Abstract
BACKGROUND
Cerebral vasospasm after aneurysmal subarachnoid hemorrhage can lead to considerable mortality and morbidity affecting the intracranial vessels, leading to delayed cerebral ischemia and stroke. Therapeutic options for patients with treatment-refractory vasospasm are limited, particularly in the setting of significant cardiopulmonary disease. Administration of nicardipine, a calcium channel blocker, into the intrathecal space may represent a potential treatment option for this population.
OBSERVATIONS
A 56-year-old woman had treatment-refractory vasospasm, severe acute respiratory distress syndrome, and Takotsubo cardiomyopathy. As an adjunct to vasopressor administration and endovascular intraarterial calcium channel blocker administration, the patient received intraventricular nicardipine. The patient demonstrated improved neurophysiology on invasive multimodality neuromonitoring, with increased cerebral blood flow and oxygenation as a result of intraventricular nicardipine administration.
LESSONS
Intraventricular nicardipine can be used as rescue therapy for patients with treatment-refractory cerebral vasospasm. This case demonstrates that intrathecal nicardipine may prevent delayed ischemic neurological deficits and improve outcomes.
Keywords: subarachnoid hemorrhage, vasospasm, nicardipine, intrathecal, intraventricular
ABBREVIATIONS : AUC = area under the curve, PbtO2 = brain tissue oxygenation, SpO2 = oxygen saturation
Aneurysmal subarachnoid hemorrhage carries considerable risk of morbidity and mortality; much of the delayed risk is related to cerebral vasospasm. Vasospasm can affect large, medium, and small vessels in the brain to varying degrees, leading to delayed cerebral ischemia and stroke. Nimodipine, a calcium channel blocker, is believed to have a neuroprotective effect secondary to antagonism of intracellular calcium. Its use orally is standard of care for patients with aneurysmal subarachnoid hemorrhage. Maintenance of euvolemia and normal circulating blood volume is additionally recommended to reduce the risk of delayed cerebral ischemia.1
When patients develop cerebral vasospasm, it can be necessary to augment cerebral perfusion with vasopressors to induce systemic hypertension. Catheter-based angiography for administration of intraarterial vasodilating medication or balloon angioplasty is commonly used as well. However, for individuals with treatment-refractory vasospasm or significant cardiopulmonary dysfunction impeding the efficacy of vasopressor therapy or for patients too ill to be transported to the neuroangiography suite, options for treatment can be limited. Use of intrathecal nicardipine has been reported as an off-label treatment option in such cases, and in small series, it has been shown to improve symptomatic vasospasm and outcome and decrease severe angiographic vasospasm and mean flow velocity.2–9
Invasive intracranial multimodality neuromonitoring in patients with aneurysmal subarachnoid hemorrhage is not standard of care but can be useful in patients with poor neurological presentations because it enhances the ability to detect subclinical changes in neurophysiological parameters and enables targeted treatment. We believe this is the first reported case in which the effect of intrathecal nicardipine administered for treatment-refractory vasospasm was correlated with improved physiological changes as measured with invasive advanced neuromonitoring in real time.
Illustrative Case
A 56-year-old woman with no significant past medical history presented with a Hunt and Hess grade 3 subarachnoid hemorrhage from a ruptured left posterior communicating artery aneurysm. After placement of a right-sided external ventricular drain, she received endovascular balloon-assisted coil embolization of the ruptured aneurysm. Her clinical course postprocedurally was initially uneventful, with normal to slightly elevated velocities on transcranial Doppler ultrasonography. Several days later, she developed Takotsubo cardiomyopathy with elevated troponin, significant ST-segment elevation in inferior electrocardiogram leads, and relative hypotension.
On postbleed day 9, the patient’s level of consciousness deteriorated, and vasopressor therapy was initiated because of concern for delayed cerebral ischemia. She was found to have severe diffuse vasospasm involving the bilateral anterior circulation, with her left middle cerebral artery being so profoundly involved that the M1 segment appeared nearly occluded on catheter angiography. Intraarterial verapamil (10 mg) was administered via the catheter in the left internal carotid artery, followed by balloon angioplasty of the left middle cerebral artery from its superior M2 division back to the M1 segment. The patient could not follow commands after the procedure, so the decision was made to place invasive advanced intracranial monitoring. A left-sided frontal burr hole was made slightly anterior and lateral to Kocher’s point, and a Licox probe (Integra LifeSciences Corp.) and QFlow 500 probe (Hemedex Inc.) were placed within the brain parenchyma. The location of the probe tips was verified on subsequent computed tomography.
The patient received hemodynamic monitoring with a Vigileo (Edwards Lifesciences Corp.) monitor and required concurrent administration of three vasopressors to achieve systolic blood pressure values in the range of 120–130 mm Hg in the context of her Takotsubo cardiomyopathy and inferior myocardial infarction. Her external ventricular drain, which had been placed on the day of admission, facilitated continuous drainage of cerebrospinal fluid, and the patient was brought to the neuroangiography suite daily for repeat cerebral angiography with administration of intraarterial verapamil because of persistent refractory severe intracranial vasospasm. Management was further complicated by the development of bilateral pulmonary infiltrates and a diagnosis of acute respiratory distress syndrome.
On day 12, the patient was judged to be too hemodynamically unstable for transport to the neuroangiography suite, and a decision was made to administer a 4-mg dose of nicardipine via the external ventricular drain every 12 hours. Injectable nicardipine was diluted with 0.9% sodium chloride for a final concentration of 2 mg/mL per a previous study by Webb et al.9 After administration of the intrathecal nicardipine, we clamped the external ventricular drain for 1 hour and then reopened it. The patient’s physiological data were captured with a CNS Monitor (Moberg Research Inc.), demonstrating the patient’s cerebral physiology before and after intraventricular administration of nicardipine.
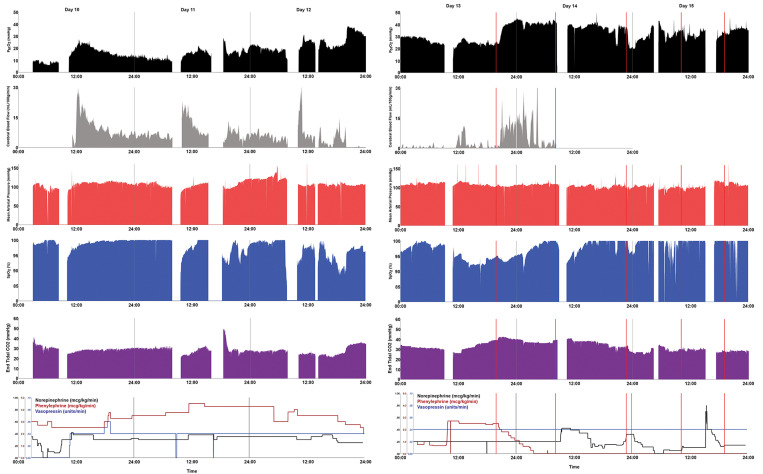
After the first dose of intrathecal nicardipine, brain tissue oxygenation (PbtO2) values approximately doubled by 30 minutes after administration despite a concurrent decrease in oxygen saturation (SpO2) values. The mean arterial blood pressure was stable over this period. Cerebral blood flow values also improved from near 0 to values approximating the treatment goal of 15 mL/100 g/min. Area under the curve (AUC) calculations were performed for 6-hour intervals after the seven intraarterial procedures and the five doses of intraventricular nicardipine administered. Notably, the marked increase in brain oxygenation and blood flow occurred despite phenylephrine being weaned off immediately after the intrathecal nicardipine dose (Fig. 1). The first dose of intrathecal nicardipine was markedly more efficacious in improving cerebral blood flow than the intraarterial infusion of verapamil performed early the same day (post-subarachnoid hemorrhage day 13). In fact, the AUC for the intrathecal infusion was 3.83 times greater than intraarterial infusion of verapamil.
FIG. 1.
Patient physiology and vasopressor dosage during treatment of vasospasm. Key physiological measures were plotted during the management of cerebral vasospasm. Values for postrupture days 10 to 15 are shown. Measures are time aligned, and 1-minute values are plotted, including cerebral PbtO2 measures (black, goal >20 mm Hg) and blood flow measures (gray, goal >15 mL/100 g/min) obtained with the Licox and QFlow 500 probes, respectively, as well as mean arterial blood pressure as measured continuously with an arterial line (red), SpO2 values measured with a pulse oximeter (blue), and end-tidal CO2 (purple). Infusion dosages of norepinephrine, phenylephrine, and vasopressin are shown in the bottom row (black, red, and blue, respectively). Vertical gray lines denote midnights. The wide gaps in the data reflect the intervals during which interventional cerebral angiography was performed, and the narrow gaps on days 12 and 15 reflect the time when computed tomography was performed. The PbtO2 values increased markedly after the first administration of intrathecal nicardipine despite dropping SpO2 and a decrease in phenylephrine dosage at this time. The perfusion increase noted after intraarterial therapy on day 13 was less than that noted with intrathecal nicardipine administration.
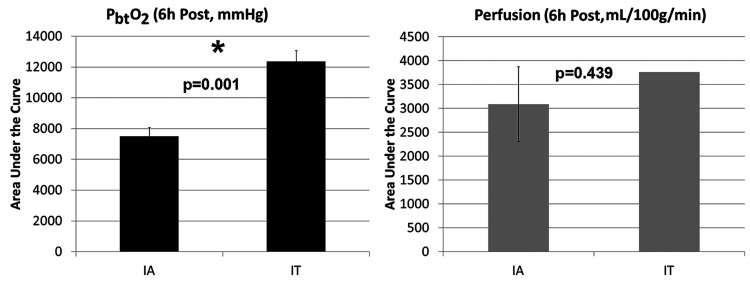
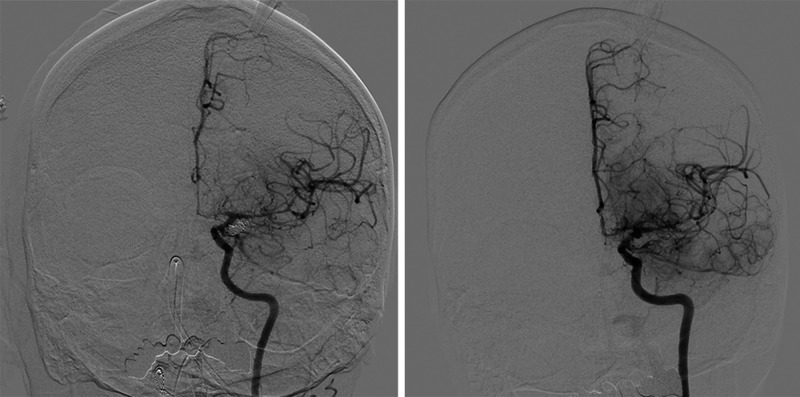
A t-test was used to compare 6-hour AUC values for brain oxygenation after the intraarterial procedures (the five performed before intrathecal nicardipine administration) with those measured after the five intraventricular nicardipine doses. The PbtO2 AUC values were nearly twice as high after intrathecal nicardipine as with intraarterial verapamil administration (p = 0.001). Cerebral blood flow AUCs after intraarterial therapy were compared with the single AUC obtained after intrathecal nicardipine by means of a t-test. Although an approximate 30% increase in cerebral blood flow AUCs was noted with the intrathecal nicardipine, the statistical comparison to intraarterial verapamil therapy did not reveal a significant difference (p = 0.438) (Fig. 2). Cerebral angiography after administration of the intraventricular nicardipine demonstrated marked improvement in the caliber of the intracranial vasculature (Fig. 3).
FIG. 2.
Improvement of brain oxygenation and blood flow after administration of intrathecal nicardipine as compared with intraarterial procedures. We performed an AUC analysis to quantitatively compare the effect of intrathecal nicardipine with intraarterial procedures on focal cerebral oxygenation and blood flow as measured with Licox and Hemedex probes, respectively. For this analysis, oxygenation and blood flow values were compared for the 6 hours after the respective interventions. A 2-sample t-test was used to compare PbtO2 values, but because perfusion monitoring was performed only after a single administration of intrathecal nicardipine, the AUC values subsequent to the intraarterial procedures were compared with the single AUC value obtained after the intrathecal nicardipine administration. For this analysis, the five intraarterial procedures performed before the initiation of intrathecal nicardipine were compared with values subsequent to the five intrathecal administrations. Both brain oxygenation and blood flow were greater after intrathecal nicardipine administration than after intraarterial procedures; this difference was significant for PbtO2 values (*p = 0.001).
FIG. 3.
Improvement of angiographic vasospasm after administration of intrathecal nicardipine. Anteroposterior cerebral angiograms of the left internal carotid artery for days 13 (left) and 14 (right) after cerebral aneurysm rupture. The severe left anterior cerebral artery and middle cerebral artery vasospasm seen on day 13 improved the day after the first administration of intrathecal nicardipine, corresponding to marked improvement in cerebral blood flow and oxygenation noted with the advanced neuromonitors.
Discussion
Observations
Cerebral vasospasm is known to occur after aneurysmal subarachnoid hemorrhage and is a key source of morbidity and mortality for patients with this challenging condition. In patients with treatment-refractory vasospasm, few options exist, particularly when concomitant cardiac and respiratory dysfunction exists, such as in our patient. Monitoring and optimization of key neurophysiological parameters such as intracranial pressure, PbtO2, and cerebral blood flow allow physicians to initiate treatment in response to subclinical trends or perturbations that might otherwise lead to secondary brain insult. In our patient with treatment-refractory cerebral vasospasm, intrathecal nicardipine was administered as a last-resort measure aimed at preventing cerebral infarction. Its administration was associated with a rapid and marked increase in cerebral oxygenation and blood flow. Alternate causes of the marked improvement in cerebral oxygenation and blood flow were not apparent, and a cause-and-effect relationship was strongly suggested in this case. Remarkably, this effect was seen despite weaning and discontinuing the phenylephrine infusion.
Cerebral PbtO2 values were generally elevated after intrathecal nicardipine administration as compared with values before the first dose. It is possible that intrathecal nicardipine has a prolonged half-life in cerebrospinal fluid because nicardipine is normally metabolized in the liver. To date, the only clinical study to report on the concentration of nicardipine after intrathecal administration was by Suzuki et al.,7 who administered 4 mg of intrathecal nicardipine twice daily and observed a mean cerebrospinal fluid concentration of 231.44 ng/mL via trough samples obtained on postbleed day 9 in 14 patients. The cerebrospinal fluid concentration observed was within the range of the median effective concentration required to cause physiological vasodilation in vitro, as demonstrated in a previous study by Yamamoto and colleagues.10
Intraarterial verapamil administration was associated with improved cerebral blood flow, and intrathecal nicardipine was associated with markedly greater improvement in cerebral blood flow. Intracellular calcium elevation is recognized as an important cellular cause of secondary injury, having numerous deleterious effects such as the activation of degradative enzymes such as calpains11 and triggering excitotoxic pathways.12 Although our data do not exclude benefit from antagonism of intraarterial calcium, our results provide clear evidence that intraarterial verapamil and intrathecal nicardipine have a vasodilatory effect.
We noted an increase in cerebral blood flow and oxygenation in focal measurements deep within the left frontal lobe. This finding suggests that nicardipine has its effect on more proximal vessels in communication with the subarachnoid space or, more likely, that intrathecal nicardipine diffuses effectively into the brain, perhaps along Virchow-Robin spaces.
Lessons
Analysis of future cases will assist in establishing a cause-and-effect relationship between intrathecal nicardipine and improvement in cerebral blood flow and oxygenation. In this instance, the improvement we observed did not appear to result from an increase in systemic oxygen saturation, systemic blood pressure, or PaCO2. Moreover, the rapid dose-response effect also makes this effect unlikely to result from the normal resolution of the vasospasm.
This is the first reported case of the use of intrathecal nicardipine for treatment of refractory vasospasm with concomitant improvement in cerebral neurophysiology based on the use of advanced multimodality intracranial monitoring. A previous study by Ko et al.4 documented the use of intraventricular nicardipine in 11 patients with advanced neuromonitoring; the authors found no improvement in PbtO2 or cerebral blood flow nor any changes in brain glucose or lactate/pyruvate ratio on cerebral microdialysis. Of note, all patients had evidence of global cerebral edema and cerebral infarctions prior to commencement of intraventricular nicardipine administration, which potentially mitigated any positive effects of the therapy. In addition, the location of the probes may have been a confounding factor because the authors placed monitors ipsilateral to the ruptured aneurysm in the frontal lobe (or in the nondominant hemisphere in the case of midline aneurysms) but stated that 73% of their patients had bilateral vasospasm.
On the basis of our experience reported herein and small prospective and retrospective series reported by others, it appears that intrathecal administration of nicardipine may represent a promising therapeutic approach for improving vasospasm. This case demonstrates that intrathecal nicardipine may prevent delayed ischemic neurological deficits and improve outcomes. This case report provides a proof of concept that warrants further exploration in a larger population of critically ill patients.
Acknowledgments
We thank Kristin Kraus, MSc, for editorial assistance.
Disclosures
The Moberg CNS Monitor is a free-standing device used in the University of Utah Neuro-Critical Care Unit to collect and present high-frequency (>1 Hz) data from many bedside devices in real time. The presentation and storage of our patient’s physiological data with this device was integral to our recognition and analysis of the effects of intrathecal nicardipine. The device manufacturer had no input or influence on this paper.
Dr. Grandhi reported personal fees from Medtronic Neurovascular, Cerenovus, and Balt Neurovascular outside the submitted work. Dr. Taussky reported personal fees from Medtronic, Cerenovus, Stryker, and Avail outside the submitted work. No other disclosures were reported.
Author Contributions
Conception and design: Grandhi, Taussky, Hawryluk. Acquisition of data: Grandhi, Condie, Hawryluk. Analysis and interpretation of data: Grandhi, Ravindra, Condie, Taussky, Hawryluk. Drafting the article: Grandhi, Menacho, Taussky, Hawryluk. Critically revising the article: Grandhi, Menacho, Ravindra, Hawryluk. Reviewed submitted version of manuscript: Grandhi, Ravindra, Condie, Hawryluk. Approved the final version of the manuscript on behalf of all authors: Grandhi. Statistical analysis: Grandhi, Hawryluk. Administrative/technical/material support: Grandhi, Condie, Hawryluk. Study supervision: Grandhi, Hawryluk.
References
- 1. Connolly ES, Jr, Rabinstein AA, Carhuapoma JR, et al. Guidelines for the management of aneurysmal subarachnoid hemorrhage: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2012;43(6):1711–1737. doi: 10.1161/STR.0b013e3182587839. [DOI] [PubMed] [Google Scholar]
- 2. Ehtisham A, Taylor S, Bayless L, Samuels OB, Klein MW, Janzen JM. Use of intrathecal nicardipine for aneurysmal subarachnoid hemorrhage-induced cerebral vasospasm. South Med J. 2009;102(2):150–153. doi: 10.1097/SMJ.0b013e31818f8ba4. [DOI] [PubMed] [Google Scholar]
- 3. Goodson K, Lapointe M, Monroe T, Chalela JA. Intraventricular nicardipine for refractory cerebral vasospasm after subarachnoid hemorrhage. Neurocrit Care. 2008;8(2):247–252. doi: 10.1007/s12028-007-9017-z. [DOI] [PubMed] [Google Scholar]
- 4. Ko SB, Choi HA, Helbok R, et al. Acute effects of intraventricular nicardipine on cerebral hemodynamics: a preliminary finding. Clin Neurol Neurosurg. 2016;144:48–52. doi: 10.1016/j.clineuro.2016.02.037. [DOI] [PubMed] [Google Scholar]
- 5. Lu N, Jackson D, Luke S, Festic E, Hanel RA, Freeman WD. Intraventricular nicardipine for aneurysmal subarachnoid hemorrhage related vasospasm: assessment of 90 days outcome. Neurocrit Care. 2012;16(3):368–375. doi: 10.1007/s12028-011-9659-8. [DOI] [PubMed] [Google Scholar]
- 6. Shibuya M, Suzuki Y, Enomoto H, Okada T, Ogura K, Sugita K. Effects of prophylactic intrathecal administrations of nicardipine on vasospasm in patients with severe aneurysmal subarachnoid haemorrhage. Acta Neurochir (Wien) 1994;131(1-2):19–25. doi: 10.1007/BF01401450. [DOI] [PubMed] [Google Scholar]
- 7. Suzuki M, Doi M, Otawara Y, Ogasawara K, Ogawa A. Intrathecal administration of nicardipine hydrochloride to prevent vasospasm in patients with subarachnoid hemorrhage. Neurosurg Rev. 2001;24(4):180–184. doi: 10.1007/s101430100152. [DOI] [PubMed] [Google Scholar]
- 8. Toyota A, Nishizawa Y. Cerebral vasospasm after subarachnoid hemorrhage, and inhibitory effect of nicardipine investigated by means of transcranial Doppler ultrasonography. Article in Japanese. No Shinkei Geka. 1991;19(12):1143–1150. [PubMed] [Google Scholar]
- 9. Webb A, Kolenda J, Martin K, Wright W, Samuels O. The effect of intraventricular administration of nicardipine on mean cerebral blood flow velocity measured by transcranial Doppler in the treatment of vasospasm following aneurysmal subarachnoid hemorrhage. Neurocrit Care. 2010;12(2):159–164. doi: 10.1007/s12028-009-9307-8. [DOI] [PubMed] [Google Scholar]
- 10. Yamamoto M, Ohta T, Toda N. Mechanisms of relaxant action of nicardipine, a new Ca++-antagonist, on isolated dog cerebral and mesenteric arteries. Stroke. 1983;14(2):270–275. doi: 10.1161/01.str.14.2.270. [DOI] [PubMed] [Google Scholar]
- 11. Cheng SY, Wang SC, Lei M, Wang Z, Xiong K. Regulatory role of calpain in neuronal death. Neural Regen Res. 2018;13(3):556–562. doi: 10.4103/1673-5374.228762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ruiz A, Alberdi E, Matute C. CGP37157, an inhibitor of the mitochondrial Na+/Ca2+ exchanger, protects neurons from excitotoxicity by blocking voltage-gated Ca2+ channels. Cell Death Dis. 2014;5:e1156. doi: 10.1038/cddis.2014.134. [DOI] [PMC free article] [PubMed] [Google Scholar]