Abstract
BACKGROUND
Cranioplasties are routinely performed to restore cosmesis and to protect intracranial contents after trauma, resection of tumors, or other pathologies. Traditionally done as a second-stage procedure, new single-stage cranioplasty protocols have been developed to minimize recovery periods, decrease complications, and improve patient satisfaction. These protocols, however, still require the use of larger than planned implants or use larger than ideal incisions to accommodate three-dimensional (3D) templates, which may not be optimal in regions with complex bony anatomy.
OBSERVATIONS
A 50-year-old woman with a painful and progressively enlarging hemangioma of the left frontal bone underwent a single-stage resection followed by custom cranioplasty using a new extended reality (XR)-based workflow. Excellent cosmetic results, decreased operative time, and a feasible workflow were achieved.
LESSONS
The use of an XR-based visualization platform allows the surgeon to treat lesions and perform custom cranioplasties in one session while avoiding common pitfalls of current single-stage workflows, such as increased operative times for tailoring implants, as well as minimizing the use of 3D overlay models, which may not appropriately conform to complex regional bony anatomy intraoperatively.
Keywords: virtual reality, augmented reality, extended reality, cranioplasty, hemangioma
ABBREVIATIONS : 3D = three-dimensional, AR = augmented reality, CAD/CAM = computer-assisted design/manufacturing, CT = computed tomography, PEEK = polyetheretherketone, VR = virtual reality, XR = extended reality
Cranioplasty after either a craniectomy or craniotomy is performed to repair the resulting defect and to restore mechanical protection to the brain, recover the original cranial aesthetics, and reverse any dysfunction associated with the defect.1 Usually, it is the second stage of a two-part procedure, the first of which is to treat the primary medical condition. Most often, this primary condition involves massive brain swelling, and for these patients, the cranioplasty frequently involves an autologous bone flap. However, when the primary condition relates to an intraosseous lesion that has to be resected, the cranioplasty uses either a titanium mesh, a synthetic bone graft, or a customized plate from various bonelike biomaterials, such as methyl methacrylate, hydroxyapatite, ceramics, and polyetheretherketone (PEEK), to replace the removed bone.2,3 The customized options provide a better “fit” but require a significant period between excision and cranioplasty, when the implant is designed using data from volumetric computed tomography (CT) and computer-assisted design/computer-assisted manufacturing (CAD/CAM) technology and is subsequently manufactured. During this period, in addition to the cosmetic “deficit” of missing a piece of one’s skull, the patient is at risk for brain injuries,4 and delayed cranioplasties have been shown to correlate with frequent complication rates approaching 35% to 40%.5,6
Since mid-2010, single-stage cranioplasty methods have been implemented for these cases.7 Custom implants can be prefabricated and used for cranioplasty immediately after lesion resection. This single-stage technique allows customizing the implant to achieve the ideal size, contour, and appearance. The technique involves manufacturing a “resection template” together with the eventual implant, and as long as the template can fit on the skull during surgery and allow the surgeon to excise the lesion area exactly like the template, this cranioplasty technique has been shown to result in improved cosmesis as well as decreased operative time and enhanced patient satisfaction.8,9
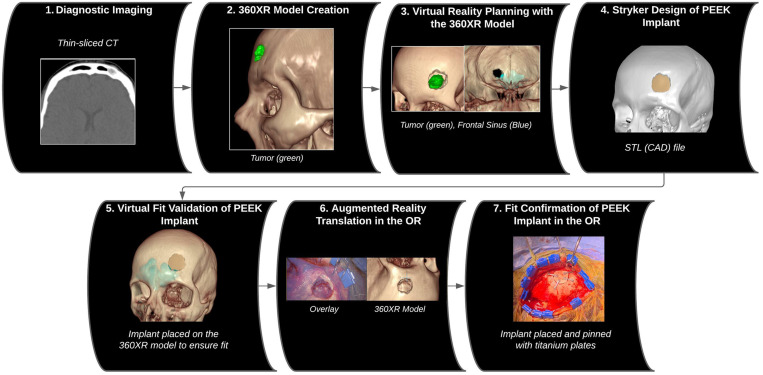
Here, we present a novel single-stage cranioplasty technique using an extended reality (XR) visualization platform to facilitate precise implant customization and placement in a patient after resection of a frontal bone lesion. The XR platform involves virtual reality (VR) and augmented reality (AR) technologies. The benefits of VR for preoperative planning and AR for translating the virtual plan onto the patient and providing intraoperative guidance have been reported for various neurosurgical procedures.10–16 In this case report, a three-dimensional (3D) model was created from the patient’s diagnostic volumetric CT scan, and a preoperative craniectomy was performed in VR. This virtual craniectomy was saved as a template and then translated into a CAD/CAM file to serve as a blueprint for manufacturing the custom-made PEEK implant. After the custom PEEK implant was fabricated, AR technology was then used intraoperatively to overlay the virtual craniectomy template onto the patient’s real-time anatomy to guide the resection of the lesion. With this, the virtual craniectomy was duplicated during surgery precisely as planned, and the custom PEEK implant fit perfectly during cranioplasty. The workflow is summarized in Fig. 1.
FIG. 1.
Image series illustrating the workflow of the XR-based single-stage cranioplasty, from preoperative planning in VR with the 360-degree XR model to implementation of the preoperative plan using AR in the operating room (OR).
Illustrative Case
A 50-year-old woman presented with a painful and progressively enlarging nodule on her left frontal bone. Her head CT scan showed a 1.2 × 0.9 × 0.9–cm skull mass abutting the left frontal sinus (Fig. 2A). The surgical recommendation was for resection of the symptomatic and expanding lesion, followed by a customized PEEK implant to repair the bony deficit.
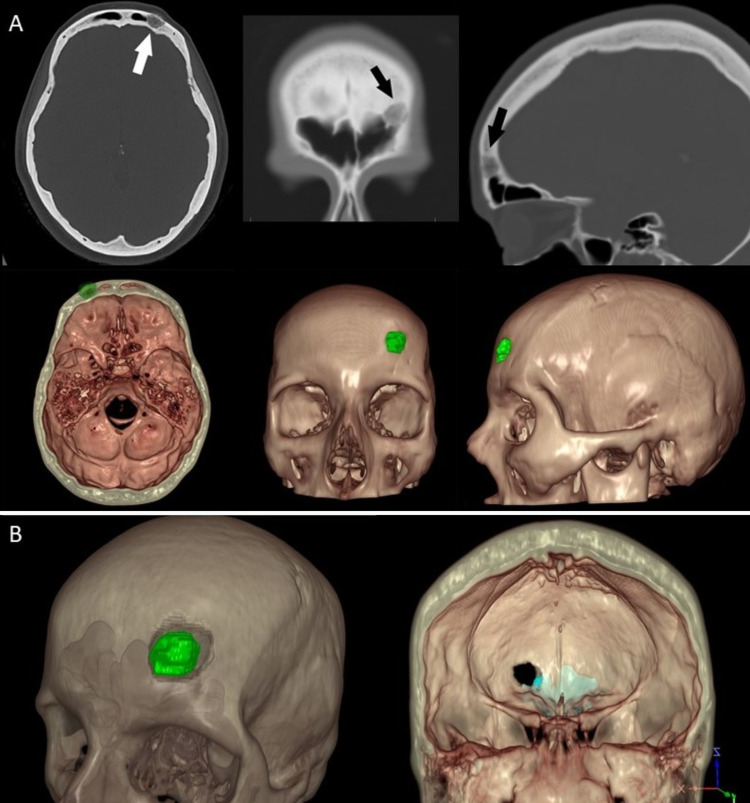
FIG. 2.
A: CT Digital Imaging and Communications in Medicine images of the skull mass in axial, coronal, and sagittal planes (arrows) and corresponding snapshots of the 3D virtual model of the CT scan. B: Different views of the 360-degree XR model: transparent virtual model displaying the tumor in green and the planned craniectomy (left), as well as a full-opacity virtual model displaying the frontal sinus (light blue) exposure of the planned craniectomy (right).
For this, a single-stage procedure was choreographed. The preoperative CT scan (0.47-mm slice thickness) was used to render a 360-degree model in the XR surgical planning platform (SRP version 7.9.0, Surgical Theater). The virtual model was adjusted to display the complete skull, frontal sinuses, and skull mass (Fig. 2). The XR model was used for surgical planning using a commercially available Oculus Rift S VR headset and controllers (Facebook Technologies). The patient-specific model was reviewed by the senior author at various angles and levels of transparency, and the XR platform’s virtual drilling tool was used to plan the craniectomy around the lesion (Fig. 2B). After determining that bone of the ideal shape and size was removed, the VR-modeled craniotomy was both exported in an STL file format (CAD) to be used as a blueprint for designing the PEEK implant (Stryker Corporation) (Fig. 3) and saved as the craniectomy template to be used later in surgery.
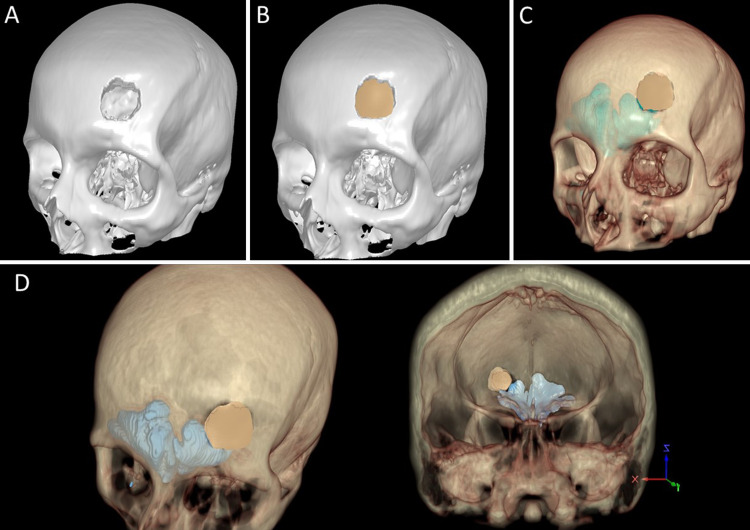
FIG. 3.
A: STL rendering of the skull to be exported as a 3D computer file. B: STL model of the customized PEEK implant (beige) onto the STL rendering of the skull (white). C: The STL model of the customized PEEK implant (beige) onto the virtual model. D: Additional views of 360-degree XR model with fitted PEEK implant.
Standard manufacturing procedures were followed in designing the implant using Geomagic Freeform 3D design software (3D Systems).8,17 A second STL file of only the PEEK implant was created, imported into the XR model, and used to verify fit (Fig. 3C). Implant fabrication was initiated once the surgeon approved the design.
In the clinic, the XR model was presented to the patient using a VR headset and virtual drilling tool to further explain the treatment plan. After the implant design was finalized, the patient was shown the XR model with the virtual customized PEEK implant during the final presurgical consultation.
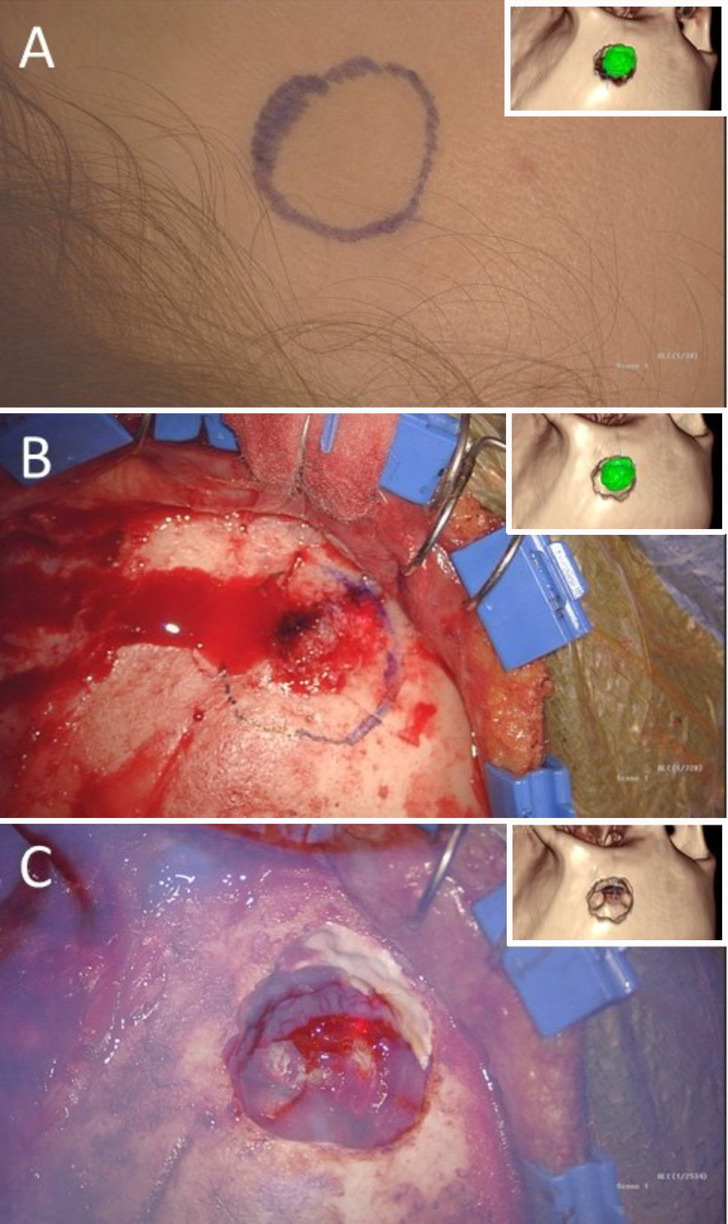
At surgery, after the anesthetized patient was secured in the pins, the craniectomy template was imported to Synchronize AR SNAP (SyncAR version 3.9.0, Surgical Theater), which was coregistered with a StealthStation S8 navigation system (Medtronic) and ARVeo microscope (Leica). The craniectomy template was projected through the navigation-tracked microscope as an AR overlay on the patient’s scalp (Fig. 4). After prepping and draping, a lateral linear incision was made behind the hairline, and the scalp flap was reflected anteriorly. A pericranial flap was harvested from the frontal bone for anticipated closure of the left frontal sinus. Once again, the AR template of the craniectomy was projected and, this time, traced onto the bone. Bone resection followed the contours of this tracing, and the tumor was resected. The exposed left frontal sinus was stripped of mucosa and closed with bone cement covered by the harvested vascularized pericranium. The custom PEEK implant was then placed into the cranial defect created by the craniectomy and attached to the skull using titanium miniplates and screws (Fig. 5). A few, 1-mm-wide gaps between the implant and the skull were filled with bone cement. No complications other than some pain in the temporalis, where muscle graft was taken from to fill the sinus, were noted. Pathologic analysis revealed an intramedullary hemangioma. At the 6-week postoperative follow-up, the forehead contour was perfect, and cosmetic results were excellent.
FIG. 4.
Microscopic images with corresponding view of the 360-degree XR model (insets) showing the translation of the planned craniectomy using the AR capabilities onto the patient’s scalp (A) and skull with a marker (B). AR overlay of the 360-degree XR model onto the patient (C) to confirm the craniectomy size and shape.
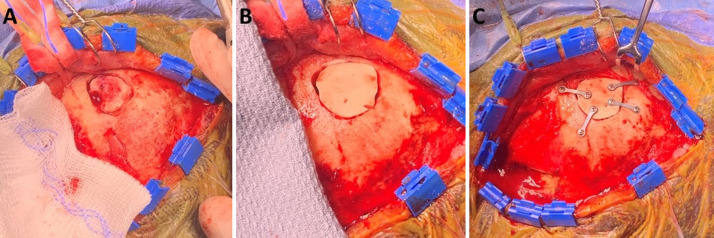
FIG. 5.
A–C: Images of the operative stages of the craniectomy drilling and PEEK implant placement of the single-stage cranioplasty.
Discussion
Observations
Currently, single-stage cranioplasty techniques use a preoperative thin-sliced CT scan to either render a virtual 3D reconstruction of the skull or generate a 3D printed model to plan the craniotomy.7,18,19 For the former technique with virtual 3D reconstructions, the craniotomy is estimated and used to create the CAD/CAM file of the custom PEEK implant design.7,19 This process requires the surgeon to review the craniotomy plan and provide feedback on the implant design. Once the craniotomy plan is approved, the custom implant may be manufactured with slightly bigger dimensions if desired. This allows room for unforeseen intraoperative modifications that would otherwise compromise an optimal fit between implant and recipient.19 For the 3D print model technique, the template for the craniectomy must be placed intraoperatively and physically secured to the skull to allow faithful duplication of the bony excision. In our case, this would have required a larger incision and exposure of the orbit, and, even with that, the template placement would not be secure, given the complex contour of the frontal bone near the sinus and orbit.
For our case, an alternative strategy was chosen, using a VR/AR platform for preoperative planning, implant verification, and intraoperative guidance of a single-stage cranioplasty immediately after an intraosseous mass resection. The patient-specific XR model that displayed the exact borders of the intraosseous tumor was used to preoperatively plan a virtual craniectomy in VR. The model allowed detailed visualization of the patient’s anatomy from any vantage point and transparency. The senior author spent approximately 5 minutes creating a virtual craniectomy in VR using the platform’s virtual drills and patient-specific VR model. The skull with the planned craniectomy was exported as a CAD/CAM file and used as a blueprint for PEEK implant design and fabrication. As a result, the exact size, location, and shape of the craniectomy were provided for designing the PEEK implant. The implant design was further verified by importing its file into the 360-degree XR model to ensure the implant fit the planned cranial defect.
Previous studies used neuronavigation guidance19–21 or an implant frame20,22 as a template to re-create the preoperative plan on the patient’s skull. Here, neuronavigation integrated with AR technology was used to provide the exact location and outline of the desired craniectomy. Because the PEEK implant was customized to fit the planned craniectomy, and AR facilitated the implementation of the plan, minimal intraoperative modification was required. This is a clear distinction from most previously published single-stage techniques that require manual modification of the implant to remove excess material for it to fit inside the bone defect. Manual modification of the implant also requires surgical expertise and can take 10 to 80 minutes to complete.19
One proof-of-concept study used a projector-based AR solution via a custom surgical workstation to guide the resizing of the implant.23 The implant was fixed with an optically tracked custom-made reference attachment, and the traced resection cut was overlaid on the implant for the surgeon to trace with a marking pen and used to modify the implant. Not including defect creation or implant fixation, total time for reference mounting, registration, defect tracing, implant tracing, and modification was approximately 15 minutes with 7.25 minutes allotted to modifying the implant. Although this technique demonstrates the use of AR for performing single-stage cranioplasty in a quick and efficient way, it only guides intraoperative implant modification. The technique presented in this report delivers a well-fitted custom implant that requires minimal modification. Furthermore, the AR overlay afforded by the seamless integration of SyncAR with surgical navigation and an operating microscope provides an interactive 3D overlay of the patient’s model with planned craniotomy directly on the patient. The AR overlay provided intraoperative guidance for craniectomy, tumor resection, and implant placement, all of which were planned and verified preoperatively.
Another observation from the cases found in the literature is that all intraosseous tumors resected were fairly large,7,19–22 with a mean dimension of 73.9 ± 24.8 mm × 69.2 ± 16.2 mm,18 and required large custom implants. In this case, we are reporting a much smaller tumor (12 mm × 9 mm) with its corresponding implant to showcase that even small lesions with a surgical indication for resection and reconstruction can be addressed using VR/AR workflows.
It is important to highlight limitations associated with the use of new VR/AR platforms. As with any new technologies, their implementation carries a high initial cost, which must be taken into account. These costs include the need to obtain high-resolution imaging in order to create each case, as well as the need to dedicate personnel and physical space to run and maintain these programs. However, as these platforms become popular, it is expected that the associated costs will decrease as the technology is refined and different platforms enter the market. This high upfront cost could potentially be offset when considering the diversity of its applications. As an example, the implementation of VR/AR has been found to be useful in planning safe surgical approaches for neuro-oncological, cerebrovascular, and spinal diseases as well as across other related specialties, such as maxillofacial and plastic surgery.11,24–27 If in the future these efforts translate to discrete reductions in complications, lengths of stay, surgical procedure times, or readmissions, then a justifiable return of investment could be proved to hospital and surgical practices alike.
Another limitation identified is the learning curve associated with adopting this technology. With time, these platforms have become more powerful, and therefore their complexity has increased as well. This translates to increased time and effort spent by surgical teams to get acquainted with the interfaces and what they can and cannot achieve with the current generation of VR/AR systems. To be able to fully control the tools and models in VR and to use the AR platform intraoperatively, surgeons need to be trained over several sessions when they acquire the technology. In addition, it is reasonable to expect an initial disruption in the usual surgical workflow as VR/AR is adopted by individual groups. As much as this represents an initial investment in effort to break the learning curve, we do not expect this to be a major limitation, because surgeons are often at the forefront of embracing new technologies that have the potential to improve their results and, in consequence, their patients’ outcomes.
In order to address the limitations associated with VR/AR use in cranial reconstruction, more studies are needed to properly evaluate objective patient outcomes as well as patients’ appraisal of the final reconstructive work. Only then will the full capabilities of VR/AR technologies be discovered and our specialty advanced further.
Lessons
Here, we present an efficient technique using XR technology for single-stage cranioplasty. This case provides evidence that VR and AR can be used to efficiently plan and execute, respectively, a cranioplasty immediately after a tumor resection procedure without the need for a second surgery. The use of this technique should be validated to verify its safety and efficacy for facilitating precise and efficient implant customization and placement in a larger and broader patient population for single-stage cranioplasties. Specifically, operative time, including setup and implant modification, should be evaluated.
Acknowledgments
We gratefully acknowledge Divya John for case building the 360-degree XR models and technical support. We also thank Kevin Cordero and Chris Noonan for assistance with the paper.
Disclosures
Ms. Barbery is a full-time employee of Surgical Theater. Dr. Dang is an employee of Surgical Theater. Dr. Jean reported personal fees from Surgical Theater LLC outside the submitted work. No other disclosures were reported.
Author Contributions
Conception and design: Jean. Acquisition of data: Jean. Analysis and interpretation of data: Jean. Drafting the article: Rios-Vicil, Barbery, Dang. Critically revising the article: all authors. Reviewed submitted version of manuscript: all authors. Approved the final version of the manuscript on behalf of all authors: Jean. Administrative/technical/material support: Rios-Vicil, Barbery, Dang.
References
- 1. Lethaus B, Safi Y, ter Laak-Poort M, et al. Cranioplasty with customized titanium and PEEK implants in a mechanical stress model. J Neurotrauma. 2012;29(6):1077–1083. doi: 10.1089/neu.2011.1794. [DOI] [PubMed] [Google Scholar]
- 2. Shah AM, Jung H, Skirboll S. Materials used in cranioplasty: a history and analysis. Neurosurg Focus. 2014;36(4):E19. doi: 10.3171/2014.2.FOCUS13561. [DOI] [PubMed] [Google Scholar]
- 3. Iaccarino C, Kolias AG, Roumy LG, Fountas K, Adeleye AO. Cranioplasty following decompressive craniectomy. Front Neurol. 2020;10:1357. doi: 10.3389/fneur.2019.01357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chang V, Hartzfeld P, Langlois M, Mahmood A, Seyfried D. Outcomes of cranial repair after craniectomy. J Neurosurg. 2010;112(5):1120–1124. doi: 10.3171/2009.6.JNS09133. [DOI] [PubMed] [Google Scholar]
- 5. Piedra MP, Nemecek AN, Ragel BT. Timing of cranioplasty after decompressive craniectomy for trauma. Surg Neurol Int. 2014;5:25. doi: 10.4103/2152-7806.127762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gooch MR, Gin GE, Kenning TJ, German JW. Complications of cranioplasty following decompressive craniectomy: analysis of 62 cases. Neurosurg Focus. 2009;26(6):E9. doi: 10.3171/2009.3.FOCUS0962. [DOI] [PubMed] [Google Scholar]
- 7. Barros A, Brauge D, Quéhan R, Cavallier Z, Roux FE, Moyse E. One-step customized PEEK cranioplasty after 3D printed resection template assisted surgery for a frontal intraosseous meningioma: a case report. Turk Neurosurg. 2021;31(1):142–147. doi: 10.5137/1019-5149.JTN.30192-20.2. [DOI] [PubMed] [Google Scholar]
- 8. Gordon CR, Fisher M, Liauw J, et al. Multidisciplinary approach for improved outcomes in secondary cranial reconstruction: introducing the pericranial-onlay cranioplasty technique. Neurosurgery. 2014;10(Suppl 2):179–190. doi: 10.1227/NEU.0000000000000296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cabraja M, Klein M, Lehmann TN. Long-term results following titanium cranioplasty of large skull defects. Neurosurg Focus. 2009;26(6):E10. doi: 10.3171/2009.3.FOCUS091. [DOI] [PubMed] [Google Scholar]
- 10. Louis RG, Steinberg GK, Duma C, et al. Early experience with virtual and synchronized augmented reality platform for preoperative planning and intraoperative navigation: a case series. Oper Neurosurg (Hagerstown) 2021;21(4):189–196. doi: 10.1093/ons/opab188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Steineke TC, Barbery D. Microsurgical clipping of middle cerebral artery aneurysms: preoperative planning using virtual reality to reduce procedure time. Neurosurg Focus. 2021;51(2):E12. doi: 10.3171/2021.5.FOCUS21238. [DOI] [PubMed] [Google Scholar]
- 12. Chugh AJ, Pace JR, Singer J, et al. Use of a surgical rehearsal platform and improvement in aneurysm clipping measures: results of a prospective, randomized trial. J Neurosurg. 2017;126(3):838–844. doi: 10.3171/2016.1.JNS152576. [DOI] [PubMed] [Google Scholar]
- 13. Jean WC. Mini-pterional craniotomy and extradural clinoidectomy for clinoid meningioma: optimization of exposure using augmented reality template: 2-dimensional operative video. Oper Neurosurg (Hagerstown) 2020;19(6):E610. doi: 10.1093/ons/opaa238. [DOI] [PubMed] [Google Scholar]
- 14. Wright JM, Raghavan A, Wright CH, et al. Back to the future: surgical rehearsal platform technology as a means to improve surgeon-patient alliance, patient satisfaction, and resident experience. J Neurosurg. 2020;135:384–391. doi: 10.3171/2020.6.JNS201865. [DOI] [PubMed] [Google Scholar]
- 15. Atli K, Selman W, Ray A. A comprehensive multicomponent neurosurgical course with use of virtual reality: modernizing the medical classroom. J Surg Educ. 2021;78(4):1350–1356. doi: 10.1016/j.jsurg.2020.11.003. [DOI] [PubMed] [Google Scholar]
- 16. Jean WC, Huang MC, Felbaum DR. Optimization of skull base exposure using navigation-integrated, virtual reality templates. J Clin Neurosci. 2020;80:125–130. doi: 10.1016/j.jocn.2020.08.018. [DOI] [PubMed] [Google Scholar]
- 17. Wolff A, Santiago GF, Belzberg M, et al. Adult cranioplasty reconstruction with customized cranial implants: preferred technique, timing, and biomaterials. J Craniofac Surg. 2018;29(4):887–894. doi: 10.1097/SCS.0000000000004385. [DOI] [PubMed] [Google Scholar]
- 18. Bianchi F, Signorelli F, Di Bonaventura R, Trevisi G, Pompucci A. One-stage frame-guided resection and reconstruction with PEEK custom-made prostheses for predominantly intraosseous meningiomas: technical notes and a case series. Neurosurg Rev. 2019;42(3):769–775. doi: 10.1007/s10143-019-01104-5. [DOI] [PubMed] [Google Scholar]
- 19. Berli JU, Thomaier L, Zhong S, et al. Immediate single-stage cranioplasty following calvarial resection for benign and malignant skull neoplasms using customized craniofacial implants. J Craniofac Surg. 2015;26(5):1456–1462. doi: 10.1097/SCS.0000000000001816. [DOI] [PubMed] [Google Scholar]
- 20. Broeckx CE, Maal TJJ, Vreeken RD, Bos RRM, Ter Laan M. Single-step resection of an intraosseous meningioma and cranial reconstruction: technical note. World Neurosurg. 2017;108:225–229. doi: 10.1016/j.wneu.2017.08.177. [DOI] [PubMed] [Google Scholar]
- 21. Marcus H, Schwindack C, Santarius T, Mannion R, Kirollos R. Image-guided resection of spheno-orbital skull-base meningiomas with predominant intraosseous component. Acta Neurochir (Wien) 2013;155(6):981–988. doi: 10.1007/s00701-013-1662-8. [DOI] [PubMed] [Google Scholar]
- 22. Carolus A, Weihe S, Schmieder K, Brenke C. One-step CAD/CAM titanium cranioplasty after drilling template-assisted resection of intraosseous skull base meningioma: technical note. Acta Neurochir (Wien) 2017;159(3):447–452. doi: 10.1007/s00701-016-3053-4. [DOI] [PubMed] [Google Scholar]
- 23. Murphy RJ, Wolfe KC, Liacouras PC, Grant GT, Gordon CR, Armand M. Computer-assisted single-stage cranioplasty. Annu Int Conf IEEE Eng Med Biol Soc. 2015;2015:4910–4913. doi: 10.1109/EMBC.2015.7319493. [DOI] [PubMed] [Google Scholar]
- 24. Luzzi S, Giotta Lucifero A, Martinelli A, et al. Supratentorial high-grade gliomas: maximal safe anatomical resection guided by augmented reality high-definition fiber tractography and fluorescein. Neurosurg Focus. 2021;51(2):E5. doi: 10.3171/2021.5.FOCUS21185. [DOI] [PubMed] [Google Scholar]
- 25. Yahanda AT, Moore E, Ray WZ, Pennicooke B, Jennings JW, Molina CA. First in-human report of the clinical accuracy of thoracolumbar percutaneous pedicle screw placement using augmented reality guidance. Neurosurg Focus. 2021;51(2):E10. doi: 10.3171/2021.5.FOCUS21217. [DOI] [PubMed] [Google Scholar]
- 26. Ayoub A, Pulijala Y. The application of virtual reality and augmented reality in oral & maxillofacial surgery. BMC Oral Health. 2019;19(1):238. doi: 10.1186/s12903-019-0937-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kim Y, Kim H, Kim YO. Virtual reality and augmented reality in plastic surgery: a review. Arch Plast Surg. 2017;44(3):179–187. doi: 10.5999/aps.2017.44.3.179. [DOI] [PMC free article] [PubMed] [Google Scholar]