specific software and hardware combination in this study. Additionally, the present study’s sample size was small and thus may not be powered to identify group differences with regard to usability or other factors.
Conclusion
Older adults with stroke generally found a smartwatch-based cardiac rhythm monitoring system to be usable, though a more streamlined system focused purely on passive cardiac monitoring, with longer battery life, would likely be more ideal for this population. However, despite these challenges, older adults highly preferred the smartwatch system compared to traditional cardiac monitoring owing to its comfort, appearance, and convenience.
Acknowledgments
Funding Sources
The Pulsewatch Study is funded by R01HL137734 from the National Heart, Lung, and Blood Institute. Eric Y. Ding’s time is supported by F30HL149335 from the National Heart, Lung, and Blood Institute. Dr Chon’s time was supported by R01 HL137734 and Dr McManus’s time is supported by R01HL126911, R01HL137734, R01HL137794, R01HL135219, R01HL136660, U54HL143541, and 1U01HL146382 from the National Heart, Lung, and Blood Institute.
Disclosures
Dr McManus has received honoraria, speaking/consulting fees, and/or grants from Flexcon, Avania, Rose Consulting, Bristol-Myers Squibb, Pfizer, Boston Biomedical Associates, Samsung, Phillips, Mobile Sense, CareEvolution, Boehringer Ingelheim, Biotronik, Otsuka Pharmaceuticals, and Sanofi; nonfinancial study support from Apple Computer and Fitbit; and financial support for serving on the Steering Committee for the GUARD-AF study (NCT04126486) and Advisory Committee for the Fitbit Heart Study (NCT04176926) and also is a member of the Heart Rhythm Society and the editor-in-chief of Cardiovascular Digital Health Journal. The other authors have no disclosures.
Authorship
All authors attest they meet the current ICMJE criteria for authorship.
Patient Consent
All patients provided written informed consent.
Ethics Statement
The authors designed the study and gathered and analyzed the data according to the Helsinki Declaration guidelines on human research. The research protocol used in this study was reviewed and approved by the institutional review board.
Disclaimer
Given his role as Editor-in-Chief, Dr David D. McManus had no involvement in the peer review of this article and has no access to information regarding its peer review. Given his role as Associate Editor, Dr Ki Chon had no involvement in the peer review of this article and has no access to information regarding its peer review. Full responsibility of the editorial process for this article was delegated to David D. Duncker, MD.
Footnotes
Supplementary data associated with this article can be found in the online version at https://doi.org/10.1016/j.cvdhj.2022.03.003.
Appendix. Supplementary data
References
- 1.January C.T., Wann L.S., Alpert J.S., et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation. J Am Coll Cardiol. 2014;64:e1–e76. doi: 10.1016/j.jacc.2014.03.022. [DOI] [PubMed] [Google Scholar]
- 2.Chugh S.S., Havmoeller R., Narayanan K., et al. Worldwide epidemiology of atrial fibrillation: a global burden of disease 2010 study. Circulation. 2014;129:837–847. doi: 10.1161/CIRCULATIONAHA.113.005119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Turakhia M.P., Shafrin J., Bognar K., et al. Estimated prevalence of undiagnosed atrial fibrillation in the United States. PLoS One. 2018;13 doi: 10.1371/journal.pone.0195088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alkhouli M., Alqahtani F., Aljohani S., Alvi M., Holmes D.R. Burden of atrial fibrillation-associated ischemic stroke in the United States. JACC Clin Electrophysiol. 2018;4:618–625. doi: 10.1016/j.jacep.2018.02.021. [DOI] [PubMed] [Google Scholar]
- 5.Jaakkola J., Mustonen P., Kiviniemi T., et al. Stroke as the first manifestation of atrial fibrillation. PLoS One. 2016;11 doi: 10.1371/journal.pone.0168010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lin H.J., Wolf P.A., Kelly-Hayes M., et al. Stroke severity in atrial fibrillation: the Framingham study. Stroke. 1996;27:1760–1764. doi: 10.1161/01.str.27.10.1760. [DOI] [PubMed] [Google Scholar]
- 7.Hart R.G., Pearce L.A., Aguilar M.I. Meta-analysis: antithrombotic therapy to prevent stroke in patients who have nonvalvular atrial fibrillation. Ann Intern Med. 2007;146:857–867. doi: 10.7326/0003-4819-146-12-200706190-00007. [DOI] [PubMed] [Google Scholar]
- 8.Ruff C.T., Giugliano R.P., Braunwald E., et al. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: a meta-analysis of randomised trials. Lancet. 2014;383:955–962. doi: 10.1016/S0140-6736(13)62343-0. [DOI] [PubMed] [Google Scholar]
- 9.Penado S., Cano M., Acha O., Hernández J.L., Riancho J.A. Atrial fibrillation as a risk factor for stroke recurrence. Am J Med. 2003;114:206–210. doi: 10.1016/s0002-9343(02)01479-1. [DOI] [PubMed] [Google Scholar]
- 10.Galli A., Ambrosini F., Lombardi F. Holter monitoring and loop recorders: from research to clinical practice. Arrhythm Electrophysiol Rev. 2016;5:136–143. doi: 10.15420/AER.2016.17.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Subbiah R., Gula L.J., Klein G.J., Skanes A.C., Yee R., Krahn A.D. Syncope: review of monitoring modalities. Curr Cardiol Rev. 2008;4:41–48. doi: 10.2174/157340308783565447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zuckerman B. De novo clearance for “Study Watch” (K182456). U.S. Food & Drug Administration. https://www.accessdata.fda.gov/cdrh_docs/pdf18/K182456.pdf Published online December 20, 2018.
- 13.Krueger A.C. De novo clearance for “Irregular Rhythm Notification Feature” (DEN 180042). U.S. Food & Drug Administration. https://www.accessdata.fda.gov/cdrh_docs/pdf18/DEN180042.pdf Published online September 11, 2018.
- 14.Smith A. Older Adults and Technology Use. Pew Research Center: Internet, Science & Tech. http://www.pewinternet.org/2014/04/03/older-adults-and-technology-use/ Published April 3, 2014.
- 15.Post-Stroke Rehabilitation Fact Sheet | National Institute of Neurological Disorders and Stroke. https://www.ninds.nih.gov/Disorders/Patient-Caregiver-Education/Fact-Sheets/Post-Stroke-Rehabilitation-Fact-Sheet
- 16.Schorr E.N., Gepner A.D., Dolansky M.A., et al. Harnessing mobile health technology for secondary cardiovascular disease prevention in older adults: a scientific statement from the American Heart Association. Circ Cardiovasc Qual Outcomes. 2021;14 doi: 10.1161/HCQ.0000000000000103. [DOI] [PubMed] [Google Scholar]
- 17.Zotz N., Saft S., Rosenlöhner J., Böhm P., Isemann D. In: Computers Helping People with Special Needs. Lecture Notes in Computer Science. Miesenberger K., Kouroupetroglou G., editors. Springer International Publishing; 2018. Identification of age-specific usability problems of smartwatches; pp. 399–406. [Google Scholar]
- 18.Khakurel J., Knutas A., Melkas H., Penzenstadler B., Fu B., Porras J. In: Universal Access in Human-Computer Interaction. Methods, Technologies, and Users. Lecture Notes in Computer Science. Antona M., Stephanidis C., editors. Springer International Publishing; 2018. Categorization framework for usability issues of smartwatches and pedometers for the older adults; pp. 91–106. [Google Scholar]
- 19.Manini T.M., Mendoza T., Battula M., et al. Perception of older adults toward smartwatch technology for assessing pain and related patient-reported outcomes: pilot study. JMIR Mhealth Uhealth. 2019;7 doi: 10.2196/10044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brooke J. Usability Evaluation in Industry. Taylor & Francis; 1996. SUS: A “Quick and Dirty” Usability Scale. [Google Scholar]
- 21.Nasreddine Z.S., Phillips N.A., Bédirian V., et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53:695–699. doi: 10.1111/j.1532-5415.2005.53221.x. [DOI] [PubMed] [Google Scholar]
- 22.Kroenke K., Spitzer R.L., Williams J.B. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16:606–613. doi: 10.1046/j.1525-1497.2001.016009606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Spitzer R.L., Kroenke K., Williams J.B.W., Löwe B. A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch Intern Med. 2006;166:1092–1097. doi: 10.1001/archinte.166.10.1092. [DOI] [PubMed] [Google Scholar]
- 24.O’Cathain A., Murphy E., Nicholl J. Three techniques for integrating data in mixed methods studies. BMJ. 2010;341:c4587. doi: 10.1136/bmj.c4587. [DOI] [PubMed] [Google Scholar]
- 25.Liang J., Xian D., Liu X., et al. Usability study of mainstream wearable fitness devices: feature analysis and system usability scale evaluation. JMIR mHealth uHealth. 2018;6 doi: 10.2196/11066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Keogh A., Dorn J.F., Walsh L., Calvo F., Caulfield B. Comparing the usability and acceptability of wearable sensors among older Irish adults in a real-world context: observational study. JMIR mHealth uHealth. 2020;8 doi: 10.2196/15704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Farivar S., Abouzahra M., Ghasemaghaei M. Wearable device adoption among older adults: a mixed-methods study. Int J Inf Manage. 2020;55:102209. doi: 10.1016/j.ijinfomgt.2020.102209. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.