Abstract
BACKGROUND
Ependymomas are the most frequent tumors of the adult spinal cord, representing 1.9% of all central nervous system tumors and 60% of spinal cord tumors. Spinal ependymomas are usually solitary, intramedullary lesions. While intradural extramedullary (IDEM) ependymomas are infrequent, multifocal IDEM ependymomas are exceptionally rare.
OBSERVATIONS
The authors reported the first case in the literature of a patient diagnosed with multifocal IDEM ependymomas who was treated with tumor resection and brain and spinal radiotherapy. The patient presented with a 10-day history of bilateral leg numbness extending to the umbilicus and gait instability. Magnetic resonance imaging (MRI) studies revealed multiple enhancing nodular nodules throughout the entire spinal canal. Brain MRI revealed no abnormal lesions. A World Health Organization grade II ependymoma was confirmed histologically. At 31 months postoperatively, the patient remained clinically asymptomatic. Although cervical and thoracic MRI revealed stable intradural nodules and several areas of leptomeningeal enhancement, no malignant cells were seen in the cerebrospinal fluid (CSF). He underwent genetic testing to determine the appropriate chemotherapeutic agent if activation of the tumor should arise.
LESSONS
Because complete resection of multifocal IDEM ependymomas is not feasible, continued monitoring with brain and spine MRI is warranted to detect potential tumor dissemination in the CSF.
Keywords: neurosurgery, ependymoma, multifocal, intradural, extramedullary
ABBREVIATIONS : ACE = angiotensin-converting enzyme, CSF = cerebrospinal fluid, IDEM = intradural extramedullary, MRI = magnetic resonance imaging, PI3K = phosphatidylinositol 3-kinase
Ependymomas are neuroectodermal tumors that arise from the ependymal lining of the ventricles and central canal of the spinal cord.1–4 Although ependymomas are more frequently located in the cranium (cranial/spinal tumor ratio 4:1), they are the most common intramedullary spinal cord neoplasms in adults and are usually solitary lesions.2,5–9 While intradural extramedullary (IDEM) ependymomas are uncommon, multifocal IDEM ependymomas are exceedingly rare. IDEM ependymomas may arise from the heterotopic ependymal cell rests that remained in the IDEM space when the neural tube closed.1,9–13 Invagination of the neuraxis into the extramedullary space has been proposed as a mechanism, as supported by tumor encapsulation of pia mater, presence of arachnoid membrane, and lack of a medullary connection.7,14 Another theory postulates that the intramedullary tumor extends to the extramedullary compartment due to the existence of a medullary connection. It has been suggested that multifocal IDEM ependymomas may be due to cerebrospinal fluid (CSF) dissemination resulting from aggressive tumor behavior or anaplastic transformation of originally benign lesions.14
We present the unique case of a patient with multifocal IDEM ependymomas who received resection followed by radiation of the brain and spine. We discuss the importance of genetic testing to decide the suitable chemotherapeutic agent that may be used as salvage therapy when surgery and radiotherapy are unsuccessful. The mechanism by which multifocal IDEM ependymomas develop and disseminate is also described.
Illustrative Case
History, Physical Examination, Radiological Imaging, and Laboratory Findings
A 45-year-old man (body mass index 32.92 kg/m2) reported a 10-day history of numbness of the lower extremities bilaterally that was described as a constant “prickling” sensation. The numbness initially extended from the bilateral knees distally and subsequently extended to the umbilicus. He also complained of gait instability. The patient attributed the numbness to buying new work boots. He had also sustained a fall from a ladder a few weeks prior to the initiation of his symptoms. On examination, the patient was able to feel pressure but no pain, vibration, or temperature sensation of the lower extremities bilaterally. Saddle anesthesia and numbness were noted in the inguinal region, and a sensory level was noted at T2. There was no weakness of the legs. The Achilles deep tendon reflex was absent on the right and 2+ on the left. The Babinski reflex was muted on the right and down on the left.
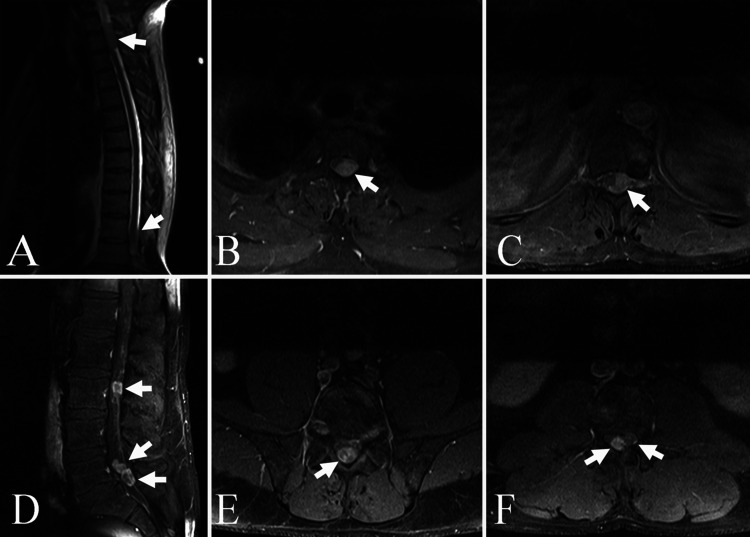
Thoracic magnetic resonance imaging (MRI) with and without gadolinium contrast demonstrated (1) a 1.2 × 1.5 × 3.5-cm enhancing mass filling the left side of the spinal canal extending from T1 to T3, resulting in marked displacement and compression of the spinal cord; (2) a 1.1 × 1.6 × 3-cm enhancing mass in the left side of the spinal canal at T11–12, causing moderate displacement and mild compression of the spinal cord; and (3) a 3.0-mm enhancing nodule within the posterior aspect of the spinal canal at T7 on the right (Fig. 1A–C). Lumbar MRI with and without gadolinium contrast revealed multiple enhancing nodular foci in the spinal canal: (1) an 8.0-mm enhancing nodule in the right posterior aspect of the spinal canal at the L3 level; (2) two 1.5-cm enhancing nodules filling most of the spinal canal at the L5–S1 level and extending superiorly to L5; and (3) a 6.0-mm enhancing nodule within the posterior aspect of the spinal canal on the left at T12 (Fig. 1D–F). Cervical MRI with and without gadolinium contrast showed the aforementioned IDEM mass from T1 to the T2–3 level as well as a 2.0- to 3.0-mm IDEM mass at the posterior right side of the cord at C7. Brain MRI with and without gadolinium contrast revealed no pathological lesions.
FIG. 1.
Gadolinium-enhanced thoracic T1-weighted MRI scans. A: Sagittal view demonstrating the T1–3 lesion (arrow). B: Axial view showing the left intradural extramedullary enhancing lesion (arrow) at T1–3 severely compressing and displacing the spinal cord to the right. C: Axial view of the tumor mass (arrow) at T11–12 displacing the spinal cord from left to right. Gadolinium-enhanced lumbar T1-weighted MRI scans. D: Sagittal view demonstrating the intradural mass at L3 (upper arrow) and two intradural deposits (lower two arrows) at L5–S1. E: Axial view of the intradural mass (arrow) at L3 in the midline. F: Axial view at L5–S1 demonstrating the two intradural lesions (arrows).
CSF findings included protein >3,000 (12 to 60 mg/dL) and angiotensin-converting enzyme (ACE) 11 U/L (0 to 2.5 U/L). Serum ACE was 23 (9 to 67 U/L). The germ cell markers beta human chorionic gonadotropin and alpha fetoprotein were both negative (<1 U/L [0 to 3 U/L] and 1 ng/mL [0 to 1 ng/mL], respectively).
Surgical Intervention
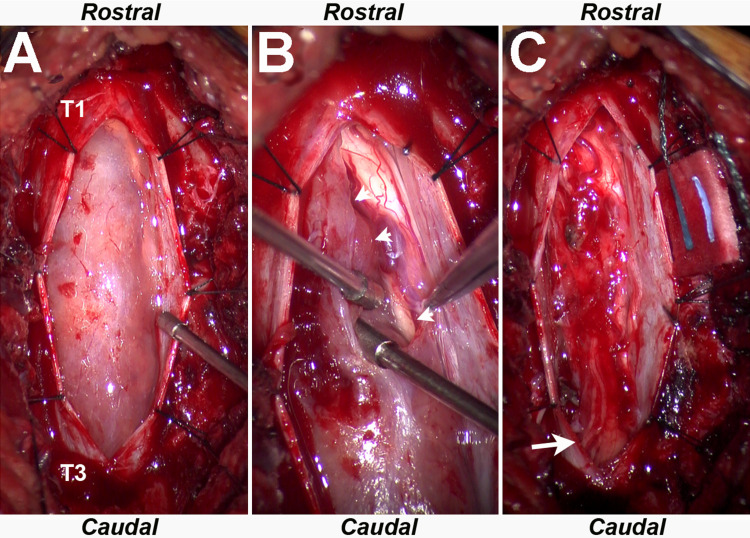
A laminectomy of T1 and T2 was performed with resection of the IDEM mass (Fig. 2). A plane was delineated between the lesion and the cord, and multiple nerve rootlets were observed between the mass and cord. The lesion was debulked, and the capsule was resected from the cord. A region of arachnoid membrane clearly distinct from and caudal to the tumor was biopsied because of its abnormal appearance.
FIG. 2.
A: Intraoperative microscopic image demonstrating a left eccentric IDEM ependymoma extending from T1 to T3. B: Intraoperative microscopic image demonstrating well-defined surgical plane (arrowheads) between the tumor capsule and spinal cord. C: Intraoperative microscopic image demonstrating resection and underlying previously compressed left-sided nerve rootlets and spinal cord (arrow).
Histopathological Findings
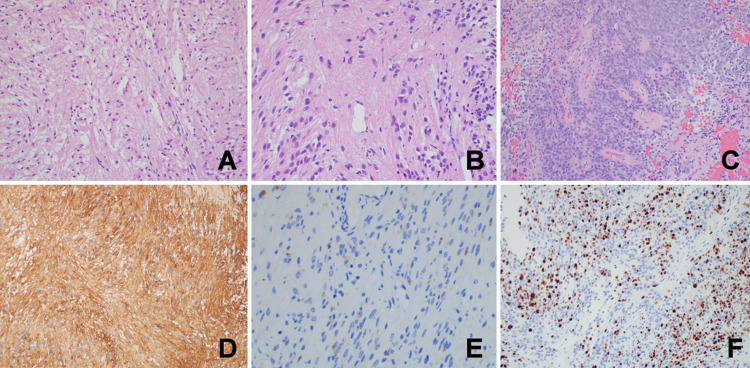
Large areas of this ependymal tumor demonstrated a tanycytic phenotype with spindled elongate cells and suggestions of perivascular pseudorosettes (Fig. 3A and B), whereas other locations were hypercellular with classic ependymal architecture (Fig. 3C). Mitotic activity was rare, there were areas of necrosis, and microvascular proliferation was not identified. Tumor cells were strongly and diffusely immunoreactive for glial fibrillary acidic protein (Fig. 3D), vimentin, and S-100. Epithelial membrane antigen labeled intracytoplasmic dot-like microlumina (Fig. 3E). The Ki-67 proliferation index was low overall, with labeling of up to 10% of tumor cell nuclei within the hypercellular nodules (Fig. 3F). The tumor showed MYCN copy number gain, and amplification of the HER-2 gene was not detected. Tumor cells were negative for somatostatin receptor 2A, SOX-10, STAT-6, and OLIG-2. A World Health Organization grade II ependymoma was confirmed. A separate biopsy site of arachnoid tissue also confirmed ependymoma infiltration.
FIG. 3.
Histopathological findings revealed tanycytic ependymal phenotype (A, hematoxylin and eosin [H&E], original magnification ×200), perivascular pseudorosette (B, H&E, original magnification ×400), ependymal architecture (C, H&E, original magnification ×200), glial fibrillary acidic protein (D, H&E, original magnification ×200), epithelial membrane antigen labeled intracytoplasmic dot-like microlumina (E, H&E, original magnification ×400), and Ki-67 in hypercellular nodules (F, H&E, original magnification ×200).
Adjuvant Therapy
The patient received intensity-modulated radiation therapy rapid arc to the brain as well as the entire spinal axis (total dose 3,060 cGy) in 17 fractions. He was also treated with an additional 5 fractions of focus radiotherapy to the areas of visible tumor (900 to 1,080 cGy). The patient did not receive chemotherapy.
Follow-Up
Within 2 weeks after surgical intervention, the patient attained significant improvement in both gait and sensation from T2 distally. At 18 months postoperatively, he reported mild cognitive decline presumably related to the cranial radiation. He denied any other neurological complaints. At 18 months after surgery, a brain MRI revealed no pathological masses. Cervical, thoracic, and lumbar MRI with and without gadolinium contrast demonstrated stable intradural nodules. Cervical and thoracic MRI showed new areas of leptomeningeal enhancement concerning for disease progression. CSF findings included protein 198 (12 to 60 mg/dL) with rare mononuclear cells and no malignant cells observed on microscopic examination. The elevated protein was attributed to the history of spinal surgery and stable spinal lesions.
The patient received genetic testing, which revealed a PIK3CA mutation (c. 1636C > A, p. Q546K, NM_006218, missense variant exon 9). PIK3CA encodes the catalytic subunit p100 alpha protein of the phosphatidylinositol 3-kinase (PI3K) enzyme.15 The p110 subunit is responsible for the enzyme’s phosphorylation activity and is involved in the PI3K-AKT-mTOR and the Ras-Raf-MEK-ERK pathways that mediate cellular growth and survival.16,17 Activating mutations, copy number gains, and overexpression of PIK3CA are associated with cancer progression. The PI3K pathway is one of the most frequently activated pathways in cancer.18 PI3Ks transduce signals from growth factors and cytokines, which results in the phosphorylation and activation of AKT, inducing changes in cell growth, proliferation, and apoptosis. In the analysis by Rogers and colleagues’ of the PI3K pathway using gene expression data and immunohistochemical analysis of phosphorylated AKT in pediatric ependymoma, they reported that the PI3K pathway could act as a biomarker to identify patients with a worse prognosis and those who could be treated with therapies targeted against the pathway.18 Butt and colleagues have also identified PIK3CA mutation patterns in a series of patients with ependymoma who received molecular profiling by next-generation sequencing.19
At 31 months postoperatively, the patient’s only complaints were increased fatigue and daytime somnolence. Cervical and thoracic MRI were stable without evidence of disease progression. The patient will continue to be monitored with brain and total spine MRI. If there is evidence of disease progression in the future, he may be a candidate for alpelisib off-label use due to his PI3CK mutation. He may also be enrolled in clinical trials or receive treatment with combination temozolomide and lapatinib for recurrent ependymoma.
Discussion
Several features commonly associated with IDEM ependymomas include diagnosis in the fifth decade, female predominance, thoracic spine location, encapsulated lesion without attachment to the central nervous system, and lack of other neoplastic processes within the brain or spinal cord.1,3,5,7,9–12,20 Patients often present with pain and progressive medullary compression. In the study by Iunes and colleagues of 19 patients with IDEM, the time elapsed from symptom onset to diagnosis ranged between 1 month and 8 years, with most patients diagnosed in less than 1 year.7 MRI is the diagnostic gold standard, with gadolinium-contrasted studies commonly demonstrating a homogenously enhanced, well-delineated tumor with a cystic, hemorrhagic, necrotic, and/or calcific component.12,21 Grade II (or “classic”) ependymomas comprise 55%–75% of spinal lesions and are characterized histologically by pseudorosettes (80%) and “true” or “ependymal” rosettes (10%).21 The tumor margins are usually well defined on gross and microscopic examination, with compression instead of invasion of the adjacent tissue. IDEM ependymomas often mimic schwannomas, meningiomas, or neuromas, which may delay their diagnosis.11–13
Although IDEM ependymomas are typically benign, they have been reported to recur, undergo anaplastic transformation, and seed in the CSF.3,4,6,9,12,21 Because IDEM ependymomas are usually solitary and isolated, complete resection is frequently performed and offers the best prognosis for a full recovery without recurrence. Adjuvant radiotherapy and chemotherapy remain controversial. However, the former has been shown to significantly improve overall survival, and the latter is usually reserved for salvage therapy if both surgery and radiotherapy fail.4,6,14,21
Only nine cases of multifocal IDEM ependymomas have been reported in the literature (Table 1).1–4,6,7,13,14 The mean age was 38 years (range, 26 to 53 years), and five (56%) patients were female. All patients had either neck and/or low back pain, paresthesia of the lower extremities, and/or gait abnormalities. MRI findings demonstrated IDEM ependymomas throughout the entire spine in most cases. Of the nine patients, two were grade I (myxopapillary), three were grade II, two were grade III (anaplastic), and one was a combination of grades II and III. All patients received at least one laminectomy, and six (67%) patients had adjuvant craniospinal or spinal radiotherapy. Only two (33%) patients were treated with chemotherapy, consisting of carboplatin in one case and temozolomide in one case. The follow-up duration ranged between 8 months and 10 years, with seven (78%) patients attaining excellent improvement of their symptoms after surgery, radiotherapy, and/or chemotherapy. One patient developed complete spinal cord syndrome 8 months after his second surgery and had an overall survival of 105 weeks. Another patient continued to experience sensory abnormalities of the legs 1 year postoperatively, which was attributed to postradiation myelopathy.
TABLE 1.
Multifocal IDEM ependymoma in the literature
| Authors & Year | Age (yrs)/ Sex | Presenting symptoms | Tumor Site/WHO Grade | Treatment | Outcome |
|---|---|---|---|---|---|
| Schuurmans et al., 20063 |
29/F |
Progressive neck pain, sensory deficit, & weakness in both arms |
C3–6, L4–S1/grade III |
C3–6 laminectomy, C4–6 corpectomies, lumbar laminectomy; RT entire spinal cord; 2 yrs later resection of tumor in Sylvian fissure |
Sensory abnormalities of legs 1 yr after spinal op, attributed to postradiation myelopathy |
| Vural et al., 20104 |
45/F |
Progressive neck & back pain, difficulty in ambulation |
4th ventricle-C2, T5–6, T8–9/grade II |
Suboccipital craniectomy & C1 laminectomy, T5–6 & T8–9 laminectomies; no adjuvant therapy |
Asymptomatic 3 yrs after op, although MRI showed multiple masses at T5, T7, T9, T12, L3, S1–2; will undergo op |
| Iunes et al., 20117 |
32/M |
Partial medullary syndrome |
Bulbomedullary junction, C2–3, T5–11, L2, L4, L5, sacrum/grade II |
T7–9 laminectomy; chemo (4 cycles carboplatin); 10 mos later T9–10 laminectomy; RT (whole brain & neuraxis) |
Complete spinal cord syndrome 8 mos after 2nd op; OS 105 wks |
| Landriel et al., 201214 |
(1) 30/M (2) 32/M |
(1) Progressive paresthesia & paresis in legs; urinary sphincter disturbances; gait instability; ataxia; chronic LBP (2) LBP; paresthesia in rt leg |
(1) C2–3, T2-T4-T5, T12–L1/grade I (2) C7, T2, T4, T5, T8, T10, T11, L1, L3, L5, S1, S2/grade I |
(1) T1–3 laminectomy; radiotherapy (2) T9–10 laminectomy |
(1) Good neurological outcome, no residual tumor on MRI at 10 yrs (2) Good neurological outcome, no residual tumor on MRI at 12 mos |
| Guarnieri et al., 20142 |
53/M |
Progressive lower leg hypoesthesia |
Lower cervical, upper & lower thoracic (unspecified levels)/grade II |
Lower thoracic laminectomy; 1 mo later lower cervical & upper thoracic laminectomy; chemo (4 cycles carboplatin) |
Improved after 2nd op, able to stand on own & walk w/o assistance |
| Vats et al., 201513 |
49/F |
Neck pain, spastic quadriparesis |
C1–2, C6–7, T4–L3/grade II |
C5–7 laminectomy, T7–9 laminectomy; RT |
Patient “doing well” at 11 mos postoperative |
| Chakravorty et al., 20171 |
47/F |
Gluteal, thigh, & groin pain; groin paresthesia |
>10 lesions in CMJ, cervical, thoracic, lumbar, sacral, cauda equina (unspecified levels)/grade III |
Sacral laminectomy; 1 yr later T4–6 laminectomy; craniospinal RT |
Asymptomatic 4 yrs after presentation |
| Honda et al., 20176 |
26/F |
Difficulty walking due to progressive paresis, pain in trunk, leg numbness |
Thoracic (7 lesions, unspecified levels)/both grades II & III |
T3–4 laminectomy (resected 2 lesions upper thoracic); 5 wks later T5–10 laminectomy (resected 5 residual lesions); craniospinal RT; chemo (temozolomide) |
Improved neurological condition & able to walk w/o support 3 yrs after 2nd op, no recurrence or dissemination on MRI |
| Present case | 45/M | Numbness both legs to umbilicus, gait instability | C7, T1–3, T7, T12, L3, L5–S1/grade II | T1–2 laminectomy; RT brain & entire spine | Improvement of gait & sensation T4 distally 2 wks after op, mild cognitive decline 18 mos after op attributed to cranial radiation, no tumor progression on cervical/thoracic MRI 31 mos postoperative |
Chemo = chemotherapy; CMJ = cervicomedullary junction; LBP = low back pain; op = surgery; OS = overall survival; RT = radiotherapy; WHO = World Health Organization.
The mechanism associated with the development of multifocal IDEM ependymomas has been posited to involve either drop metastasis or CSF migration cranially from a more distal location.3,4,6 Schurmans and colleagues surmised that the process in their case was either due to drop metastasis from the cervical IDEM ependymoma or that the lumbar IDEM ependymomas may have undergone anaplastic transformation with the cells migrating cranially in the CSF to form the cervical subdural and intracranial metastases.3 Vural and colleagues as well as Honda and colleagues speculated that the IDEM ependymomas may have disseminated to other spinal cord levels via the CSF or that the tumors may have occurred at multicentric foci.4,6 Vural and colleagues further investigated this concept by performing cytogenetic analysis to confirm the pathogenesis of tumor multiplicity.4 They determined that the tumors at different locations originated from the same primary tumor, specifically, the one at the cervicomedullary junction in their particular case. The tumor at this site was strongly enhancing radiologically, and the thoracic tumors were weakly enhancing, suggesting that poor vascularization of the latter may reflect an early stage of a disseminated tumor.
Observations
The present case is the first to describe a patient diagnosed with a multifocal IDEM ependymoma who was treated with a tumor resection followed by radiation and received genetic testing to ascertain an appropriate chemotherapeutic agent. It has been reported that trauma in multifocal IDEM is known to cause hemorrhage, tumor rupture, and dissemination.4 It is unknown whether the fall from a ladder that our patient sustained weeks before the initiation of his symptoms may have contributed to the CSF dissemination of the ependymal cells, although this mechanism is possible. Because the patient had several IDEM ependymomas throughout his entire spine, the tumor resection was performed at the spinal level that localized to the patient’s neurological signs and symptoms, specifically, a T1–2 laminectomy. Although the brain MRI demonstrated no pathologic lesions, craniospinal radiation was performed in case there was a subclinical cerebral ependymoma. Intracranial ependymomas tend to be friable with a propensity to seed tumor cells in a cranial-caudal direction to the thoracic and lumbar subarachnoid space. Occult intracranial lesions could conceivably have been present in our case leading to these multiple drop metastases. We propose that the large lesion at T1–3 most likely had friable surface tumor cells that seeded caudally, causing the multifocal IDEM ependymomas.
While previous patients with multifocal IDEM ependymomas have been treated with carboplatin and temozolomide, our patient’s genetic testing revealed a PIK3CA mutation, enabling him to be a candidate for alpelisib. This personalized chemotherapy determination after testing for cancer gene alterations may prove invaluable when chemotherapy is deemed necessary. At 18 months postoperatively, the patient denied any neurological complaints apart from mild cognitive decline attributed to cranial radiation. Although cervical and thoracic MRI revealed stable intradural nodules and new areas of leptomeningeal enhancement, no malignant cells were seen in the CSF. At 31 months postoperatively, his only complaints were increased fatigue and daytime somnolence. Cervical and thoracic MRIs were stable without evidence of disease progression.
Lessons
Although a complete resection is the preferred treatment for a solitary IDEM ependymoma, it is not the most practical surgical option for multifocal IDEM ependymomas. Decompression of the lesion causing mass effect on the spinal cord is the ideal treatment, followed by adjunct radiotherapy and/or chemotherapy. Patients with multifocal IDEM ependymomas necessitate close monitoring with brain and total spine MRI studies to detect anaplastic transformation, recurrence, or CSF dissemination of these lesions.
Acknowledgments
We acknowledge Norton Healthcare for their continued support.
Disclosures
The authors report no conflict of interest concerning the materials or methods used in this study or the findings specified in this paper.
Author Contributions
Conception and design: CB Shields, LBE Shields, Sun, Spalding, Sinicrope. Acquisition of data: CB Shields, LBE Shields, Highfield, LaRocca, Sun. Analysis and interpretation of data: CB Shields, LBE Shields, Highfield, Spalding, Sun. Drafting the article: LBE Shields. Critically revising the article: all authors. Reviewed submitted version of manuscript: all authors. Approved the final version of the manuscript on behalf of all authors: CB Shields, LBE Shields. Administrative/technical/material support: CB Shields, LBE Shields. Study supervision: CB Shields.
References
- 1. Chakravorty A, Frydenberg E, Shein TT, Ly J, Earls P, Steel T. Multifocal intradural extramedullary anaplastic ependymoma of the spine. J Spine Surg. 2017;3(4):727–731. doi: 10.21037/jss.2017.11.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Guarnieri G, Tecame M, Izzo R, Zeccolini F, Genovese L, Muto M. Multisegmental diffuse intradural extramedullary ependymoma. An extremely rare case. Neuroradiol J. 2014;27(2):179–185. doi: 10.15274/NRJ-2014-10018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Schuurmans M, Vanneste JA, Verstegen MJ, van Furth WR. Spinal extramedullary anaplastic ependymoma with spinal and intracranial metastases. J Neurooncol. 2006;79(1):57–59. doi: 10.1007/s11060-005-9114-9. [DOI] [PubMed] [Google Scholar]
- 4. Vural M, Arslantas A, Ciftci E, Artan S, Adapinar B. Multiple intradural-extramedullary ependymomas: proven dissemination by genetic analysis. J Neurosurg Spine. 2010;12(5):467–473. doi: 10.3171/2009.11.SPINE08780. [DOI] [PubMed] [Google Scholar]
- 5. Ali AS, Qureshi MS, Ahmed J, et al. Intradural extramedullary ependymoma at lumbar (L1-L4 level) spine: a suspicious case and literature review. J Clin Case Rep. 2015;S3:4. [Google Scholar]
- 6. Honda A, Iizuka Y, Hirato J, Kiyohara H, Iizuka H. Multiple intradural-extramedullary spinal ependymomas including tumors with different histological features. Eur Spine J. 2017;26(suppl 1):222–224. doi: 10.1007/s00586-017-5055-1. [DOI] [PubMed] [Google Scholar]
- 7. Iunes EA, Stávale JN, de Cássia Caldas Pessoa R, et al. Multifocal intradural extramedullary ependymoma. Case report. J Neurosurg Spine. 2011;14(1):65–70. doi: 10.3171/2010.9.SPINE09963. [DOI] [PubMed] [Google Scholar]
- 8. Kaliaperumal C, Suttner N, Herron B, Choudhari KA. Rare case of primary spinal ependymomatosis occurring in a 26-year-old man: a case report. J Med Case Reports. 2009;3:72. doi: 10.1186/1752-1947-3-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Moriwaki T, Iwatsuki K, Ohnishi Y, Umegaki M, Ishihara M, Yoshimine T. Intradural extramedullary spinal ependymoma: a case report of malignant transformation occurring. Asian Spine J. 2013;7(2):139–142. doi: 10.4184/asj.2013.7.2.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Benzagmout M, Boujraf S, Oulali N, et al. Intradural extramedullary ependymoma: is there constantly a hormonal relationship? Surg Neurol. 2008;70(5):536–538. doi: 10.1016/j.surneu.2007.05.055. [DOI] [PubMed] [Google Scholar]
- 11. Duffau H, Gazzaz M, Kujas M, Fohanno D. Primary intradural extramedullary ependymoma: case report and review of the literature. Spine (Phila Pa 1976) 2000;25(15):1993–1995. doi: 10.1097/00007632-200008010-00021. [DOI] [PubMed] [Google Scholar]
- 12. Son DW, Song GS, Han IH, Choi BK. Primary extramedullary ependymoma of the cervical spine: case report and review of the literature. J Korean Neurosurg Soc. 2011;50(1):57–59. doi: 10.3340/jkns.2011.50.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Vats A, Ramdasi R, Zaveri G, Pandya S. Multicentric intradural extramedullary ependymoma: report of a rare case. J Craniovertebr Junction Spine. 2015;6(3):134–136. doi: 10.4103/0974-8237.161596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Landriel F, Ajler P, Tedesco N, Bendersky D, Vecchi E. Multicentric extramedullary myxopapillary ependymomas: two case reports and literature review. Surg Neurol Int. 2012;3:102. doi: 10.4103/2152-7806.100859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Karakas B, Bachman KE, Park BH. Mutation of the PIK3CA oncogene in human cancers. Br J Cancer. 2006;94(4):455–459. doi: 10.1038/sj.bjc.6602970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Castellano E, Downward J. RAS interaction with PI3K: more than just another effector pathway. Genes Cancer. 2011;2(3):261–274. doi: 10.1177/1947601911408079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hemmings BA, Restuccia DF. PI3K-PKB/Akt pathway. Cold Spring Harb Perspect Biol. 2012;4(9):a011189. doi: 10.1101/cshperspect.a011189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Rogers HA, Mayne C, Chapman RJ, Kilday JP, Coyle B, Grundy RG. PI3K pathway activation provides a novel therapeutic target for pediatric ependymoma and is an independent marker of progression-free survival. Clin Cancer Res. 2013;19(23):6450–6460. doi: 10.1158/1078-0432.CCR-13-0222. [DOI] [PubMed] [Google Scholar]
- 19. Butt E, Alyami S, Nageeti T, et al. Mutation profiling of anaplastic ependymoma grade III by Ion Proton next generation DNA sequencing. F1000 Res. 2019;8:613. doi: 10.12688/f1000research.18721.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Weinstein GM, Arkun K, Kryzanski J, Lanfranchi M, Gupta GK, Bedi H. Spinal intradural, extramedullary ependymoma with astrocytoma component: a case report and review of the literature. Case Rep Pathol. 2016;2016:3534791. doi: 10.1155/2016/3534791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Celano E, Salehani A, Malcolm JG, Reinertsen E, Hadjipanayis CG. Spinal cord ependymoma: a review of the literature and case series of ten patients. J Neurooncol. 2016;128(3):377–386. doi: 10.1007/s11060-016-2135-8. [DOI] [PMC free article] [PubMed] [Google Scholar]