Abstract
Worldwide, a huge amount of agricultural food wastes and byproducts containing valuable bioactive compounds are generated, especially throughout the entire supply chain. Minimizing food wastes and byproducts is the first option to avoid environmental problems, and to help the economy and the society. Although many countries implement policies to reduce food wastes and byproducts, and different management methods are available to utilize agricultural food wastes, they are still produced annually. Nanotechnological and biotechnological approaches are recently used as novel and green applications to valorize agricultural food wastes and improve their stability and applicability. In this Review, these approaches are covered in detail with given examples. Another valorization way of consumable food waste is using it for functional food production. This Review focuses on specific examples of functional foods with food waste as an ingredient. In addition, the problems and limitations of waste management and valorization methods are investigated, considering future perspectives.
Keywords: agricultural food waste, agricultural byproduct, waste management, waste valorization, nanotechnology, biotechnology, food application
1. Introduction
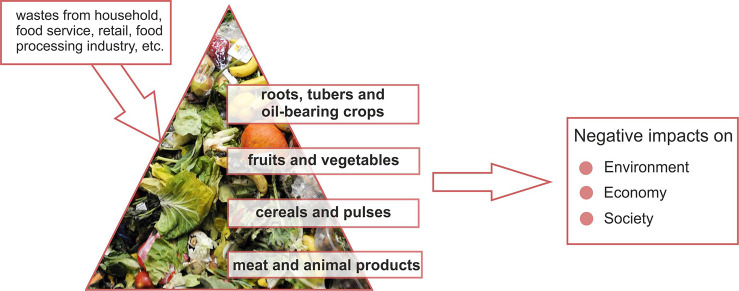
Agricultural and food wastes constitute a significant problem worldwide, because of the adverse effects that agricultural waste has on the environment, economy, and society. Various scientific studies focus on the management of food waste. Food loss is defined as a decrease in food quantity and quality that occurs due to decisions and actions of the suppliers in the food chain.1 On the other hand, food waste is defined as a decrease in food quantity and quality due to the decisions and actions of retailers, food service providers, and consumers.1 The main reason for food waste and loss is rapid global population growth and food consumption behavior.2 The environmental effects occurring during food production are increased by food loss in the food system.3 The total global food loss and waste, ∼1.3 billion tons annually, corresponds to one-third of the food produced for human consumption. The food waste is made by using nearly 30% of the agricultural land area in the world.4 The Food Waste Index Report stated that ∼931 million tons of food waste, including 61% household, 26% food service, and 13% retail waste, were generated in 2019.5 The generation of food waste, primarily from the household, is related to purchasing power of consumers determined by the income levels of countries.3,5 Latin America and Europe have the highest consumer waste with 200 and 180 kg per capita per year, respectively. Each of North America and Oceania, North Africa, and West and Central Asia have 175 kg consumer waste per capita per year. They are followed by the consumer waste of industrialized Asia (155 kg), sub-Saharan Africa (150 kg), and south and southeast Asia (110 kg) per capita per year.2 In 2016, Central and Southern Asia, Northern America, and Europe were identified as the first three regions with the highest food loss, considering the entire supply chain from post-harvest to distribution.1 The approximate food waste is 307 g per capita per day for high-income countries, corresponding to twice that of the upper-middle income countries.3 Food waste is obtained primarily at later stages of the supply chain in industrialized countries. However, because of lacking financial and technical properties during harvesting, storage, and cooling, most of the waste in developing countries is obtained at early stages in the food supply chain.6
The residues of raw agricultural products constitute the agricultural wastes.7 Such residues include manure and animal carcasses (animal waste); corn stalks, sugar cane bagasse, drops and culls from fruits and vegetables, pruning (crop waste); pesticides, insecticides, and herbicides (hazardous and toxic agricultural waste); and food processing waste obtained during growing and processing in liquid, slurry, or solid forms. The main food groups contributing to nutrient and food waste or loss are cereals and pulses, fruits and vegetables, meat and animal products, roots, tubers, and oil-bearing crops.1,3,6 Among these food groups, roots, tubers, and oil-bearing crops with almost 26% and fruits and vegetables with nearly 22% are the first two groups, based on food loss.1 The cereals, root crops/fruits/vegetables, and oilseeds compose the global annual food waste with 30%, 40–50%, and 20%, respectively.6 Without considering the agricultural food losses, food waste is produced within the food chain, including 42% of households, 38% of food processing, and 20% of other processes. Considering the food supply chain, the beverage industry produces food waste with 26%. It is followed by the dairy and ice cream industry (21.3%), fruit and vegetable production and preservation (14.8%), the manufacture of grain and starch products (12.9%), meat production, processing, and conservation (8%), the manufacture of vegetable and animal oils and fats (3.9%), the production and preservation of fish and fish products (0.4%), and the manufacture of other food products (12.7%).8 The animal-derived food wastes contain fats, lard, blood, internal organs of farm animals, offal, head, tails, scales, shells of marine animals, and dairy products such as cheese whey, curd, and milk sludge. The wastes of vegetables, fruits, cereals, roots and tubers, oil crops, and pulses consist of peels, stems, seeds, shells, bran, germs, cull, pomace, pulp, and other residues obtained from processing.8,9 The estimated wastes arising from food supply chains based on geographical locations mentioned in several studies can be summarized with some examples given for annual waste amount in tons as the following: 50 000–100 000 vegetable oil waste in the UK, 4 000 000 tomato pomace in Europe, 57 000 wheat straw in the USA, 40 000–45 000 cereal waste in Europe, 700 orange peel in the USA, 700 grape pomace in France, 2 881 500 olive pomace worldwide, 3 000 000–4 200 000 apple pomace worldwide, and 70–140 potato peel worldwide.10
This paper emphasizes the significance of food wastes and their effects on nutrition, environment, economy, and management systems. On the other hand, different methods used to valorize agricultural food wastes and improve their stability and applicability are reviewed. Besides, some specific examples of applying ingredients obtained from waste materials in functional foods are provided. Finally, the limitations and problems encountered during the management and application of these wastes, together with future perspectives, are mentioned.
2. Significance of Food Wastes from Nutritional, Environmental, and Economical Points of View
Food waste is considered a significant source of complex carbohydrates, proteins, lipids, and phytochemicals, because of its high contents of polysaccharides, dietary fibers, oils, vitamins, phenolics, carotenoids, and other pigments. Therefore, the potential health benefits of wasted foods rely on their high contents of biologically active compounds.10,11 Food waste has social impacts associated with nutrient loss and world hunger. The wasted foods can theoretically fill nutritional gaps for millions of people. For example, the annual amount of food loss and waste can provide a diet of 2100 kcal per day for 2 billion people. According to FAO records, this potential of food loss and waste is crucial since 690 million people were estimated to be sufffering from hunger in 2019. This number has increased dramatically during the COVID-19 pandemic, and it is expected to increase further more.5,12 Food waste of the U.S. food supply in 2012 at the retail and consumer levels consists of 33 g of protein, 5.9 g of dietary fiber, 1.7 μg of vitamin D, 286 mg of calcium, 880 mg of potassium, and 1217 kcal per capita per day.13 Also, it has been reported that the wasted calcium, choline, riboflavin, zinc, and vitamin B12 especially arise from the loss of meat, dairy, and eggs.3 The daily food waste per person corresponds to the food of 795–840 kcal. And, carotenoids have the highest value with 31%, and vitamin D has the lowest value with 25% within all wasted nutrients.14 Because of these wasted nutritious foods, food waste reduction can provide more available nutrients for human consumption.12 If the necessary precautions for food waste reduction are not taken, and food consumption and production are not planned, the World in 2050, with a population of more than nine billion, will need 60% more food, which equals at least 2 billion tons. The predictions show that the food gap in 2050 can be reduced by ∼20% via decreasing the global food waste by half.15
Food waste has environmental and economic impacts right along with its social impacts (Figure 1). Food waste has devastating effects on climate change.16 The estimated carbon footprint contribution of food waste to greenhouse gas (GHG) emissions equals ∼3.3 billion tons of CO2 accumulation in the atmosphere per year.17 The food consumption and waste generation trends of 17 110 family members in China, a highly populated country, have been investigated based on the household’s carbon, water, and ecological footprint quantification. The annual consumption of food at home (415 kg) resulted with 1080 kg CO2 eq of carbon, 673 m3 of water, and 4956 gm2 of ecological footprints, whereas the annual wastage of food at home (16 kg) causes 40 kg CO2 eq of carbon, 18 m3 of water, and 173 gm2 of ecological footprints.18 Food waste can also create many other environmental problems, since it is disposed without any appropriate pretreatment by landfill or incineration in the dumping sites.2 Some environmental issues causing respiration difficulties for living organisms and air pollution due to dioxin, ash, and flue gas released to the atmosphere are created by the incineration of food wastes.2,17 As is the case with the incineration of food waste, toxic byproducts contaminate groundwater and cause corrosive gases, such as methane and hydrogen sulfide, to be generated by food waste landfilling.2 The landfilling of food waste reduces its energy content. The energy loss at landfill sites is equivalent to 43% of the delivered energy for food preparation in the U.S. and more than 100% of the current annual renewable energy demand of industries in the U.K.16
Figure 1.
Impacts of agricultural food wastes and byproducts.
Another important issue related to food waste and loss is economic problems. The approximate global cost of food waste equals 1000 billion dollars annually. This number can increase up to 2600 billion dollars when ignored environmental costs are taken into account.19 FAO reported that the cost of total food waste amount in 2007 was almost 750 billion dollars, which is approximately equal to the gross domestic product (GDP) of Turkey and Switzerland in 2011. Vegetables primarily contribute to the economic cost of food waste and loss with 23%, and also meat, fruits, and cereals contribute to the total cost with the percentages of 21%, 19%, and 18%, respectively.4 Annually, more than 55 million metric tons of avoidable food wastes are produced in the U.S., corresponding to almost 29% of the annual production. The cost of these wasted foods equals 198 billion dollars.20 At least 18.6 billion dollars are lost in the U.K. with 8.3 billion metric tons of annual household food waste.20,21 Therefore, since food waste reduction can help providing sufficient food for the increasing population globally, it is a critical issue in terms of economy, environment, and society.22
3. Food Waste Management
The first option to manage food waste is to prevent waste generation. Reusing and recycling are considered as secondary options in food waste management.23,24 Other methods, such as reduce–reuse–recycle, extended producer responsibility, and sustainable management to reduce the wasted food, have been developed to manage the food wastage.23 Food losses can also be prevented by local investments, educations, ensuring the cold chain, improving packaging and market facilities in low-income countries. For high-income countries, enhancing communication in the supply chain, improving purchase/consumption planning, and awareness of the best-before-dates can be options for food loss prevention.8,25 To manage or reduce food waste, the countries apply several policies for individuals, organizations, and businesses, depending on their consumer behaviors, income levels, and development levels.22,26 The European Union Waste Directive supports its members for preparing required programs to manage and reduce their food waste by 30% by 2025. Italy and France apply the same programs countrywide, whereas Austria, Czech Republic, Poland, Netherlands, Sweden, and Scotland, apply their programs at the municipal level.24 Also, in the U.S., Food Waste Challenge (FWC) and EPA Food Recovery Challenge (FRC) are preferred as waste reduction recognition programs.22
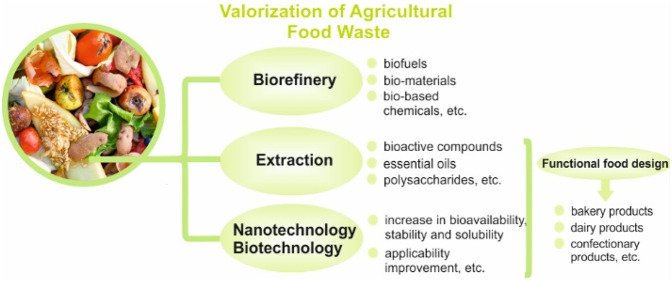
Although the best options are prevention or reduction of the food waste, according to the food waste management methods, the valorization of food waste can be considered one of the best methods when prevention or reduction is not possible. Valorization refers to the diversion of former food waste to food and feed products. It also includes converting food waste to extracted food and feed ingredients, considering food waste’s quality, robustness, and composition. The conversion of food waste requires evaluating the market conditions regarding technological feasibility, economic viability, legislative reportability, and environmental sustainability and utility.27 Food wastes, which potentially contain carbohydrates, proteins, lipids, and nutraceuticals, are composed of different constituents.10,17 Carbohydrates are mostly derived from food waste containing rice and vegetables, whereas proteins and lipids mostly derive from meat and egg wastes.17 The food waste has started to be assessed as valuable biomass that can be utilized as profitable products instead of an uncontrollable discard. Since food waste is renewable and inexpensive, it can be beneficial for obtaining energy, biofuels, enzymes, antioxidant extracts, novel biodegradable materials, and other commercial products.9 Although it is stated that the governments in the European Union (EU) will struggle with the valorization of food waste at a larger scale within the next decades, the EU defined the concept of biowaste valorization to obtain more valuable products in 2010. Currently, landfilling, composting, and incineration are three popular choices for most food waste produced in the EU. However, the increase in power purchasing and the worsening of food management have led to the rise in food waste generation in recent decades, making food valorization a crucial subject for society.28
4. Novel Approaches for the Valorization of Agricultural Food Wastes/Byproducts
4.1. Valorization Methods of Agricultural Food Wastes/Byproducts
The value-added products, which are fine chemicals, nutraceuticals, antioxidants, bioactives, biopolymers, biopeptides, antibiotics, industrial enzymes, bionanocomposites, single-cell proteins, polysaccharides, activated carbon adsorbent, chitosan, corrosion inhibitors, organic acids, pigments, sugars, wax esters, and xanthan gum, can be recovered by using food wastes as a substrate.29,30 The conventional treatment of food waste such as landfilling and incineration leads to environmental, economic, and social problems. Thus, several available valorization methods, which are more sustainable and profitable to manage food waste, arise as alternative options to obtain the value-added products mentioned above. Also, special chemicals refined from food waste, varying from solvent to antioxidant materials, are essential for nutraceutical and biomaterial applications.30 The combined methods, including biochemical, chemical, and physical steps, should be applied to separate the potentially marketable compounds found in food wastes and byproducts to selectively extract and modify the preferred components and change them to higher-value food products and additives. These methods should be applied carefully to avoid microbiological hazards and ensure that the final products are suitable for consumers’ taste and produced by following the food regulations.8
Only the effective utilization of renewable resources and the exploitation of renewable carbon can replace fossil resources to produce chemicals, materials, polymers, fuels, and energy. The industrial development will be sustainable through specialty product extraction, conversion by green chemical or biotechnological processes, integrated biorefining, industrial symbiosis, cascade processing, and on-site processing of seasonal waste streams, obtained by the effective exploitation of agricultural and forestry residues, aquatic biomass, and different waste streams.31 The coproducts, which are not appropriate for food exploitation, should be utilized as energy sources after the application of fermentation, biogas production, and composting, indicating that an integrated biorefinery approach can provide the valorization of food waste for bioactive molecule production for pharmaceutical, cosmetic, food, and nonfood applications.8
4.1.1. Biorefinery for Agricultural Food Wastes/Byproducts
The effective valorization of byproducts obtained during biomass production, such as agricultural residues, food processing waste, and food residuals, can contribute to the global bioeconomy.32 The concept of biorefinery, rapidly accepted as a sustainable alternative by the scientific community, involves energy and commercial production by recycling food waste.33 The biotechnological techniques, including anaerobic digestion, fermentation, and composting, transform the abundant and low-cost waste biomass into biorefinery products such as biofuels and biomass biofertilizers, bioplastics, and secondary chemicals.32−34 In addition, these biotechnological techniques can convert the agro-food wastes into efficient biobased adsorbents used in the bioremediation of various pollutants found in wastewaters.33 The public perception of food waste will be changed by utilizing the food wastes more in chemical synthesis to create a closed-loop economy by providing a renewable supply chain. For example, a recent provisional agreement on the renewable energy policy, which is also targeting to obligate the development of waste-derived biofuels, is done by the EU. The agreement defining a biorefinery based on food wastes is planned to have a crucial role in contributing to a more sustainable and greener society in the future.30 Consequently, food waste management with the concept of biorefinery has positive environmental effects, because of less greenhouse gas emissions, environmental burden reduction of their disposal, and being more independent about the use of fossil-based sources for fuel generation.33,34
Biofuel
Biofuel, which can be in solid, liquid, and gaseous forms, is defined as the energy originated from biomass and refined products of biomass, consisting of bioethanol, biodiesel, biokerosene, natural gas, etc. From the beginning of human civilization, biofuel has been widely used in daily human activities like cooking, lighting, and heating.35 The production of biofuel, as an alternative fuel, is increasingly supported worldwide, because of the problems regarding the production and permanence of petroleum and coal-based fuels.36 Nowadays, countries are working on the utilization of their food wastes as fuels. For example, a project including Nordic countries focuses on the policies to increase the use of food wastes and the investigation of new technologies to transform their wastes into transport fuels. Denmark, Finland, and Sweden are currently using their food wastes such as fruit andvegetable wastes, animal-based wastes, bakery wastes, and biowastes from households, agricultural byproducts, industrial and commercial origins in biodiesel, bioethanol, and biogas production.37 Some of the most common biofuels and their applications are summarized in the following paragraphs.
Biodiesel, which is fatty acid methyl ester, is produced from several plant oils, including soybean, rapeseed, and canola, by direct or indirect transesterification.2,36 The study of Karmee and Lin exemplified the use of low-cost food waste in biodiesel production.36 They obtained the lipids by the fungal hydrolysis of food wastes, and these lipids are transesterified to produce biodiesel. Biogas like biomethane/hydrogen/hythane, another biofuel and renewable energy source, is obtained by anaerobic digestion of agro-food biomass residuals, considering the renewable energy legislation of the EU.2,38 However, some components in biogas, such as hydrogen sulfide,carbon dioxide, nitrogen, hydrogen, oxygen, and water vapor, cause a decrease in calorific value compared to natural gas. Some physicochemical and biological technologies include cleaning and upgrading techniques to evaluate the raw biogas quality. Biological methods, cryogenic separation, hydrate separation, membrane enrichment, in situ upgrading, multistage, and high-pressurized anaerobic digestion can be given as examples of advanced modern biogas upgrading techniques.39 As an example of biogas production from food waste, a study focused on improving the anaerobic digestion process used in biogas production.40 They concluded that the practical usage of ultrasound during the pretreatment of food wastes and the anaerobic digestion increase the yield of biogas within a shorter time range. Recently, an innovative solid-state microanaerobic digestion process has been developed to valorize food waste by degrading them. This technology makes anaerobic digestion a compact process that requires low water and energy. A quantity of 143 L/kg methane was produced via the solid-state microanaerobic digestion process, which is planned to be improved to increase its applicability and optimize its process conditions.41 Another popular biofuel is bioalcohol, which is considered as an emerging alternative liquid fuel, because of its petroleum-like characteristics.33 It represents the most commercialized transportation fuel, which achieves carbon neutrality and is compatible with an internal combustion engine.42 Ethanol is produced by the microbial fermentation of various feedstocks, including potatoes, molasses, corn, stover, wheat, sugar cane, bagasse, sugar beet, grain, switchgrass, barley, and many other carbohydrate-rich sources, and is the most common bioalcohol.33,36 In addition, fast food wastes, as a good source of carbohydrates, were used for bioethanol production. For instance, ethanol was produced by enzymatic hydrolysis with α-amylase and fermentation process from the waste of pizza with the highest yield of 0.292 g/g waste of pizza43 and waste of hamburger with the highest output of 0.271 g/g waste of hamburger.44 Biochar, a carbon-rich biofuel, is produced with a charring (or pyrolysis) process, by heating the biomass above 250 °C under limited or no air conditions.45 It is used as a renewable carbon material in many areas, especially soil amendment and environmental management.30,45 For instance, along with the benefits of biochar in mediating soil acidity, water holding capacity, cation exchange capacity, and nutrient retention, it is considered a suitable electrode in supercapacitors, which are used within green energy storage devices.30
Valuable Biomaterials
Biopolymers, bioplastics, biofertilizers, enzymes, organic acids, single-cell protein (microbial biomass) are also obtained from agricultural food wastes/byproducts with the application of different treatments like fermentation and composting. These valuable biomaterials are used in the cosmetics, pharmaceutical, chemical, food, and beverage industries.33 Enzymes, which are significant ingredients for different products and processes, have a massive significance in the industry, since they exhibit specificity against substrate and product, moderate reaction conditions, formation of byproducts in a minimum amount, and high yield. The raw material costs are responsible for up to 30% of the total production cost of enzymes.46 Therefore, the usage of food wastes and byproducts is a good option for reducing the raw material costs for the production of enzymes. It also reduces the waste amount and prevents its negative impacts on the environment. There are several studies about the recovery of enzymes from food wastes and byproducts in the literature. For example, α-amylase from coffee wastes by solid-state fermentation with a fungal strain of Neurospora crassa CFR 308,47 glucoamylase from food waste by submerged fermentation with Aspergillus niger UV-60,48 and lipase from melon wastes by solid-state fermentation with Bacillus coagulans,49 were recovered. Lactic, succinic, citric, 3-hydroxy propionic, acetic, and butyric acids are among the organic acids produced from food wastes, and organic acid production by acidogenesis is influenced by the composition of the food waste.2 In the study of Kim et al., kimchi cabbage waste was used in organic acid production with lactic acid bacteria. The results showed that organic acids including 12.1 and 12.7 g/L lactic acid, 7.4 and 7.1 g/L fumaric acid, 4.5 and 4.6 g/L acetic acid from the waste of kimchi cabbage with Lactobacillus sakei WiKim31 and L. curvatus WiKim38, respectively, were obtained by the simultaneous application of saccharification and fermentation for 48 h.50 Also, in another study, 47.3 g/L succinic acid was obtained from bread waste by solid-state fermentation with Aspergillus awamori and Aspergillus oryzae, which can produce complex enzymes containing a high amount of amylolytic and proteolytic enzymes, respectively.51
Another valuable product obtained from waste materials of foods with microbial fermentation is single-cell protein.52 The demand to formulate innovative and alternative proteinaceous food sources is increasing, because of concerns about population growth and the increasing number of hungry and chronically malnourished people. The most crucial step to respond to this demand is single-cell protein production.53 Single-cell protein, the extracted protein from microbial biomass like bacteria, yeast, algae, and fungi, can be used as a supplement protein source instead of conventional high-cost protein sources in the staple human diet to alleviate problems related to protein scarcity.33,53,54 Besides the nutritional benefits of using single-cell proteins in human or animal diet, another advantage is reducing the costs of final products during the formulation of food and fodder stocks, rich in protein, by using bioconversion products from wastes of agriculture and industry.53 As an example of innovative biotechnology, several species of insects have been employed to valorize the residual biomasses. The insects can incorporate the nutrients of organic wastes into their bodies. This ability of insects can reduce the amount of waste material, creating more valuable and homogeneous biomass.55 For example, the biotreatment of food waste by black soldier fly (Hermetia illucens) larvae provides volume reduction of the wastes and production of high-quality animal feed. It can recover, recycle, and valorize the food waste materials as constituents of animal feed and grass fertilizers.56 Biopolymers, other important products obtained from food wastes and byproducts, include a wide variety of products. These biopolymers are used in critical applications in different industries like medicine, cosmetics, pharmaceutical and food industries, water treatment, production and development of biosensors, industrial plastics, and clothing fabrics, because of their biodegradability, biofunctionality, biostability, and biocompatibility.57 Food waste is also utilized for bioplastic production includingpolyhydroxyalkanoates (PHA) and polyhydroxybutyrate (PHB) as organic polymers that can completely degrade into carbon dioxide and water within months after they are buried.2 Therefore, bioplastic production from food waste contributes to reducing both plastic waste and food waste.58
4.1.2. Extraction Methods of Valuable Compounds from Agricultural Food Wastes/Byproducts
The biobased molecules, natural biopolymers, and phytochemicals can be obtained directly by extraction instead of synthesizing them from petroleum-based chemicals.32 Once these valuable biobased products are extracted, they can be considered high-value products such as food additives, nutraceuticals, therapeutics, and cosmetics.33 However, the economic feasibility of the extraction of the high-value components must be considered. In order to provide this economic feasibility, the desired components should be extracted by an appropriate method to obtain all of the valued components for full exploitation of the waste.8
The applied extraction techniques show differences based on the nature of the food matrix and the bioactive food ingredient, which will be extracted.33 Also, the extraction method used and the cellular matrix of the byproduct dominantly affect the recovery rate of a chemical entity.59 The extraction techniques can be divided into conventional and nonconventional.60 The conventional methods, consisting of solvent extraction, Soxhlet, maceration, and hydrodistillation, are characterized by temperature, agitation, and organic solvents like methanol, ethanol, and acetone.33,60 To maximize the resistance of the bioactive components, operating parameters including temperature, contact time, pH, particle size, solid-to-liquid ratio, and the stirring rate should be chosen appropriately. Longer extraction time, high volume of solvent use causing the generation of a high amount of toxic waste, and the need for the application of isolation or clarification technique as a final step for obtaining the extract without any solvent residues or impurities because of the use of toxic and expensive organic solvents are the disadvantages of these conventional techniques.33,61 The reduction of solvent consumption and extraction time, the improvement of extraction efficiency, and the use of greener solvents lead to more effective, cleaner, and greener modern or nonconventional techniques with decreased energy usage and organic solvent implementation are environmentally beneficial.60,61 Microwave-assisted, ultrasound-assisted, pressurized liquid, supercritical fluid, pulsed electric field-assisted, and enzyme-assisted extractions are highly examined novel thermal and nonthermal extraction techniques. Compared to conventional methods, the advanced extraction techniques listed above are more effective when their higher extraction efficiency, lower solvent consumption, lower extraction time, and energy cost are considered.8,33,62,63 Since the conventional solvents used in waste valorization have some disadvantages, because of their high price, high toxicity, and melting points, the search for a green and cost-effective technique has led to the emergence of deep eutectic solvents (DESs) and their bioanalogs, natural deep eutectic solvents (NADESs).64,65 The DESs and NADESs are considered environmentally friendly and novel solvents, which have a high capacity of dissolving biomasses to valorize food wastes effectively. They have been used to extract valuable components from wastes of vegetable oils, dairy, beverage products, and also from several natural raw materials such as lignocellulosic biomass, bark, wood, andalgae.64 For example, a simple, nonexpensive, and eco-friendly NADES has been designed to extract phenolic compounds from agro-food industrial byproducts. This eutectic solvent, which was prepared by combining lactic acid, glucose, and water, has been effectively applied to onion, olive, tomato, and pear byproducts, indicating the versatility of the technique.66 The usage of deep eutectic solvents can increase the interest in green extraction of food wastes to recover new high-quality products and functional ingredients in the food industry.64
As mentioned above, agricultural food wastes may be good sources of valuable compounds. Therefore, different extraction methods are widely investigated in the scientific community valorizing these beneficial compounds and reducing their harmful effects on the environment. Different types of extracted compounds from agricultural food wastes and byproducts by diverse extraction techniques applied in the last years are summarized in Table 1. The information about the extraction processes and their optimization can provide an excellent opportunity to design and improve innovative and functional products at the industrial level to valorize valuable compounds in agricultural food wastes.
Table 1. Examples of Extraction Methods and Extracted Valuable Compounds from Agricultural Food Wastes and Byproducts.
| agricultural food waste/byproduct | extraction method | optimal conditions of the extraction methods providing the best yield | extracted valuable compounds | ref(s) |
|---|---|---|---|---|
| kiwi juice pomace | microwave-assisted extraction with different conditions | optimal conditions for extraction at a microwave power of 400 W and pressure of 350 psi → solvent composition: 50% ethanol:water, solid-to-solvent ratio: 1:15 at 75 °C for 15 min | bioactive compounds based on total phenolic content, flavan-3-ol content and ascorbic acid content (high bioactive compound content and antioxidant potential of extract with optimal conditions) | (67) |
| optimized extract (total polyphenol content: 4.8 ± 0.1 mg GAE g–1, total flavonoid content: 1.38 ± 0.01 mg CAT g–1, ascorbic acid content: 120.6 ± 0.5 mg 100 g–1) | ||||
| pistachio hard shells | extraction with different solvents/microwave-assisted extraction | optimal conditions for microwave-assisted extraction → using EtOH at 1000 W for 270 s | bioactive compounds based on total phenolic content and total flavonoid content (the highest yield (3.00% w/w) with microwave-assisted extraction at optimum conditions) | (68) |
| the highest bioactive compounds in the microwave-assisted extract: gallic acid, monogalloylglucose isomer, pentagalloylglucose, and kaempferol | ||||
| extract by microwave-assisted extraction at optimum conditions (total phenolic content (332 ± 11 mg GAE/g), total flavonoid content (376 ± 22 mg/CatEg), antioxidant activity by DPPH, TEAC, and ORAC (6.1 ± 0.9 μg/mL, 4001 ± 7.5 μmol TE/g, 879 ± 17 μmol TE/g, respectively) | ||||
| tomato processing waste | ultrasound-assisted extraction/conventional organic solvent extraction | optimal conditions for ultrasound-assisted extraction → solvent: hexane:acetone:ethanol (2:1:1 v/v/v) including 0.05% (w/v) butylated hydroxy toluene (BHT), solid liquid ratio (1:35 w/v) at 15 °C, 90 W for 30 or 15 min | lycopene and β-carotene (maximum lycopene and β-carotene yields with ultrasound-assisted extraction at 90 W for 30 and 15 min, respectively) | (69) |
| lycopene (76.87 mg/kg dry weight) by ultrasound-assisted extraction at 90 W for 30 min | ||||
| β-carotene (6.12 mg/kg dry weight) by ultrasound-assisted extraction at 90 W for 15 min | ||||
| pulp of hot pepper paste | ultrasound-assisted extraction/maceration extraction | optimal conditions for ultrasound-assisted extraction → 60% amplitude and 60 °C for 5 min | higher amount of capsaicin and phenolic content in a shorter time by ultrasound-assisted extraction | (70) |
| optimal condition for maceration extraction for 8 h → 50 °C | content of the extract by maceration extraction at optimal conditions (β-carotene content: 192.85 mg β-carotene/100 g dry matter, capsaicin content: 299.49 μg capsaicin/g dry matter, total phenolic content: 277.23 mg GAE/kg dry matter, and total antioxidant activity: 170.59 μM TEAC/g dry matter) | |||
| content of the extract by ultrasound-assisted extraction at optimal conditions (β-carotene content: 230.544 mg β-carotene/100g dry matter, capsaicin content: 781.42 μg capsaicin/g dry matter, total phenolic content: 710.78 mg GAE/kg dry matter, and total antioxidant activity: 189.25 μmol TEAC/g dry matter) | ||||
| beetroot waste | pressurized liquid extraction (at 40 °C and 7.5, 10, and 12.5 MPa for 90 min, flow rate of 3 mL min–1) | optimal conditions for pressurized liquid extraction at 40 °C for 90 min → the mixture of ethanol:water 70/30 at 10 MPa | the highest global yield of extract from leaves (36.0% w/w) with pressurized liquid extraction under optimum conditions | (71) |
| extract from leaves with pressurized liquid extraction at optimum conditions (total phenolic content (7 ± 1 mgGAE gextract–1) | ||||
| bioactive compounds (the most abundantly detected phenolic compounds: ferulic acid, vitexin, and sinapaldehyde) | ||||
| grape pomace | ultrasound-assisted extraction/microwave-assisted extraction | optimal conditions for microwave-assisted extraction → with 2% citric acid at 1000 W for 10 min | bioactive compounds (the recoveryof anthocyanins → 45%, total phenolic content (6.68 ± 0.05 mgGAE g–1 (dry basis)), total monomeric anthocyanins (1.32 ± 0.03 mg malvidin-3,5-diglycoside g–1 (dry basis)), antioxidant activity by ABTS and DPPH (23.84 ± 0.57 μmolTE g–1 (dry basis), and 33.27 ± 2.00 μmolTE g–1 (dry basis), respectively with microwave-assisted extraction at optimum conditions) | (72) |
| sesame bran | enzyme-assisted extraction/ultrasound-assisted extraction/ultrasound-assisted enzymatic extraction | optimal conditions for ultrasound-assisted enzymatic extraction → pH (9.8), and 1.248 AU (Anson unit)/100 g enzyme concentration at 836 W, 43 °C for 98 min | protein and antioxidant compounds (total phenolic contents (3.82–6.03 mg GAE/g) antioxidant capacity based on DPPH (1.24–3.55 μmol TE/g), and antioxidant capacity based on ABTS (37.9–42.3 μmol TE/g) by ultrasound-assisted enzymatic extraction designs) | (73) |
| the highest protein yield, total phenolic content, and antioxidant capacities with ultrasound-assisted enzymatic extraction under optimum conditions | ||||
| coffee husk | supercritical CO2 extraction | optimal conditions for supercritical CO2 extraction → solvent to raw material mass ratio: 197 kg CO2/kg husks at 373 K and 300 bar | caffeine (the extract yield (59%), the purity of caffeine (77%), and the caffeine yield (84%) with extraction under optimum conditions) | (74) |
| pumpkin seeds | ultrasound-assisted three-phase partitioning extraction | optimal conditions for ultrasound-assisted three-phase partitioning extraction → (NH4)2SO4 addition:30 g/100 mL, a t-butanol to slurry ratio of 1.0:1.0 (mL:mL), pH 5, and a duty cycle of 60% at 118 W for 20 min irradiation time | oil, protein, and polysaccharide (the optimal yields (39.79%, 14.30%, and 1.97%, respectively) with extraction under optimum conditions) | (75) |
| fatty acid composition of extracted oil (palmitic acid (12.27%), oleic acid (32.35%), linoleic acid (48.58%), saturated fatty acids (18.73%), monounsaturated fatty acids (32.35%), and polyunsaturated fatty acids (48.91%)) | ||||
| rice bran | conventional organic solvent-based Soxhlet extraction/subcritical CO2 Soxhlet extraction | optimal conditions for subcritical CO2 Soxhlet extraction → solvent-to-feed ratio: 24:1 at 68–70 bar and 27–29 °C | Oil (the yields (22% for conventional Soxhlet extraction and 13%–14.5% for subcritical CO2 Soxhlet extraction)) | (76) |
| nearly 10 times more thermolabile compounds, such as tocopherols (45.40–64.37 μg/g of oil), tocotrienols (157.89–198.31 μg/g of oil), and oryzanols (1.33–2.18 mg/g of oil), lower free fatty acid and peroxide values in extracted oil with subcritical CO2 Soxhlet extraction | ||||
| wheat bran | ultrasound-assisted enzymatic extraction | optimal conditions for ultrasound-assisted enzymatic extraction → raw material concentration: 50 g/L, enzyme dose: 4.5 g/L, at 180 W, 50 °C for 70 min | Arabinoxylan (the experimental yield (142.6 mg/g of wheat bran)) | (77) |
| green plantain peels | wet extraction | optimal conditions for wet extraction → 5% w/v ascorbic acid concentration | starch (the average yield based on dry mass (29%) with nearly 70% purity) | (78) |
| increase in starch yield with increase in antioxidant concentration whereas no significant effect of immersion time on final yield | ||||
| broccoli stalk | extraction with 0.1 M nitric acid | optimal conditions for extraction with 0.1 M nitric acid → liquid-to-solid ratio of 25 (v/w), 30 min | extracted pectin (the main neutral sugars:rhamnose and galactose, homogalacturonan and rhamnogalacturonan I substituted with β-1,4-D-galactan detected in extract based on analyses of nuclear magnetic resonance spectroscopy) | (79) |
| 75% galacturonic acid with a degree of methyl-esterification of 56%, and an acetyl content of 1.1% found in the pectin fraction of extract | ||||
| pectic fraction of extract (yield 18%) | ||||
| watermelon rind | microwave-assisted extraction with different acid solutions (for 15 min at 39.9 W) | optimal conditions for microwave-assisted extraction → 1 N sulfuric acid for 15 min at 39.9 W | pectin (the highest yield (18%) with extraction under optimum conditions) | (80) |
| banana peels | extraction with 0.5 N hydrochloric acid/citric acid (at 90 °C for 1, 2, 3, 4 h) | optimal conditions for extraction → 0.5 N HCl at 90 °C for 4 h | pectin (the highest yield (17.05% dry basis) with extraction under optimum conditions) | (81) |
| characteristics of extracted pectin from ripe banana peels using 0.5 N HCl for 3 h (moisture content (10.00%), ash (11.15%), methoxyl content (6.40%), anhydrouronic acid (57.32%), and degree of esterification (63.37%)) | ||||
| characteristics of extracted pectin from unripe banana peels using 0.5 N HCl for 3 h (moisture content (14.13%), ash (13.83%), methoxyl content (5.25%), anhydrouronic acid (39.68%), and degree of esterification (75.03%)) | ||||
| orange peels | hot-water extraction/rapid solid liquid dynamic (RSLD) extraction/microwave-assisted extraction | optimal conditions for acidic hot-water extraction → liquid-to-solid ratio (20), pH (1.5) at 70 °C for 60 min | pectin (the highest yield (21%) by acidic hot-water extraction under optimum conditions) | (82) |
| extracted pectin by acidic hot-water extraction at optimum conditions (esterification degree (82.5), morphology (stressed surface), thermogravimetric properties (the temperature at which the 50% of the mass loss occurs: 304 °C, the temperature that corresponds to the maximum decomposition rate: 243 °C, the total mass loss at 650 °C: 74%)) | ||||
| kiwi seeds | conventional Soxhlet extraction/microwave-assisted extraction/supercritical CO2 extraction/ultrasound assisted extraction/microwave integrated Soxhlet extraction | optimal conditions for ultrasound-assisted extraction → 10 mg seed powder in 400 mL n-hexane at 50 °C, 80 W for 30 min | oil (the highest yield (28.9 oil/seed wt %) with ultrasound-assisted extraction at optimum conditions) | (83) |
| composition of extracted oil by ultrasound-assisted extraction at optimum conditions (total saturated fatty acids (8.94%), total monounsaturated fatty acids (14.79%), and total polyunsaturated fatty acids (76.27%)) | ||||
| the presence of off-flavors in oil samples with conventional Soxhlet extraction and ultrasound-assisted extraction | ||||
| lemon peels | sequential extraction with a combination of microwave-assisted extraction and hydrodistillation and microwave-assisted extraction | optimal conditions for combination of microwave-assisted extraction and hydrodistillation → water-to-solid ratio: 0.3 mL/g, 1st step (irradiation power: 1.2 W/g for 5 min) and 2nd step (irradiation power: 0.7 W for 15 min) | essential oil extracted by a combination of microwave-assisted extraction and hydrodistillation: limonene (65.082 wt %), β-pinene (14.517 wt %), and γ-terpinene (9.743 wt %) | (84) |
| essential oil (potent inhibition against E. coli and S. aureus bacteria) | ||||
| pigment extracted by microwave-assisted extraction: eriocitrin (1.096 wt %), diosmin (1.645 wt %), and hesperidin (0.529 wt %) in the initial extract | ||||
| optimal conditions for microwave-assisted extraction → 80% (v/v) ethanol, 80 °C and 50 min, with a liquid-to-solid ratio of 1:10 | ||||
| yield of essential oil and pigment: ∼2 and 6 wt %, respectively | ||||
| lemon peels | conventional Soxhlet extraction/high-pressure–high-temperature extraction | optimal conditions for high-pressure–high-temperature extraction → at 150 °C for 30 min and matrix solvent ratio: 1:15 | d-limonene (the highest yield (3.56%) with high-pressure–high-temperature extraction under optimal conditions) | (85) |
| citrus peels | conventional hydrodistillation/microwave-assisted hydrodistillation | optimal conditions for microwave-assisted hydrodistillation at constant pressure (300 mbar), solid-to-liquid ratio (1:1.5) → 1st step (irradiated with 785 W for 5 min) and 2nd step (irradiated with 250 W for 15 min) | essential oil from Navel Navelate oranges (monoterpenes, oxygenated monoterpenes, and sesquiterpenes) chemical composition of essential oil from Navel Navelate oranges by conventional hydrodistillation (monoterpenes (98.56%) and oxygenated monoterpenes (0.14%)) chemical composition of essential oil from Navel Navelate oranges by microwave assisted hydrodistillation (monoterpenes (99.34%), oxygenated monoterpenes (0.14%), and sesquiterpenes (0.01%)) the most abundant chemical of essential oil from Navel Navelate oranges: d-limonene (96.75% for conventional hydrodistillation and 97.38% for microwave assisted hydrodistillation) the yield based on dry basis for Navel Navelate oranges (1.8% with microwave assisted hydrodistillation and 1.7% with conventional hydrodistillation) | (86) |
4.2. Improving the Stability and Bioavailability/Accessibility of Bioactive Compounds Derived from Agricultural Food Wastes/Byproducts
The valorization of biomass by conventional methods such as solid–liquid extraction after maceration and novel and green methods such as extraction with sonication, supercritical fluids, microwaves, and pulsed electric fields recently attracted attention, because of their high phytochemical content, which provides antioxidant, anti-inflammatory, and antibacterial properties leading to potential health benefits.87,88 For instance, these health-promoting bioactive compounds in fruit and vegetable wastes play an essential role in their anticancer, antimutagenic, antiviral, antioxidant, antitumor activities and their ability to reduce the risks of cardiometabolic diseases. Also, recent studies focusing on phytochemicals state that the high antioxidant properties of polyphenols from plant sources, including skins, seeds, pulp, or pomace, make polyphenols one of the primary phytochemicals important for health aspects.89
Although the demand and consumer acceptance for natural antioxidants in the food industry are constantly increasing to prevent harmful chemical additives and inhibit the oxidation processes in the final product, there are several problems with the utilization of natural antioxidants in various food products.87,88 Most of them are challenged by chemical degradation in foods and the gastrointestinal tract (GIT), reducing their bioavailability and bioactivity.87 In addition, many of them have a poor solubility problem, leading to a restriction of their direct incorporation in some foods, and many of them struggle with sensitivity to oxygen, light, heat, enzymes, salts, and acid or alkaline media, causing losses in their beneficial effects and activity.88 In recent years, scientists have performed extensive research to increase the amount and bioavailability of the components obtained from food wastes/byproducts and improve their stability during food processing and gastrointestinal digestion. The most common novel techniques to overcome these limitations are applied by nanotechnological approaches such as encapsulation, nanoemulsion, and biotechnological processes such as fermentation and enzyme use.
Nanotechnology can be defined as a technique developed to study, design, create, synthesize, apply and manipulate materials, devices, and functional systems by using nanoscale control of materials.90 Recently, microencapsulation and nanoencapsulation techniques have been applied for biomass wastes to find solutions to previously explained problems by increasing their stability, solubility, and bioavailability.87,88 Within the scope of nanoencapsulation, edible nanoparticles, with a maximum particle size of 500 nm and composed of proteins, carbohydrates, lipids, phospholipids, or surfactants, trap the phytochemicals inside with different applications like spray drying, freeze-drying, coacervation, crystallization, molecular encapsulation, extrusion, and electrostatic extrusion to increase the efficiency and management of the phytochemicals by enhancing their stability, bioavailability, bioactivity, and dispersibility.87,89
Since nanotechnological approaches provide higher stability, bioaccessibility, and bioavailability for bioactive compounds, they can potentially lead to innovative and functional products, especially in the food and pharmaceutical industries. The studies in the last five years about the application of different encapsulation techniques and their effects on the extracts derived from agricultural food wastes or byproducts are summarized in Table 2.
Table 2. Encapsulation Methods and Their Effects for Extracted Valuable Compounds from Agricultural Food Wastes.
| extract | encapsulation method | wall material | encapsulation efficiency | effects of encapsulation | references |
|---|---|---|---|---|---|
| carotenoids from carrot processing waste | spray drying/freeze-drying | whey protein/maltodextrin/Inulin | freeze-drying with pure whey protein (63.69 g/100 g) | freeze-dried encapsulate (best hygroscopicity, oxidative stability, antioxidant capacity, and color properties) | (93) |
| spray drying with the mixture of 71 g/100 g whey protein and 29 g/100 g inulin (53.78 g/100 g) | |||||
| spray-dried encapsulate (lowest water activity, moisture content, and particle size) | |||||
| carotenoids from tomato peels | electrospinning | Zein fibers | >90% | nanoencapsulation process (an increase of 11-fold antioxidant activity) | (94) |
| encapsulated extract (better retention of lycopene and antioxidant activity during 14-days storage time than nonencapsulated extract) | |||||
| phenolics and carotenoids from red pepper waste | spray drying/freeze-drying | whey protein | slightly better results for freeze-drying than spray drying method | encapsulation process (a protective effect against pH changes and enzymatic activities along with digestion and increase in bioaccessibility in the gut) (an efficient method for improvements in nutrition, color, and bioactive properties) | (95) |
| phenolic compounds from winemaking waste | extrusion | alginate and chitosan | between 55% and 79% | encapsulation process (retention of chemical stability and biological activities) | (96) |
| more suitable and efficient wall material (the mixture of alginate and chitosan at concentrations of 1% w/v and 3% w/v, respectively, and encapsulating about 80% of the extracts) | |||||
| phenolic compounds from cornsilk | spray drying/freeze-drying/microwave drying | maltodextrin | freeze-drying (99.84%) | freeze-drying with 100% maltodextrin (the highest recovery of phenolic compounds and the highest retention of antioxidant activity) | (97) |
| microwave drying (99.83%) | |||||
| spray drying (99.65%) | |||||
| phenolic compounds from unused chokeberries | spray drying/co-crystallization/ionic gelation | maltodextrin/skim milk powder/whey protein/alginate/sucrose syrup | spray drying (99.6%) | spray-dried encapsulate (higher phenolic content and better stability, but lower radical scavenging activity than encapsulates by other methods) | (98) |
| co-crystallization (96.3%) | |||||
| ionic gelation (94.2%) | |||||
| encapsulated extracts (reduction of 6.07%, 24.75%, and 52.97% was observed in total phenolic content by spray drying, co-crystallization, and ionic gelation, respectively, during storage time at 5 °C) | encapsulated extracts (reduction of 6.07%, 24.75%, and 52.97% was observed in total phenolic content by spray drying, co-crystallization, and ionic gelation, respectively, during storage time at 5 °C) | ||||
| phenolic compounds from golden apple and red grape pomace | nanoemulsification | chitosan/soy protein | nanoemulsification with soy protein (95%) | encapsulation process (improvement of antioxidant activity of phenolic extracts) | (99) |
| nanoemulsification with chitosan (75%) | |||||
| carotenoids and phenolic compounds from sweet potato peels | spray drying/freeze-drying | whey protein | spray drying for carotenoids and phenolics (60.0% and 61.9%, respectively) | encapsulation process (better quality of encapsulates based on water activity, moisture content, hygroscopicity, and encapsulation efficiency of phenolics by freeze-drying and smaller particle size, better flow properties, and encapsulation efficiency of carotenoids by spray drying) | (100) |
| freeze-drying for carotenoids and phenolics (9.34% and 64.3%, respectively) | |||||
| spray drying (retention of carotenoid and phenolic compounds and prolonged shelf life under light and dark storage conditions) | |||||
| blueberry residue | ionotropic gelation | sodium alginate | - | encapsulation process (67.01% retention of the phenolic compounds and 68.2% release of phenolics (120 min) after in vitro dissolution) | (101) |
| bioactive compounds from lemon byproducts | spray drying/freeze-drying | maltodextrin/soybean protein/ ι-carrageenan | freeze-drying with the mixture of maltodextrin and soybean protein (more efficient technique than spray drying) | encapsulation process (the highest retention of total phenolic content, total flavonoid content, and ferric ion reducing antioxidant power by freeze-drying with the mixture of maltodextrin and soybean protein) | (102) |
| freeze-dried encapsulate (lower moisture content and water activity than those produced by spray-drying) | |||||
| bioactive compounds from beetroot pomace | freeze-drying | soy protein | 86.14% | encapsulation process (retention of polyphenols (76.67%), betalain pigments, betacyanins (17.77%), and betaxanthins (17.72%) during storage time (three months) at room temperature) | (103) |
| encapsulated extracts (higher release of polyphenolic compounds in simulated intestinal fluid than in gastric fluid during in vitro digestion) |
In addition to the encapsulation methods provided in Table 2, emulsion-based systems can also be applied to valorize and improve compounds derived from food waste efficiency and stability. For example, extracts rich in mango peel phenolics were encapsulated in water-in-oil-in-water emulsions with different surfactants (Tween 20, Tween 80, and lecithin). Water-in-oil-in-water emulsion with Tween 20 had the highest encapsulation efficiency (98.65% ± 1.14%). In contrast, the best physical and encapsulation stability within the storage period was obtained in emulsions with Tween 80. These results showed that the application of efficient and stable emulsion-based systems with suitable surfactants could successfully encapsulate phenolic compounds.91 Also, another study indicated that mixing excipient emulsion (oil-in-water) prepared by microfluidics and tomato pomace has increased the total phenolic content of tomato pomace and lycopene bioaccessibility in tomato pomace.92 The study of Zhu et al. indicated that the nanoencapsulation systems are commercially used in several countries worldwide. The NanoCeuticals Supplements—RBC Life Sciences in Germany, consisting of NANOCLUSTERS technology, use nanosized powders to improve organoleptic properties and increase bioavailability. Super Nano Green Tea, which consists of nanometric particles (200 nm) with increased bioavailability, and Nano-Selenium Rich Black Tea with improved selenium bioavailability, can be given as examples of commercial nanoencapsulation applications in China. Furthermore, as another example, Nano Gold (NGT) edible gold is produced with physical methods in Taiwan as gold nanometric particles, with diameters of 0.5100 nm.90 Although some countries commercially use nanotechnological approaches, some regulations and further in vivo studies to determine their long-term effects are necessary for their extensive usage.
Biotechnological approaches, the most well-known of which is fermentation, have been used to valorize the food wastes and byproducts by converting them into functional components.104 Therefore, in addition to the above-mentioned nanotechnological approaches, numerous studies focus on fermentation to increase the content of bioactive compounds and their bioavailability. Fermentation, which appears as one of the oldest processes used to convert products to value-added products, occurs by breaking down the organic compounds to obtain energy through anaerobic metabolism.105,106 The fermentation process is highly preferred in scientific and industrial fields, since it substantially satisfies the need to limit the amount of waste produced. It consumes less energy, generates a small amount of water, and has a low cost.106 Three types of fermentation processes, solid-state, submerged, and liquid fermentation are applied, depending on the product type. Solid-state and submerged fermentation are the two most commonly used processes in novel research and industry to obtain bioactive compounds.105 Recently, the fermentation process, in which desired bioactive compounds such as antioxidants are produced, is becoming more prevalent in scientific and industrial fields, regarding nutritional and health issues.107 Different examples of the effects of fermentation (especially solid-state fermentation) on the bioactive compounds from agricultural food wastes/byproducts are summarized in Table 3.
Table 3. Effects of Fermentation on the Bioactive Compounds from Agricultural Food Waste/Byproducts.
| agricultural food waste/byproduct | fermentation method | microorganisms | effects of fermentation | reference |
|---|---|---|---|---|
| mango seeds | solid-state fermentation | Aspergillus niger | mobilization of polyphenolic compounds | (110) |
| improvement of nutraceutical properties | ||||
| practical method for releasing the bound phenolics | ||||
| apricot pomace | solid-state fermentation | Aspergillus niger and Rhizopus oligosporus | increase of total phenolic and flavonoid contents (over 70% for total phenolics and 38% for total flavonoids with R. oligosporus, and more than 30% for total phenolics and 12% for total flavonoids with A. niger) | (111) |
| improvement of free radical scavenging capacities | ||||
| plum fruit (Prunus domestica L.) byproducts | solid-state fermentation | Aspergillus niger and Rhizopus oligosporus | significant increase of total phenolic and antioxidant levels (for total phenolic content: higher than 30% with R. oligosporus and higher than 21% with A. niger) | (112) |
| achievement of higher lipid recovery from plum kernels | ||||
| enrichment of polar lipids with n-3 polyunsaturated fatty acids | ||||
| spent coffee grounds | solid-state fermentation | Bacillus clausii | increase of total phenolic and flavonoid contents and antioxidant capacity (36%, 13%, and 15%, respectively) | (113) |
| improvement of antimicrobial activity against gram (+) and gram (−) bacteria | ||||
| spent coffee grounds | fermentation | Bacillus clausii | increase of total proteins, soluble proteins, and protein hydrolysates amounts (2.7-, 2.2-, and 1.2-fold, respectively) | (114) |
| tomato seed meal extract | fermentation | Lactobacillus plantarum | reduction of crude and soluble proteins contents (18.44% and 68.99%, respectively) due to L. plantarum growth on the substrate after 24 h | (115) |
| increase of radical scavenging activity after 24 h (87%) due to different bioactive peptides production | ||||
| significant reduction of total amino acids (specifically glutamic acid and aspartic acid) concentration | ||||
| improvement of new amides and aromatic compounds formation | ||||
| peanut press cake | solid-state fermentation | Aspergillus awamori | improvement of phenolic and antioxidant properties | (116) |
| improvement of functional properties (apart from bulk density) | ||||
| improvement of morphological characteristics and mineral content | ||||
| Jussara pulp | Lactobacillus fermentation | Lactobacillus and Bifidobacterium strains | increase in antioxidant activity | (117) |
| more extensive changing of jussara anthocyanins by Lactobacillus deubruekii | ||||
| the main bioconversion products from anthocyanins → protocatechuic acid | ||||
| rice bran | solid-state fermentation | Rizhopus oryzae | increase of phenolic compound content (more than 2-fold) | (118) |
| the highest increase of phenolic acid → gallic and ferulic acid | ||||
| phenolic extract from fermented rice bran → inhibition of the peroxidase enzyme |
Other innovative biotechnological approaches to valorize high-value ingredients from fruit and vegetable wastes are present along with fermentation. They are primarily used in drug and functional food formulation as natural bioactive compounds.108 For example, enzyme complexes can hydrolyze materials, releasing desired products in a wide range of food waste. The studies have focused on using several enzyme complexes, mainly α-amylase, cellulases, xylanases, pectinases, proteases, chitinase, according to the composition of a specific substance. The wide range of products derived from food wastes with the help of these enzyme complexes include antioxidants, protein hydrolysates, pigments, oligosaccharides, growth-promoting substrates, etc.104 For example, the effects of enzymatic treatment using only tannase, pectinase, and cellulase, or a mixture of these three enzymes on the phenolic compounds of grape pomace were investigated. The results showed that grape pomace’s total polyphenol content and antioxidant activity were increased with enzymatic treatments, which released phenolic acids and aglycones from grape pomace, and tannase109 obtained the greatest hydrolytic efficacy. It is advantageous to prefer biotechnological approaches, especially fermentation, for food waste valorization at an industrial level, because of their low energy requirement and low cost. In addition, biotechnological approaches are good options for food waste valorization to design innovative functional foods, because of their nutritional and health benefits. The food wastes that cannot be used directly in food products can be integrated with nanotechnological and biotechnological approaches.
5. Use of Agricultural Food Wastes/Byproducts and Their Extracts As Additives in Food Products
The market currently deals with discussions on synthetic additives without reaching a consensus.90 This situation leads the food industry to search for natural food additives produced using novel, natural, and economical protein sources, dietary fiber, flavoring agents, colorants, antioxidants, and antimicrobials.90,119 Since the environmental problems can be reduced, and food additives or supplements with high economic and nutritional value can be utilized, byproducts as a source for natural food additives emerge as a good option. Therefore, the companies can choose to transform these valuable byproducts into a value-added product which allows them to decrease their treatment cost, create additional profit, and thus make them more competitive in the market.119 Also, some biotechnological processes are available to diminish the antinutritional factors found in some of the byproducts and, therefore, allow them to be a source of food additives or be part of a balanced food formulation. Although the local communities in poor regions utilize their residues and byproducts by using novel food formulations, most small traders in those regions dispose of their wastes to the environment. Therefore, the Environmental Protection Agency (EPA) determined the order of priority to ensure that the surplus is used to feed hungry people first and then animals. The order of priority indicated that the rest of the surplus should be utilized in industrial uses. If they cannot be used, they should be composted and incinerated.120
With the contribution of food waste as natural additives into food products to provide nutrients and bioactive compounds, the sensory properties and consumer acceptance are also essential criteria for food products.120 For example, two different flavors—pineapple and white chocolate—are used to produce the jelly samples with the collagen extracted from chicken feet. This study demonstrated that both products have good sensory acceptance by tasters, and consumers can willingly consume them.121 However, another study on the acceptability of cookies enriched with antioxidant fiber using a blueberry pomace byproduct was conducted. The tasters were given a reference vanilla cookie and a new cookie elaborated with blueberry pomace. Although they liked the labels of the new cookie before tasting it, they did not find it better than the reference cookie.122 Several studies on the use of agricultural wastes in food formulations and their effects on the final products are listed in Table 4.
Table 4. Agricultural Food Wastes and by-Products As Natural Food Additives for Different Types of Food Products.
| functional additives | food product | final product quality | reference |
|---|---|---|---|
| encapsulated bioactives from red pepper waste | yogurt | retention of carotenoid (71.43%) and polyphenols (increase up to 123.73%) and higher pigment retention during 21 days of storage time | (125) |
| higher sensory and general acceptance due to better color, appearance, and flavor | |||
| encapsulated bioactives from grape skin | whole wheat cocoa biscuits (with 1.2, 2.3, and 3.5% addition of encapsulated extract on dough weight) | increase of phenolic content (up to 134%) and antioxidant capacity (up to 244%) | (126) |
| reduction of phenolic content (16%) during cooking | |||
| change of color and not relevant positive impact on oxidative stability | |||
| encapsulated extract addition level (significant influence on the sensory acceptance) | |||
| free and encapsulated phenolic extract from olive leaf | full-fat mayonnaise (∼80% oil) | addition of both types of extract (improvement of dispersion degree of the sample, and causing lower spreadability, higher salty and bitter taste) | (127) |
| addition of encapsulated extract (improvement of physical properties) | |||
| enriched mayonnaise with free or encapsulated extract (lower overall acceptance) | |||
| encapsulated black mulberry waste extract | dark chocolate | chitosan coated liposomal powders (better anthocyanin protection than spray-dried extract) | (128) |
| encapsulation in liposomes (improvement of in vitro bioaccessibility of anthocyanins) | |||
| maximum fortification with encapsulated anthocyanins (76.8% based on conching temperature and pH) | |||
| onion skin powder | bread | enhancement of bioaccessible lipid oxidation preventers and compounds having chelating and antioxidant abilities | (129) |
| higher antioxidant activity | |||
| 2%–3% of onionskin powder addition (significant development of the antioxidant capabilities) | |||
| up to 3% of onionskin powder addition instead of wheat flour (satisfactory consumers’ acceptance) | |||
| lettuce waste flour | bread | reduction of dough leavening capacity, and increase of bread moisture and firmness, phenolic content (up to 3.4 g GAE kg–1), and antioxidant capacity (200%) | (130) |
| addition of lettuce waste flour (at least 170 g kg–1) (improved fiber content (>30 g kg–1), reduction of yeast odor and flavor intensity, and increase of silage and herbaceous scent and flavor, dried fruit flavor, acid, and sour taste) | |||
| enriched bread with 170 and 575 g kg–1 addition of lettuce flour (comparable sensory properties and consumer acceptability with commercial wholemeal bread containing similar rye bran content) | |||
| olive paste flour | durum wheat spaghetti | the best addition amount of olive paste flour for enrichment (10% (w/w)) | (131) |
| addition of 10% olive paste flour (an increase of total polyphenol content [from 82.39 μg/g DW to 245.08 μg/g DW], 15 times higher in apigenin, luteolin, and quercetin levels) | |||
| addition of transglutaminase (0.6%) to enriched pasta with 10% olive paste flour (increase of overall pasta quality) | |||
| enriched spaghetti with 10% olive paste flour and 0.6% transglutaminase (very similar quality with the control sample for uncooked and cooked spaghetti) | |||
| carrot pomace powder and dushab (a traditional grape juice concentrate) | cakes | addition of carrot pomace powder and dushab (reduction of specific volume of cakes and increase of moisture content, color difference, browning index, firmness, and reduction of cohesiveness with increase of added amount) | (132) |
| increase of carrot pomace powder addition (increase of chewiness and gumminess) | |||
| addition of dushab (not effective on chewiness and gumminess, and decrease of cake springiness) | |||
| grape residue flour | grape ice cream | addition of 2% of grape residue flour (increase of protein, lipids, ash, dietary fiber, total energy value, reducing sugars, total phenolics, flavonoids, flavonols, and anthocyanins, and higher radical scavenging ability) (satisfactory sensory attributes and the best formulation among other ice creams containing grape residue flour in different levels) | (133) |
| apple pomace powder | stirred-type yogurt | addition of apple pomace powder (2%–3%) (alteration on the structure of stirred yogurt) | (134) |
| apple pomace powder addition into the diluted yogurt system (potential stabilization of acid drink and reduction of protein aggregates sedimentation) | |||
| coffee pulp extract | probiotic beverage with or without kefir culture | probiotic beverage from coffee pulp extract by steam pretreatment with kefir culture (improvement of phytochemicals, physicochemical properties, and enhancement of organoleptic properties, the best overall acceptance according to the control beverage without kefir cultures) | (135) |
Furthermore, the industry can have economic gains, the nutritional problems can be diminished, the environmental implications generating mismanagement of waste can be reduced, and some beneficial health effects can be produced if the food waste and byproducts are used adequately as sources of food additives. Recently, the industries are investigating innovative methods to achieve zero waste, which refers to generated waste as raw material for new products and applications. The Millennium Development Goals, the upcoming Sustainable Development Goals, and the Zero Hunger Challenge of the United Nations can be directly affected by these innovative methods designed to obtain zero waste.120 For example, the company GEA is running the Pro-Enrich project, which aims to investigate new approaches to valorize fruit and vegetable residues as functional proteins, phenols with antioxidant and antimicrobial properties, dietary fibers, and pigments in various applications. The trend to extract plant-based functional proteins and other valuable compounds from plants has increased due to environmental and economic issues of animal-based applications. Within this project’s scope, the excess of biomass generated after processing fruits and vegetables is provided by suppliers from various parts of the supply chain from the EU and the world. Pro-Enrich’s project focuses on new ways to extract valuable compounds while protecting their purity, functionality, and quality with energy-efficient and cost-effective methods. These valuable compounds extracted from the tomato residues, citrus fruit residues, olive pomace, olive mill wastewater, and rapeseed (canola) meal/press-cake are used in several industries such as dietary supplements, pet food, cosmetics, food ingredients, and adhesives.123 Another project conducted by the Food Innovation Centre of CSIRO (Commonwealth Scientific and Industrial Research Organization), an Australian government agency, focused on developing a new method to valorize biomass as value-added ingredients and food products. The project investigated a new process to stabilize the apple pomace, which was chosen as a model food source, to protect it from physical, chemical, and microbial degradation. The results showed that this process helps create a functional and nutritious ingredient. Therefore, it can be used for other fruits, vegetables, and horticultural products such as broccoli, carrots, olives, and grapes.124
6. Limitations and Future Perspectives
Food waste is considered a nutritional, functional, and nutraceutical raw material that can be used in various applications in food formulations, making it a potential solution for economic, social, and environmental problems. They can be used directly as food components or proteins, lipids, vitamins, fibers, starch, minerals, and antioxidants. Other biomolecules within the food waste can be physically or chemically extracted and used as nutritional and functional components. To maintain the physicochemical and microbiological stability of biomaterials during the valorization of these food wastes, the application of the unitary drying operation is necessary to prevent microbial risks. Thus, the governments should support installing the infrastructure and technology, which make the utilization of food wastes and byproducts in the production and storage areas possible.120 On the other hand, eliminating other risks, including toxic materials and antinutritional factors, should also be considered.
Another critical limitation about using food wastes/byproducts as functional food ingredients for the development and design of new food products is the sensory quality of the food materials and consumer acceptance. The use of food wastes/byproducts as functional ingredients in foods produced at the industrial level is limited compared to their use as biofuel in the industry. Therefore, more-detailed studies about the formulation of new functional foods are needed to achieve higher quality and consumer acceptance. In addition, the companies should try to valorize their food wastes and byproducts by integrating them into new industrial products. Indeed, changes in the processing steps and designs may be considered, for example, to reintegrate the wastes of their products to the original food at an industrial scale (i.e., tomato skin and seeds may be processed and restored into tomato paste/sauce). On the other hand, probably the intermediary companies (waste brokers) who collect wastes and direct them to specific points for processing into new products/components will increase in number in the future, which will be beneficial from the waste valorization point of view.
The authors declare no competing financial interest.
References
- FAO (Food and Agriculture Organization of the United Nations) . The State of Food and Agriculture 2019: Moving Forward on Food Loss and Waste Reduction; Rome, Italy, 2019. [Google Scholar]
- Yukesh Kannah R.; Merrylin J.; Poornima Devi T.; Kavitha S.; Sivashanmugam P.; Kumar G.; Rajesh Banu J. Food waste valorization: Biofuels and value added product recovery. Bioresource Technol. Rep. 2020, 11, 100524. 10.1016/j.biteb.2020.100524. [DOI] [Google Scholar]
- Chen C.; Chaudhary A.; Mathys A. Nutritional and environmental losses embedded in global food waste. Resources; Conserv. Recycling 2020, 160, 104912. 10.1016/j.resconrec.2020.104912. [DOI] [Google Scholar]
- FAO (Food and Agriculture Organization of the United Nations) . Food Wastage Footprint: Impacts on Natural Resources, 2013.
- United Nations Environment Programme . Food Waste Index Report; 2021, Nairobi.
- Rao P.; Rathod V. Valorization of food and agricultural waste: a step towards greener future. Chem. Rec. 2019, 19 (9), 1858–1871. 10.1002/tcr.201800094. [DOI] [PubMed] [Google Scholar]
- Obi F. O.; Ugwuishiwu B. O.; Nwakaire J. N. Agricultural waste concept; generation; utilization and management. Nigerian J. Technol. 2016, 35 (4), 957–964. 10.4314/njt.v35i4.34. [DOI] [Google Scholar]
- Baiano A. Recovery of biomolecules from food wastes-a review. Molecules. 2014, 19 (9), 14821–14842. 10.3390/molecules190914821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plazzotta S.; Manzocco L.. Food waste valorization. In Saving Food, Galanakis C. M., Ed.; Academic Press: New York, 2019; pp 279–313. [Google Scholar]
- Ravindran R.; Jaiswal A. K. Exploitation of food industry waste for high-value products. Trends Biotechnol. 2016, 34 (1), 58–69. 10.1016/j.tibtech.2015.10.008. [DOI] [PubMed] [Google Scholar]
- Dueñas M.; García-Estévez I. Agricultural and food waste: Analysis, characterization and extraction of bioactive compounds and their possible utilization. Foods 2020, 9, 817. 10.3390/foods9060817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrad Z.; Blackstone N. T. Identifying the links between consumer food waste; nutrition; and environmental sustainability: a narrative review. Nutr. Rev. 2021, 79 (3), 301–314. 10.1093/nutrit/nuaa035. [DOI] [PubMed] [Google Scholar]
- Spiker M. L.; Hiza H. A.; Siddiqi S. M.; Neff R. A. Wasted food; wasted nutrients: nutrient loss from wasted food in the United States and comparison to gaps in dietary intake. J. Acad. Nutr. Dietetics 2017, 117 (7), 1031–1040. 10.1016/j.jand.2017.03.015. [DOI] [PubMed] [Google Scholar]
- Conrad Z.; Niles M. T.; Neher D. A.; Roy E. D.; Tichenor N. E.; Jahns L. Relationship between food waste; diet quality; and environmental sustainability. PloS one 2018, 13 (4), e0195405 10.1371/journal.pone.0195405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Searchinger T.; Hanson C.; Ranganathan J.; Lipinski B.; Waite R.; Winterbottom R.; Dinshaw A.; Heimlich R.. Creating a Sustainable Food Future: Interim Findings. World Resources Rep. 2013–14, 2013. [Google Scholar]
- Melikoglu M.; Lin C.; Webb C. Analysing global food waste problem: pinpointing the facts and estimating the energy content. Central Eur. J. Eng. 2013, 3 (2), 157–164. 10.2478/s13531-012-0058-5. [DOI] [Google Scholar]
- Paritosh K.; Kushwaha S. K.; Yadav M.; Pareek N.; Chawade A.; Vivekanand V. Food waste to energy: an overview of sustainable approaches for food waste management and nutrient recycling. BioMed. Res. Int. 2017, 2017, 1–19. 10.1155/2017/2370927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song G.; Li M.; Semakula H. M.; Zhang S. Food consumption and waste and the embedded carbon; water and ecological footprints of households in China. Sci. Total Environ. 2015, 529, 191–197. 10.1016/j.scitotenv.2015.05.068. [DOI] [PubMed] [Google Scholar]
- Seberini A.Economic; social and environmental world impacts of food waste on society and zero waste as a global approach to their elimination. In SHS Web of Conferences, EDP Sciences; 2020; Vol. 74, 03010.
- Venkat K. The climate change and economic impacts of food waste in the United States. Int. J. Food Syst. Dynam. 2011, 2 (4), 431–446. [Google Scholar]
- Chapagain A.; James K.. The water and carbon footprint of household food and drink waste in the UK. A Report Containing Quantification and Analysis of the Water and Carbon. WRAP: Banbury, U.K., 2011. [Google Scholar]
- Chen C. R.; Chen R. J. Using two government food waste recognition programs to understand current reducing food loss and waste activities in the US. Sustainability 2018, 10 (8), 2760. 10.3390/su10082760. [DOI] [Google Scholar]
- Papargyropoulou E.; Lozano R.; Steinberger J. K.; Wright N.; bin Ujang Z. The food waste hierarchy as a framework for the management of food surplus and food waste. J. Cleaner Prod. 2014, 76, 106–115. 10.1016/j.jclepro.2014.04.020. [DOI] [Google Scholar]
- Giordano C.; Falasconi L.; Cicatiello C.; Pancino B. The role of food waste hierarchy in addressing policy and research: A comparative analysis. J. Cleaner Prod. 2020, 252, 119617. 10.1016/j.jclepro.2019.119617. [DOI] [Google Scholar]
- Vilariño M. V.; Franco C.; Quarrington C. Food loss and waste reduction as an integral part of a circular economy. Front. Environ. Sci. 2017, 5, 21. 10.3389/fenvs.2017.00021. [DOI] [Google Scholar]
- Aschemann-Witzel J.; De Hooge I.; Amani P.; Bech-Larsen T.; Oostindjer M. Consumer-related food waste: Causes and potential for action. Sustainability 2015, 7 (6), 6457–6477. 10.3390/su7066457. [DOI] [Google Scholar]
- Metcalfe P.Role of food waste valorisation potential. REFRESH 2019, D6.13.
- Cecilia J. A.; García-Sancho C.; Maireles-Torres P. J.; Luque R. Industrial food waste valorization: a general overview. Biorefinery 2019, 253–277. 10.1007/978-3-030-10961-5_11. [DOI] [Google Scholar]
- Kavitha S.; Kannah R. Y; Kumar G.; Gunasekaran M.; Banu J. R.. Introduction: sources and characterization of food waste and food industry wastes. In Food Waste to Valuable Resources, Banu J. R., Kumar G., Gunasekaran M., Kavitha S., Eds.; Academic Press: New York, 2020; pp 1–13. [Google Scholar]
- Xiong X.; Yu I. K. M.; Tsang D. C.; Bolan N. S.; Ok Y. S.; Igalavithana A. D.; Kirkham M. B.; Kim K.; Vikrant K. Value-added chemicals from food supply chain wastes: State-of-the-art review and future prospects. Chem. Eng. J. 2019, 375, 121983. 10.1016/j.cej.2019.121983. [DOI] [Google Scholar]
- Lin C. S. K.; Koutinas A. A.; Stamatelatou K.; Mubofu E. B.; Matharu A. S.; Kopsahelis N.; Pfaltzgraff L. A.; Clark J. H.; Papanikolaou S.; Kwan T. H.; Luque R. Current and future trends in food waste valorization for the production of chemicals; materials and fuels: a global perspective. Biofuels; Bioproducts Biorefining 2014, 8 (5), 686–715. 10.1002/bbb.1506. [DOI] [Google Scholar]
- Kover A.; Kraljić D.; Marinaro R.; Rene E. R. Processes for the valorization of food and agricultural wastes to value-added products: recent practices and perspectives. Syst. Microbiol. Biomanuf. 2022, 2, 50. 10.1007/s43393-021-00042-y. [DOI] [Google Scholar]
- Nayak A.; Bhushan B. An overview of the recent trends on the waste valorization techniques for food wastes. J. Environ. Manage. 2019, 233, 352–370. 10.1016/j.jenvman.2018.12.041. [DOI] [PubMed] [Google Scholar]
- O’Connor J.; Hoang S. A.; Bradney L.; Dutta S.; Xiong X.; Tsang D. C.; Ramadass K.; Vinu A.; Kirkham M. B.; Bolan N. S. A review on the valorisation of food waste as a nutrient source and soil amendment. Environ. Pollut. 2021, 272, 115985. 10.1016/j.envpol.2020.115985. [DOI] [PubMed] [Google Scholar]
- Isah S.; Ozbay G. Valorization of food loss and wastes: Feedstocks for biofuels and valuable chemicals. Front. Sustain. Food Syst. 2020, 4, 82. 10.3389/fsufs.2020.00082. [DOI] [Google Scholar]
- Karmee S. K.; Lin C. S. K. Valorisation of food waste to biofuel: current trends and technological challenges. Sustain. Chem. Process. 2014, 2 (1), 1–4. 10.1186/s40508-014-0022-1. [DOI] [Google Scholar]
- Nordic Energy Research . Food Waste to Biofuels. 2019.
- Caruso M. C.; Braghieri A.; Capece A.; Napolitano F.; Romano P.; Galgano F.; Altieri G.; Genovese F. Recent updates on the use of agro-food waste for biogas production. Appl. Sci. 2019, 9 (6), 1217. 10.3390/app9061217. [DOI] [Google Scholar]
- Korbag I.; Omer S. M. S.; Boghazala H.; Abusasiyah M. A. A.. Recent Advances of Biogas Production and Future Perspective. In Biogas-Recent Advances and Integrated Approaches, Abomohra A. E., Elsayed M., Qin Z., Ji H., Liu Z., Eds.; IntechOpen; United Kingdom, 2020; pp 1–3. [Google Scholar]
- Joshi S. M.; Gogate P. R. Intensifying the biogas production from food waste using ultrasound: Understanding into effect of operating parameters. Ultrasonics Sonochem. 2019, 59, 104755. 10.1016/j.ultsonch.2019.104755. [DOI] [PubMed] [Google Scholar]
- Degueurce A.; Dabert P.; Argence V.; Blondel L.; Le Bihan A.; Lebreton M.; Peu P.; Sarrazin M.; Picard S.; Trémier A. An Innovative Solid-State Micro-Anaerobic Digestion Process to Valorize Food Waste: Technical Development Constraints and Consequences on Biological Performances. Waste Biomass Valor. 2022, 13, 617–630. 10.1007/s12649-021-01555-2. [DOI] [Google Scholar]
- Jung S.; Kim H.; Tsang Y. F.; Lin K. Y. A.; Park Y. K.; Kwon E. E. A new biorefinery platform for producing (C2–5) bioalcohols through the biological/chemical hybridization process. Bioresour. Technol. 2020, 311, 123568. 10.1016/j.biortech.2020.123568. [DOI] [PubMed] [Google Scholar]
- Liu Y.; Han W.; Xu X.; Chen L.; Tang J.; Hou P. Ethanol production from waste pizza by enzymatic hydrolysis and fermentation. Biochem. Eng. J. 2020, 156, 107528. 10.1016/j.bej.2020.107528. [DOI] [Google Scholar]
- Han W.; Liu Y.; Xu X.; Huang J.; He H.; Chen L.; Qiu S.; Tang J.; Hou P. Bioethanol production from waste hamburger by enzymatic hydrolysis and fermentation. J. Cleaner Prod. 2020, 264, 121658. 10.1016/j.jclepro.2020.121658. [DOI] [Google Scholar]
- Lehmann J.; Joseph S.; Biochar for environmental management: an introduction. In Biochar for Environmental Management: Science; Technology and Implementation, Lehmann J.; Joseph S., Eds.; Routledge: Taylor and Francis Group; New York, 2015; pp 1–14. [Google Scholar]
- Ravindran R.; Jaiswal A. K. Microbial enzyme production using lignocellulosic food industry wastes as feedstock: a review. Bioengineering 2016, 3 (4), 30. 10.3390/bioengineering3040030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murthy P. S.; Madhava Naidu M.; Srinivas P. Production of α-amylase under solid-state fermentation utilizing coffee waste. J. Chem. Technol. Biotechnol.: Int. Res. Process; Environ. Clean Technol. 2009, 84 (8), 1246–1249. 10.1002/jctb.2142. [DOI] [Google Scholar]
- Wang Q.; Wang X.; Wang X.; Ma H. Glucoamylase production from food waste by Aspergillus niger under submerged fermentation. Process Biochem. 2008, 43 (3), 280–286. 10.1016/j.procbio.2007.12.010. [DOI] [Google Scholar]
- Alkan H.; Baysal Z.; Uyar F.; Dogru M. Production of lipase by a newly isolated Bacillus coagulans under solid-state fermentation using melon wastes. Appl. Biochem. Biotechnol. 2007, 136 (2), 183–192. 10.1007/BF02686016. [DOI] [PubMed] [Google Scholar]
- Kim H. M.; Park J. H.; Choi I. S.; Wi S. G.; Ha S.; Chun H. H.; Hwang I. M.; Chang J. Y.; Choi H.; Kim J.; Park H. W. Effective approach to organic acid production from agricultural kimchi cabbage waste and its potential application. PloS one 2018, 13 (11), e0207801 10.1371/journal.pone.0207801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung C. C. J.; Cheung A. S. Y.; Zhang A. Y. Z.; Lam K. F.; Lin C. S. K. Utilisation of waste bread for fermentative succinic acid production. Biochem. Eng. J. 2012, 65, 10–15. 10.1016/j.bej.2012.03.010. [DOI] [Google Scholar]
- Spalvins K.; Ivanovs K.; Blumberga D. Single cell protein production from waste biomass: review of various agricultural by-products. Agronomy Res. 2018, 16 (S2), 1493–1508. [Google Scholar]
- Anupama; Ravindra P. Value-added food:: Single cell protein. Biotechnol. Adv. 2000, 18 (6), 459–479. 10.1016/S0734-9750(00)00045-8. [DOI] [PubMed] [Google Scholar]
- Nasseri A. T.; Rasoul-Ami S.; Morowvat M. H.; Ghasemi Y. Single cell protein: production and process. Am. J. Food Technol. 2011, 6 (2), 103–116. 10.3923/ajft.2011.103.116. [DOI] [Google Scholar]
- Leni G.; Caligiani A.; Sforza S.. Bioconversion of agri-food waste and by-products through insects: a new valorization opportunity. InValorization of Agri-Food Wastes and By-Products. Academic Press; 2021; pp 809–828. [Google Scholar]
- Magee K.; Halstead J.; Small R.; Young I. Valorisation of Organic Waste By-Products Using Black Soldier Fly (Hermetia illucens) as a Bio-Convertor. Sustainability 2021, 13 (15), 8345. 10.3390/su13158345. [DOI] [Google Scholar]
- Ranganathan S.; Dutta S.; Moses J. A.; Anandharamakrishnan C. Utilization of food waste streams for the production of biopolymers. Heliyon 2020, 6 (9), e04891 10.1016/j.heliyon.2020.e04891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramadhan M. O.; Handayani M. N.. The potential of food waste as Bioplastic material to promote environmental sustainability: A review. In IOP Conference Series: Materials Science and Engineering, IOP Publishing; 2020; Vol. 980, p. 012082.
- Gençdaǧ E.; Görgüç A.; Yılmaz F. M. Recent advances in the recovery techniques of plant-based proteins from agro-industrial by-products. Food Rev. Int. 2021, 37 (4), 447–468. 10.1080/87559129.2019.1709203. [DOI] [Google Scholar]
- Soquetta M. B.; Terra L. D. M.; Bastos C. P. Green technologies for the extraction of bioactive compounds in fruits and vegetables. CyTA-J. Food 2018, 16 (1), 400–412. 10.1080/19476337.2017.1411978. [DOI] [Google Scholar]
- Torres-Valenzuela L. S.; Ballesteros-Gómez A.; Rubio S. Green solvents for the extraction of high added-value compounds from agri-food waste. Food Eng. Rev. 2020, 12 (1), 83–100. 10.1007/s12393-019-09206-y. [DOI] [Google Scholar]
- Galanakis C. M. Recovery of high added-value components from food wastes: Conventional; emerging technologies and commercialized applications. Trends Food Sci. Technol. 2012, 26 (2), 68–87. 10.1016/j.tifs.2012.03.003. [DOI] [Google Scholar]
- Zia S.; Khan M. R.; Shabbir M. A.; Aslam Maan A.; Khan M. K. I.; Nadeem M.; Khalil A. A.; Din A.; Aadil R. M. An inclusive overview of advanced thermal and nonthermal extraction techniques for bioactive compounds in food and food-related matrices. Food Rev. Int. 2020, 1–31. 10.1080/87559129.2020.1772283. [DOI] [Google Scholar]
- Jablonský M.; Škulcová A.; Malvis A.; Šima J. Extraction of value-added components from food industry based and agro-forest biowastes by deep eutectic solvents. J. Biotechnol. 2018, 282, 46–66. 10.1016/j.jbiotec.2018.06.349. [DOI] [PubMed] [Google Scholar]
- Florindo C.; Oliveira M. M.; Branco L. C.; Marrucho I. M. Carbohydrates-based deep eutectic solvents: thermophysical properties and rice straw dissolution. J. Mol. Liq. 2017, 247, 441–447. 10.1016/j.molliq.2017.09.026. [DOI] [Google Scholar]
- de los Ángeles Fernández M.; Espino M.; Gomez F. J.; Silva M. F. Novel approaches mediated by tailor-made green solvents for the extraction of phenolic compounds from agro-food industrial by-products. Food Chem. 2018, 239, 671–678. 10.1016/j.foodchem.2017.06.150. [DOI] [PubMed] [Google Scholar]
- Carbone K.; Amoriello T.; Iadecola R. Exploitation of kiwi juice pomace for the recovery of natural antioxidants through microwave-assisted extraction. Agriculture 2020, 10 (10), 435. 10.3390/agriculture10100435. [DOI] [Google Scholar]
- Cardullo N.; Leanza M.; Muccilli V.; Tringali C. Valorization of Agri-Food Waste from Pistachio Hard Shells: Extraction of Polyphenols as Natural Antioxidants. Resources 2021, 10 (5), 45. 10.3390/resources10050045. [DOI] [Google Scholar]
- Yilmaz T.; Kumcuoglu S.; Tavman S. Ultrasound-assisted extraction of lycopene and [Beta]-carotene from tomato processing wastes. Italian J. Food Sci. 2017, 29 (1), 186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Civan M.; Kumcuoglu S. Green ultrasound-assisted extraction of carotenoid and capsaicinoid from the pulp of hot pepper paste based on the bio-refinery concept. LWT-Food Sci. Technol. 2019, 113, 108320. 10.1016/j.lwt.2019.108320. [DOI] [Google Scholar]
- Battistella Lasta H. F.; Lentz L.; Goncalves Rodrigues L. G.; Mezzomo N.; Vitali L.; Salvador Ferreira S. R. Pressurized liquid extraction applied for the recovery of phenolic compounds from beetroot waste. Biocatal. Agric. Biotechnol. 2019, 21, 101353. 10.1016/j.bcab.2019.101353. [DOI] [Google Scholar]
- da Rocha C. B.; Noreña C. P. Z.. Microwave-assisted extraction and ultrasound-assisted extraction of bioactive compounds from grape pomace. Int. J. Food Eng. 2020, 16( (1–2), ). 10.1515/ijfe-2019-0191 [DOI] [Google Scholar]
- Görgüç A.; Bircan C.; Yılmaz F. M. Sesame bran as an unexploited by-product: Effect of enzyme and ultrasound-assisted extraction on the recovery of protein and antioxidant compounds. Food Chem. 2019, 283, 637–645. 10.1016/j.foodchem.2019.01.077. [DOI] [PubMed] [Google Scholar]
- Tello J.; Viguera M.; Calvo L. Extraction of caffeine from Robusta coffee (Coffea canephora var. Robusta) husks using supercritical carbon dioxide. J. Supercritical Fluids 2011, 59, 53–60. 10.1016/j.supflu.2011.07.018. [DOI] [Google Scholar]
- Wang H.; Chen K.; Cheng J.; Jiang L.; Yu D.; Dai Y.; Wang L. Ultrasound-assisted three phase partitioning for simultaneous extraction of oil; protein and polysaccharide from pumpkin seeds. LWT 2021, 151, 112200. 10.1016/j.lwt.2021.112200. [DOI] [Google Scholar]
- Chia S. L.; Boo H. C.; Muhamad K.; Sulaiman R.; Umanan F.; Chong G. H. Effect of subcritical carbon dioxide extraction and bran stabilization methods on rice bran oil. J. Am. Oil Chem. Soc. 2015, 92 (3), 393–402. 10.1007/s11746-015-2596-5. [DOI] [Google Scholar]
- Wang J.; Sun B.; Liu Y.; Zhang H. Optimisation of ultrasound-assisted enzymatic extraction of arabinoxylan from wheat bran. Food Chem. 2014, 150, 482–488. 10.1016/j.foodchem.2013.10.121. [DOI] [PubMed] [Google Scholar]
- Hernández-Carmona F.; Morales-Matos Y.; Lambis-Miranda H.; Pasqualino J. Starch extraction potential from plantain peel wastes. J. Environ. Chem. Eng. 2017, 5 (5), 4980–4985. 10.1016/j.jece.2017.09.034. [DOI] [Google Scholar]
- Petkowicz C. L.; Williams P. A. Pectins from food waste: Characterization and functional properties of a pectin extracted from broccoli stalk. Food Hydrocolloids 2020, 107, 105930. 10.1016/j.foodhyd.2020.105930. [DOI] [Google Scholar]
- Hartati I.; Riwayati I.; Subekti E.. Microwave assisted extraction of watermelon rind pectin with different kind of acid solution. In Proceedings of the International Conference on Engineering Technology and Industrial Application, 1st ICETIA 2014; Publikasi Ilmiah, 2014, pp 27–30. [Google Scholar]
- Castillo-Israel K. A. T.; Baguio S. F.; Diasanta M. D. B.; Lizardo R. C. M.; Dizon E. I.; Mejico M. I. F.. Extraction and characterization of pectin from Saba banana [Musa’saba’(Musa acuminata x Musa balbisiana)] peel wastes: A preliminary study. Int. Food Res. J. 2015, 22 ( (1), ). [Google Scholar]
- Benassi L.; Alessandri I.; Vassalini I. Assessing Green Methods for Pectin Extraction from Waste Orange Peels. Molecules 2021, 26 (6), 1766. 10.3390/molecules26061766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cravotto G.; Bicchi C.; Mantegna S.; Binello A.; Tomao V.; Chemat F. Extraction of kiwi seed oil: Soxhlet versus four different non-conventional techniques. Natural Prod. Res. 2011, 25 (10), 974–981. 10.1080/14786419.2010.524162. [DOI] [PubMed] [Google Scholar]
- Martínez-Abad A.; Ramos M.; Hamzaoui M.; Kohnen S.; Jiménez A.; Garrigós M. C. Optimisation of sequential microwave-assisted extraction of essential oil and pigment from lemon peels waste. Foods 2020, 9 (10), 1493. 10.3390/foods9101493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopresto C. G.; Petrillo F.; Casazza A. A.; Aliakbarian B.; Perego P.; Calabrò V. A non-conventional method to extract D-limonene from waste lemon peels and comparison with traditional Soxhlet extraction. Sep. Purif. Technol. 2014, 137, 13–20. 10.1016/j.seppur.2014.09.015. [DOI] [Google Scholar]
- Bustamante J.; van Stempvoort S.; García-Gallarreta M.; Houghton J. A.; Briers H. K.; Budarin V. L.; Matharu A. S.; Clark J. H. Microwave assisted hydro-distillation of essential oils from wet citrus peel waste. J. Cleaner Prod. 2016, 137, 598–605. 10.1016/j.jclepro.2016.07.108. [DOI] [Google Scholar]
- McClements D. J.; Öztürk B.. Utilization of Nanotechnology to Improve the Application and Bioavailability of Phytochemicals Derived from Waste Streams. J. Agric. Food Chem. 2021. 10.1021/acs.jafc.1c03020 [DOI] [PubMed] [Google Scholar]
- Valdés García A.; Garrigós Selva M. D. C.. Microencapsulation of Natural Antioxidant Compounds Obtained from Biomass Wastes: A Review. In Materials Science Forum, Vol. 875; Trans Tech Publications, Ltd., 2016; pp 112–126. [Google Scholar]
- Thakur J.; Borah A. Microcapsules of bioactive compounds from fruits and vegetables waste and their utilization: A review. Pharma Innovation 2021, 10 (5), 151–157. 10.22271/tpi.2021.v10.i5c.6191. [DOI] [Google Scholar]
- Zhu Z.; Gavahian M.; Barba F. J.; Roselló-Soto E.; Kovačević D. B.; Putnik P.; Denoya G. I.. Valorization of waste and by-products from food industries through the use of innovative technologies. In Agri-Food Industry Strategies for Healthy Diets and Sustainability ;Academic Press; New York, 2020; pp 249–266. [Google Scholar]
- Velderrain-Rodríguez G. R.; Acevedo-Fani A.; González-Aguilar G. A.; Martín-Belloso O. Encapsulation and stability of a phenolic-rich extract from mango peel within water-in-oil-in-water emulsions. J. Functional Foods 2019, 56, 65–73. 10.1016/j.jff.2019.02.045. [DOI] [Google Scholar]
- Nemli E.; Ozakdogan S.; Tomas M.; McClements D. J.; Capanoglu E. Increasing the Bioaccessibility of Antioxidants in Tomato Pomace Using Excipient Emulsions. Food Biophys. 2021, 16, 355. 10.1007/s11483-021-09674-y. [DOI] [Google Scholar]
- Šeregelj V.; Ćetković G.; Čanadanović-Brunet J.; Šaponjac V. T.; Vulić J.; Lević S.; Nedović V.; Brandolini A.; Hidalgo A. Encapsulation of carrot waste extract by freeze and spray drying techniques: An optimization study. LWT 2021, 138, 110696. 10.1016/j.lwt.2020.110696. [DOI] [Google Scholar]
- Horuz T. İ.; Belibaǧlı K. B. Nanoencapsulation of carotenoids extracted from tomato peels into zein fibers by electrospinning. J. Sci. Food Agric. 2019, 99 (2), 759–766. 10.1002/jsfa.9244. [DOI] [PubMed] [Google Scholar]
- Vulić J.; Šeregelj V.; Kalušević A.; Lević S.; Nedović V.; Tumbas Šaponjac V.; Čanadanović-Brunet J.; Ćetković G. Bioavailability and bioactivity of encapsulated phenolics and carotenoids isolated from red pepper waste. Molecules 2019, 24 (15), 2837. 10.3390/molecules24152837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moschona A.; Liakopoulou-Kyriakides M. Encapsulation of biological active phenolic compounds extracted from wine wastes in alginate-chitosan microbeads. J. Microencapsulation 2018, 35 (3), 229–240. 10.1080/02652048.2018.1462415. [DOI] [PubMed] [Google Scholar]
- Pashazadeh H.; Zannou O.; Ghellam M.; Koca I.; Galanakis C. M.; Aldawoud T. M. S. Optimization and Encapsulation of Phenolic Compounds Extracted from Maize Waste by Freeze-Drying; Spray-Drying; and Microwave-Drying Using Maltodextrin. Foods 2021, 10, 1396. 10.3390/foods10061396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzatsi P.; Goula A. M. Encapsulation of Extract from Unused Chokeberries by Spray Drying; Co-crystallization; and Ionic Gelation. Waste Biomass Valor. 2021, 12, 4567. 10.1007/s12649-020-01316-7. [DOI] [Google Scholar]
- Gaber Ahmed G. H.; Fernandez-Gonzalez A.; Diaz Garcia M. E. Nano-encapsulation of grape and apple pomace phenolic extract in chitosan and soy protein via nanoemulsification. Food Hydrocolloids 2020, 108, 105806. 10.1016/j.foodhyd.2020.105806. [DOI] [Google Scholar]
- Šeregelj V.; Ćetković G.; Čanadanović-Brunet J.; Šaponjac V. T.; Vulić J.; Stajčić S. Encapsulation and degradation kinetics of bioactive compounds from sweet potato peel during storage. Food Technol. Biotechnol. 2020, 58 (3), 314. 10.17113/ftb.58.03.20.6557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Azevedo Bittencourt L. L.; Silva K. A.; de Sousa V. P.; Fontes-Sant’Ana G. C.; Rocha-Leão M. H. Blueberry residue encapsulation by ionotropic gelation. Plant Foods Human Nutr. 2018, 73 (4), 278–286. 10.1007/s11130-018-0685-y. [DOI] [PubMed] [Google Scholar]
- Papoutsis K.; Golding J. B.; Vuong Q.; Pristijono P.; Stathopoulos C. E.; Scarlett C. J.; Bowyer M. Encapsulation of citrus by-product extracts by spray-drying and freeze-drying using combinations of maltodextrin with soybean protein and ι-Carrageenan. Foods 2018, 7 (7), 115. 10.3390/foods7070115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tumbas Šaponjac V.; Čanadanović-Brunet J.; Ćetković G.; Jakišić M.; Djilas S.; Vulić J.; Stajčić S. Encapsulation of beetroot pomace extract: RSM optimization; storage and gastrointestinal stability. Molecules 2016, 21 (5), 584. 10.3390/molecules21050584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres-León C.; Chávez-González M. L.; Hernández-Almanza A.; Martínez-Medina G. A.; Ramírez-Guzmán N.; Londoño-Hernández L.; Aguilar C. N. Recent advances on the microbiological and enzymatic processing for conversion of food wastes to valuable bioproducts. Curr. Opin. Food Sci. 2021, 38, 40–45. 10.1016/j.cofs.2020.11.002. [DOI] [Google Scholar]
- Sadh P. K.; Kumar S.; Chawla P.; Duhan J. S. Fermentation: a boon for production of bioactive compounds by processing of food industries wastes (by-products). Molecules 2018, 23 (10), 2560. 10.3390/molecules23102560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadj Saadoun J.; Bertani G.; Levante A.; Vezzosi F.; Ricci A.; Bernini V.; Lazzi C. Fermentation of agri-food waste: A promising route for the production of aroma compounds. Foods 2021, 10 (4), 707. 10.3390/foods10040707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martí-Quijal F. J.; Khubber S.; Remize F.; Tomasevic I.; Roselló-Soto E.; Barba F. J. Obtaining antioxidants and natural preservatives from food by-products through fermentation: A review. Fermentation 2021, 7 (3), 106. 10.3390/fermentation7030106. [DOI] [Google Scholar]
- Sabater C.; Ruiz L.; Delgado S.; Ruas-Madiedo P.; Margolles A. Valorization of Vegetable Food Waste and By-Products Through Fermentation Processes. Front. Microbiol. 2020, 11, 2604. 10.3389/fmicb.2020.581997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martins I. M.; Roberto B. S.; Blumberg J. B.; Chen C. Y. O.; Macedo G. A. Enzymatic biotransformation of polyphenolics increases antioxidant activity of red and white grape pomace. Food Res. Int. 2016, 89, 533–539. 10.1016/j.foodres.2016.09.009. [DOI] [PubMed] [Google Scholar]
- Torres-León C.; Ramírez-Guzmán N.; Ascacio-Valdes J.; Serna-Cock L.; dos Santos Correia M. T.; Contreras-Esquivel J. C.; Aguilar C. N. Solid-state fermentation with Aspergillus niger to enhance the phenolic contents and antioxidative activity of Mexican mango seed: A promising source of natural antioxidants. LWT 2019, 112, 108236. 10.1016/j.lwt.2019.06.003. [DOI] [Google Scholar]
- Dulf F. V.; Vodnar D. C.; Dulf E. H.; Pintea A. Phenolic compounds; flavonoids; lipids and antioxidant potential of apricot (Prunus armeniaca L.) pomace fermented by two filamentous fungal strains in solid state system. Chem. Central J. 2017, 11 (1), 1–10. 10.1186/s13065-017-0323-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dulf F. V.; Vodnar D. C.; Socaciu C. Effects of solid-state fermentation with two filamentous fungi on the total phenolic contents; flavonoids; antioxidant activities and lipid fractions of plum fruit (Prunus domestica L.) by-products. Food Chem. 2016, 209, 27–36. 10.1016/j.foodchem.2016.04.016. [DOI] [PubMed] [Google Scholar]
- Rochín-Medina J. J.; Ramírez K.; Rangel-Peraza J. G.; Bustos-Terrones Y. A. Increase of content and bioactivity of total phenolic compounds from spent coffee grounds through solid state fermentation by Bacillus clausii. J. Food Sci. Technol. 2018, 55 (3), 915–923. 10.1007/s13197-017-2998-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramírez K.; Pineda-Hidalgo K. V.; Rochín-Medina J. J. Fermentation of spent coffee grounds by Bacillus clausii induces release of potentially bioactive peptides. LWT 2021, 138, 110685. 10.1016/j.lwt.2020.110685. [DOI] [Google Scholar]
- Mechmeche M.; Kachouri F.; Ksontini H.; Hamdi M. Production of bioactive peptides from tomato seed isolate by Lactobacillus plantarum fermentation and enhancement of antioxidant activity. Food Biotechnol. 2017, 31 (2), 94–113. 10.1080/08905436.2017.1302888. [DOI] [Google Scholar]
- Sadh P. K.; Chawla P.; Duhan J. S. Fermentation approach on phenolic; antioxidants and functional properties of peanut press cake. Food Bioscience 2018, 22, 113–120. 10.1016/j.fbio.2018.01.011. [DOI] [Google Scholar]
- Braga A. R. C.; de Souza Mesquita L. M.; Martins P. L. G.; Habu S.; de Rosso V. V. Lactobacillus fermentation of jussara pulp leads to the enzymatic conversion of anthocyanins increasing antioxidant activity. J. Food Compos. Anal. 2018, 69, 162–170. 10.1016/j.jfca.2017.12.030. [DOI] [Google Scholar]
- Schmidt C. G.; Gonçalves L. M.; Prietto L.; Hackbart H. S.; Furlong E. B. Antioxidant activity and enzyme inhibition of phenolic acids from fermented rice bran with fungus Rizhopus oryzae. Food Chem. 2014, 146, 371–377. 10.1016/j.foodchem.2013.09.101. [DOI] [PubMed] [Google Scholar]
- Gowe C. Review on potential use of fruit and vegetables by-products as a valuable source of natural food additives. Food Sci. Qual. Manage. 2015, 45, 47–61. [Google Scholar]
- Torres-León C.; Ramírez-Guzman N.; Londoño-Hernandez L.; Martinez-Medina G. A.; Díaz-Herrera R.; Navarro-Macias V.; Alvarez-Pérez O. B.; Picazo B.; Villarreal-Vázquez M.; Ascacio-Valdes J.; Aguilar C. N. Food waste and byproducts: An opportunity to minimize malnutrition and hunger in developing countries. Front. Sustain. Food Syst. 2018, 2, 52. 10.3389/fsufs.2018.00052. [DOI] [Google Scholar]
- de Almeida P. F.; de Araújo M. G. O.; Santana J. C. C. Collagen extraction from chicken feet for jelly production. Acta Sci. Technol. 2012, 34 (3), 345–351. 10.4025/actascitechnol.v34i3.10602. [DOI] [Google Scholar]
- Curutchet A.; Cozzano S.; Tárrega A.; Arcia P. Blueberry pomace as a source of antioxidant fibre in cookies: Consumer’s expectations and critical attributes for developing a new product. Food Sci. Technol. Int. 2019, 25 (8), 642–648. 10.1177/1082013219853489. [DOI] [PubMed] [Google Scholar]
- Garcia-Garcia G.; Stone J.; Rahimifard S. Opportunities for waste valorisation in the food industry – A case study with four UK food manufacturers. J. Cleaner Prod. 2019, 211, 1339–1356. 10.1016/j.jclepro.2018.11.269. [DOI] [Google Scholar]
- Commonwealth Scientific and Industrial Research Organization . Converting food waste into nutritious ingredients URL (https://www.csiro.au/en/research/production/food/converting-food-waste-into-nutritious-ingredients) (30.10.2021) https://www.csiro.au/en/research/production/food/converting-food-waste-into-nutritious-ingredients.
- Šeregelj V.; Tumbas Šaponjac V.; Lević S.; Kalušević A.; Ćetković G.; Čanadanović-Brunet J.; Nedović V.; Stajčić S.; Vulić J.; Vidaković A. Application of encapsulated natural bioactive compounds from red pepper waste in yogurt. J. Microencapsulation 2019, 36 (8), 704–714. 10.1080/02652048.2019.1668488. [DOI] [PubMed] [Google Scholar]
- Dordoni R.; Duserm Garrido G.; Marinoni L.; Torri L.; Piochi M.; Spigno G. Enrichment of whole wheat cocoa biscuits with encapsulated grape skin extract. Int. J. Food Sci. 2019, 2019, 1–11. 10.1155/2019/9161840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flamminii F.; Di Mattia C. D.; Sacchetti G.; Neri L.; Mastrocola D.; Pittia P. Physical and sensory properties of mayonnaise enriched with encapsulated olive leaf phenolic extracts. Foods 2020, 9 (8), 997. 10.3390/foods9080997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gültekin-Özgüven M.; Karadaǧ A.; Duman Ş.; Özkal B.; Özçelik B. Fortification of dark chocolate with spray dried black mulberry (Morus nigra) waste extract encapsulated in chitosan-coated liposomes and bioaccessability studies. Food Chem. 2016, 201, 205–212. 10.1016/j.foodchem.2016.01.091. [DOI] [PubMed] [Google Scholar]
- Gawlik-Dziki U.; Świeca M.; Dziki D.; Baraniak B.; Tomiło J.; Czyż J. Quality and antioxidant properties of breads enriched with dry onion (Allium cepa L.) skin. Food Chem. 2013, 138 (2–3), 1621–1628. 10.1016/j.foodchem.2012.09.151. [DOI] [PubMed] [Google Scholar]
- Plazzotta S.; Sillani S.; Manzocco L. Exploitation of lettuce waste flour to increase bread functionality: effect on physical; nutritional; sensory properties and on consumer response. Int. J. Food Sci. Technol. 2018, 53 (10), 2290–2297. 10.1111/ijfs.13820. [DOI] [Google Scholar]
- Padalino L.; D’Antuono I.; Durante M.; Conte A.; Cardinali A.; Linsalata V.; Mita g.; Logrieco A. F.; Del Nobile M. A. Use of olive oil industrial by-product for pasta enrichment. Antioxidants 2018, 7 (4), 59. 10.3390/antiox7040059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohtarami F. Effect of carrot pomace powder and Dushab (traditional grape juice concentrate) on the physical and sensory properties of cakes: a combined mixtures design approach. Curr. Nutr. Food Sci. 2019, 15 (6), 572–582. 10.2174/1573401314666180525111901. [DOI] [Google Scholar]
- Nascimento E. D. A.; Melo E. D. A.; Lima V. L. A. G. D. Ice cream with functional potential added grape agro-industrial waste. J. Culinary Sci. Technol. 2018, 16 (2), 128–148. 10.1080/15428052.2017.1363107. [DOI] [Google Scholar]
- Wang X.; Kristo E.; LaPointe G. Adding apple pomace as a functional ingredient in stirred-type yogurt and yogurt drinks. Food Hydrocolloids 2020, 100, 105453. 10.1016/j.foodhyd.2019.105453. [DOI] [Google Scholar]
- Patil S.; Pimpley V.; Warudkar K.; Murthy P. S. Valorisation of Coffee Pulp for Development of Innovative Probiotic Beverage Using Kefir: Physicochemical; Antioxidant; Sensory Analysis and Shelf Life Studies. Waste Biomass Valor. 2022, 13, 905–916. 10.1007/s12649-021-01554-3. [DOI] [Google Scholar]